Abstract
Plant leaf cuticle is related to the prevention of moisture loss, transpiration, and diffusion of light reflection. The purpose of this study was to examine the morphological characteristics of ginseng leaves in ginseng plants resistant and susceptible to high temperature injury (HTI) to be related with the leaf-burning. For the HTI resistant lines Yunpoong, high-temperature injury resistance (HTIR) 1, HTIR 2, and HTIR 3, and the HTI-susceptible line Chunpoong, the cuticle densities were 53.0%, 46.2%, 44.9%, 48.0%, and 17.0%; the adaxial leaf cuticle layers were 141.3, 119.7, 119.7, 159.4, and 85.0 nm in thickness; the abaxial leaf cuticle layers were 153.6, 165.8, 157.9, 199.6, and 119.4 nm in thickness; and the stomtal lengths were 21.7, 32.4, 29.4, 30.9, and 21.8 μm, respectively. All of these aspects suggest that HTI resistant lines have higher cuticle density, thicker adaxial and abaxial leaf cuticle layers, and longer of stomta length than the HTI-susceptible line, protecting leaves from moisture loss and excessive transpiration under high temperatures to be resistant against the leaf-burning.
Keywords: Panax ginseng, Ginseng leaves, Leaf-burning, Cuticular wax, High-temperature injury
INTRODUCTION
Panax ginseng Meyer, unlike other crops, sprouts only once a year but not after the death and defoliation of the aerial parts due to diseases, insects, and physiological disorders. This leads an insufficient accumulation of nutrients for the growth of underground parts, rendering ginseng roots to be dormant for one year. Ginseng cultivation is often affected by high-temperature injury (HTI) due to global warming and changes in sun-shading materials. Currently, new varieties of ginseng have been selected and registered in Korea, including Chunpoong, Yunpoong, Gumpoong, Gopoong, Sunun, Sunone, Sunpoong, Sunhyang, and Cheongsun, each of which has unique characteristics. Chunpoong yields good quality of ginseng root suitable for manufacturing Korean red ginseng, but shows poor seed dehiscence ratios, yields small quantities, and is susceptible to the leaf-burning. On the other hand, Yunpoong has high-yielding ability and is resistant to the leaf-burning, but does not yield quality red ginseng [1].
Plants are subjected to non-biological stresses, such as dryness, high or low temperatures, freezing, UV radiation, nutrition and moisture in soil, and environmental pollution. The role of cuticle layer is important for enduring such environmental stresses [2]. Lee et al. [3] reported the characteristics of cuticles on the leaves of Chunpoong and Yunpoong, and Lee [4] reported the degree of development of cuticles on leaves for different varieties of ginseng. Lee et al. [5] developed four ginseng lines with resistance to the leaf-burning, and photoinhibition of these lines was studied. For other crops, several studies were conducted on the topics such as the relationship between the amount of cuticles on mulberry leaves and moisture retention capacity [6], wax accumulation on peanut leaves and moisture deficiency [7], wax synthesis on leaf surface in case of moisture stress on cotton [8], and formation of wax on Pinus palustris in Pinaceae in relation to nitrogen content in soil, and CO2 content in the atmosphere in relation to moisture stress [9].
However, there are very few reports on the relationship between the development of cuticle layer on ginseng leaves and leaf-burning phenomena. This experiment was conducted to examine cuticle distribution, thickness, and porosity of leaves in four ginseng lines that are resistant to the leaf-burning – Yunpoong and HTI resistant lines such as high-temperature injury resistance (HTIR) 1, HTIR 2, and HTIR 3 – and of a leaf-burning-susceptible line Chunpoong, to specify the characteristics resistant and sensitive to the leaf-burning and to give basic information for selecting lines resistant to the leaf-burning in the future.
MATERIALS AND METHODS
For this study, five leaves of two-year-old plants were collected from HTI resistant (Yunpoong, HTIR 1, HTIR 2, and HTIR 3) and susceptible (Chunpoong) lines cultivated in an experimental farm of KT&G Central Research (Daejeon, Korea) in 2008 under the shade with less than 20% light transmission ratio (Fig. 1). In order to examine the distribution, thickness, and porosity of cuticle layer, only the smaller leaf part in the center was used.
