Abstract
The major commercial ginsengs are Panax ginseng Meyer (Korean ginseng), P. quinquifolium L. (American ginseng), and P. notoginseng (Burk.) FH Chen (Notoginseng). P. ginseng is the most commonly used as an adaptogenic agent and has been shown to enhance physical performance, promote vitality, increase resistance to stress and aging, and have immunomodulatory activity. These ginsengs contain saponins, which can be classified as dammarane-type, ocotillol-type and oleanane-type oligoglycosides, and polysaccharides as main constituents. Dammarane ginsenosides are transformed into compounds such as the ginsenosides Rg3, Rg5, and Rk1 by steaming and heating and are metabolized into metabolites such as compound K, ginsenoside Rh1, protoand panaxatriol by intestinal microflora. These metabolites are nonpolar, pharmacologically active and easily absorbed from the gastrointestinal tract. However, the activities metabolizing these constituents into bioactive compounds differ significantly among individuals because all individuals possess characteristic indigenous strains of intestinal bacteria. To overcome this difference, ginsengs fermented with enzymes or microbes have been developed.
Keywords: Panax ginseng, Panax quinquifolium, Panax notoginseng, Constituents, Biotransformation
INTRODUCTION
The term ginseng refers to the dried roots of several plants of the species Panax sp. (Family Araliaceae). The three major commercial ginsengs are P. ginseng Meyer (Korean ginseng), which has been used as an herbal medicine for more than 2000 years [1], P. quinquifolium L. (American ginseng), and P. notoginseng (Burk.) FH Chen (Notoginseng) [2,3]. These ginsengs have been used worldwide for thousands of years as either food or herbal medicines. P. ginseng has been the most commonly used as an adaptogenic agent and has been shown to enhance physical performance, promote vitality, increase resistance to stress and aging, and have immunomodulatory activity [4,5].
The roots of these ginsengs have been used as herbal medicines in Asian countries, and their bioactive chemicals have been isolated. In 1854, Garriques performed the first chemical studies on ginseng [6] and separated a saponin fraction from P. quinquifolium. Despite these findings, the components of ginseng were not studied again until 1963. Shibata et al. [7], Shibata et al. [8], and Shibata et al. [9] isolated ginseng saponins from the root of P. ginseng and identified their structures in 1963. These saponins were called ginsenosides. Since the reports of Shibata et al., many researchers have isolated the components of Korean ginseng, American ginseng, and Notoginseng. Today, approximately 200 substances, such as ginsenosides, polysaccharides, polyacetylenes, peptides, and amino acids, have been isolated from Korean ginseng [2], and more than 100 substances have been isolated from American ginseng and Notoginseng. Among the substances isolated from ginseng, the major and most unique components are the ginseng saponins (ginsenosides), which can be classified as dammarane-type, ocotillol-type and oleanane-type oligoglycosides (Fig. 1), and polysaccharides [10]. Furthermore, the dammaranetype saponins are classified into protopanaxadiol and protopanaxatriol types. The protopanaxadiol-type has sugar moieties attached to OH at C-3 and/or C-20, and the protopanaxatriol-type has sugar moieties attached to OH at C-3, C-6, and/or C-20. The ocotillol-type has a five-membered epoxy ring at C-20, and the oleanane-type has a modified C-20 side chain. With the development of instrumental analysis, minor constituents are constantly being isolated from these ginsengs.
Fig. 1. The structures of representative ginseng saponins. PPD, protopanaxadiol; PPT, protopanaxatriol.
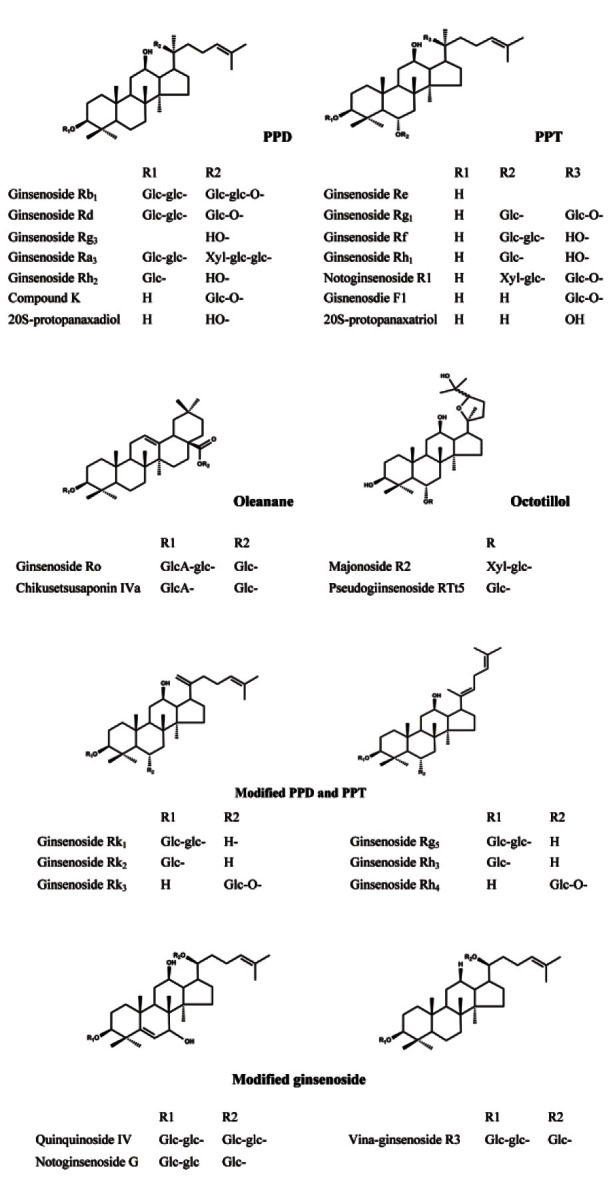
Chemically, several differences exist among Korean ginseng, American ginseng, and Notoginseng. An important parameter used for differentiation is the presence of the ginsenoside Rf in Korean ginseng and Notoginseng, the pseudoginsenoside F11 in American ginseng [11], and the notoginsenoside R1 in Korean ginseng and Notoginseng. In addition, the ratio of Rg1/ Rb1 has been widely used to differentiate between these ginsengs. Ratios of less than 0.4 are indicative of American ginseng, whereas a high value ratio is characteristic of Korean ginseng and Notoginseng [12]. Although notoginsenosides are not isolated from the root of Korean ginseng, many notoginsenosides are found in Notoginseng (Table 1).
Table 1.
Comparison of typical ginsenoside composition of Korean ginseng (Panax ginseng), American ginseng (P. quinquefolius) and Notoginseng (P. notogisneng)
| Chemical composition | Korean ginseng | American ginseng | Notoginseng |
|---|---|---|---|
|
| |||
| Major ginsenosides | Rb1, Rg1, Rb2 | Rb1, Re, Rd | Rb1, Rg1, Ra3, R1 |
| PPD-group to PPT-group | <2.0 | >2.0 | <2.0 |
| Rb1: Rg1 | <5.0 | >5.0 | <1 |
PPD, protopanaxadiol; PPT, protopanaxatriol.
Recent studies have reported the constituents of the roots of Korean ginseng, American ginseng and Notoginseng as well as their leaves and stem, flower buds, and fruits. Based on these findings, the leaves and stem, flower buds, fruits, and roots have recently been used as functional food, cosmetics, and herbal medicines. Furthermore, immunopotentiating polysaccharides have been isolated from these ginsengs. Acidic polysaccharides are more abundant in Korean ginseng compared with those in American ginseng and Notoginseng. However, the structures of many polysaccharides that are isolated from ginsengs have not been clearly identified.
CHEMICAL DIVERSITY OF PANAX GINSENG
Today, approximately 200 substances, such as ginsenosides, polysaccharides, polyacetylenes, peptides, and amino acids, have been isolated from Korean ginseng [2]. Of these, ginsenosides and polysaccharides are the major and most unique constituents.
Saponins
Saponins are the major constituents isolated from the root of Korean ginseng. Because P. ginseng has been historically used as an herbal medicine, extensive chemical studies have focused on the root of P. ginseng. Recent studies are focusing on the constituents of the leaves and stem, flower buds, fruits, and roots of ginseng and their pharmacological activities. Since Shibata et al. isolated prosapogenins in 1963, many kinds of saponins have been isolated from its roots, leaves and stem, flower buds, fruits, and seeds. Shibata et al. [7] established the chemical structures of main the prosapogenins 20Sprotopanaxadiol, 20S-protopanaxatriol, prosapgenin, and ginsenoside Rg1, which were extracted from the dried root of ginseng [8,9,13]. Kitagawa et al. [14] and Kitagawa et al. [15] isolated malonyl ginsenosides Rb1, Rb2, Rc, and Rd from the dried root of ginseng. Subsequently, the ginsenosides Ro, Ra1, Ra2, Ra3 Re, Rf, Rg1, Rg2, Rg3, Rh1, the notoginsenosides R4, 20-gluco-ginsenoside Rf, koryoginsenoside R1, and R2, and the ginsenosides Rb1, Rb2, Rc, and Rd, have been reported [11,16].
Recently, Ruan et al. [17] isolated the malonylginsenoside Ra3 from the root of P. ginseng. Zhu et al. [18] isolated 6 new protopanaxatriol-type ginsenosides, the ginsenosides Re1, Re2, Re3, Re4, Re5, Re6, and 10 known protopanaxatriol ginsenosides, including the ginsenoside Rg1 from the root of P. ginseng.
Approximately one thousand years ago, red (steamed) ginseng was developed to enhance the storage and the pharmacological effect of ginseng in Korea. Until recently, red ginseng had been widely made and largely consumed. Many scientists have isolated and identified its constituents to understand the pharmacological activities and bioactive components of red ginseng. Hiromichi et al. [19] isolated the ginsenosides Ra1, Ra2, and Ra3, and the notoginsenoside R4 from the steamed root of ginseng (red ginseng). Kasai et al. [20] isolated the ginsenosides Ra1, Ra2, Ra3, Rs1, and Rs2, the notoginsenoside R1, and the quinquenoside R1. Thereafter, the ginsenosides Ro, Rb1, Rb2, Tc, Rd, Re, Rf, Rg1, Rg2, Rg3, Rh1, the ginsenosides Rh2, 20R-ginsenoside Rh1, 20S-ginsenoside Rg3, and 20R-ginsenoside Rg2 were isolated from red ginseng. Ryu et al. [21] isolated the ginsenoside Rg6 and 20(E)-ginsenoside F4 from red ginseng. Baek et al. [22] isolated the ginsenoside Rh4. Then, the ginsenosides Rs1, Rs2, Rs3, and Rs4, the quinoginsenoside R4, the ginsenosides Rg3, Rg5, Rg6, F4, and Rf2 were isolated from red ginseng [23,24]. In particular, large quantities of the ginsenosides Rh1, Rg3 and Rg2 were found in red ginseng. Under intense steaming or heating, ginsenoside Rg3 may be further transformed to 20S-Rh2 and 20R-Rh2, and these ginsenosides subsequently become aglycone 20S-protopanaxadiol, 20R-protopanaxadiol, or even 20-dehydroprotopanaxadiol through chemical degradation. Ginsenosides Rk1 and Rg5 are again transformed into the degradation products Rk2 and Rh3. Rh1 can be changed into the aglycones 20S-protopanaxatriol, 20R-protopanaxatriol, and 20-dehydropropanaxatriol.
