Abstract
Ginseng, the root of Panax ginseng Meyer, has been used frequently in traditional oriental medicine and is popular globally. Ginsenosides, which are the saponins in ginseng, are the major components having pharmacological and biological activities, including anti-diabetic and anti-tumor activities. In this study, we investigated the effects of total saponin from Korean red ginseng (TSKRG) on thrombin-produced thromboxane A2 (TXA2), an aggregating thrombogenic molecule, and its associated microsomal enzymes cyclooxygenase (COX)-1 and TXA2 synthase (TXAS). Thrombin (0.5 U/mL) increased TXA2 production up to 169 ng/108 platelets as compared with control (0.2 ng/108 platelets). However, TSKRG inhibited potently TXA2 production to the control level in a dose-dependent manner, which was associated with the strong inhibition of COX-1 and TXAS activities in platelet microsomes having cytochrome c reductase activity. The results demonstrate TSKRG is a beneficial traditional oriental medicine in platelet-mediated thrombotic diseases via suppression of COX-1 and TXAS to inhibit production of TXA2.
Keywords: Total saponin from Korean red ginseng, Cyclooxygenase 1, Thromboxane A2 synthase
INTRODUCTION
Platelet aggregation is essential for normal hemostatic process when blood vessels are injured. However, it can also cause cardiovascular diseases such as thrombosis, atherosclerosis and myocardial infarction [1]. Hence, inhibition of platelet aggregation might be a promising target to the development of anti-thrombotic drugs and an approach for the prevention of cardiovascular disease. When platelets are activated by agonists such as collagen, thrombin and adenosine diphosphate (ADP), phosphatidylinositol 4, 5-bisphosphate (PIP2) is broken down by phospholipase C which is activated through Gprotein coupled receptor (GPCR) or glycoprotein VI. Diacylglycerol (DG) and inositol-1,4,5-trisphosphate (IP3) are generated from PIP2 [2,3]. IP3 mobilizes Ca2+ from the dense tubular system into the cytoplasm. Ca2+ calmodulin complex activates myosin light chain kinase, which in turn phosphorylates the myosin light chain [4,5]. Moreover, DG phosphorylates pleckstrin through protein kinase C (PKC) and it is also used as a donor of arachidonic acid (AA) [6]. AA is a precursor of prostaglandin G2/prostaglandin H2 (PGH2) and thromboxane A2 (TXA2) [7]. TXA2 interacts with membrane receptors of other platelets in a autacoidal reaction, which results in elevation of intracellular free Ca2+, a platelet aggregation-inducing molecule, via Gαq of GPCR [3,8,9]. Inhibition of TXA2 production leads to anti-thrombosis.
Anti-thrombotic drugs such as aspirin, imidazole and indomethacin have anti-thrombotic effects by inhibiting the generation of TXA2. The mechanisms are concerned with cyclooxygenase (COX)-1 and TXA2 synthase (TXAS), which convert AA to TXA2 [10,11]. Therefore, the inhibition of the medicated COX-1 and TXAS is very important to prevent platelet-induced thrombosis. For instance, aspirin, COX-1 inhibitor, and ozagrel, TXAS inhibitor are being used as anti-thrombotic agents [12,13].
It has been reported that nonsaponin fraction [14-16], aqueous fraction [17], polyacetylene compound [18], acidic polysaccharide [19], panaxatriol and panaxadiol [20-22], ginsenoside Rg1 [17,23], and dihydroginsenoside Rg3 [24] from Panax ginseng have anti-platelet effects on agonist-induced platelet aggregation. However, there are no reports concerning the involvement of ginseng compounds in the inhibition of COX-1 or TXAS to suppress platelet aggregation. In this study, we investigated whether total saponin from Korean red ginseng (TSKRG) inhibits the specific activities of COX-1 and TXAS to suppress TXA2 production, and this provide new information of TSKRG-mediated anti-platelet activity.
MATERIALS AND METHODS
Materials
Total saponin from Korean red ginseng was obtained from KT&G Central Research Institute (Daejeon, Korea). Thrombin was purchased from Chrono-Log Corporation (Havertown, PA, USA). TXB2 enzyme immunoassay kits were purchased from GE Healthcare (Buckinghamshire, UK). COX-fluorescent activity assay kit, ozagrel and prostaglandin H2 were purchased from Cayman Chemical (Ann Arbor, MI, USA). Cytochrome c reductase (NADPH) assay kit and other reagents were obtained Sigma-Aldrich (St. Louis, MO, USA). All other chemicals and reagents used in the present study were of analytical grade.
