Abstract
Atopic dermatitis (AD) is an allergic, inflammatory skin disease characterized by chronic eczema and mechanical injury to the skin, caused by scratching. Korean red ginseng (RG) has diverse biological activities, but the molecular effects of RG on allergic diseases, like AD, are unclear. The present study was designed to investigate whether RG inhibits 1-chloro-2,4-dinitrobenzene (DNCB)-induced AD in a mouse model. DNCB was applied topically on the dorsal surface of Balb/c mice to induce AD-like skin lesions. We observed the scratching behavior and examined the serum IgE level and interleukin (IL)-4 and IL-10 in splenocytes compared with dexamethasone. We also evaluated the DNCB-induced mitogen-activated protein kinases (MAPKs), NF-κB, and Ikaros activities after RG treatment using reverse transcriptase-polymerase chain reaction, Western blotting, and ELISA. Our data showed that the topical application of RG significantly improved the AD-like skin lesions and scratching behavior. RG decreased not only the mRNA expression of IL-4 and IL-10, but also the secretion of IL-4 protein and serum IgE in mice. Additionally, RG treatment decreased the DNCB-induced MAPKs activity and subsequent Ikaros translocation irrespective of NF-κB. We suggest that RG may be useful as a therapeutic nutrition for the treatment of AD.
Keywords: Panax ginseng, Red ginseng, Atopic dermatitis, Ikaros
INTRODUCTION
Ginseng, the root of Panax ginseng Meyer (Araliaceae), is a popular natural health food in Asia. During heat processing for the preparation of Korean red ginseng (RG), it undergoes changes in its ginsenoside composition and biological effects. For example, ginsenosides Rh2, RS4, and Rg3 are found only in RG and are hydrolyzed products, derived from saponin by heating. They can inhibit the growth of cancer cells via the induction of apoptosis [1].
In a previous study, we prepared red ginseng from ginseng (Korea) and found that it inhibited the antigen-induced β-hexosaminidase release in RBL-2H3 mast cells and reduced the ICAM-1 expression in A549 human epithelial cells induced by TNF-α [2]. Several studies have reported that ginsenoside Rg3 or Rh2, produced by a steaming process, suppressed histamine release from mast cells due to stimulation with compound 48/80 in vitro [3-5].
Atopic dermatitis (AD) is a chronic, allergic inflammatory skin disease characterized by pruritic eczema, mechanical injury to the skin, caused by scratching, an important feature of AD [6], elevated serum IgE levels, and markedly increased levels of inflammatory cells, including eosinophils, mast cells, and lymphocytes [7,8]. Various cells involved in the allergic reaction infiltrate the lesions. Among the infiltrating cells, T helper 2 (Th2) cell is one of the most important cell types involved in AD development [8]. Th2 cells release cytokines such as interleukin (IL)-4, IL-5, IL-13, and IL-10 in allergic inflammation and AD. These released cytokines result in the proliferation and activation of both mast cells and eosinophils in allergic inflammation, consequently leading to scratching of the skin and impaired skin barrier function [9]. Excessive production of IL-4, IL-5, IL-10, IL-13, and serum IgE is thought to be important in the development of allergic diseases, including AD [10-12].
Here, we investigated the inhibitory effects of RG on AD, or skin allergic inflammation, in mice by observing the degree of recovery of 1-chloro-2,4-dinitrobenzene (DNCB)-induced AD-like skin lesions, by counting the frequency of scratching and detecting representative allergic markers, such as the serum IgE levels and IL-4, IL-5, and IL-10 concentrations.
NF-κB activity is increased in allergic asthma, and inhibition of NF-κB activity improved the symptoms of asthma [13]. Additionally, Ikaros transcription factor has a suppressive role in mast cell-derived IL-4 gene transcription, because Ikarosnull mast cells showed elevated IL-4 mRNA expression and chromatin accessibility across the Th2 locus [14,15]. We therefore identified NF-κB and Ikaros as possible transcription factors and mitogen-activated protein kinases (MAPKs) and casein kinase 2 (CK2) as intracellular signal pathways triggered by RG in DNCB-treated primary splenocytes in mice.