Fig. 1. Growth status of ginseng lines resistant to high-temperature injury (HTI; Yunpoong, high-temperature injury resistance [HTIR] 1, HTIR 2, and HTIR 3) and susceptible to HTI (Chunpoong) on August 28, 2008.

For observation under scanning electron microscope (SEM), the leaves were cut into 0.2×0.2 mm segments under a dissecting microscope, fixed in 4% glutaraldehyde in room temperature for 2 h, and cleansed with buffer solution 3 times for 20 min each. Then, they were fixed in 2% osmium tetroxide in room temperature for 1 h and washed with distilled water 3 times for 20 min each. Then each segment was dehydrated in an ethanol series of 50%, 70%, 80%, 90%, 95%, and 100% ethanol for 1 h. Dehydrated segments were stored in isoamylacetate and the tissues were dried in critical point dryer (BioRadical E3000; Nakahara, Tokyo, Japan) set at 1,000 psi and critical temperature. Dried tissues were mounted onto stubs, gold-coated for 10 minutes using an ion coater (JFC 1110E; JEOL Ltd., Tokyo, Japan), and observed under SEM (20 kV; S-3500N, Hitachi, Tokyo, Japan) in terms of cuticle distribution on adaxial surfaces and porosity on abaxial surfaces.
For observation under transmission electron microscope (TEM), the above method of preliminary fixing, follow-up, and dehydrating was also applied. Dehydrated tissues were transferred into propylene oxide, embedded in Spurr’s epoxy resin, and polymerized 60℃ for 24 h in an incubator. Embedded materials were cut into 0.4 mm-thick semisections using glass blade in a ultramicrotome (Reichert Jung Ultracut S; Leica, Cambridge, UK), dyed in toluidine blue-basic fuchsin, and observed under bright-field microscope (Axiphot 2; Carl Zeiss, Jena, Germany). The observed parts were refined to acquire 60 nm-thick silver sections and attached them onto 300-mesh copper grids for double-dying in 1% uranyl acetate and lead citrate to observe the thickness of cuticles on adaxial and abaxial surfaces under TEM (JEM 2000 EX II, 80 kV; Oxford, UK).
RESULTS AND DISCUSSION
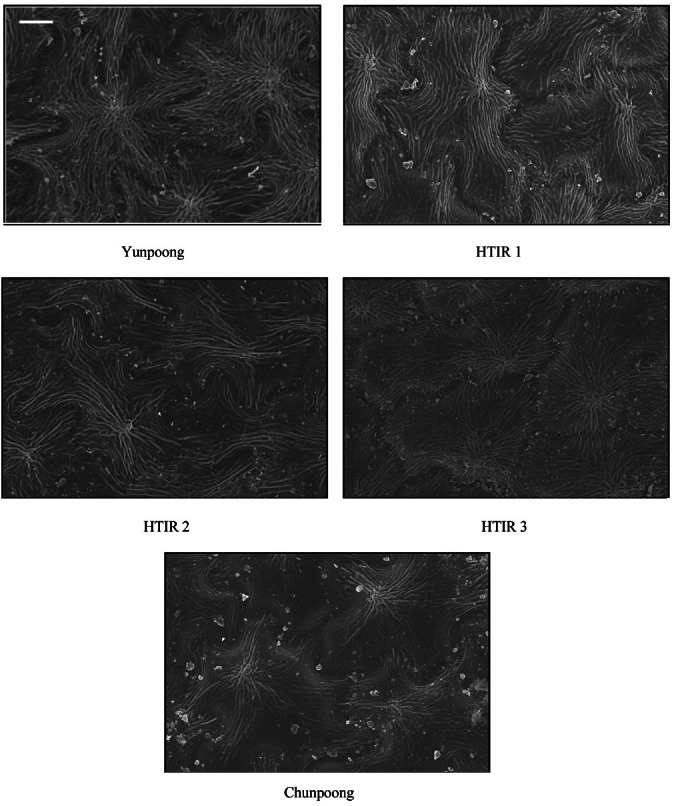
The distribution of cuticles on adaxial surfaces of the resistance lines - Yunpoong, HTIR 1, HTIR 2, and HTIR 3 – was 53.0%, 46.2%, 44.9%, and 48.0%, respectively. For the susceptible line – Chunpoong – it was 17.0% (Table 1 and Fig. 2). Cuticle distribution was 3.1, 2.7, 2.6, and 2.8 times higher for the resistance lines compared with the susceptible line.