Preparation of washed rat platelets
Whole blood was obtained from the abdominal vein of normal Sprague-Dawley rats (150-200 g), anti-coagulated with 0.38% sodium citrate solution and centrifuged at 220 ×g for 10 min to obtain the platelet-rich plasma. After removing the red blood cells, the preparation was centrifuged again at 1650 × g for 10 min to obtain platelet pellets. The platelets were washed twice with a washing buffer (138 mM NaCl, 2.7 mM KCl, 12 mM NaHCO3, 0.36 mM NaH2PO4, 5.5 mM glucose, 1 mM EDTA, pH 6.5). Washed platelets were suspended in a buffer consisting of 138 mM NaCl, 2.7 mM KCl, 12 mM NaHCO3, 0.36 mM NaH2PO4, 5.5 mM glucose, 0.49 mM MgCl2 and 0.25% gelatin, pH 7.4, and adjusted to a final concentration of 5×108 platelets/mL. All of the procedures were carried out at room temperature (25℃) to avoid platelet aggregation from cooling [25]. Animals were maintained as per the principles and guidelines of the Ethics Committee of the Animal Care of Inje University.
Measurement of platelet aggregation
The washed platelets (108/mL) were pre-incubated at 37℃ in the presence of 2 mM CaCl2 with or without TSKRG. After 3 min, platelets were further stimulated with thrombin (0.5 U/mL) for 5 min. The aggregation was monitored using an aggregometer (Chrono-Log Corporation) with gentle stirring. Each aggregation rate was calculated as a percent of light transmission. The suspending buffer was used as a reference (transmission 0). TSKRG was dissolved in water; this had no effect on the results.
Measurement of thromboxane B2
Because TXA2 is unstable and quickly converted to thromboxane B2 (TXB2), the amounts of TXA2 were evaluated by determining TXB2 [7]. Washed platelets (108/mL) were pre-incubated at 37℃ in the presence of 2 mM CaCl2 with or without TSKRG or other reagents. After 3 min, platelets were further stimulated with thrombin (0.5 U/mL) for 5 min. The reaction was terminated by adding ice-cold 5 mM EDTA and 0.2 mM indomethacin to inhibit subsequent metabolism of AA to TXA2. The amounts of TXB2, a stable metabolite of TXA2, were determined using a TXB2 EIA kit according to the procedure described by the manufacturer. The assay is based on the competition between unlabelled TXB2 and a fixed quantity of peroxidase-labelled TXB2 for a limited number of binding sites on a TXB2 specific antibody.
Isolation of microsomal fraction
Washed platelets (108 platelets/mL) in suspending buffer (pH 7.4) containing 1% protease inhibitor were sonicated once at a sensitivity of 100% for 20 s and 10 times on ice with a model HD2070 sonicator (Bandelin Electronic, Bandelin, Germany) to obtain platelet lysates. Each homogenate was ultracentrifuged at 105,000 ×g for 1 h at 4℃ to obtain a microsomal fraction containing endoplasmic reticulum (ER) membrane. The supernatant was considered as a cytosolic fraction. All of the separated fractions were identified by cytochrome c reductase (an ER membrane marker enzyme) [26] and used as enzyme source in Western blots as described below.
Cytochrome c reductase activity assay
Cytochrome c reductase is a flavoprotein localized in the ER. Cytochrome c reductase activity of the fractions (homogenates, cytosols and microsomes of platelets) was assayed using a cytochrome c reductase (NADPH) assay kit (Sigma-Aldrich). The reaction was initiated by addition of NADH and the reduction of cytochrome c was monitored by the increase of absorbance at 550 nm for 7 min with a kinetic program.
Western blot analysis
Protein concentrations were measured using the Bradford method. Protein samples (30 μg) from each treatment platelets were separated by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The separated proteins were electrophoretically transferred to a polyvinylidene fluoride membrane (GE Healthcare, Franklin Lakes, NJ, USA). An immunoblot was prepared using primary antibody (COX-1, 1:200 dilution; TXAS, 1:1000 dilution) and alkaline phosphatease-conjugated secondary antibody at a dilution of 1:10,000 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). After treatment with secondary antibody (anti-mouse of anti-goat IgG-horseradish peroxidase conjugate), detection of antibody-bound protein in the membrane was performed by enhanced chemiluminescence (GE Healthcare, Buckinghamshire, UK).
Cyclooxygenase-1 activity assay
For the measurement of COX-1 activity, the microsomal fraction of platelets was pre-incubated with aspirin (500 μM), a positive control as a COX inhibitor, with or without various concentrations of TSKRG at 37℃ for 30 min. COX-1 activity of the treated microsomal fraction was assayed with COX-1 fluorescent assay kit (Cayman Chemical).