Shoji et al. [25] and Shoji et al. [26] were interested in the constituents of the leaves of P. ginseng. They isolated the ginsenosides Rb1, Rb2, Rc, Rd, Re, Rg1, F1, F2, and F4 from the leaves and stems. The ginsenosides F4, 20R-protopanaxadiol, 20R-protopanaxatriol, ginsenoside Rh3, 20R-ginsenoside Rh2, 20S-ginsenoside Rh2, ginsenosides Rh1, Rg3, Rg2, Rg1, Re, Rd, Rc, Rb2, and Rb1 were isolated from the flower buds of P. ginseng [27]. Ginsenosides Rh5, Rh6, Rh7, Rh8, Rh9 and Rg7, the majoroside F1, F2, the notoginsenoside Fe, the majoroside F4, and chikusetsusaponin L8 were also isolated [28,29].
Recently, Tung et al. [30] isolated two new ginsenosides Ki and Km from the leaves of P. ginseng. Liu et al. [31] isolated three new ginsenosides, 20S-3β,6α,12b-,20-tetrahydroxydammara-25-ene-24- one 20-O-β-glucopyranoside, 20S-3β,6α,12β-,20,24,25-pentahydroxydammarane 20-O-β-D-glucopyranoside, 20S,23E-3β,6α,12b,20,25-tetrahydroxydammara-23-ene 20-O-β-glucopyranoside and six known compounds from the leaves of P. ginseng. Wu et al. [32] isolated 3β,6α,12β-triol-22,23,23,25,26,27-hexanordammaran- 20-one and dammar-20(22),24-diene-3β,6α,12β-triol from the leaves of P. ginseng. Huang et al. [33] isolated new 20R,22(xi),24(S)-dammar-25(26)-ene-3β,6α,12β,20,22,24-hexanol from the leaves of P. ginseng [34]. Tung et al. steamed the leaves of P. ginseng, and then investigated their constituents. They isolated new ginsenosides: SL-1, SL-2, SL3, and 11 known compounds [35].
Shoji et al. [26] also investigated the constituents of the flower buds of P. ginseng. They isolated ginsenosides Rb1, Rb2, Rc, Rd, Re, Rg1, F3, and M7cd from the flower buds of P. ginseng. They also isolated ginsenosides Rb2, Rd, Rd, Re, Rg2, 20R-protopanaxatriol, 20Rginsenoside Rh1, 20R-ginsenoside Rg2, notoginsenoside-E, and gypenoside XVII from the flower buds of P. ginseng [25]. Yoshikawa et al. [36] isolated new floralginsenosides A, B, C, D, E, and F from the flower buds of P. ginseng. They also isolated new floralginsenosides-M, N, O, and P and the ginsenosides Rd and Re [37]. Nguyen et al. [38] isolated new floralginsenosides-Ta, Tb, Tc, and 6 known dammarane-type saponins from the flower buds of P. ginseng. Tung et al. [39] isolated antioxidant floralginsenosides Ka, Kb, and Kc from the flower buds of P. ginseng. Floralginsenosides Ka-Kc and majoroside F1 were also isolated from the flower buds of P. ginseng [39]. Recently, many researchers have been interested in the fruits and seeds of P. ginseng. Zhao et al. [40] isolated three new ginsenosides, 20R-25 methoxyldammarane-3β,6α,12β,20-triol, 20R-25-methoxyldammarane-3β,6α,12b,20-tetrol, and 20R-20,25 dimethoxyldammarane-3β,6α,12b,20-diol, and 2 known ginsenosides from the fruit of P. ginseng. Sugimoto et al. [41] isolated a new type of saponin, called panaxadione, from the seeds of P. ginseng.
Polysaccharides
Many kinds of polysaccharides have been isolated from Korean ginseng. For example, Konno et al. [42], Konno et al. [43], and Ohshima et al. [44] isolated the hypoglycemic glycans, panasans A - E, poanaxans F - H, panaxans I - L, panaxans M - P, and panaxans Q - U from the root of P. ginseng. Tomoda et al. [45] and Tomoda et al. [46] isolated the immunomodulating glycans ginsenan PA and ginsenan PB from the root of P. ginseng. These immunomodulationg glycans are composed of L-arabinose, D-galactose, L-rhamnose, D-galacturonic acid, and D-glucuronic acid. However, the intact structures of these glycans have not been identified. Immunomodulating glycans, such as acidic polysaccharide ginsenan S-IA and ginsenan S-II A (MW 5.6×104, 1.0×105), have been continually reported.
Others
Favonoids and polyacetylenes, such as kaempferol, trifolin and panasenoside, were also isolated from the roots of P. ginseng. Matsunaga et al. [47] isolated polyacetylenes, panaxynol, and panaxydol.
Iwabuchi et al. [48] isolated two sesquierpene alcohols, pansinsanol A and panasinsanol B. Sesquiterpene hydrocarbons, α-panasinene, β-panasinsene, α-neoclovene, and β-panasinsene were also isolated from the rootlet. Furthermore, two new sesquitene alcohols, ginsenol and senecrassidiol, were found [49,50]. In addition, ginsenoynes A, B, C, D, E, F, G, H, I, J, and K have been reported.
CHEMICAL DIVERSITY OF PANAX QUINQUIFOLIUM
American ginseng (P. quinquifolium L., family Araliaceae), a plant native to North America, is now cultivated in many countries. P. quinquifolium also contains saponins, flavonoids, polyacetylenes, polysaccharides, amino acids, fatty acids, and peptides.
Saponins
Saponins, particularly ginsenosides, are constituents of American ginseng (P. quinquifolium). More than 60 ginsenosides, including dammarane-, ocotillol-, and oleanane-types, have been isolated from the roots, leaves, stems, flower buds and berries of P. quinquifolium. Chen et al. [51] isolated quiquenosides L10 and L16 from the leaves and stems of P. quinquifolium. Jiang et al. [52] isolated two new saponins, 3β,12β,20S trihydroxydammar- 23-ene-3-O-{[β-D-glucopyranosyl(1->2)-β-Dglucopyranosyl]- 20-O-[α-L-arabinopyranosyl(1->)]-β- D-glucopyranoside and 3β-20S-dihydroxy-12β-23Repoxydammar- 24-ene-3-O-{[β-D-glucopyranosyl(1- >2)-β-D-glucopyranosyl]-20-O-[β-D-xylosyl(1->6)]-β- D-glucopyranoside from the leaves of P. quinquifolium. Many ginsenosides have been isolated from P. quinquifolium. Protopanaxadiol-type ginsenosides isolated from the roots, leaves and stems, and flower buds of American ginseng are the ginsenosides Rb1, Rc, Rb2, Rb3, Rd, Rg3, F2, Rs1, quinquenosides I, II, III, V, L10, L14, pseudoginsenosides RC1, F8, and gypenoside XVII [51-56]. Protopanaxatriol-type ginsenosides found in American ginseng are the ginsenosides Rg1, Rg2, Re, Rg2, F3, Ia, F1, Rh1, 20S-acetyl ginsenoside Rg2, 20Racetyl ginsenoside Rg2, floralquinquenoside E, 20Rginsenoside Rg2, Rh1, 20S-protopanxatriol, and 20Rprotopanaxatriol [52,54,56]. Du et al. [57] isolated four malonyl-ginsenosides, Rb1, Rc, Rb2, and Rd. In addition, the minor ginsenosides 4 ocotillol-type ginsenosides (24Rpseudoginsenoside F11, pseudoginsenoside RT5, F-11, 24R-vina-ginsenoside R1), 2 oleanane-type ginsenosides (ginsenoside Ro, chikusetsusaponin Iva), and 3 dammarane saponins with a modified steroid skeleton (vinaginsenoside R3, quinquenoside IV, and notoginsenoside G) were isolated from P. quinquifolium [54,56].
Minor saponins modified in C-20 side-chain (quinquenosides L1, L2, L8, L11, L3, L7, L9, L16, I, gypenosides LXIX, LXXI, majoroside-F1, notoginsenosides A, C, E, K, ginsenosides I, III, vina-ginsenoside R8, floralquinquenosides B, D, A, C, quinquefolosides La, Lc) were also isolated [52,54,58]. American red ginseng was also experimentally prepared under the same steam and heat treatments as Korean ginseng [59]. Then, the chemical composition of American red ginseng was studied. The constituents of the steamed American ginseng were largely different from those of the untreated ginseng. The steaming process induced obvious chemical degradation and conversion of original saponins to some newly occurring compounds. The polar ginsenosides, including Rg1, Re, Rb1, Rc, Rb2, Rb3, and Rd, decreased remarkably; however, less polar ginsenosides, including Rg2, Rg3, Rg5, Rh2, Rk1, and Rs4, increased [60,61]. The 20S and 20R-ginsenoside are typical stereoisomers formed by the selective attack of the hydroxyl group after elimination of the glycosyl residue at C-20. Additionally, Rk1/ Rg5 and Rk3/Rh4, isomers of the double bond at C-20/21 or C-20/22, were identified. This result was similar to the transformation of ginsenosides seen in Korean red ginseng.
Nakamura et al. [54] isolated five new dammaranetype triterpene glycosides, floralquinquenosides A, B, C, D, and E, and 18 known dammarane-type triterpene glycosides, ginsenoside Rb3, ginsenoside Rd, ginsenoside Rs1, pseudo-ginsenoside-RC1, pseudo-ginsenoside- F8, quinquenoside III, ginsenoside I, notoginsenoside- E, ginsenoside Re, ginsenoside Rg1, ginsenoside Rg2, ginsenoside-F3, ginsenoside Ia, quinquenoside L9, pseudo-ginsenoside RT5, pseudo-ginsenoside F11, and 24(S)-pseudo-ginsenoside F11 from the flower buds of P. quinquifolium L. Qiu et al. [62] isolated a new saponin quinquefoloside-L(c) (3β,12β,20Strihydroxy- 25-methoxy-25-methoxydammar-23-ene-3-O-β-Dglucopyranosyl( 1->2)β-D-glucopyranosyl-20-O-β- xylopyranosyl(1->6)-β-D-glucopyranoside) from the leaves of P. quinquifolium. This saponin was found to be cytotoxic against tumor cells.
Polysaccharides
Many kinds of polysaccharides were also isolated from American ginseng. Immunomodulating glycans, such as water-soluble COLD-FX (CVT-E002), were isolated from the roots of P. quinquifolium [63]. These glycans are poly furanosyl-pyranosylsaccharides. Hypoglycemic glycans, such as quinquefolans A, B, and C, were isolated from the roots of P. quinquifolium [64]. These glycans displayed hypoglycemic effects in normal and alloxan-induced hyperglycemic mice.