Table 1.
Comparison of cuticular and stomatal morphological characteristics of leaves in selected two-year-old ginseng lines
| Lines | Culticle density (%) | Thickness of cuticle (nm) | Stomata | ||
|---|---|---|---|---|---|
|
| |||||
| Adaxial | Abaxial | No. (ea/mm2) | Major axis length (μm) | ||
|
| |||||
| Yunpoong | 53.0±0.80 | 141.3±1.83 | 153.6±3.46 | 15.2±0.76 | 21.7±0.16 |
| HTIR 1 | 46.2±2.36 | 119.7±1.38 | 165.8±2.82 | 16.8±1.11 | 32.4±0.58 |
| HTIR 2 | 44.9±1.40 | 119.7±1.30 | 157.9±3.82 | 15.4±0.92 | 29.4±1.91 |
| HTIR 3 | 48.0±1.64 | 159.4±2.26 | 196.6±2.29 | 18.9±1.10 | 30.9±1.71 |
| Chunpoong | 17.9±1.76 | 585.0±1.31 | 119.4±1.82 | 16.9±0.88 | 21.8±0.86 |
Values are presented as means±SE.
HTIR, high-temperature injury resistance.
Fig. 2. Scanning electron micrographs of cuticle density of adaxial leaf surfaces in selected two-year-old ginseng lines resistant to high-temperature injury (HTI; Yunpoong, high-temperature injury resistance [HTIR] 1, HTIR 2, and HTIR 3) and susceptible to HTI (Chunpoong). Bar=50 μm.
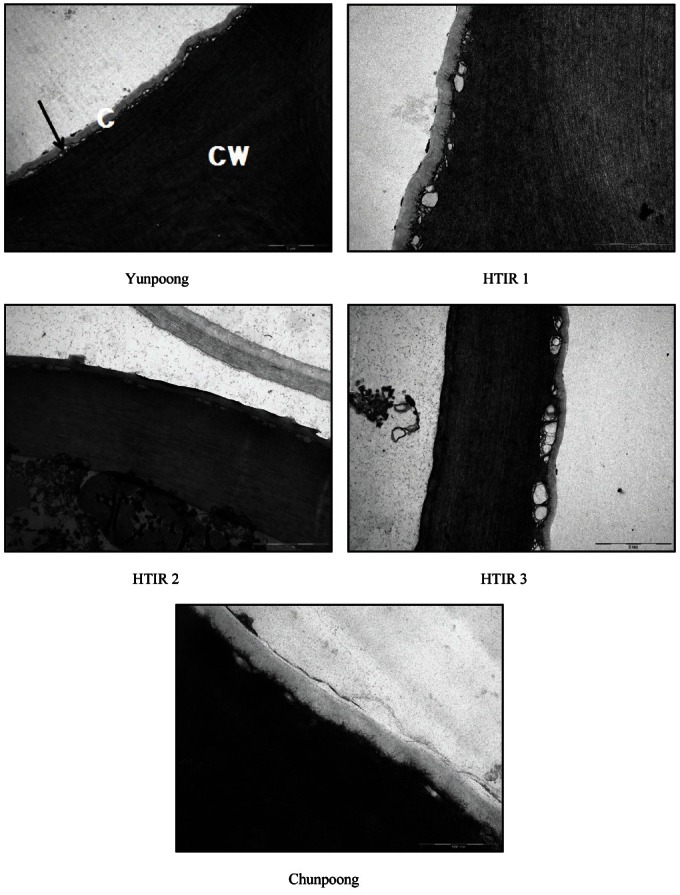
The thickness of cuticle layer on adaxial surfaces of the resistance lines of Yunpoong, HTIR 1, HTIR 2, and HTIR 3 was 141.3, 119.7, 119.7, and 159.4 nm, respectively. However, it was 85.0 nm for the susceptible line of Chunpoong (Table 1 and Fig. 3). The thickness of cuticles on adaxial surfaces of the resistance lines was 1.7, 1.4, 1.4, and 1.9 times higher than that of the susceptible line. The thickness of cuticle layer on abaxial surfaces of the resistance lines of Yunpoong, HTIR 1, HTIR 2, and HTIR 3 was 153.6, 165.8, 157.9, and 199.6 nm, respectively, and 119.4 nm for the susceptible line of Chunpoong (Table 1 and Fig. 4), indicating the cuticles on abaxial surfaces of the resistance lines were thicker 1.3, 1.4, 1.3, and 1.7 times than that of the susceptible line. The correlation coefficients indicated there was highly significant correlation for the leaf-burning with cuticle distribution (r=-0.974**), but not with with the cuticle thickness on adaxial (r=-0.841) and abaxial (r=-0.801) surfaces.