Thromboxane A2 synthase activity
For the measurement of TXAS activity, the microsomal fraction was pre-incubated with ozagrel (11 nM, IC50), a positive control as a TXAS inhibitor, with or without various concentrations of TSKRG at 37℃ for 5 min. The reaction was initiated by the addition of PGH2. After incubation for 1 min at 37℃, the reaction was terminated by the addition of 1 M citric acid. After neutralization with 1 N NaOH, the amount of TXB2, a stable metabolite of TXA2, was determined using a TXB2 enzyme immunoassay kit according to the procedure described by the manufacturer.
Statistical analyses
The experimental results are expressed as the mean± SEM. accompanied by the number of observations. Data were assessed by analysis of variance. If this analysis indicated significant differences among the group means, then each group was compared by the Newman-Keuls method. A p-value <0.05 was considered statistically significant.
RESULTS
Threshold concentration of thrombin causing platelet aggregation in a maximum
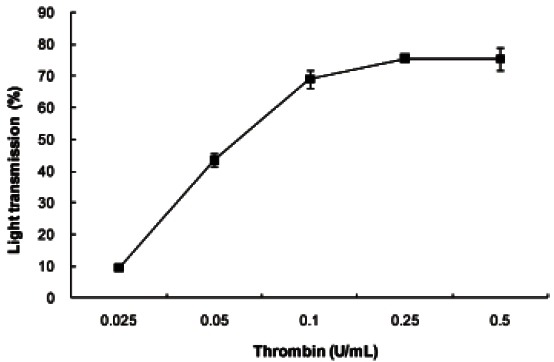
When washed platelets (108 platelets/mL) were incubated with various concentrations of thrombin, light transmission (%) indicating platelet aggregation was maximally increased by 0.25 U/mL of thrombin (Fig. 1). However, we used the 0.5 U/mL of thrombin.
Fig. 1. The concentration threshold of thrombin on platelet aggregation. Washed platelets (108/mL) were stimulated with various doses of thrombin for 5 min in the presence of 2 mM CaCl2 at 37℃. Platelet aggregation (%) was recorded as an increase in light transmission. Data are expressed as mean±SD (n=4).
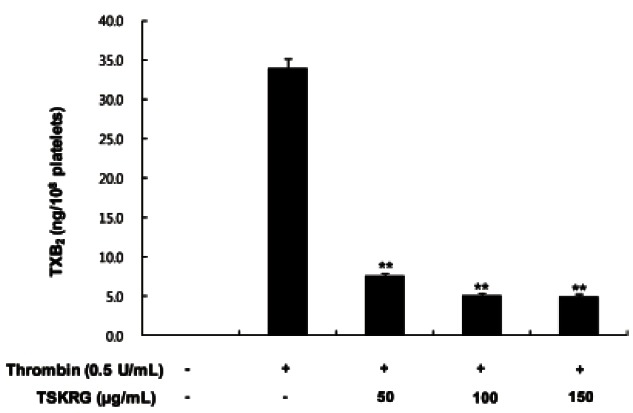
Effect of total saponin from Korean red ginseng on thromboxane A2 production
The amount of TXA2 (determined as TXB2) in intact platelets was 0.2 ng/108 platelets, which was markedly increased to 33.93±1.22 ng/108 platelets when platelets were stimulated with thrombin (0.5 U/mL). TSKRG (50- 150 μg/mL), however, powerfully reduced the production of TXA2 (Fig. 2). These results show that TSKRG may inhibit the activity of COX-1 or TXAS in a dosedependent manner to suppress the production of TXA2 in thrombin-induced platelet aggregation.
Fig. 2. Effect of total saponin from Korean red ginseng (TSKRG) on thrombin-induced thromboxane B2 (TXB2) generation. Washed platelets (108/mL) were pre-incubated for 3 min at 37℃ in the presence of 2 mM CaCl2 with or without TSKRG, and then thrombin (0.5 U/mL) was added. The reactions were terminated by adding ice-cold 5 mM EDTA and 0.2 mM indomethacin. The amounts of TXB2 were determined using a TXB2 enzyme immunoassay kit. Data are expressed as mean±SD (n=4). **p<0.001 compared with that of thrombin-induced platelets without TSKRG.