Others
Polyacetylenes, such as polyacetylene PQ-1, PQ- 2, PQ-3, panaxynol, panaxydol, and 1,8-heptadecadiene- 4,6-diyne-3,10-diol, were isolated from the roots of P. quinquifolium [65-67]. Nakamura et al. [54] isolated three flavonoids and 3 flavonoid glycosides, kaempferol 3-O-β-D-sophoroside-7-O-α-Lrhamnopyranoside, kaempferol-O-(2,3-di-E-p-coumaroyl-α-Lrhamnopyranoside), and kaempferol-3-O-α -L-rhamnopyranoside.
CHEMICAL DIVERSITY OF PANAX NOTOGINSENG
Notoginseng is also commonly used as a traditional Chinese medicine. Notoginseng contains saponins, flavonoids, polyacetylenes, polysaccharides, amino acids, fatty acids, and peptides. Ginsenosides and polysaccharides are notable components in P. notoginseng, just as they are in P. ginseng and P. quinquifolium [68,69].
Saponins
More than 60 dammarane saponins have been isolated from the roots, rhizome, rootlets, fibers, leaves, flower buds, seeds, and fruit pedicels of Notoginseng. Most of these saponins are ginsenosides and notoginsenosides.
However, an oleanolic acid saponin has not been found in Notoginseng. Oleanolic acid saponin occurs commonly in the plant kingdom and can be isolated from both Asian ginseng and American ginseng. The chemical constituents of American ginseng can be distinguished from those of Notoginseng.Yoshikawa et al. [70] and Yoshikawa et al. [71] isolated 9 dammaranetype triterpene glycosides, notoginsenosides-A, -B, -C, -D, -E, -G, -H, -I, and -J, and an acetylenic fatty acid glycoside, notoginsenic acid β-sophoroside. Chen and Sorensen [72] isolated ginsenosides Rb1, Rb2, Rc, Rd, Re, Rf, and Rg. Wei et al. [73], Wei et al. [74], and Wei et al. [75] subsequently reported the isolation of ginsenoside Rb1, notoginsenosides-Fc and -Fa, and gypenosides XV and XVII. The genuine aglycones of these ginsenosides are hydrolyzed to panaxadiol and panaxatriol (dammar-20(22)-en-3β,12β,26-triol, and 20R-dammaran-3β,12β,20,25-tetrol) by acidic hydrolysis [76]. Cui et al. [76] isolated two new ginsenosides, notoginsenoside Rw1 [6-O-β-D-xylopranosyl-20-O-β- D-xylopyranosyl-(1-˃6)-β-D-glucopyranosyl-dammar- 24-ene-3b,6a,12b,20(S)tetraol] and Rw2 [6-O-β- Dxylopyranosyl-(1->2)-β-D-glucopyranosyl-dammar- 22-ene-(trans)-3β,6α,12βb,20(S),25-pentaol], from the roots of P. notoginseng. Wan et al. [77] isolated 5,6-didehydroginsenoside Rd, 5,6-didehydroginsenoside Rb1 from the root of P. notoginseng. Liu et al. [78] identified 151 saponins in the root of notoginseng by Esi-Ms, HPLC/ESI-MS(n). Of these, 56 new compounds were identified, although these compounds were not isolated.
Recently, Wang et al. [79] isolated 20S-25-methoxyldammarane- 3β,12β,20-trio, a compound that exhibits potent cytotoxicity against tumor cells. Zhao et al. [80] isolated 20(S)-25-OCH3-protopanaxadiol, another cytotoxic compound against tumor cells, from the leaves of P. notoginseng. Wang et al. [81] isolated floranotoginsenosides- A, B, C, and D and 5 known compounds from the flower buds of P. notoginseng.
Sun et al. [82] steamed the root of notoginseng and isolated ginsenosides Rg1, Re, Rb1, Rd and notoginsenoside R1 from Notoginseng. As seen on the steaming process of red ginseng, steaming notoginseng increased the contents of ginsenosides Rh1, Rg2, Rg3, Rh2, and 20Rginsenoside Rg2. Teng et al. [83] treated notoginseng roots under a specific mild acidic condition and isolated twenty saponins from them. Five isolated saponins were new dammarane glycosides, and these saponins were named as notoginsenosides T1-T5. Other saponins were the ginsenosides Rg5, Rg2, Rg1, Re, Rh4, Rd, 20Rginsenoside Rg3, ginsenoside Rg3, 20R-ginsenoside Rh1, ginsenoside Rh1, the notoginsenosides E, R2, and R1, the gypenoside XVII, 20R-25-hydroxyginsenoside Rh1, and 20S-25-hydroxyginsenoside Rh1.
Polysaccharides
Sanchinan-A (1.5x106), PF3111, PF3112, PBGA11, and PBGA12 were isolated from Notoginseng. These compounds showed immunostimulating activity [84].
Others
Choi et al. [85] isolated the β-amyloid-induced neurotoxicity-ameliorating constituent, quercetin-3-β-xylopyranosyl-β-D-galactopyranoside, from the root of Notoginseng. Wei et al. [73] isolated quercetin-3-Osophoroside, kaempferol-3-O(2”-β-D-glucopyranosyl)- β-D-galactopyranoside, and quercetin-3-O(2”-β-Dglucopyranosyl)-β-D-galactopyranoside from the leaves of P. notoginseng. Liu et al. [86] isolated polyacetylene compounds, and Chan et al. [87] isolated trilinolein from the roots of P. notoginseng.
METABOLITES OF MAIN CONSTITUENTS AND THEIR BIOLOGICAL ACTIVITIES
When ginseng is orally administered to humans, its main constituents (ginseng saponins and polysaccharides) cannot be easily absorbed from the intestine due to their hydrophilicity. Therefore, these constituents inevitably come into contact with intestinal microflora in the alimentary tract and can be metabolized by intestinal microflora [88,89]. The metabolites are then easily absorbed from the gastrointestinal tract because most of the metabolites are nonpolar. These absorbed metabolites may express pharmacological actions. For example, when the extract of the root of P. ginseng was orally administered to humans, compound K and ginsenosides Rh1 and F1 were detected in the blood [90,91]. Ginsenosides Rb1 and Rb2 were not detected. When ginsenoside Rb1, a main constituent of P. ginseng, was orally administered to conventional rats, compound K was detected in the intestinal contents, blood, and urine [92,93]. Ginsenoside Rb1 was not detected. Furthermore, compound K was detected in the blood and intestinal contents when ginsenoside Rb1 was orally administered to gnotobiotic rats [94]. However, when ginsenoside Rb1 was orally administered to germ-free rats, compound K and ginsenoside Rb1 were not detected in the blood and intestinal contents. When Notoginseng extract, whose main constituents are ginsenoside Ra3, Rb1, Rd, Re, and Rg1 and notoginsenoside R1, was orally administered to rats, the compounds that were mainly absorbed into the blood were ginsenoside Ra2, Rb1, and Rd and compound K [95]. These results are not consistent. Nevertheless, compound K, a metabolites of protopanaxadiol ginsenosides, always are absorbed into the blood. Furthermore, of parental ginsenosides and their metabolites, compound K, ginsenoside Rh1, Rh2, and protopanaxatriol showed the most potent biological effects compared with those of parental compounds. For example, compound K and 20(S)-ginsenoside Rh2 exhibited the most potent cytotoxicity against tumor cells [96,97]. Ginsenoside Rb1 and Rb2 did not exhibit cytotoxicity against the tumor cell lines. However, most ginsenosides have anti-tumor activities in vivo [98,99]. Therefore, the metabolism of ginseng constituents by intestinal microflora is likely to play an important role in the pharmacological activity of ginseng. Protopanaxadiol ginsenosides are metabolized to compound K by human intestinal microflora, such as Bifidobacterium K-110, Bifidobacterium H-1, Provetellaoris, Fusobacterium K-60, Bacteroides JY-6, Eubacterium A-44, and Bifidobacterium K-506 [89]. Protopanaxatriol saponin ginsenosides Re and Rg1 were easily transformed to ginsenoside Rh1 or protopanaxatriol by human intestinal bacteria, such as Fusobacterium K-60, Bacteroides JY-6, Eubacterium A-44, and Bacteroides HJ-15 [100,101].
Compound K
Compound K exhibits chemopreventive [97], chemotherapeutic [102], anti-inflammatory [103], hepatoprotective [92], anti-pruritic [104], antiallergic [105], and hypoglycemic [106] effects in vitro and in vivo. Compound K also reduced stress in mice [107].
Ginsenoside Rh2
Ginsenoside Rh2 showed anti-tumor [99], anti-inflammatory [108], anti-pruritic [109], antiallergic [106], hepatoprotective [110], hypoglycemic, and hypolipidemic effects in mice [111,102]. Ginsenoside Rh2 effectively inhibited adipocyte differentiation via PPAR-γ inhibition [112] and improved insulin sensitivity [113]. Ginsenoside Rh2 ameliorated transient focal ischemia in rats [114].
Ginsenoside Rh1
Ginsenoside Rh1 exhibited anti-inflammatory [115], antiallergic [106], anti-dermatitic [116] effects in vitro and in vivo. Ginsenoside Rh1 showed anticarcinogenic and estrogenic effects [117] and stimulated the secretion of lipoprotein lipase in 3T3-L1 adipocytes [118].
Protopanaxatriol
Protopanaxatriol binds to glucocorticoid and estrogen receptors in endothelial cells and stimulates these receptors [119]. Protopanaxatriol (PPT) also has an estrogenic effect in MCF9 cells [120]. PPT has an adjuvant effect and activates PPARγ in 3T3-L1 adipocytes [121]. PPT also exhibits anti-inflammatory and antiangiogenic effects [122].
ACIDIC POLYSACCHARIDES
Ginseng polysaccharides are also degraded to low molecular weights by heat/steaming processing and intestinal microflora. However, the properties of the degraded molecules have not been clearly studied. Water-soluble polysaccharides and oligosaccharides from Korean ginseng have a number of effects on immune and host defense functions [123]. These glycans activate macrophages, induce IFN-γ and TNF-α production in immune cells, stimulate phagocytosis [124], stimulate natural killer-cell activity [125], and activate components of cell-mediated immunity [126]. Red ginseng acidic polysaccharides restored the proliferation of splenocytes and NK cell activity that had been suppressed by paclitaxel. Additionally, the synergistic effect of RGAP and paclitaxel increased the tumoricidal activity of macrophages [127]. Antirotaviral pectic polysaccharide was isolated from heat-processed ginseng [128].
Industrial application
When ginseng is orally administered to humans, its hydrophilic components inevitably come into contact with intestinal microflora in the alimentary tract and undergo transformation prior to absorption from the gastrointestinal tract. The pharmacological activities of these compounds are then expressed.