Fig. 3. Transmission electron micrographs of cuticle thickness of adaxial leaf surfaces in selected two-year-old ginseng lines resistant to high-temperature injury (HTI; Yunpoong, high-temperature injury resistance [HTIR] 1, HTIR 2, and HTIR 3) and susceptible to HTI (Chunpoong). Arrow indicates vesicle. Bars=500 nm. C, cuticle; CW, cell wall.
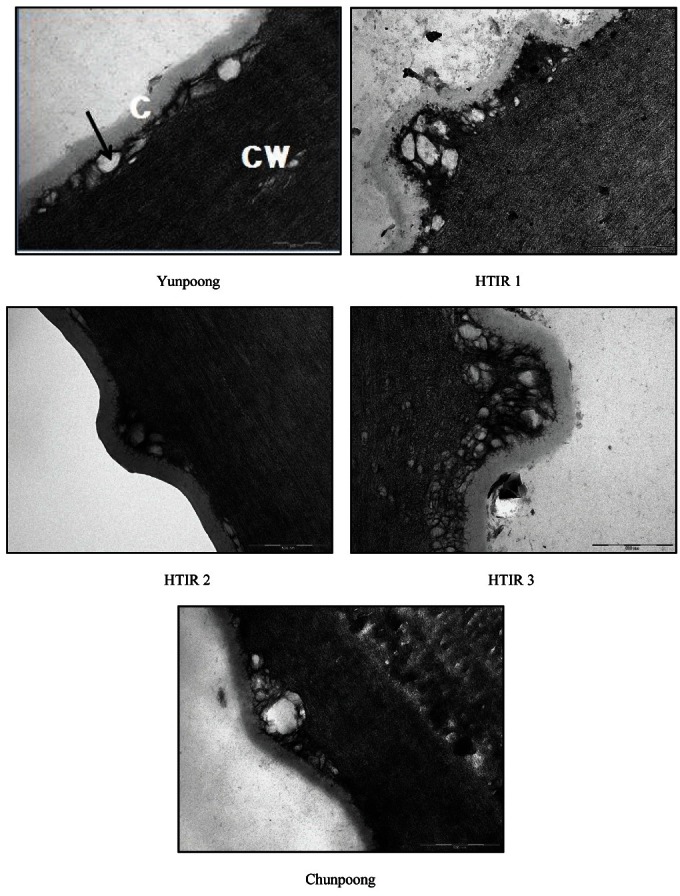
Fig. 4. Transmission electron micrographs of cuticle thickness of abaxial leaf surfaces in selected two-year-old ginseng lines resistant to high-temperature injury (HTI; Yunpoong, high-temperature injury resistance [HTIR] 1, HTIR 2, and HTIR 3) and susceptible to HTI (Chunpoong). Arrow indicates vesicle. Bar in HTI resistant lines=2 nm; HTI-susceptible line=500 nm. C, cuticle; CW, cell wall.