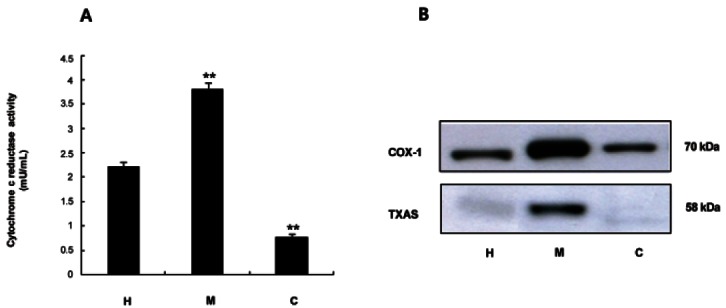
Determination of enzyme source of cyclooxygenase- 1 and thromboxane A2 synthase
To determine whether TSKRG is involved in the inhibition of COX-1 and TXAS, enzyme sources having COX-1 or TXAS were needed. We measured the activity of cytochrome c reductase, an ER marker enzyme, in homogenates, microsomes and cytosols of platelets. As shown in Fig. 3A, the microsomal fraction had the highest specific activity of cytochrome c reductase. Next, we determined the fraction from platelets that expressed COX-1 and TXAS. Remarkably high expressions of COX-1 (70 kDa) and TXAS (58 kDa) were observed in the microsomal fraction (Fig. 3B). These observations were consistent with the view that the microsomal fraction from platelets had the highest activity of cytochrome c reductase. Thus, microsomal fractions were used to determine the activities of COX-1 and TXAS.
Fig. 3. Determination of enzyme source on cyclooxygenase (COX)-1 and thromboxane A2 synthase (TXAS) activity. (A) Detection of cytochrome c reductase activities in homogenates, microsomes, and cytosols of platelets. NADPH-cytochrome c reductase, the marker enzyme for microsomes in platelets, was assayed by using a NADHP cytochrome c reductase assay kit. One hundred micrograms of protein were used. (B) Western blot analysis of COX-1 and TXAS in homogenates, microsomes, and cytosols of platelets. The 30 μg (TXAS, COX-1) of total protein on homogenates, microsomes, and cytosols of platelets was separated by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the resolved proteins were transferred to polyvinylidene fluoride membranes and detected by enhanced chemiluminescence. H, homogenates; M, microsomes; C, cytosols. **p<0.001 compared with that of total saponin from Korean red ginseng-non-treated microsomes.
Effect of total saponin from Korean red ginseng on cyclooxygenase-1 activity
COX-1 converts AA to PGH2. To determine if the inhibitory effect of TSKRG on TXA2 production was due to the direct suppression of COX-1, a cell-free enzyme assay method with the microsomal fraction of platelets was used. As shown in Fig. 4A, COX-1 activity in the absence of TSKRG (control) was 900.7±40.7 pmol/min/ mg protein. However, TSKRG produced dose-dependent inhibition of COX-1 activity; at 150 μg/mL, COX-1 activity was reduced to 397.0±11.5 pmol/min/mg protein. As a positive control, 500 μM of aspirin was used, which inhibited COX-1 activity to 340.1±31.1 nmol/min/mg protein (Fig. 4A). The 56% inhibition by 150 μg/mL TSKRG as compared with that of control (900.7±40.07 pmol/min/mg protein) was almost equal to the 62% inhibition elicited by 500 μM of aspirin.
Fig. 4. Inhibitory effect of total saponin from Korean red ginseng (TSKRG) on cyclooxygenase (COX)-1 and thromboxane A2 synthase (TXAS). (A) Effect of TSKRG on COX-1 activity in microsomes of platelets. The microsomal fraction (10 μg of total protein) of platelets was pre-incubated with or without various concentrations of TSKRG for 30 min at 37℃. The TSKRG-induced COX-1 activity was assayed with COX-1 fluorescent assay kit. (B) Effect of TSKRG on TXAS activity in platelet microsomes. The microsomal fraction (20 μg total protein) of platelets was pre-incubated for 5 min at 37℃ with or without various concentrations of TSKRG. The reaction was initiated by adding 5 μM prostaglandin H2 (PGH2) and terminated by adding 1 M citric acid. The activity was measured as the production of PGH2- mediated thromboxane B2 (TXB2) by TXB2 EIA kit. The data are given as the mean±SD (n=4). **p<0.001 compared with that of TSKRG-nontreated microsomal fraction of platelets.