All individuals possess characteristic indigenous strains of intestinal bacteria. The activities that metabolize these constituents into bioactive compounds that are absorbable from the intestine into the blood differ significantly between individuals. Ginsengs containing bioactive and absorbable metabolites are valuable for treating various diseases. Therefore, fermented and heat-processed ginseng products have recently been released onto the market. Fermentation and heat processing transform hydrophilic components to hydrophobic compounds that can be easily absorbed from the gastrointestinal tract. To develop fermented ginseng, edible microbes, such as probiotics, should be used. Currently, there are few developed fermented ginsengs.
Therefore, research related to the biotransformation of ginsenosides is focused on bioactive production.
These metabolites, such as ginsenoside compound K, Rh1, Rh2, Rk1, Rh3, Rh4, and protopanaxadiol, are readily absorbed into the blood and express pharmacological effects. These ginsenosides have demonstrated excellent potential for pharmacological use. To develop these ginsenosides, many kinds of microbes isolated from soils or intestinal microflora have been used (Table 2) [129,130].
Table 2.
Ginsenosides transformation by hydrolyzing the sugar moieties in ginsenosides using microbial glycosidases
| Substrate | Product | Microorganism | Enzyme | Reference |
|---|---|---|---|---|
|
| ||||
| Ginseng extract | C-K | Bifidobacterium longum H-1 | β-D-Glucosidase/α-L-Arabinosidase | [131] |
| Ginseng extract | Sulfolobus solfataricus | β-D-Glycosidase (recombinant: 83) | [132] | |
| Ginseng extract | Aspergillus niger | Pectinase (commercial) | [133] | |
| Ginseng extract | Rh2 | Bifidobacterium longum H-1 | β-D-Glucosidase (crude) | [134] |
| Ginseng extract | Rg3 | Trichoderma reesie | Cellulase (commercial) | [135] |
| Ra1, Ra2 | Rb2, Rc | Bifidobacterium breve | β-D-Xylosidase (purified) | [136] |
| Gypenoside-5 | Rd | Absidia sp. | α-L-Rhamnosidase (purified) | [137] |
| Rb2 | Bifidobacterium breve | α-L-Arabinopyranosidase (purified) | [138] | |
| Rc | Bifidobacterium breve | α-L-Arabinopyranosidase (purified) | [136] | |
| Rb1 | Chloroflexus aurantiacus | β-D-Glucosidase (recombinant) | [139] | |
| Rb1 | Cladosprorium fulvum | β-D-Glucosidase (purified) | [140] | |
| Rb1, Rb2, Rc | Thermus caldophilus | β-D-Glycosidase (recombinant) | [141] | |
| PPD mixture | Trichoderma viride | Cellulase (commercial) | [142] | |
| Rb1, Rb2, Rc | Rg3 | Penicillium sp. | Lactase (commercial) | [143] |
| Rb1 Rd | Paecilomyces bainier | β-D-Glucosidase (purified) | [144] | |
| Rb1, Rb2, Rc | F2 | Aspergillus oryzae | Lactase (commercial) | [142] |
| Rb1, Rb2, Rc | Penicillium sp. | Lactase (commercial) | [143] | |
| Rb1, Rb, Rc | C-K, C-Y, C-Mc | Sulfolobus acidocaldarius | β-D-Glycosidase (recombinant) | [132] |
| Rb1, Rb, Rc, | Paecilomyces bainier | β-D-Glucosidase (purified) | [145] | |
| Rb1 | C-K | Fusobacterium sp. | β-D-Glucosidase (purified) | [146] |
| Rb1, Rb2, Rc | Aspergillus oryzae | β-D-Galactosidase (commercial) | [142] | |
| Rb1, Rb2, Rc | Penicillium sp. | Lactase (commercial) | [143] | |
| Rb1, Rb2, Rb3, Rc | Aspergillus sp. | β-D-Glycosidase (purified) | [147] | |
| Rg3 | Rh2 | Fusarium proliferatum | β-D-Glucosidase (purified) | [148] |
| Re | Rg1 | Penicillium sp. | Hesperidinase (commercial) | [143] |
| Re | Bacterodies sp. | α-D-Rhamnosidase (purified) | [149] | |
| Re | Rg2 | Aspergillus oryzae | β-Galactosidase (commercial) | [142] |
| Re | Bacterodies sp. | β-D-Glucosidase (purified) | [150] | |
| Rg1 | F1 | Bacterodies sp. | β-D-Glucosidase (purified) | [150] |
| Re, Rg1 | Penicillium decumbens | Naringinase (commercial) | [142] | |
| Rg1 | Rh1 | Bacterodies sp. | β-D-Glucosidase (purified) | [150] |
| Rg2 | Aspergillus oryzae | β-Galactosidase (commercial) | [143] | |
| Rg2 | Absidia sp. | α-L -Rhamnosidase (purified) | [151] | |
| Rg1, Rg2, Rf | Penicillium sp. | Lactase (commercial) | [143] | |
| Rf | Aspergillus niger | β-D-Glucosidase (recombinant) | [152] | |
C-K, compound K; PPD, protopanaxadiol; C-Mc, compound Mc; C-Y, compound Y.
However, most of these biotransformed ginseng extracts are inedible, and most of these microbes may not be safe. Therefore, to develop fermented ginseng extract, we must consider fermented bacteria (Are these bacteria edible?) and safe metabolites (Are these metabolites produced by industrial applied microbes similar to metabolites produced by human intestinal microflora?). Nevertheless, these metabolites are good candidates for new drugs.
CONCLUSION
Korean ginseng, American ginseng, and Notoginseng contain saponins, which are found to be dammarane-type, ocotillol-type and oleanane-type oligoglycosides, and polysaccharides. Of these constituents, dammarane ginsenosides can be metabolized into compound K, ginsenoside Rh1, and protopanaxatriol by intestinal microflora. These metabolites, such as compound K and protopanaxatriol, are pharmacologically active and easily absorbed from the gastrointestinal tract. However, the activities that metabolize these constituents into bioactive compounds differ significantly among individuals. To overcome this challenge, ginsengs have been fermented with enzymes or microbes to produce such metabolites. However, before using these enzymes and probiotics, the safety of these microbes and metabolites must be assessed. The safe ginseng bioproducts produced by enzymes or microbes are valuable for the development of new drugs and/or functional foods.
References
- 1.Li CP, Li RC. An introductory note to ginseng. Am J Chin Med (Gard City N Y) 1973;1:249–261. doi: 10.1142/S0192415X73000279. [DOI] [PubMed] [Google Scholar]
- 2.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/S0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy DO, Scholey AB. Ginseng: potential for the enhancement of cognitive performance and mood. Pharmacol Biochem Behav. 2003;75:687–700. doi: 10.1016/S0091-3057(03)00126-6. [DOI] [PubMed] [Google Scholar]
- 4.Singh VK, Agarwal SS, Gupta BM. Immunomodulatory activity of Panax ginseng extract. Planta Med. 1984;50:462–465. doi: 10.1055/s-2007-969773. [DOI] [PubMed] [Google Scholar]
- 5.Scaglione F, Ferrara F, Dugnani S, Falchi M, Santoro G, Fraschini F. Immunomodulatory effects of two extracts of Panax ginseng C.A. Meyer. Drugs Exp Clin Res. 1990;16:537–542. [PubMed] [Google Scholar]
- 6.Garriques SS. On panaquilon, a new vegetable substance. Ann Chem Pharm. 1854;90:231–234. doi: 10.1002/jlac.18540900216. [DOI] [Google Scholar]
- 7.Shibata S, Tanaka O, NagaI M, Ishit T. Studies on the constituents of Japanese and Chinese crude drugs. XII. Panaxadiol, a sapogenin of ginseng roots. Chem Pharm Bull (Tokyo) 1963;11:762–765. doi: 10.1248/cpb.11.762. [DOI] [PubMed] [Google Scholar]
- 8.Shibata S, Ando T, Tanaka O, Meguro Y, Sôma K, Iida Y. Saponins and sapogenins of Panax ginseng C.A. Meyer and some other Panax spp. Yakugaku Zasshi. 1965;85:753–755. [PubMed] [Google Scholar]
- 9.Shibata S, Tanaka O, Soma K, Aando T, Iida Y, Nakamura H. Studies on saponins and sapogenins of ginseng. The structure of panaxatriol. Tetrahedron Lett. 1965;42:207–213. doi: 10.1016/s0040-4039(01)99595-4. [DOI] [PubMed] [Google Scholar]
- 10.Lu JM, Yao Q, Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi KT. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 2008;29:1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 12.Yuan CS, Wang CZ, Wicks SM, Qi LW. Chemical and pharmacological studies of saponins with a focus on American ginseng. J Ginseng Res. 2010;34:160–167. doi: 10.5142/jgr.2010.34.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibata S, Fujita M, Itokawa H, Tanaka O, Ishii T. Studies on the constituents of Japanese and Chinese crude drugs. XI. Panaxadiol, a sapogenin of ginseng roots. Chem Pharm Bull (Tokyo) 1963;11:759–761. doi: 10.1248/cpb.11.759. [DOI] [PubMed] [Google Scholar]
- 14.Kitagawa I, Taniyama T, Shibuya H, Noda T, Yoshikawa M. Chemical studies on crude drug processing. V. On the constituents of ginseng radix rubra (2): comparison of the constituents of white ginseng and red ginseng prepared from the same Panax ginseng root. Yakugaku Zasshi. 1987;107:495–505. doi: 10.1248/yakushi1947.107.7_495. [DOI] [PubMed] [Google Scholar]
- 15.Kitagawa I, Yoshikawa M, Yoshihara M, Hayashi T, Taniyama T. Chemical studies of crude drugs (1). Constituents of ginseng radix rubra. Yakugaku Zasshi. 1983;103:612–622. [PubMed] [Google Scholar]