Cuticle layer protects leaves from moisture loss when the temperature of leaves increase. It was reported that the resistance line of Yunpoong has wider distribution of cuticles than Chunpoong to be capable of preventing moisture loss, providing resistance characteristics to the leaf-burning [3]. Lee [4] also reported that cuticle distribution on adaxial surfaces was 31.2% for Yunpoong and 19.8% for Chunpoong. These aspects were also agreed with the HTI resistant resistance lines of HTIR 1, HTIR 2, and HTIR 3 used in our study. Mamrutha et al. [6] reported that average moisture retention capacity of mulberry leaves with high cuticle wax content was still about 55% after five hours from harvesting, but those with high cuticle wax content only had about 10% average moisture retention capacity, suggesting a positive correlation between cuticle wax content and moisture retention capacity (R2=0.71). In processing the ginseng leaves at 30℃ for 5 h , the moisture retention capacity was 43.5% for Chunpoong and 53.5% for Yunpoong, respectively. Therefore, it was thought that there is a close relationship between the density of cuticle layer and moisture retention capacity (data not shown). When peanuts were cultivated with sufficient irrigation, accumulated wax was about 1.46 to 2.06 mg-2, whereas in dry condition, accumulated wax increased to 1.70 to 2.44 mg-2, showing that peanuts endure dryness by decreasing moisture evaporation [7].
Plant leaves absorb light with the wavelength of 400 to 700 nm in photosynthetically active region, and their leaf hairs and cuticle layer on the surface of leaves play critical roles in protecting them from harmful UV rays (UV-A, B 330 nm). When wax layer was removed from the leaves of Eucalyptus spp., a succulent plant, its ability to reflect harmful wavelengths decreased greatly and its ability to absorb visible wavelengths and near-infrared wavelengths increased, showing that wax layer is critical for reflecting light in plants. Also, transpiration from leaves occurs through their pores and cuticle layer and is affected by the thickness, amount, and micro-structure of cuticles. In case of moisture deficiency, cuticle layer affects the opening and closing of leaf pores, reduction of stomatal conductance, and prevention of moisture loss. The plant leaves under and in response to moisture stress increase wax accumulation in a few days to prevent loss of moisture [3]. Cuticle density and accumulation of Pinus palustris in the family Pinaceae increases on the surface of leaves when the plant grows in the soil with a lack of nitrogen and moisture. In case of nitrogen deficiency, there is not enough nutrition on the top of leaves and cuticles develop to protect leaves from diseases and insects. In case of moisture deficiency, cuticles develop largely to prevent loss of moisture [9]. Cuticle layer has been observed to rise from leaves (Fig. 2), which matches with the reports of Lee [4] and Prior et al. [9]. Lee [4] reported that vesicles are observed when cuticles form ridges and these vesicle cause the cuticle ridges to rise. These vesicles were also found in this study, and more vesicles were observed on the resistant lines than on the sensitive line. HTIR 3 showed vesicles not only on cuticle layer, but also on cell membranes (Figs. 3 and 4). Lee [4] reported findings that are different from those of this study; the thickness of cuticles on adaxial surfaces was 115.0 nm for Yunpoong and 133.6 nm for Chunpoong, while the thickness of cuticles on abaxial surfaces was 110.8 nm for Yunpoong and 124.7 nm for Chunpoong.
This is because the leaves used were from the plants cultivated under the shade with about 10% light transmission, whereas in our study the plants were cultivated under the shade with the increased light transmission of 20% from the early stage of growth, increasing the thickness of cuticle layers to endure the stresses of strong light and high temperature. Ku et al. [10] reported that the thickness of ginseng leaves increases by about 1.2 times when light transmission is increased from 5% to 20% or 35%, while Shepherd and Wynne Griffiths [2] reported that the density of wax on the surface of barley leaves grown under light for 4 d is 2.5 times higher than that of barley leaves grown in dark for the same period of time. Also, the cuticle layer on abaxial surfaces was thicker than that of adaxial surfaces, probably because ginseng has pores only on abaxial surfaces so as to prevent moisture evaporation through pores by the thickening of chticle layers .