MATERIALS AND METHODS
Animals and reagents
Six-week-old male Balb/c mice were obtained from Dae-Han Bio Link (Eumseong, Korea) and housed with access to food and water ad libitum. Mice were maintained in the well-documented Experimental Animal Facility and protocols were approved by the Animal Care Committee at Sungkyunkwan University. The studies were performed in accordance with the Institutional Animal Care and Use Committee Guidelines. During the experiment, all animals were kept at a temperature of 23±1℃ and humidity of 55±5% with 10-18 circulations/h under a 12/12-h light/dark cycle. The animals were divided randomly into five groups of five mice each: 1) normal control group; 2) vehicle-treated group; 3) dexamethasone-treated group; and 4) and 5) RG-treated groups. All reagents, including dexamethasone, were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless stated otherwise.
Red ginseng extract preparation
Total RG extract was purchased from Central Research Institute, KT&G (Daejeon, Korea). This extract was processed with 70% alcohol once for 8 h and then with 15% alcohol twice. After a concentration process (60-64 cmHg, 65 brix) to remove the alcohol, five-times diluted product was prepared. RG was obtained by filtration with centrifugation (Continuous Centrifuge, Kansai, Japan), followed by vacuum evaporation (60-64 cmHg, 67 brix) at 60℃.
Induction and treatment of atopic dermatitis-like skin lesions in mice
DNCB was used to induce AD-like skin lesions according to the method of Yun et al. [16] with modifications. Briefly, 0.5 mL of 1% DNCB, freshly dissolved in acetone:olive oil (4:1), applied topically on a shaved area of the dorsal surface twice per wk for two weeks (four times in total). After inducing the AD-like skin lesions, the lesions were treated with 0.5 mL of PBS (vehicle), dexamethasone (positive control; 10 μM), or RG (10 and 100 μg/mL) every other day (four times in total).
Measuring scratching behavior frequency
Scratching behavior was observed after the completion of all treatments. Briefly, the mice were placed in cages and the number of scratching behaviors was counted for 10 min. This was repeated five times (50 min in total). Scratching behavior was defined in this experiment as movement with the hind paws.
Measuring the serum IgE level
After finishing the treatment, blood samples were collected from the inferior vena cava and serum was separated. The serum IgE concentration was measured using an ELISA kit purchased from BioLegend (San Diego, CA, USA).
Determining interleukin-4 secretion
The spleen was removed aseptically and total splenocytes were prepared. Splenocytes (5×105 cells/well) were plated in 96-well plates and incubated for 24 h in RPMI 1640 medium (GIBCO, Grand Island, NY, USA) containing 10% FBS (HyClone, Logan, UT, USA), penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37℃ in a 5% CO2 humidified incubator. Culture supernatants were collected and the amount of secreted IL-4 was measured using an ELISA kit (BioLegend).
Reverse transcriptase-polymerase chain reaction analysis of gene expression
The expression of IL-4 and IL-10 mRNA was determined using the reverse transcriptase-polymerase chain reaction (PCR). Total RNA was extracted from splenocytes using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and processed to cDNA with RevertAid (Fermentas, Vilnius, Lithuania). The Solgent™ EF-Taq DNA Polymerase kit was used for PCR and cDNA was amplified using primers.
Subcellular fractionation
Cytosolic and nuclear extracts were prepared. Briefly, 5×107 splenocytes/mL were plated in 100-mm dishes and treated with RG (100 μg/mL) and DNCB (1%) for 4 h. Harvested cells were resuspended in 0.2 mL of buffer A (10 mM HEPES at pH 7.5, 1.5 mM MgCl2, 10 mM KCl, 1 mM DDT, 0.1% NP-40, 0.2 mM PMSF). The cells were lysed on ice for 15 min, and centrifuged (5,000 g, 5 min, 4℃). The supernatant was collected as cytosolic extracts. The nucleic pellet was washed with buffer A lacking NP-40, and resuspended in 0.025 mL of buffer C (20 mM HEPES at pH 7.5, 25% glycerol, 0.42 M NaCl, 0.2 mM EDTA, 1.5 mM MgCl2, 1 mM DDT, 0.2 mM PMSF). After incubation on ice for 30 min, the nuclear debris was spun down (13,000 g, 10 min, 4℃). The supernatant was collected as nuclear extracts. The protein concentration was measured using a Bio-Rad protein as say kit (Hercules, CA, USA).