- 16.Haruyo K, Shuichi S, Yoshiteru I, Junzo S. Studies on the saponins of ginseng. IV. On the structure and enzymatic hydrolysis of ginsenoside Ra1. Chem Pharm Bull. 1982;30:2393–2398. doi: 10.1248/cpb.30.2393. [DOI] [Google Scholar]
- 17.Ruan CC, Liu Z, Li X, Liu X, Wang LJ, Pan HY, Zheng YN, Sun GZ, Zhang YS, Zhang LX. Isolation and characterization of a new ginsenoside from the fresh root of Panax ginseng. Molecules. 2010;15:2319–2325. doi: 10.3390/molecules15042319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu GY, Li YW, Hau DK, Jiang ZH, Yu ZL, Fong WF. Protopanaxatriol-type ginsenosides from the root of Panax ginseng. J Agric Food Chem. 2011;59:200–205. doi: 10.1021/jf1037932. [DOI] [PubMed] [Google Scholar]
- 19.Hiromichi B, Ryoji K, Yuhichiro S, Tohru F. Ginsenoside Ra1 and ginsenoside Ra2, new dammarane-saponins of ginseng roots. Chem Pharm Bull. 1982;30:2380–2385. doi: 10.1248/cpb.30.2380. [DOI] [Google Scholar]
- 20.Kasai R, Besso H, Tanaka O, Saruwatani Y, Fuwa T. Saponins of red ginseng. Chem Pharm Bull. 1983;31:2120–2125. doi: 10.1248/cpb.31.2120. [DOI] [Google Scholar]
- 21.Ryu JH, Park JH, Kim TH, Sohn DH, Kim JM, Park JH. A genuine dammarane glycoside, (20E)-ginsenoside F4 from Korean red ginseng. Arch Pharm Res. 1996;19:335–336. doi: 10.1007/BF02976251. [DOI] [Google Scholar]
- 22.Baek NI, Kim DS, Lee YH, Park JD, Lee CB, Kim SI. Ginsenoside Rh4, a genuine dammarane glycoside from Korean red ginseng. Planta Med. 1996;62:86–87. doi: 10.1055/s-2006-957816. [DOI] [PubMed] [Google Scholar]
- 23.Baek NI, Kim JM, Park JH, Ryu JH, Kim DS, Lee YH, Park JD, Kim SI. Ginsenoside Rs(3), a genuine dammarane- glycoside from Korean red ginseng. Arch Pharm Res. 1997;20:280–282. doi: 10.1007/BF02976158. [DOI] [PubMed] [Google Scholar]
- 24.Park JD, Lee YH, Kim SI. Ginsenoside Rf2, a new dammarane glycoside from Korean red ginseng (Panax ginseng). Arch Pharm Res. 1998;21:615–617. doi: 10.1007/BF02975384. [DOI] [PubMed] [Google Scholar]
- 25.Shoji Y, Kiyoko K, Osamu T. Further study on dammarane- type saponins of roots, leaves, flower-buds, and fruits of Panax ginseng C.A. Meyer. Chem Pharm Bull. 1979;27:88–92. doi: 10.1248/cpb.27.88. [DOI] [Google Scholar]
- 26.Shoji Y, Kiyoko M, Ryoji K, Osamu T. Saponins of buds and flowers of Panax ginseng C. A. Meyer. (1). Isolation of ginsenosides-Rd, -Re, and -Rg1. Chem Pharm Bull. 1976;24:3212–3213. doi: 10.1248/cpb.24.3212. [DOI] [Google Scholar]
- 27.Zhang S, Takeda T, Zhu T, Chen Y, Yao X, Tanaka O, Ogihara Y. A new minor saponin from the leaves of Panax ginseng. Planta Med. 1990;56:298–300. doi: 10.1055/s-2006-960963. [DOI] [PubMed] [Google Scholar]
- 28.Dou DQ, Chen YJ, Liang LH, Pang FG, Shimizu N, Takeda T. Six new dammarane-type triterpene saponins from the leaves of Panax ginseng. Chem Pharm Bull (Tokyo) 2001;49:442–446. doi: 10.1248/cpb.49.442. [DOI] [PubMed] [Google Scholar]
- 29.Qiu F, Ma ZZ, Xu S, Yao XS, Chen YJ, Che ZT. Studies on dammarane-type saponins in the flower-buds of Panax ginseng C.A. Meyer. J Asian Nat Prod Res. 1998;1:119–123. doi: 10.1080/10286029808039853. [DOI] [PubMed] [Google Scholar]
- 30.Tung NH, Song GY, Park YJ, Kim YH. Two new dammarane- type saponins from the leaves of Panax ginseng. Chem Pharm Bull. 2009;57:1412–1414. doi: 10.1248/cpb.57.1412. [DOI] [PubMed] [Google Scholar]
- 31.Liu GY, Li XW, Wang NB, Zhou HY, Wei W, Gui MY, Yang B, Jin YR. Three new dammarane-type triterpene saponins from the leaves of Panax ginseng C.A. Meyer. J Asian Nat Prod Res. 2010;12:865–873. doi: 10.1080/10286020.2010.508035. [DOI] [PubMed] [Google Scholar]
- 32.Wu LJ, Wang LB, Gao HY, Wu B, Song XM, Tang ZS. A new compound from the leaves of Panax ginseng. Fitoterapia. 2007;78:556–560. doi: 10.1016/j.fitote.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Huang J, Tang XH, Ikejima T, Sun XJ, Wang XB, Xi RG, Wu LJ. A new triterpenoid from Panax ginseng exhibits cytotoxicity through p53 and the caspase signaling pathway in the HepG2 cell line. Arch Pharm Res. 2008;31:323–329. doi: 10.1007/s12272-001-1159-8. [DOI] [PubMed] [Google Scholar]
- 34.Tung NH, Song GY, Minh CV, Kiem PV, Jin LG, Boo HJ, Kang HK, Kim YH. Steamed ginseng-leaf components enhance cytotoxic effects on human leukemia HL-60 cells. Chem Pharm Bull (Tokyo) 2010;58:1111–1115. doi: 10.1248/cpb.58.1111. [DOI] [PubMed] [Google Scholar]
- 35.Tung NH, Quang TH, Son JH, Koo JE, Hong HJ, Koh YS, Song GY, Kim YH. Inhibitory effect of ginsenosides from steamed ginseng-leaves and flowers on the LPS-stimulated IL-12 production in bone marrow-derived dendritic cells. Arch Pharm Res. 2011;34:681–685. doi: 10.1007/s12272-011-0419-2. [DOI] [PubMed] [Google Scholar]
- 36.Yoshikawa M, Sugimoto S, Nakamura S, Sakumae H, Matsuda H. Medicinal flowers. XVI. New dammaranetype triterpene tetraglycosides and gastroprotective principles from flower buds of Panax ginseng. Chem Pharm Bull (Tokyo) 2007;55:1034–1038. doi: 10.1248/cpb.55.1034. [DOI] [PubMed] [Google Scholar]
- 37.Yoshikawa M, Sugimoto S, Nakam S, Matsuda H. Medicinal flowers. XI. Structures of new dammarane-type triterpene diglycosides with hydroperoxide group from flower buds of Panax ginseng. Chem Pharm Bull (Tokyo) 2007;55:571–576. doi: 10.1248/cpb.55.571. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen HT, Song GY, Kim JA, Hyun JH, Kang HK, Kim YH. Dammarane-type saponins from the flower buds of Panax ginseng and their effects on human leukemia cells. Bioorg Med Chem Lett. 2010;20:309–314. doi: 10.1016/j.bmcl.2009.10.110. [DOI] [PubMed] [Google Scholar]
- 39.Tung NH, Song GY, Nhiem NX, Ding Y, Tai BH, Jin LG, Lim CM, Hyun JW, Park CJ, Kang HK, et al. Dammarane-type saponins from the flower buds of Panax ginseng and their intracellular radical scavenging capacity. J Agric Food Chem. 2010;58:868–874. doi: 10.1021/jf903334g. [DOI] [PubMed] [Google Scholar]
- 40.Zhao JM, Li N, Zhang H, Wu CF, Piao HR, Zhao YQ. Novel dammarane-type sapogenins from Panax ginseng berry and their biological activities. Bioorg Med Chem Lett. 2011;21:1027–1031. doi: 10.1016/j.bmcl.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 41.Sugimoto S, Nakamura S, Matsuda H, Kitagawa N, Yoshikawa M. Chemical constituents from seeds of Panax ginseng: structure of new dammarane-type triterpene ketone, panaxadione, and hplc comparisons of seeds and flesh. Chem Pharm Bull (Tokyo) 2009;57:283–287. doi: 10.1248/cpb.57.283. [DOI] [PubMed] [Google Scholar]
- 42.Konno C, Sugiyama K, Kano M, Takahashi M, Hikino H. Isolation and hypoglycaemic activity of panaxans A, B, C, D and E, glycans of Panax ginseng roots. Planta Med. 1984;50:434–436. doi: 10.1055/s-2007-969757. [DOI] [PubMed] [Google Scholar]
- 43.Konno C, Murakami M, Oshima Y, Hikino H. Isolation and hypoglycemic activity of panaxans Q, R, S, T and U, glycans of Panax ginseng roots. J Ethnopharmacol. 1985;14:69–74. doi: 10.1016/0378-8741(85)90030-3. [DOI] [PubMed] [Google Scholar]
- 44.Oshima Y, Konno C, Hikino H. Isolation and hypoglycemic activity of panaxans I, J, K and L, glycans of Panax ginseng roots. J Ethnopharmacol. 1985;14:255–259. doi: 10.1016/0378-8741(85)90091-1. [DOI] [PubMed] [Google Scholar]
- 45.Tomoda M, Takeda K, Shimizu N, Gonda R, Ohara N, Takada K, Hirabayashi K. Characterization of two acidic polysaccharides having immunological activities from the root of Panax ginseng. Biol Pharm Bull. 1993;16:22–25. doi: 10.1248/bpb.16.22. [DOI] [PubMed] [Google Scholar]
- 46.Tomoda M, Hirabayashi K, Shimizu N, Gonda R, Ohara N, Takada K. Characterization of two novel polysaccharides having immunological activities from the root of Panax ginseng. Biol Pharm Bull. 1993;16:1087–1090. doi: 10.1248/bpb.16.1087. [DOI] [PubMed] [Google Scholar]
- 47.Matsunaga H, Katano M, Yamamoto H, Mori M, Takata K. Studies on the panaxytriol of Panax ginseng C. A. Meyer. Isolation, determination and antitumor activity. Chem Pharm Bull (Tokyo) 1989;37:1279–1281. doi: 10.1248/cpb.37.1279. [DOI] [PubMed] [Google Scholar]
- 48.Iwabuchi H, Yoshikura M, Ikawa Y, Kamisako W. Studies on the sesquiterpenoids of Panax ginseng C. A. Meyer. Isolation and structure determination of sesquiterpene alcohols, panasinsanols A and B. Chem Pharm Bull (Tokyo) 1987;35:1975–1981. doi: 10.1248/cpb.35.1975. [DOI] [PubMed] [Google Scholar]
- 49.Iwabuchi H, Yoshikura M, Kamisako W. Studies on the sesquiterpenoids of Panax ginseng C. A. Meyer. II. Isolation and structure determination of ginsenol, a novel sesquiterpene alcohol. Chem Pharm Bull (Tokyo) 1988;36:2447–2451. doi: 10.1248/cpb.36.2447. [DOI] [PubMed] [Google Scholar]