The number of stomata per 1 mm2 was 15.2, 16.8, 15.4, and 18.9 for the resistant lines of Yunpoong, HTIR 1, HTIR 2, and HTIR 3 and 16.9 for the susceptible line of Chunpoong, not showing a significant difference between the resistant and susceptible lines (Table 1 and Fig. 5). However, Lee [11] reported that 4-year-old leaves of Yunpoong have 69.2 stomata per 1 mm2, and of Chunpoong have 44.8. Lee [4] also reported that the number of stomata per 1 cm2 is 51 for Yunpoong and 30 for Chunpoong and that because Yunpoong has more stomata to accelerate transpiration in high temperature and reduce the temperature of leaves to be more resistant to the leaf burning than Chunpoong. Lee [12] stated that the number of stomata increases by about 3 per 1 mm2 as light transmission increases, but there is no significant difference and that the stomatal length decreases by 1.0-1.2 times as light transmission increases. It is thought that the number of stomata is increased and the length of stomata is decreased to minimize loss of moisture when light transmission increases. Based on these results, it is concluded that Lee [4] and Lee [11] set light transmission to about 10%, whereas this study was conducted at 20% light transmission to indicate no difference in the number of stomata between the two lines. The correlation coefficient for the leaf-burning and the number of stomata was r= 0.082, for which no significance was found.
Fig. 5. Scanning electron micrographs for the number and size of stomata of the abaxial leaf surfaces in selected two-year-old ginseng lines resistant to high-temperature injury (HTI; Yunpoong, high-temperature injury resistance [HTIR] 1, HTIR 2, and HTIR 3) and susceptible to HTI (Chunpoong). Arrow indicates longitudinal stomatal length. Bar= 50 μ m.
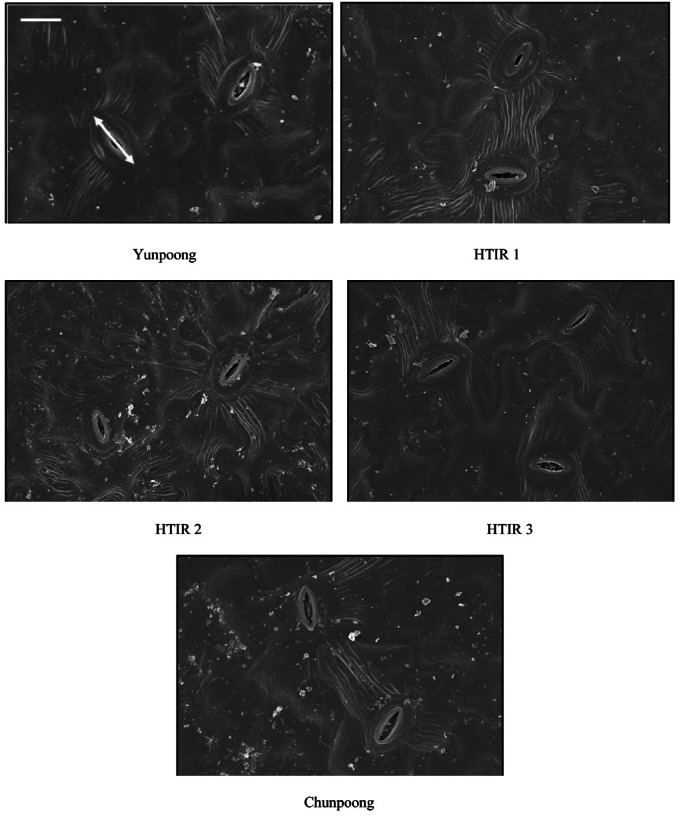
The longitudinal stomatal lengths were 21.7, 32.4, 29.4, and 30.9 μ m for the resistance lines of Yunpoong, HTIR 1, HTIR 2, and HTIR 3, respectively, and 21.8 μm for the susceptible line of Chunpoong (Table 1 and Fig. 5). This indicates there was no significant difference in stomatal length between the resistance Yunpoong and the susceptible Chunpoong, but that the stomatal lengths in the HTI resistant lines of HTIR 1, HTIR 2, and HTIR 3 increased by 1.5, 1.3, and 1.4 times as in HTI-susceptible line Chunpoong. The correlation coefficiencts for the cross and longitudinal stomatal lengths were r= -0.633 and r= -0.612, respectively, showing no significance. In particular, the distribution of cuticle layer on abaxial surfaces was clustered around stomata (Fig. 5), and it has been reported that this is also observed in Pinus palustris [9]. This is because cells related to guard cells are developed through a physiologically unique process of synthesis and the plasticity of abaxial cuticles is lower than that of adaxial cuticles when the cells respond to environmental changes, such as moisture deficiency or light irradiation.