Western blotting
Total splenocytes were plated at 3×107 cells/ml and treated with RG (100 μg/mL) and DNCB (1%) for 4 h and then harvested and lysed with lysis buffer containing 20 mM Tris, pH 7.6, 150 mM NaCl, 1% Triton X-100, and protease inhibitor cocktail. The protein content was measured using a protein assay kit (Hercules). Samples were diluted with lysis buffer containing 1% β-mercaptoethanol. Equal amounts of cellular protein (50 μg) were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. After blocking, the membranes were incubated with the targeted antibody and then with horseradish peroxidase-conjugated secondary antibody to IgG. Immunoreactive proteins were visualized using the ECL Western blot detection system. The protein level was compared to a loading control, such as β-actin or non-phosphorylated protein.
Statistical analyses
Each experiment was repeated three or four times, and the results of a representative experiment are shown. The results are expressed as mean±SEM and were analyzed using Student’s t-test. A statistical probability of p<0.05 was considered significant (#p<0.05, ##p<0.01, *p<0.05, and **p<0.01 ).
RESULTS
Effect of red ginseng on the atopic dermatitis-like skin lesions
The topical application of DNCB induced AD-like skin lesions in Balb/c mice, compared with the untreated group. Erythema was seen on the backs of the DNCB-treated mice after the second treatment and eczema with keratosis followed the final application of DNCB. The skin lesions of the RG-treated mice improved markedly compared with the vehicle-treated mice (Fig. 1).
Fig. 1. Atopic dermatitis (AD)-like skin lesion and improvement of skin condition after treatment with Korean red ginseng (RG). AD-like skin lesion were induced by topical application of 1% 1-chloro-2,4-dinitrobenzene (DNCB) for four times in twice a week, and RG application on the skin lesions improved the skin condition. Dex, dexamethasone.

Effect of red ginseng on scratching behavior frequency
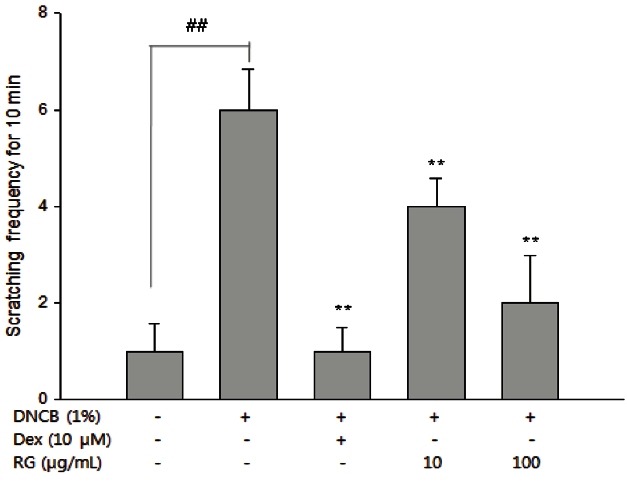
DNCB application substantially increased the frequency of scratching behavior, while RG treatment significantly suppressed the DNCB-mediated scratching behavior (Fig. 2).
Fig. 2. Inhibitory effect of Korean red ginseng (RG) on 1-chloro-2,4-dinitrobenzene (DNCB)-induced scratching behavior. Scratching behavior was observed after completion of all treatments. In brief, the number of scratching behaviors was counted for 10 min. Measurement was repeated for five times (50 min in total). Each bar shows the mean±SEM of three independent experiments. Dex, dexamethasone. ##p<0.01, significantly different from control group; **p<0.01, significantly different from DNCB-alone.
Effect of red ginseng on serum IgE levels
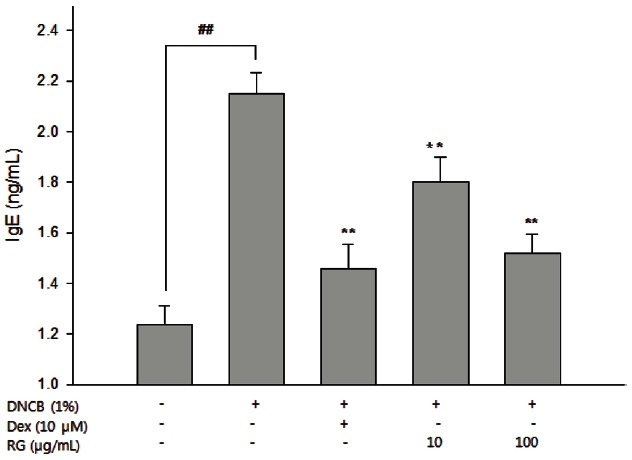
The serum IgE level was decreased significantly in the RG-treated mice. In particular, 100 μg/mL RG had the same effect on the IgE level as dexamethasone (Fig. 3).