- 50.Iwabuchi H, Kato N, Yoshikura M. Studies on the sesquiterpenoids of Panax ginseng C. A. Meyer. IV. Chem Pharm Bull (Tokyo) 1990;38:1405–1407. doi: 10.1248/cpb.38.1405. [DOI] [PubMed] [Google Scholar]
- 51.Chen J, Zhao R, Zeng YM, Meng H, Zuo WJ, Li X, Wang JH. Three new triterpenoid saponins from the leaves and stems of Panax quinquefolium. J Asian Nat Prod Res. 2009;11:195–201. doi: 10.1080/10286020802682734. [DOI] [PubMed] [Google Scholar]
- 52.Jiang HP, Qiu YK, Cheng DR, Kang TG, Dou DQ. Structure elucidation and complete NMR spectral assignments of two new dammarane-type tetraglycosides from Panax quinquefolium. Magn Reson Chem. 2008;46:786–790. doi: 10.1002/mrc.2247. [DOI] [PubMed] [Google Scholar]
- 53.Li GY, Zeng YM, Meng H, Li X, Wang JH. A new triterpenoid saponin from the leaves and stems of Panax quinquefolium L. Chin Chem Lett. 2009;20:1207–1210. doi: 10.1016/j.cclet.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 54.Nakamura S, Sugimoto S, Matsuda H, Yoshikawa M. Medicinal flowers. XVII. New dammarane-type triterpene glycosides from flower buds of American ginseng, Panax quinquefolium L. Chem Pharm Bull (Tokyo) 2007;55:1342–1348. doi: 10.1248/cpb.55.1342. [DOI] [PubMed] [Google Scholar]
- 55.Wang JH, Li W, Sha Y, Tezuka Y, Kadota S, Li X. Triterpenoid saponins from leaves and stems of Panax quinquefolium L. J Asian Nat Prod Res. 2001;3:123–130. doi: 10.1080/10286020108041379. [DOI] [PubMed] [Google Scholar]
- 56.Yoshikawa M, Murakami T, Yashiro K, Yamahara J, Matsuda H, Saijoh R, Tanaka O. Bioactive saponins and glycosides. XI. Structures of new dammarane-type triterpene oligoglycosides, quinquenosides I, II, III, IV, and V, from American ginseng, the roots of Panax quinquefolium L. Chem Pharm Bull (Tokyo) 1998;46:647–654. doi: 10.1248/cpb.46.647. [DOI] [PubMed] [Google Scholar]
- 57.Du XW, Wills RB, Stuart DL. Changes in neutral and malonyl ginsenosides in American ginseng (Panax quinquefolium) during drying, storage and ethanolic extraction. Food Chem. 2004;86:155–159. doi: 10.1016/j.foodchem.2003.11.003. [DOI] [Google Scholar]
- 58.Xie G, Plumb R, Su M, Xu Z, Zhao A, Qiu M, Long X, Liu Z, Jia W. Ultra-performance LC/TOF MS analysis of medicinal Panax herbs for metabolomic research. J Sep Sci. 2008;31:1015–1026. doi: 10.1002/jssc.200700650. [DOI] [PubMed] [Google Scholar]
- 59.Ren G, Chen F. Degradation of ginsenosides in American ginseng (Panax quinquefolium) extracts during microwave and conventional heating. J Agric Food Chem. 1999;47:1501–1505. doi: 10.1021/jf980678m. [DOI] [PubMed] [Google Scholar]
- 60.Wang CZ, Zhang B, Song WX, Wang A, Ni M, Luo X, Aung HH, Xie JT, Tong R, He TC, et al. Steamed American ginseng berry: ginsenoside analyses and anticancer activities. J Agric Food Chem. 2006;54:9936–9942. doi: 10.1021/jf062467k. [DOI] [PubMed] [Google Scholar]
- 61.Wang CZ, Aung HH, Ni M, Wu JA, Tong R, Wicks S, He TC, Yuan CS. Red American ginseng: ginsenoside constituents and antiproliferative activities of heat-processed Panax quinquefolius roots. Planta Med. 2007;73:669–674. doi: 10.1055/s-2007-981524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qiu YK, Dou DQ, Cai LP, Jiang HP, Kang TG, Yang BY, Kuang HX, Li MZ. Dammarane-type saponins from Panax quinquefolium and their inhibition activity on human breast cancer MCF-7 cells. Fitoterapia. 2009;80:219–222. doi: 10.1016/j.fitote.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 63.Wang M, Guilbert LJ, Li J, Wu Y, Pang P, Basu TK, Shan JJ. A proprietary extract from North American ginseng (Panax quinquefolium) enhances IL-2 and IFN-gamma productions in murine spleen cells induced by Con-A. Int Immunopharmacol. 2004;4:311–315. doi: 10.1016/j.intimp.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 64.Oshima Y, Sato K, Hikino H. Isolation and hypoglycemic activity of quinquefolans A, B, and C, glycans of Panax quinquefolium roots. J Nat Prod. 1987;50:188–190. doi: 10.1021/np50050a010. [DOI] [PubMed] [Google Scholar]
- 65.Christensen LP, Jensen M, Kidmose U. Simultaneous determination of ginsenosides and polyacetylenes in American ginseng root (Panax quinquefolium L.) by high-performance liquid chromatography. J Agric Food Chem. 2006;54:8995–9003. doi: 10.1021/jf062068p. [DOI] [PubMed] [Google Scholar]
- 66.Baranska M, Schulz H, Christensen LP. Structural changes of polyacetylenes in American ginseng root can be observed in situ by using Raman spectroscopy. J Agric Food Chem. 2006;54:3629–3635. doi: 10.1021/jf060422d. [DOI] [PubMed] [Google Scholar]
- 67.Washida D, Kitanaka S. Determination of polyacetylenes and ginsenosides in Panax species using high performance liquid chromatography. Chem Pharm Bull (Tokyo) 2003;51:1314–1317. doi: 10.1248/cpb.51.1314. [DOI] [PubMed] [Google Scholar]
- 68.Wang CZ, McEntee E, Wicks S, Wu JA, Yaun CS. Phytochemical and analytical studies of Panax notoginseng (Burk.) F.H. Chen. J Nat Med. 2006;60:97–106. doi: 10.1007/s11418-005-0027-x. [DOI] [Google Scholar]
- 69.Zhu S, Zou K, Fushimi H, Cai S, Komatsu K. Comparative study on triterpene saponins of ginseng drugs. Planta Med. 2004;70:666–677. doi: 10.1055/s-2004-827192. [DOI] [PubMed] [Google Scholar]
- 70.Yoshikawa M, Morikawa T, Yashiro K, Murakami T, Matsuda H. Bioactive saponins and glycosides. XIX. Notoginseng (3): immunological adjuvant activity of notoginsenosides and related saponins: structures of notoginsenosides-L, -M, and -N from the roots of Panax notoginseng (Burk.) F. H. Chen. Chem Pharm Bull (Tokyo) 2001;49:1452–1456. doi: 10.1248/cpb.49.1452. [DOI] [PubMed] [Google Scholar]
- 71.Yoshikawa M, Murakami T, Ueno T, Yashiro K, Hirokawa N, Murakami N, Yamahara J, Matsuda H, Saijoh R, Tanaka O. Bioactive saponins and glycosides. VIII. Notoginseng (1): new dammarane-type triterpene oligoglycosides, notoginsenosides-A, -B, -C, and -D, from the dried root of Panax notoginseng (Burk.) F.H. Chen. Chem Pharm Bull (Tokyo) 1997;45:1039–1045. doi: 10.1248/cpb.45.1039. [DOI] [PubMed] [Google Scholar]
- 72.Chen Y, Sorensen LK. Determination of marker constituents in radix Glycyrrhizae and radix Notoginseng by near infrared spectroscopy. Fresenius J Anal Chem. 2000;367:491–496. doi: 10.1007/s002160000356. [DOI] [PubMed] [Google Scholar]
- 73.Wei JX, Wang LA, Du H, Li R. Isolation and identifi cation of sanchinoside B1 and B2 from rootlets of Panax notoginseng (Burk.) F. H. Chen. Yao Xue Xue Bao. 1985;20:288–293. [PubMed] [Google Scholar]
- 74.Wei JX, Wang JF, Chang LY, Du YC. Chemical studies of san-chi Panax notoginseng (Burk.) F. H. Chen. I. Studies on the constituents of San-Chi root hairs. Yao Xue Xue Bao. 1980;15:359–364. [PubMed] [Google Scholar]
- 75.Wei JX, Liangyu C, Jufen W, Friedrichs E, Jores M, Puff H, Breitmaier E. Two new dammaran sapogenins from leaves of Panax notoginseng. Planta Med. 1982;45:167–171. doi: 10.1055/s-2007-971367. [DOI] [PubMed] [Google Scholar]
- 76.Cui XM, Jiang ZY, Zeng J, Zhou JM, Chen JJ, Zhang XM, Xu LS, Wang Q. Two new dammarane triterpene glycosides from the rhizomes of Panax notoginseng. J Asian Nat Prod Res. 2008;10:845–849. doi: 10.1080/10286020802144776. [DOI] [PubMed] [Google Scholar]
- 77.Wan JB, Zhang QW, Hong SJ, Guan J, Ye WC, Li SP, Lee MY, Wang YT. 5,6-Didehydroginsenosides from the roots of Panax notoginseng. Molecules. 2010;15:8169–8176. doi: 10.3390/molecules15118169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Y, Li J, He J, Abliz Z, Qu J, Yu S, Ma S, Liu J, Du D. Identification of new trace triterpenoid saponins from the roots of Panax notoginseng by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2009;23:667–679. doi: 10.1002/rcm.3917. [DOI] [PubMed] [Google Scholar]
- 79.Wang W, Rayburn ER, Hang J, Zhao Y, Wang H, Zhang R. Anti-lung cancer effects of novel ginsenoside 25-OCH(3)- PPD. Lung Cancer. 2009;65:306–311. doi: 10.1016/j.lungcan.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao Y, Wang W, Han L, Rayburn ER, Hill DL, Wang H, Zhang R. Isolation, structural determination, and evaluation of the biological activity of 20(S)-25-methoxyldammarane- 3beta, 12beta, 20-triol [20(S)-25-OCH3- PPD], a novel natural product from Panax notoginseng. Med Chem. 2007;3:51–60. doi: 10.2174/157340607779317508. [DOI] [PubMed] [Google Scholar]
- 81.Wang JR, Yamasaki Y, Tanaka T, Kouno I, Jiang ZH. Dammarane-type triterpene saponins from the flowers of Panax notoginseng. Molecules. 2009;14:2087–2094. doi: 10.3390/molecules14062087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun S, Wang CZ, Tone R, Li XL, Fishbein A, Wang Q, He TC, Du W, Yuan CS. Effects of steaming the root of Panax notoginseng on chemical composition and anticancer activities. Food Chem. 2010;118:307–314. doi: 10.1016/j.foodchem.2009.04.122. [DOI] [Google Scholar]