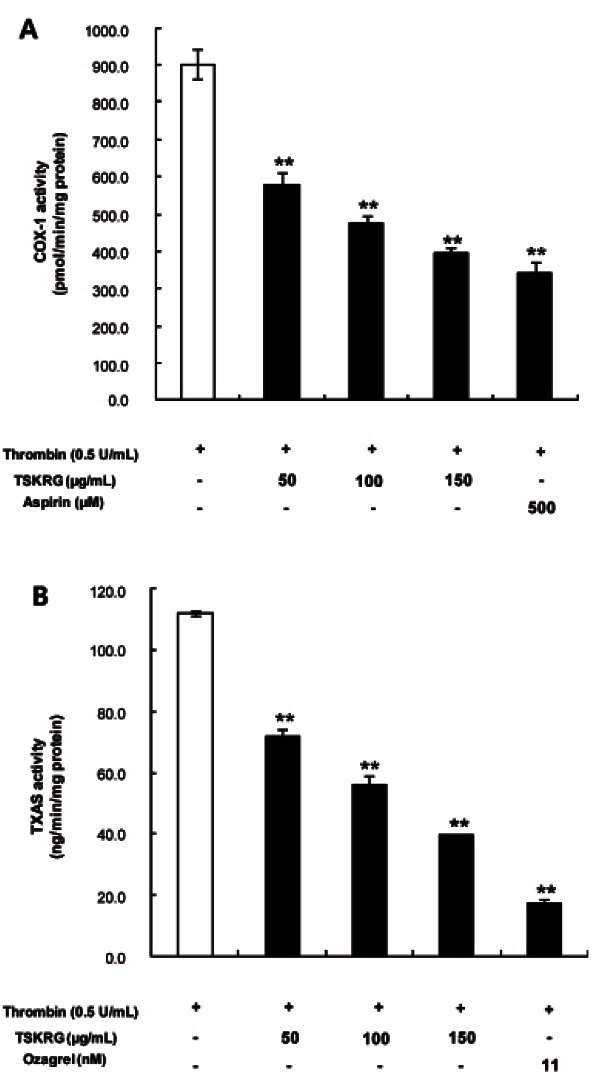
Effect of total saponin from Korean red ginseng on thromboxane A2 synthase activity
To determine whether the inhibitory effect on TXA2 production of TSKRG was due to the direct suppression of TXAS, the aforementioned cell-free enzyme assay method with the microsomal fraction of platelets was also used. In the TSKRG-untreated control, TXAS activity was 112.1±0.7 ng/min/mg protein. However, TSKRG inhibited TXAS activity in a dose-dependent fashion, to 39.6±0.3 ng/min/mg protein at 150 μg/mL. In addition, 11 nM ozagrel as a positive control was used; TXAS activity was 17.5±0.9 ng/min/mg protein (Fig. 4B). The 64.7% inhibition by 150 μg/mL TSKRG as compared with 112.1±0.7 ng/min/mg protein of the control (approximately 77%) to that (84.4%) by 11 nM of ozagrel.
DISCUSSION
TXA2 acts as a positive feedback promoter on activated platelets and is a strong agonist on resting platelets [3,27]. TXA2 is also a vasoconstrictor and a bronchoconstrictor [28]. Thus, a compound that can inhibit TXA2 action or production has potential application as an antithrombotic agent [29,30]. Because ginsenosides from P. ginseng do not inhibit TXA2 production in collagen-, ADP-, and AA-activated platelets [31], we investigated if TSKRG containing ginsenosides lacked inhibitory activity on agonist-produced TXA2. TSKRG decreased thrombin-elevated TXA2 level in a dose-dependent manner (Fig. 2), which is consistent with the report that panaxadial and panaxatriol inhibitor of thrombin- or adrenaline-induced TXA2 production [21,22].
Although these results indicate that TSKRG inhibits a pathway involved in TXA2 production, it is insufficient for a complete understanding of the inhibitory action of ginseng compounds on platelet aggregation. Presently, we tried to explain the inhibitory mechanism of TSKRG on TXA2-production by assaying the activities of the TXA2 production-associated microsomal enzymes, COX-1 and TXAS. Because COX-1 and TXAS are localized in the endoplasmic reticulum [32,33], we isolated microsomes from platelets, determined its marker enzyme, cytochrome c reductase, and observed the expression of COX-1 and TXAS. The activity of cytochrome c reductase was highest in the microsomal fraction rather than the cytosolic fraction or homogenates (Fig. 3A). The results mean that the microsomal fractions from platelet lysates contain COX-1 and TXAS.