Fig. 3. Effect of Korean red ginseng (RG) on level of IgE in serum. After completion of all treatments, blood samples were collected from the inferior vena cava and serum was separated. Level of serum IgE was measured by ELISA. The data are presented as the mean±SEM of a representative experiment of triplicates. Dex, dexamethasone. ##p<0.01, significantly different from control group; **p<0.01, significantly different from 1-chloro-2,4-dinitrobenzene (DNCB)-alone.
Effect of red ginseng on interleukin-4 and interleukin-10 production
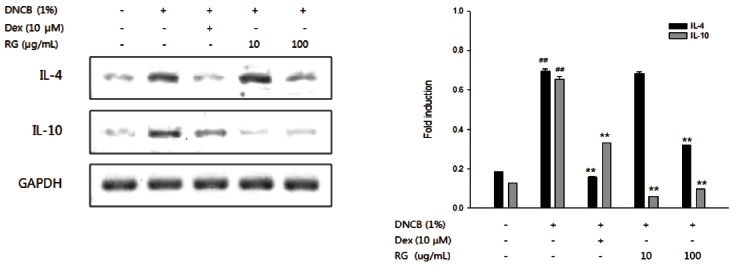
To investigate whether RG is involved in regulating the Th2 cytokine gene, the expression of IL-4 and IL-10 production in splenocytes was examined. As shown in Fig. 4, DNCB-induced AD mice showed much higher level of IL-4 secreted than that of control mice, while RG treatment suppressed IL-4 protein secretion. DNCB also significantly increased the mRNA levels of both IL-4 and IL-10, while RG suppressed the DNCB-imduced mRNA levels of IL-4 (only at 100 μg/mL) and IL-10 (10, 100 μg/mL) mRNA (Fig. 5).
Fig. 4. Inhibitory effect of Korean red ginseng (RG) on 1-chloro-2,4-dinitrobenzene (DNCB)-induced interleukin (IL)-4 secretion in splenocytes. After completion of all treatments, splenocytes (5×105 cells/well) were incubated for 24 h. Culture supernatants were collected and level of secreted IL-4 was determined using ELISA. The data are presented as the mean±SEM of a representative experiment of triplicates. Dex, dexamethasone. ##p<0.01, significantly different from control group; **p<0.01, significantly different from DNCB-alone.
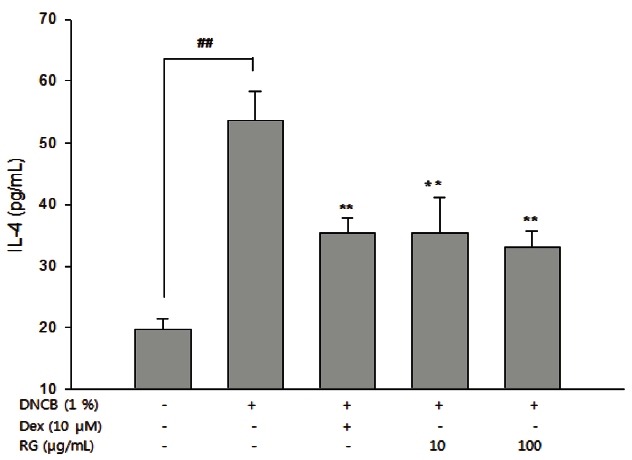
Fig. 5. Suppressive effect of Korean red ginseng (RG) on mRNA expressions of interleukin (IL)-4, IL-10. After completion of all treatments, total RNA was extracted from splenocytes. Reverse transcriptase-polymerase chain reaction analysis was performed to measure mRNA levels of IL-4, IL-10, respectively. The results illustrated are from a single experiment, and are representative of three separate experiments. Dex, dexamethasone. ##p<0.01, significantly different from control group; **p<0.01, significantly different from 1-chloro-2,4-dinitrobenzene (DNCB)-alone.
Effects of Korean red ginseng on MAPKs activation in DNCB-treated splenocytes in mice
To elucidate the cellular signaling pathway for the anti-inflammatory atopic effects of RG, we have to determine the proper time to detection. Therefore, we used splenocyte primary cells as in vitro system similar with in vivo conditions. Cells were treated with DNCB (1 μg/mL), RG (100 μg/mL), or DNCB/RG, then examined the effects of RG on the phosphorylation of ERK1/2, JNKs, and p38 kinases by Western blotting. RG treatment suppressed the DNCB-induced phosphorylation of ERK1/2, JNKs, and p38 MAP kinases (Fig. 6A).