- 83.Teng RW, Li HZ, Wang DZ, Yang CR. Hydrolytic reaction of plant extracts to generate molecular diversity: new dammarane glycosides from the mild acid hydrolysate of root saponins of Panax notoginseng. Helv Chim Acta. 2004;87:1270–1278. doi: 10.1002/hlca.200490116. [DOI] [Google Scholar]
- 84.Ohtani K, Mizutani K, Hatono S, Kasai R, Sumino R, Shiota T, Ushijima M, Zhou J, Fuwa T, Tanaka O. Sanchinan-A, a reticuloendothelial system activating arabinogalactan from sanchi-ginseng (roots of Panax notoginseng). Planta Med. 1987;53:166–169. doi: 10.1055/s-2006-962664. [DOI] [PubMed] [Google Scholar]
- 85.Choi RC, Zhu JT, Leung KW, Chu GK, Xie HQ, Chen VP, Zheng KY, Lau DT, Dong TT, Chow PC, et al. A flavonol glycoside, isolated from roots of Panax notoginseng, reduces amyloid-beta-induced neurotoxicity in cultured neurons: signaling transduction and drug development for Alzheimer’s disease. J Alzheimers Dis. 2010;19:795–811. doi: 10.3233/JAD-2010-1293. [DOI] [PubMed] [Google Scholar]
- 86.Liu JH, Lee CS, Leung KM, Yan ZK, Shen BH, Zhao ZZ, Jiang ZH. Quantification of two polyacetylenes in Radix Ginseng and roots of related Panax species using a gas chromatography-mass spectrometric method. J Agric Food Chem. 2007;55:8830–8835. doi: 10.1021/jf070735o. [DOI] [PubMed] [Google Scholar]
- 87.Chan P, Thomas GN, Tomlinson B. Protective effects of trilinolein extracted from Panax notoginseng against cardiovascular disease. Acta Pharmacol Sin. 2002;23:1157–1162. [PubMed] [Google Scholar]
- 88.Kobashi K, Akao T. Relation of intestinal bacteria to pharmacological effects of glycosides. Bioscience Microflora. 1997;16:1–7. [Google Scholar]
- 89.Kim DH. Herbal medicines are activated by intestinal microflora. Nat Prod Sci. 2002;8:35–43. [Google Scholar]
- 90.Tawab MA, Bahr U, Karas M, Wurglics M, Schubert-Zsilavecz M. Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos. 2003;31:1065–1071. doi: 10.1124/dmd.31.8.1065. [DOI] [PubMed] [Google Scholar]
- 91.Karikura M, Miyase T, Tanizawa H, Taniyama T, Takino Y. Studies on absorption, distribution, excretion and metabolism of ginseng saponins. VII. Comparison of the decomposition modes of ginsenoside-Rb1 and -Rb2 in the digestive tract of rats. Chem Pharm Bull (Tokyo) 1991;39:2357–2361. doi: 10.1248/cpb.39.2357. [DOI] [PubMed] [Google Scholar]
- 92.Lee HU, Bae EA, Han MJ, Kim NJ, Kim DH. Hepatoprotective effect of ginsenoside Rb1 and compound K on tert-butyl hydroperoxide-induced liver injury. Liver Int. 2005;25:1069–1073. doi: 10.1111/j.1478-3231.2005.01068.x. [DOI] [PubMed] [Google Scholar]
- 93.Akao T, Kida H, Kanaoka M, Hattori M, Kobashi K. Intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng. J Pharm Pharmacol. 1998;50:1155–1160. doi: 10.1111/j.2042-7158.1998.tb03327.x. [DOI] [PubMed] [Google Scholar]
- 94.Akao T, Kanaoka M, Kobashi K. Appearance of compound K, a major metabolite of ginsenoside Rb1 by intestinal bacteria, in rat plasma after oral administration: measurement of compound K by enzyme immunoassay. Biol Pharm Bull. 1998;21:245–249. doi: 10.1248/bpb.21.245. [DOI] [PubMed] [Google Scholar]
- 95.Liu H, Yang J, Du F, Gao X, Ma X, Huang Y, Xu F, Niu W, Wang F, Mao Y, et al. Absorption and disposition of ginsenosides after oral administration of Panax notoginseng extract to rats. Drug Metab Dispos. 2009;37:2290–2298. doi: 10.1124/dmd.109.029819. [DOI] [PubMed] [Google Scholar]
- 96.Shibata S. Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J Korean Med Sci. 2001;16(Suppl):S28–S37. doi: 10.3346/jkms.2001.16.S.S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shin JE, Park EK, Kim EJ, Hong YH, Lee KT, Kim DH. Cytotoxicity of compound K (IH-901) and ginsenoside Rh2, main biotransformants of ginseng saponins by bifidobacteria, against some tumor cells. J Ginseng Res. 2003;27:129–134. doi: 10.5142/JGR.2003.27.3.129. [DOI] [Google Scholar]
- 98.Choo MK, Sakurai H, Kim DH, Saiki I. A ginseng saponin metabolite suppresses tumor necrosis factor-alpha-promoted metastasis by suppressing nuclear factor-kappaB signaling in murine colon cancer cells. Oncol Rep. 2008;19:595–600. [PubMed] [Google Scholar]
- 99.Nakata H, Kikuchi Y, Tode T, Hirata J, Kita T, Ishii K, Kudoh K, Nagata I, Shinomiya N. Inhibitory effects of ginsenoside Rh2 on tumor growth in nude mice bearing human ovarian cancer cells. Jpn J Cancer Res. 1998;89:733–740. doi: 10.1111/j.1349-7006.1998.tb03278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bae EA, Park SY, Kim DH. Constitutive beta-glucosidases hydrolyzing ginsenoside Rb1 and Rb2 from human intestinal bacteria. Biol Pharm Bull. 2000;23:1481–1485. doi: 10.1248/bpb.23.1481. [DOI] [PubMed] [Google Scholar]
- 101.Bae EA, Han MJ, Kim EJ, Kim DH. Transformation of ginseng saponins to ginsenoside Rh2 by acids and human intestinal bacteria and biological activities of their transformants. Arch Pharm Res. 2004;27:61–67. doi: 10.1007/BF02980048. [DOI] [PubMed] [Google Scholar]
- 102.Lee SJ, Sung JH, Lee SJ, Moon CK, Lee BH. Antitumor activity of a novel ginseng saponin metabolite in human pulmonary adenocarcinoma cells resistant to cisplatin. Cancer Lett. 1999;144:39–43. doi: 10.1016/S0304-3835(99)00188-3. [DOI] [PubMed] [Google Scholar]
- 103.Park EK, Shin YW, Lee HU, Kim SS, Lee YC, Lee BY, Kim DH. Inhibitory effect of ginsenoside Rb1 and compound K on NO and prostaglandin E2 biosyntheses of RAW264.7 cells induced by lipopolysaccharide. Biol Pharm Bull. 2005;28:652–656. doi: 10.1248/bpb.28.652. [DOI] [PubMed] [Google Scholar]
- 104.Shin YW, Kim DH. Antipruritic effect of ginsenoside rb1 and compound k in scratching behavior mouse models. J Pharmacol Sci. 2005;99:83–88. doi: 10.1254/jphs.FP0050260. [DOI] [PubMed] [Google Scholar]
- 105.Choo MK, Park EK, Han MJ, Kim DH. Antiallergic activity of ginseng and its ginsenosides. Planta Med. 2003;69:518–522. doi: 10.1055/s-2003-40653. [DOI] [PubMed] [Google Scholar]
- 106.Han GC, Ko SK, Sung JH, Chung SH. Compound K enhances insulin secretion with beneficial metabolic effects in db/db mice. J Agric Food Chem. 2007;55:10641–10648. doi: 10.1021/jf0722598. [DOI] [PubMed] [Google Scholar]
- 107.Kim DH, Jung JS, Moon YS, Sung JH, Suh HW, Kim YH, Song DK. Inhibition of intracerebroventricular injection stress-induced plasma corticosterone levels by intracerebroventricularly administered compound K, a ginseng saponin metabolite, in mice. Biol Pharm Bull. 2003;26:1035–1038. doi: 10.1248/bpb.26.1035. [DOI] [PubMed] [Google Scholar]
- 108.Bae EA, Kim EJ, Park JS, Kim HS, Ryu JH, Kim DH. Ginsenosides Rg3 and Rh2 inhibit the activation of AP-1 and protein kinase A pathway in lipopolysaccharide/interferon-gamma-stimulated BV-2 microglial cells. Planta Med. 2006;72:627–633. doi: 10.1055/s-2006-931563. [DOI] [PubMed] [Google Scholar]
- 109.Park EK, Choo MK, Kim EJ, Han MJ, Kim DH. Antiallergic activity of ginsenoside Rh2. Biol Pharm Bull. 2003;26:1581–1584. doi: 10.1248/bpb.26.1581. [DOI] [PubMed] [Google Scholar]
- 110.Lee HU, Bae EA, Han MJ, Kim DH. Hepatoprotective effect of 20(S)-ginsenosides Rg3 and its metabolite 20(S)- ginsenoside Rh2 on tert-butyl hydroperoxide-induced liver injury. Biol Pharm Bull. 2005;28:1992–1994. doi: 10.1248/bpb.28.1992. [DOI] [PubMed] [Google Scholar]
- 111.Trinh HT, Han SJ, Kim SW, Lee YC, Kim DH. Bifidus fermentation increases hypolipidemic and hypoglycemic effects of red ginseng. J Microbiol Biotechnol. 2007;17:1127–1133. [PubMed] [Google Scholar]
- 112.Hwang JT, Kim SH, Lee MS, Kim SH, Yang HJ, Kim MJ, Kim HS, Ha J, Kim MS, Kwon DY. Anti-obesity effects of ginsenoside Rh2 are associated with the activation of AMPK signaling pathway in 3T3-L1 adipocyte. Biochem Biophys Res Commun. 2007;364:1002–1008. doi: 10.1016/j.bbrc.2007.10.125. [DOI] [PubMed] [Google Scholar]
- 113.Lee WK, Kao ST, Liu IM, Cheng JT. Increase of insulin secretion by ginsenoside Rh2 to lower plasma glucose in Wistar rats. Clin Exp Pharmacol Physiol. 2006;33:27–32. doi: 10.1111/j.1440-1681.2006.04319.x. [DOI] [PubMed] [Google Scholar]
- 114.Park EK, Choo MK, Oh JK, Ryu JH, Kim DH. Ginsenoside Rh2 reduces ischemic brain injury in rats. Biol Pharm Bull. 2004;27:433–436. doi: 10.1248/bpb.27.433. [DOI] [PubMed] [Google Scholar]
- 115.Jung JS, Shin JA, Park EM, Lee JE, Kang YS, Min SW, Kim DH, Hyun JW, Shin CY, Kim HS. Anti-inflammatory mechanism of ginsenoside Rh1 in lipopolysaccharide-stimulated microglia: critical role of the protein kinase A pathway and hemeoxygenase-1 expression. J Neurochem. 2010;115:1668–1680. doi: 10.1111/j.1471-4159.2010.07075.x. [DOI] [PubMed] [Google Scholar]