As shown in Fig. 3B, COX-1 (70 kDa) and TXAS (50 kDa) were potently expressed in the microsomal fraction than in other cellular fractions. We determined the effects of TSKRG on the activities of COX-1 and TXAS in the microsomal fraction. TSKRG inhibited COX-1 and TXAS activities. In the TSKRG-untreated control, COX-1 activity was 900.7±40.7 pmol/min/mg protein. But, activity was decreased in the presence of TSKRG to 397.0±11.5 pmol/min/mg protein. As a positive control, 500 μM aspirin also inhibited COX-1 activity to 340.1±31.1 pmol/min/mg protein (Fig. 4A). These results suggest that TSKRG may be used as a COX-1 inhibitor with aspirin. In addition, TXAS activity in the absence of TSKRG was 112.1±0.7 ng/min/mg protein (Fig. 4B), while TSKRG inhibited TXAS activity to 39.6±0.3 ng/ min/mg protein. As a positive control, 11 nM ozagrel also inhibited TXAS activity to 17.5±0.9 ng/min/mg protein. TSKRG may be used as a TXAS inhibitor, similar to ozagrel. TSKRG that we used contained ginsenoside (G)-Rg1, G-Re, G-Rb1, G-Rc, G-Rg2, G-Rb2, G-Rd, and G-Rg3, which were analyzed with HPLC-ELSD method (Zorbax ODS C18 column [250×4.6 mm id, 5 μm], Zorbax ODS C18 guard column [12.5×4.6 mm id, 5 μm]). Unfortunately, in the present study, we don’t know which ginsenoside of TSKRG has inhibitory effect on thrombin- stimulated COX-1 and TXAS activities, these should be investigated in the future.
Thrombin is a strong stimulator of platelet aggregation, and activates phospholipase Cβ via protease-activated receptors (PARs)-1 and PAR-4 of GPCR, which supplies TXA2 precursor AA from phosphoinositides such as PIP2, phosphatidylinositol 4-monophosphate (PIP), and phosphatidylinositol (PI) [3,34]. Accordingly, it cannot not excluded that TSKRG could inhibit phospholipase C activity to suppress the thrombin-mediated hydrolysis of phosphoinositides such as PI, PIP, and PIP2. It can be inferred that panaxatriol from P. ginseng inhibited the thrombin-stimulated hydrolysis of PIP2, PIP, and PI [20]. At present, although ginsenoside Rg1, ginsenoside Rg3, and dihydroginsenoside Rg3 are known to have an anti-platelet effect [17,23,24,35], we are unable to discern which TSKRG ginsenoside is involved in the inhibition of COX-1 or TXAS activities to suppress TXA2 production. Hepoxilin cyclopropane, which inhibits TXA2 production by suppressing TXAS activity, inhibits the binding of TXA2 to the TXA2 receptor (TP) [36]. TXA2 is a platelet agonist that binds to TP linked to both Gαq and G12/G13 of GPCR [3]. Gi-stimulated phospholipase Cβ hydrolyses PIP2, PIP, and PI, which produces IP3 that in turn mobilizes Ca2+ and DG to activate PKC. These signaling pathways are involved in facilitating platelet aggregation. G12/G13 mediates the small GTPase Rho/ Rho-kinase, which stimulates phosphorylation of myosin light chain to fully activate platelet aggregation and degranulation [37]. Considering previous reports [3,36,37], TXAS synthase activity inhibition by TSKRG may be involve in the down-regulation of Gi- and G12/G13-mediated platelet aggregation pathway. In addition, spinach saponin and hepoxilin cyclopropane that inhibit TXA2 production increase cyclic adenosine monophosphate (cAMP) [36,38], which suppresses cytosolic free Ca2+, an aggregation-inducing molecule. Because TSKRG inhibits TXA2 production as well as spinach saponin and hepoxilin cyclopropane, TSKRG may be involved in increasing the production of cAMP. In conclusion, TSKRG has a beneficial anti-platelet effect within thrombotic diseases via the inhibition of COX-1 and TXAS activities.
Acknowledgments
This study was supported by a grant (20110233 to PHJ) from the National Research Foundation, and supported in part by a grant (2010-0024028) from Basic Science Research Program through the National Research Foundation funded by the Ministry of Education, Science and Technology, Korea, and supported in part by grant (20100420) from Inje University, Korea.