Fig. 6. Effect of Korean red ginseng (RG) on activated mitogen-activated protein kinases (MAPKs) and translocation of Ikaros. (A) Suppressive effect of RG on activated MAPKs. RG were treated to splenocytes with or without 1-chloro-2,4-dinitrobenzene (DNCB). The phosphorylations of MAPKs such as p38, JNK and ERK1/2 were assessed by western blotting described in methods. (B) Suppressive effect of RG on Ikaros translocation. After isolation of cytosolic and nuclear fractions, the translocation of NF-kB (p65), casein kinase 2 (CK2) and Ikaros were assessed by western blotting described in methods respectively. The results illustrated are from a single experiment, and are representative of three separate experiments. #p<0.05, significantly different from control group; *p<0.05, significantly different from DNCB-alone.
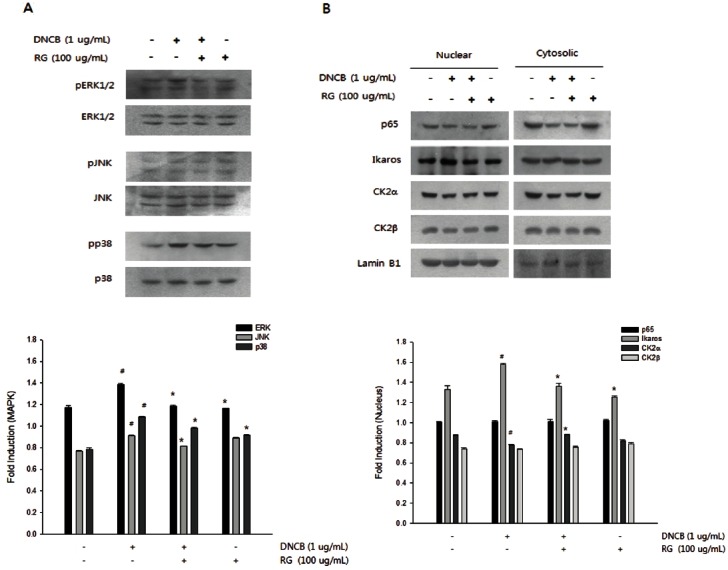
Effects of RG on translocation of NF-κB and Ikaros in DNCB-treated splenocytes in mice
To identify possible transcription factors triggered by RG, the translocation of NF-κB and Ikaros was determined. Ikaros translocation induced by DNCB was suppressed by RG treatment, whereas DNCB did not affect NF-κB translocation. Since protein kinase CK2 shuttles between cytosol and nucleus, we have undertaken the study of the intracellular distribution of CK2 in splenocytes in response to DNCB stimulation. CK2α, the catalytic subunit of CK2, can deactivate the transcriptional function of Ikaros by phosphorylation in the nucleus. DNCB increased Ikaros translocation with decreased CK2α activity in the nucleus. The protein levels of CK2β, the regulatory subunit, were also measured by western blots and the significant changes in the nucleus were not observed. The DNCB-induced changes in Ikaros and CK2α in the nucleus were restored by RG treatment (Fig. 6B).
DISCUSSION
AD is a chronic, allergic inflammatory skin disease characterized by eczema and scratching behavior, which can induce skin injury. The skin sensitivity and inflammation induced by DNCB, a potential allergen, are characterized by elevated levels of IgE and cytokines, such as IL-4 and IL-10 [16,17]. The topical application of RG improved the AD-like skin lesions and the frequency of scratching behavior in DNCB-induced AD mice.
Of the numerous cells that infiltrate AD skin lesions, Th2 cells play important roles in producing major cytokines in allergic inflammation and AD, such as IL-4, IL-5 and IL-10. Based on previous studies, over-expression of IL-4, IL-5, IL-10, and serum IgE is a marker of allergic diseases, including asthma and AD [18,19]. In particular, IL-4 contributes to the expansion of the Th2 cell subset from naive T cells and the isotype switching of B cells to produce IgE antibodies against specific environmental allergens [20,21]. IL-4 is also essential in the switch from naive T cells to allergic Th2 cells [22]. IL-10 acts in down-regulating IL-12 production by antigen-presenting cells and promotes the differentiation of naive Th0 cells toward the Th2 profile [11,12]. The up-regulation of total serum IgE is a clear indicator of AD [10]. Thus, we examined whether RG suppressed the serum IgE in DNCB-induced AD-like skin lesions.