Based on these results, it was concluded that the distribution of cuticle layers, thickness of cuticles on adaxial and abaxial surfaces, and the longitudinal stomatal lengths are more profoundly developed on the lines resistance to the leaf-burning than on the susceptible one. It is thought that these characteristics of leaf tissues increase UV reflection and prevent moisture loss in July and August when ginseng leaves are exposed to high temperature, strong light, and moisture deficiency in soil, decreasing leaf-burning frequencies.
Acknowledgments
This study was supported by research fund of Biogreen21.
References
- 1.Lee SS. Research of ginseng cultivation. In: Korean Society of Ginseng. Reviews in ginseng research I. Korean Society of Ginseng Press; Seoul: 2007. pp. 3–7. [Google Scholar]
- 2.Shepherd T, Wynne Griffiths D. The effects of stress on plant cuticular waxes. New Phytol. 2006;171:469–499. doi: 10.1111/j.1469-8137.2006.01826.x. [DOI] [PubMed] [Google Scholar]
- 3.Lee SS, Ahn IO, Lee JH, Park DW. Study of disease resistance and high quality to ginseng breeding. Bio-resources Research Center, KT&G Central Research Institute; Daejeon: 2004. [Google Scholar]
- 4.Lee KH. Cythohistological comparative study of Panax ginseng C. A. Meyer in roots and leaves pertaining to their ages, grades, and cultivars. Konkuk University; Seoul: 2008. [dissertation] [Google Scholar]
- 5.Lee JS, Lee JH, Ahn IO. Characteristics of resistant lines to high-temperature injury in ginseng (Panax ginseng C. A. Meyer). J Ginseng Res. 2010;34:274–281. doi: 10.5142/jgr.2010.34.4.274. [DOI] [Google Scholar]
- 6.Mamrutha HM, Mogili T, Jhansi Lakshmi K, Rama N, Kosma D, Udaya Kumar M, Jenks MA, Nataraja KN. Leaf cuticular wax amount and crystal morphology regulate post-harvest water loss in mulberry (Morus species). Plant Physiol Biochem. 2010;48:690–696. doi: 10.1016/j.plaphy.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Samdur MY, Manivel P, Jain VK, Chikani BM, Gor HK, Desai S, Misra JB. Genotypic differences and water-deficit induced enhancement in epicuticular wax load in peanut. Crop Sci. 2003;43:1294–1299. doi: 10.2135/cropsci2003.1294. [DOI] [Google Scholar]
- 8.Weete JD, Leek GL, Peterson CM, Currie HE, Branch WD. Lipid and surface wax synthesis in water-stressed cotton leaves. Plant Physiol. 1978;62:675–677. doi: 10.1104/pp.62.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prior S, Pritchard S, Runion G, Rogers H, Mitchell R. Influence of atmospheric CO2 enrichment, soil N, and water stress on needle surface wax formation in Pinus palustris (Pinaceae). Am J Bot. 1997;84:1070–1077. doi: 10.2307/2446150. [DOI] [PubMed] [Google Scholar]
- 10.Ku K, Zhang Z, Wang Y. Effects of light intensity on microstructure and ultrastructure of Panax ginseng leaves under field condition. Acta Bot Sin. 1994;36:23–27. [Google Scholar]
- 11.Lee SS. Characteristics of photosynthesis among new cultivars of ginseng (Panax ginseng C. A. Meyer). J Ginseng Res. 2002;26:85–88. doi: 10.5142/JGR.2002.26.2.085. [DOI] [Google Scholar]
- 12.Lee CH. Effect of light intensity and temperature on the physiological characteristics and growth of Panax spp. leaves. Korean J Ginseng Sci. 1988;12:30–39. [Google Scholar]