References
- 1.Schwartz SM, Heimark RL, Majesky MW. Developmental mechanisms underlying pathology of arteries. Physiol Rev. 1990;70:1177–1209. doi: 10.1152/physrev.1990.70.4.1177. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- 3.Jennings LK. Role of platelets in atherothrombosis. Am J Cardiol. 2009;103(3 Suppl):4A–10A. doi: 10.1016/j.amjcard.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Pasqui AL, Capecchi PL, Ceccatelli L, Mazza S, Gistri A, Laghi Pasini F, Di Perri T. Nitroprusside in vitro inhibits platelet aggregation and intracellular calcium translocation. Effect of haemoglobin. Thromb Res. 1991;61:113–122. doi: 10.1016/0049-3848(91)90238-r. [DOI] [PubMed] [Google Scholar]
- 5.Nishikawa M, Tanaka T, Hidaka H. Ca2+-calmodulin-dependent phosphorylation and platelet secretion. Nature. 1980;287:863–865. doi: 10.1038/287863a0. [DOI] [PubMed] [Google Scholar]
- 6.Murakami K, Chan SY, Routtenberg A. Protein kinase C activation by cis-fatty acid in the absence of Ca2+ and phospholipids. J Biol Chem. 1986;261:15424–15429. [PubMed] [Google Scholar]
- 7.Hamberg M, Svensson J, Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975;72:2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cattaneo M, Tenconi PM, Lecchi A, Mannucci PM. In vitro effects of picotamide on human platelet aggregation, the release reaction and thromboxane B2 production. Thromb Res. 1991;62:717–724. doi: 10.1016/0049-3848(91)90375-7. [DOI] [PubMed] [Google Scholar]
- 9.Moriyama T, Takamura H, Narita H, Tanaka K, Matsuura T, Kito M. Elevation of cytosolic free Ca2+ is directly evoked by thromboxane A2 in human platelets during activation with collagen. J Biochem. 1988;103:901–902. doi: 10.1093/oxfordjournals.jbchem.a122383. [DOI] [PubMed] [Google Scholar]
- 10.Patrono C. Aspirin as an antiplatelet drug. N Engl J Med. 1994;330:1287–1294. doi: 10.1056/NEJM199405053301808. [DOI] [PubMed] [Google Scholar]
- 11.Cipollone F, Patrignani P, Greco A, Panara MR, Padovano R, Cuccurullo F, Patrono C, Rebuzzi AG, Liuzzo G, Quaranta G, et al. Differential suppression of thromboxane biosynthesis by indobufen and aspirin in patients with unstable angina. Circulation. 1997;96:1109–1116. doi: 10.1161/01.cir.96.4.1109. [DOI] [PubMed] [Google Scholar]
- 12.Patrono C. Aspirin: new cardiovascular uses for an old drug. Am J Med. 2001;110:62S–65S. doi: 10.1016/S0002-9343(00)00645-8. [DOI] [PubMed] [Google Scholar]
- 13.Ichikawa K, Tazawa S, Hamano S, Kojima M, Hiraku S. Effect of ozagrel on locomotor and motor coordination after transient cerebral ischemia in experimental animal models. Pharmacology. 1999;59:257–265. doi: 10.1159/000028328. [DOI] [PubMed] [Google Scholar]
- 14.Park HJ, Lee JH, Song YB, Park KH. Effects of dietary supplementation of lipophilic fraction from Panax ginseng on cGMP and cAMP in rat platelets and on blood coagulation. Biol Pharm Bull. 1996;19:1434–1439. doi: 10.1248/bpb.19.1434. [DOI] [PubMed] [Google Scholar]
- 15.Park HJ, Rhee MH, Park KM, Nam KY, Park KH. Effect of non-saponin fraction from Panax ginseng on cGMP and thromboxane A2 in human platelet aggregation. J Ethnopharmacol. 1995;49:157–162. doi: 10.1016/0378-8741(95)01317-2. [DOI] [PubMed] [Google Scholar]
- 16.Rhee MH, Park KM, Park HJ, Nam KY, Park KH. Inhibition of serotonin release by lipophilic fraction from Korean red ginseng. Korean J Ginseng Sci. 1993;17:127–130. [Google Scholar]
- 17.Park HJ, No YH, Rhee MH, Park KM, Park KH. Effects of protein fractions and ginsenosides from Panax ginseng C.A. Meyer on substrate phosphorylation by a catalytic fragment of protein kinase. Korean Biochem J. 1994;27:280–283. [Google Scholar]
- 18.Kuo SC, Teng CM, Lee JC, Ko FN, Chen SC, Wu TS. Antiplatelet components in Panax ginseng. Planta Med. 1990;56:164–167. doi: 10.1055/s-2006-960916. [DOI] [PubMed] [Google Scholar]
- 19.Lee WM, Kamruzzaman SM, Song YB, Cho JY, Park HJ, Rhee MH. Inhibitory activities of red ginseng acidic polysaccharide in platelet aggregation. J Ginseng Res. 2008;32:73–78. doi: 10.5142/JGR.2008.32.1.073. [DOI] [Google Scholar]