In this study, the RG-treated groups had significantly lower levels of total serum IgE and showed IL-10 mRNA expression compared with the DNCB-induced AD mice. We also observed an effect of RG on IL-4 protein secretion and mRNA expression. RG at 100 μg/mL decreased the level of both IL-4 mRNA and protein. RG at 10 μg/mL however decreased IL-4 protein secretion, without suppressing IL-4 mRNA expression, indicating that RG affects the expression of IL-4 not at the transcriptional level, but apparently via a post-translational modification in a certain or low-concentrations. RG at 100 μg/mL seems to be able to affect the DNCB-induced IL-4 transcript levels, which suggests that a proper RG concentration is needed to limit IL-4 expression from the transcriptional stage of cellular signaling induced by DNCB.
According to recent articles, topically or orally applied RG not only has effects in recovery from skin inflammation and ear thickness, but also benefits IL-4 production in NC/Nga mice, which concurs with our results. However, in regulation of serum IgE level, a hallmark of AD, only oral administration of RG shows significant effect on reduction of IgE whereas for topical application has not much effect [10,23,24]. In our condition, topically application of RG has significant reduction in serum IgE levels. It means that appropriate dose and treatment period were very important in application of RG for obtaining therapeutic effects of atopic skin disease.
Although dexamethasone is one of the most effective steroid drugs for treating inflammatory diseases, including contact dermatitis and AD, the use of topical steroids is often avoided due to their many undesirable side effects. Our data showed that RG appears to exert beneficial effects on DNCB-treated AD mice equivalent to those of dexamethasone. Some studies have shown that ginseng is safe. In a 4-week-placebo-controlled study involving 77 women, no side effects were observed for the supplemented with a mixture of ginseng [25]. Ding et al. [26] also reported that RG was an effective and safe adjuvant for the treatment of congestive heart failure without any side effects [26]. This suggests that RG has anti-atopic and anti-allergic effects equal to currently used steroids, without undesirable side effects.
Cells recognize and respond to extracellular stimuli by engaging specific intracellular programs, such as the signaling cascade that leads to activation of the MAPKs. The most widely studied groups of MAPKs to date are the ERK1/2, JNKs, and p38 kinases [27]. To investigate intracellular signaling by RG, we further examined the phosphorylation of ERK1/2, JNKs, and p38 kinases on exposure to RG. We found that RG decreased the DNCB-induced activation of MAPKs, such as ERK1/2, JNKs, and p38 MAPK.
Because NF-κB is activated in allergic disease [14] and recent studies have suggested that Ikaros is associated with IL-4 gene transcription [15], we investigated the effects of RG on the translocation of NF-κB and Ikaros as a possible transcription factor related to IL-4, IL-10, and IgE production. We determined that DNCB-induced Ikaros translocation was suppressed by RG, which is consistent with the reversed patterns of CK2α translocation in the nucleus. The CK2-mediated phosphorylation of Ikaros in the nucleus leads to the degradation of Ikaros via the ubiquitin pathway [28]. Conversely, NF-κB activation by DNCB was not observed in our model, which implies that DNCB might modulate MAPKs differently and that NF-κB pathways depend on the origins of cells [29,30].
There are reports that Ikaros plays an important role in the differentiation of the CD4+ T cells through by IL-4 regulation [31,32]. Ikaros directly activates Th2 gene, IL-4, by promoting local accessibility during CD4+ T cell differentiation, and IL-4 production is increased by following Ikaros expression in Ikarosnull T cells. It also has been reported that IL-10 expression is regulated by Ikaros in CD4+ T cells [31].
In our study, RG had significant effects in the DNCB-induced AD-like mouse model. In AD-like mice, the topical application of RG improved the skin lesions and reduced the Th2-activated cytokines, such as IL-4, IL-10, and the serum IgE levels.
These anti-atopic effects of RG seem to be mediated through the inhibition of MAPK (ERK1/2, JNKs, and p38 MAPK) signaling pathways and the activation of CK2α, which further inhibits transcriptional activity of Ikaros. This suppressive effect of RG on Ikaros activity seems to inhibit both IL-4 and IL-10 exprssions in splenocytes. Based on these results, we suggest that RG may be useful as a therapeutic nutrition or an adjuvant to avoid the undesirable side effects of steroids in the treatment of AD.
References
- 1.Nam KY. The comparative understanding between red ginseng and white ginsengs, processed ginseng (Panax ginseng C.A. Meyer). J Ginseng Res. 2005;29:1–18. doi: 10.5142/JGR.2005.29.1.001. [DOI] [Google Scholar]
- 2.Park HJ, Jung DH, Joo HM, Kang NS, Jang SA, Lee JG, Sohn EH. The comparative study of anti-allergic and anti-inflammatory effects by fermented red ginseng and red ginseng. Korean J Plant Resour. 2010;23:415–422. [Google Scholar]
- 3.Kim WY, Kim JM, Han SB, Lee SK, Kim ND, Park MK, Kim CK, Park JH. Steaming of ginseng at high temperature enhances biological activity. J Nat Prod. 2000;63:1702–1704. doi: 10.1021/np990152b. [DOI] [PubMed] [Google Scholar]
- 4.Tachikawa E, Kudo K, Harada K, Kashimoto T, Miyate Y, Kakizaki A, Takahashi E. Effects of ginseng saponins on responses induced by various receptor stimuli. Eur J Pharmacol. 1999;369:23–32. doi: 10.1016/S0014-2999(99)00043-6. [DOI] [PubMed] [Google Scholar]
- 5.Park EK, Choo MK, Kim EJ, Han MJ, Kim DH. Antiallergic activity of ginsenoside Rh2. Biol Pharm Bull. 2003;26:1581–1584. doi: 10.1248/bpb.26.1581. [DOI] [PubMed] [Google Scholar]
- 6.Nickoloff BJ, Naidu Y. Perturbation of epidermal barrier function correlates with initiation of cytokine cascade in human skin. J Am Acad Dermatol. 1994;30:535–546. doi: 10.1016/S0190-9622(94)70059-1. [DOI] [PubMed] [Google Scholar]
- 7.Akdis CA, Akdis M, Trautmann A, Blaser K. Immune regulation in atopic dermatitis. Curr Opin Immunol. 2000;12:641–646. doi: 10.1016/S0952-7915(00)00156-4. [DOI] [PubMed] [Google Scholar]
- 8.Leung DY, Bieber T. Atopic dermatitis. Lancet. 2003;361:151–160. doi: 10.1016/S0140-6736(03)12193-9. [DOI] [PubMed] [Google Scholar]
- 9.Homey B, Steinhoff M, Ruzicka T, Leung DY. Cytokines and chemokines orchestrate atopic skin inflammation. J Allergy Clin Immunol. 2006;118:178–189. doi: 10.1016/j.jaci.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 10.Lester MR, Hofer MF, Gately M, Trumble A, Leung DY. Down-regulating effects of IL-4 and IL-10 on the IFN-gamma response in atopic dermatitis. J Immunol. 1995;154:6174–6181. [PubMed] [Google Scholar]
- 11.Muraille E, Leo O. Revisiting the Th1/Th2 paradigm. Scand J Immunol. 1998;47:1–9. doi: 10.1111/j.1365-3083.1998-47-1.00383.x. [DOI] [PubMed] [Google Scholar]
- 12.Moverare R, Elfman L, Stalenheim G, Bjornsson E. Study of the Th1/Th2 balance, including IL-10 production, in cultures of peripheral blood mononuclear cells from birch-pollen-allergic patients. Allergy. 2000;55:171–175. doi: 10.1034/j.1398-9995.2000.00244.x. [DOI] [PubMed] [Google Scholar]
- 13.Barnes PJ. Role of GATA-3 in allergic diseases. Curr Mol Med. 2008;8:330–334. doi: 10.2174/156652408785160952. [DOI] [PubMed] [Google Scholar]
- 14.Yang L, Cohn L, Zhang DH, Homer R, Ray A, Ray P. Essential role of nuclear factor kappaB in the induction of eosinophilia in allergic airway inflammation. J Exp Med. 1998;188:1739–1750. doi: 10.1084/jem.188.9.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregory GD, Raju SS, Winandy S, Brown MA. Mast cell IL-4 expression is regulated by Ikaros and influences encephalitogenic Th1 responses in EAE. J Clin Invest. 2006;116:1327–1336. doi: 10.1172/JCI27227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yun JW, Kim H, Kang HJ, Koh JY, Kim BH. Comparative evaluation of animal models for atopic dermatitis in NC/Nga mice. Lab Anim Res. 2008;24:59–66. [Google Scholar]