- 20.Park JM, Rhee MH, Shin HJ, Song YB, Hyun HC, Park KH, Cho HJ, Choi SA, Kang HC, Kim KJ, et al. Inhibitory effects of Panaxatriol from Panax ginseng C.A. Meyer on phosphoinositide breakdown induced by thrombin in platelets. J Ginseng Res. 2008;32:107–113. doi: 10.5142/JGR.2008.32.2.107. [DOI] [Google Scholar]
- 21.Park KM, Rhee MH, Park HJ. Panaxadiol and panaxatriol from Panax ginseng C.A. Meyer inhibit the synthesis of thromboxane A2 in adrenaline-stimulated human platelet aggregation. Korean J Ginseng Sci. 1994;18:44–48. [Google Scholar]
- 22.Park HJ, Rhee MH, Park KM, Nam KY, Park KH. Panaxadiol from Panax ginseng C.A. Meyer inhibits synthesis of thromboxane A2 in platelet aggregation induced by thrombin. Korean J Ginseng Sci. 1993;17:131–134. [Google Scholar]
- 23.Kimura Y, Okuda H, Arichi S. Effects of various ginseng saponins on 5-hydroxytryptamine release and aggregation in human platelets. J Pharm Pharmacol. 1988;40:838–843. doi: 10.1111/j.2042-7158.1988.tb06285.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee WM, Kim SD, Park MH, Cho JY, Park HJ, Seo GS, Rhee MH. Inhibitory mechanisms of dihydroginsenoside Rg3 in platelet aggregation: critical roles of ERK2 and cAMP. J Pharm Pharmacol. 2008;60:1531–1536. doi: 10.1211/jpp.60.11.0015. [DOI] [PubMed] [Google Scholar]
- 25.Reuter H, Niemeyer G, Gross R. Studies of the aggregation of human blood platelets. 3. On the inhibition of platelet aggregation in EDTA plasma following incubation at 37 degrees C. Klin Wochenschr. 1967;45:1147–1149. doi: 10.1007/BF01727398. [DOI] [PubMed] [Google Scholar]
- 26.Lagarde M, Menashi S, Crawford N. Localisation of phospholipase A2 and diglyceride lipase activities in human platelet intracellular membranes. FEBS Lett. 1981;124:23–26. doi: 10.1016/0014-5793(81)80045-2. [DOI] [PubMed] [Google Scholar]
- 27.Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 28.FitzGerald GA. Mechanisms of platelet activation: thromboxane A2 as an amplifying signal for other agonists. Am J Cardiol. 1991;68:11B–15B. doi: 10.1016/0002-9149(91)90379-Y. [DOI] [PubMed] [Google Scholar]
- 29.Clutton P, Folts JD, Freedman JE. Pharmacological control of platelet function. Pharmacol Res. 2001;44:255–264. doi: 10.1006/phrs.2001.0861. [DOI] [PubMed] [Google Scholar]
- 30.Halushka PV, Allan CJ, Davis-Bruno KL. Thromboxane A2 receptors. J Lipid Mediat Cell Signal. 1995;12:361–378. doi: 10.1016/0929-7855(95)00023-J. [DOI] [PubMed] [Google Scholar]
- 31.Teng CM, Kuo SC, Ko FN, Lee JC, Lee LG, Chen SC, Huang TF. Antiplatelet actions of panaxynol and ginsenosides isolated from ginseng. Biochim Biophys Acta. 1989;990:315–320. doi: 10.1016/S0304-4165(89)80051-0. [DOI] [PubMed] [Google Scholar]
- 32.Samuelsson B. Biosynthesis and metabolism of prostaglandins. Prog Biochem Pharmacol. 1967;3:59–70. [Google Scholar]
- 33.Sun FF, Chapman JP, McGuire JC. Metabolism of prostaglandin endoperoxide in animal tissues. Prostaglandins. 1977;14:1055–1074. doi: 10.1016/0090-6980(77)90285-4. [DOI] [PubMed] [Google Scholar]
- 34.Savage B, Cattaneo M, Ruggeri ZM. Mechanisms of platelet aggregation. Curr Opin Hematol. 2001;8:270–276. doi: 10.1097/00062752-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Lee SR, Park JH, Choi KJ, Kim ND. Inhibitory effects of ginsenoside Rg3 on platelet aggregation and its mechanism of action. Korean J Ginseng Sci. 1997;21:132–140. [Google Scholar]
- 36.Pace-Asciak CR, Reynaud D, Demin P, Aslam R, Sun A. A new family of thromboxane receptor antagonists with secondary thromboxane synthase inhibition. J Pharmacol Exp Ther. 2002;301:618–624. doi: 10.1124/jpet.301.2.618. [DOI] [PubMed] [Google Scholar]
- 37.Moers A, Wettschureck N, Offermanns S. G13-mediated signaling as a potential target for antiplatelet drugs. Drug News Perspect. 2004;17:493–498. doi: 10.1358/dnp.2004.17.8.863692. [DOI] [PubMed] [Google Scholar]
- 38.Cho HJ, Choi SA, Kim CG, Hong JH, Park HJ, Park HJ. Dietary spinach saponin-enriched lipophilic fraction inhibits platelet aggregation and blood coagulation. J Med Food. 2011;14:784–791. doi: 10.1089/jmf.2010.1411. [DOI] [PubMed] [Google Scholar]