- 17.Izuhara K, Yanagihara Y, Hamasaki N, Shirakawa T, Hopkin JM. Atopy and the human IL-4 receptor alpha chain. J Allergy Clin Immunol. 2000;106(1 Pt 2):S65–S71. doi: 10.1067/mai.2000.106776. [DOI] [PubMed] [Google Scholar]
- 18.Maggi E, Del Prete G, Macchia D, Parronchi P, Tiri A, Chretien I, Ricci M, Romagnani S. Profiles of lymphokine activities and helper function for IgE in human T cell clones. Eur J Immunol. 1988;18:1045–1050. doi: 10.1002/eji.1830180712. [DOI] [PubMed] [Google Scholar]
- 19.Romagnani S. Type 1 T helper and type 2 T helper cells: functions, regulation and role in protection and disease. Int J Clin Lab Res. 1991;21:152–158. doi: 10.1007/BF02591635. [DOI] [PubMed] [Google Scholar]
- 20.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 21.Georas SN, Guo J, De Fanis U, Casolaro V. T-helper cell type-2 regulation in allergic disease. Eur Respir J. 2005;26:1119–1137. doi: 10.1183/09031936.05.00006005. [DOI] [PubMed] [Google Scholar]
- 22.Hines C. The diverse effects of mast cell mediators. Clin Rev Allergy Immunol. 2002;22:149–160. doi: 10.1385/CRIAI:22:2:149. [DOI] [PubMed] [Google Scholar]
- 23.Kim HS, Kim DH, Kim BK, Yoon SK, Kim MH, Lee JY, Kim HO, Park YM. Effects of topically applied Korean red ginseng and its genuine constituents on atopic dermatitis-like skin lesions in NC/Nga mice. Int Immunopharmacol. 2011;11:280–285. doi: 10.1016/j.intimp.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Lee JH, Cho SH. Korean red ginseng extract ameliorates skin lesions in NC/Nga mice: an atopic dermatitis model. J Ethnopharmacol. 2011;133:810–817. doi: 10.1016/j.jep.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 25.Ito TY, Trant AS, Polan ML. A double-blind placebo-controlled study of ArginMax, a nutritional supplement for enhancement of female sexual function. J Sex Marital Ther. 2001;27:541–549. doi: 10.1080/713846828. [DOI] [PubMed] [Google Scholar]
- 26.Ding DZ, Shen TK, Cui YZ. Effects of red ginseng on the congestive heart failure and its mechanism. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1995;15:325–327. [PubMed] [Google Scholar]
- 27.Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dovat S, Payne KJ. Tumor suppression in T cell leukemia--the role of Ikaros. Leuk Res. 2010;34:416–417. doi: 10.1016/j.leukres.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aiba S, Manome H, Nakagawa S, Mollah ZU, Mizuashi M, Ohtani T, Yoshino Y, Tagami H. p38 Mitogen-activated protein kinase and extracellular signal-regulated kinases play distinct roles in the activation of dendritic cells by two representative haptens, NiCl2 and 2,4-dinitrochlorobenzene. J Invest Dermatol. 2003;120:390–399. doi: 10.1046/j.1523-1747.2003.12065.x. [DOI] [PubMed] [Google Scholar]
- 30.Ade N, Antonios D, Kerdine-Romer S, Boisleve F, Rousset F, Pallardy M. NF-kappaB plays a major role in the maturation of human dendritic cells induced by NiSO(4) but not by DNCB. Toxicol Sci. 2007;99:488–501. doi: 10.1093/toxsci/kfm178. [DOI] [PubMed] [Google Scholar]
- 31.Quirion MR, Gregory GD, Umetsu SE, Winandy S, Brown MA. Cutting edge: Ikaros is a regulator of Th2 cell differentiation. J Immunol. 2009;182:741–745. doi: 10.4049/jimmunol.182.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Umetsu SE, Winandy S. Ikaros is a regulator of Il10 expression in CD4+ T cells. J Immunol. 2009;183:5518–5525. doi: 10.4049/jimmunol.0901284. [DOI] [PMC free article] [PubMed] [Google Scholar]