- 116.Park EK, Choo MK, Han MJ, Kim DH. Ginsenoside Rh1 possesses antiallergic and anti-inflammatory activities. Int Arch Allergy Immunol. 2004;133:113–120. doi: 10.1159/000076383. [DOI] [PubMed] [Google Scholar]
- 117.Lee Y, Jin Y, Lim W, Ji S, Choi S, Jang S, Lee S. A ginsenoside-Rh1, a component of ginseng saponin, activates estrogen receptor in human breast carcinoma MCF- 7 cells. J Steroid Biochem Mol Biol. 2003;84:463–468. doi: 10.1016/S0960-0760(03)00067-0. [DOI] [PubMed] [Google Scholar]
- 118.Masuno H, Kitao T, Okuda H. Ginsenosides increase secretion of lipoprotein lipase by 3T3-L1 adipocytes. Biosci Biotechnol Biochem. 1996;60:1962–1965. doi: 10.1271/bbb.60.1962. [DOI] [PubMed] [Google Scholar]
- 119.Leung KW, Leung FP, Mak NK, Tombran-Tink J, Huang Y, Wong RN. Protopanaxadiol and protopanaxatriol bind to glucocorticoid and oestrogen receptors in endothelial cells. Br J Pharmacol. 2009;156:626–637. doi: 10.1111/j.1476-5381.2008.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bae EA, Shin JE, Kim DH. Metabolism of ginsenoside Re by human intestinal microflora and its estrogenic effect. Biol Pharm Bull. 2005;28:1903–1908. doi: 10.1248/bpb.28.1903. [DOI] [PubMed] [Google Scholar]
- 121.Han KL, Jung MH, Sohn JH, Hwang JK. Ginsenoside 20S-protopanaxatriol (PPT) activates peroxisome proliferator-activated receptor gamma (PPARgamma) in 3T3-L1 adipocytes. Biol Pharm Bull. 2006;29:110–113. doi: 10.1248/bpb.29.110. [DOI] [PubMed] [Google Scholar]
- 122.Usami Y, Liu YN, Lin AS, Shibano M, Akiyama T, Itokawa H, Morris-Natschke SL, Bastow K, Kasai R, Lee KH. Antitumor agents. 261. 20(S)-protopanaxadiol and 20(s)-protopanaxatriol as antiangiogenic agents and total assignment of (1)H NMR spectra. J Nat Prod. 2008;71:478–481. doi: 10.1021/np070613q. [DOI] [PubMed] [Google Scholar]
- 123.Wang M, Guilbert LJ, Ling L, Li J, Wu Y, Xu S, Pang P, Shan JJ. Immunomodulating activity of CVT-E002, a proprietary extract from North American ginseng (Panax quinquefolium). J Pharm Pharmacol. 2001;53:1515–1523. doi: 10.1211/0022357011777882. [DOI] [PubMed] [Google Scholar]
- 124.Hu S, Concha C, Cooray R, Holmberg O. Ginseng-enhanced oxidative and phagocytic activities of polymorphonuclear leucocytes from bovine peripheral blood and stripping milk. Vet Res. 1995;26:155–161. [PubMed] [Google Scholar]
- 125.Kim JY, Germolec DR, Luster MI. Panax ginseng as a potential immunomodulator: studies in mice. Immunopharmacol Immunotoxicol. 1990;12:257–276. doi: 10.3109/08923979009019672. [DOI] [PubMed] [Google Scholar]
- 126.Hwang I, Ahn G, Park E, Ha D, Song JY, Jee Y. An acidic polysaccharide of Panax ginseng ameliorates experimental autoimmune encephalomyelitis and induces regulatory T cells. Immunol Lett. 2011;138:169–178. doi: 10.1016/j.imlet.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 127.Shin HJ, Kim YS, Kwak YS, Song YB, Kim YS, Park JD. Enhancement of antitumor effects of paclitaxel (taxol) in combination with red ginseng acidic polysaccharide (RGAP). Planta Med. 2004;70:1033–1038. doi: 10.1055/s-2004-832643. [DOI] [PubMed] [Google Scholar]
- 128.Baek SH, Lee JG, Park SY, Bae ON, Kim DH, Park JH. Pectic polysaccharides from Panax ginseng as the antirotavirus principals in ginseng. Biomacromolecules. 2010;11:2044–2052. doi: 10.1021/bm100397p. [DOI] [PubMed] [Google Scholar]
- 129.Kim DH. Metabolism of ginsenosides to bioactive compounds by intestinal microflora and its industrial application. J Ginseng Res. 2009;33:165–176. doi: 10.5142/JGR.2009.33.3.165. [DOI] [Google Scholar]
- 130.Park CS, Yoo MH, Noh KH, Oh DK. Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases. Appl Microbiol Biotechnol. 2010;87:9–19. doi: 10.1007/s00253-010-2567-6. [DOI] [PubMed] [Google Scholar]
- 131.Lee JH, Hyun YJ, Kim DH. Cloning and characterization of α-L-arabinofuranosidase and bifunctional α-L-arabinopyranosidase/β-D-galactopyranosidase from Bifidobacterium longum H-1. J Appl Microbiol. 2011;111:1097–1107. doi: 10.1111/j.1365-2672.2011.05128.x. [DOI] [PubMed] [Google Scholar]
- 132.Noh KH, Son JW, Kim HJ, Oh DK. Ginsenoside compound K production from ginseng root extract by a thermostable beta-glycosidase from Sulfolobus solfataricus. Biosci Biotechnol Biochem. 2009;73:316–321. doi: 10.1271/bbb.80525. [DOI] [PubMed] [Google Scholar]
- 133.Kim BH, Lee SY, Cho HJ, You SN, Kim YJ, Park YM, Lee JK, Baik MY, Park CS, Ahn SC. Biotransformation of Korean Panax ginseng by pectinex. Biol Pharm Bull. 2006;29:2472–2478. doi: 10.1248/bpb.29.2472. [DOI] [PubMed] [Google Scholar]
- 134.Park EK, Choo MK, Oh JK, Ryu JH, Kim DH. Ginsenoside Rh2 reduces ischemic brain injury in rats. Biol Pharm Bull. 2004;27:433–436. doi: 10.1248/bpb.27.433. [DOI] [PubMed] [Google Scholar]
- 135.Chang KH, Jee HS, Lee NK, Park SH, Lee NW, Paik HD. Optimization of the enzymatic production of 20(S)-ginsenoside Rg(3) from white ginseng extract using response surface methodology. N Biotechnol. 2009;26:181–186. doi: 10.1016/j.nbt.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 136.Shin HY, Lee JH, Lee JY, Han YO, Han MJ, Kim DH. Purification and characterization of ginsenoside Ra-hydrolyzing beta-D-xylosidase from Bifidobacterium breve K-110, a human intestinal anaerobic bacterium. Biol Pharm Bull. 2003;26:1170–1173. doi: 10.1248/bpb.26.1170. [DOI] [PubMed] [Google Scholar]
- 137.Yu H, Liu H, Zhang C, Tan D, Lu M, Jin F. Purification and characterization of gypenoside-alpha-L-rhamnosidase hydrolyzing gypenoside-5 into ginsenoside Rd. Process Biochem. 2004;39:861–867. doi: 10.1016/S0032-9592(03)00196-1. [DOI] [Google Scholar]
- 138.Shin HY, Park SY, Sung JH, Kim DH. Purification and characterization of alpha-L-arabinopyranosidase and alpha-L-arabinofuranosidase from Bifidobacterium breve K-110, a human intestinal anaerobic bacterium metabolizing ginsenoside Rb2 and Rc. Appl Environ Microbiol. 2003;69:7116–7123. doi: 10.1128/AEM.69.12.7116-7123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim SJ, Lee CM, Kim MY, Yeo YS, Yoon SH, Kang HC, Koo BS. Screening and characterization of an enzyme with beta-glucosidase activity from environmental DNA. J Microbiol Biotechnol. 2007;17:905–912. [PubMed] [Google Scholar]
- 140.Zhao X, Gao L, Wang J, Bi H, Gao J, Du X, Zhou Y, Tai G. A novel ginsenoside Rb1-hydrolyzing beta-D-glucosidase from Cladosprium fulvum. Process Biochem. 2009;44:612–618. doi: 10.1016/j.procbio.2009.01.016. [DOI] [Google Scholar]
- 141.Son JW, Kim HJ, Oh DK. Ginsenoside Rd production from the major ginsenoside Rb1 by beta-glucosidase from Thermus caldophilus. Biotechnol Lett. 2008;30:713–716. doi: 10.1007/s10529-007-9590-4. [DOI] [PubMed] [Google Scholar]
- 142.Ko SR, Suzuki Y, Suzuki K, Choi KJ, Cho BG. Marked production of ginsenosides Rd, F2, Rg3, and compound K by enzymatic method. Chem Pharm Bull (Tokyo) 2007;55:1522–1527. doi: 10.1248/cpb.55.1522. [DOI] [PubMed] [Google Scholar]
- 143.Ko SR, Choi KJ, Suzuki K, Suzuki Y. Enzymatic preparation of ginsenosides Rg2, Rh1, and F1. Chem Pharm Bull (Tokyo) 2003;51:404–408. doi: 10.1248/cpb.51.404. [DOI] [PubMed] [Google Scholar]
- 144.Yan Q, Zhou W, Li X, Feng M, Zhou P. Purification method improvement and characterization of a novel ginsenoside-hydrolyzing beta-glucosidase from Paecilomyces bainier sp. 229. Biosci Biotechnol Biochem. 2008;72:352–359. doi: 10.1271/bbb.70425. [DOI] [PubMed] [Google Scholar]
- 145.Yan Q, Zhou XW, Zhou W, Li XW, Feng MQ, Zhou P. Purification and properties of a novel beta-glucosidase, hydrolyzing ginsenoside Rb1 to CK, from Paecilomyces bainier. J Microbiol Biotechnol. 2008;18:1081–1089. [PubMed] [Google Scholar]
- 146.Park SY, Bae EA, Sung JH, Lee SK, Kim DH. Purification and characterization of ginsenoside Rb1-metabolizing beta-glucosidase from Fusobacterium K-60, a human intestinal anaerobic bacterium. Biosci Biotechnol Biochem. 2001;65:1163–1169. doi: 10.1271/bbb.65.1163. [DOI] [PubMed] [Google Scholar]
- 147.Yu H, Zhang C, Lu M, Sun F, Fu Y, Jin F. Purification and characterization of new special ginsenosidase hydrolyzing multi-glycisides of protopanaxadiol ginsenosides, ginsenosidase type I. Chem Pharm Bull (Tokyo) 2007;55:231–235. doi: 10.1248/cpb.55.231. [DOI] [PubMed] [Google Scholar]
- 148.Su JH, Xu JH, Lu WY, Lin GQ. Enzymatic transformation of ginsenoside Rg3 to Rh2 using newly isolated Fusarium proliferatum ECU 2042. J Mol Catal B Enzym. 2006;38:113–118. doi: 10.1016/j.molcatb.2005.12.004. [DOI] [Google Scholar]
- 149.Bae EA, Choo MK, Park EK, Park SY, Shin HY, Kim DH. Metabolism of ginsenoside R(c) by human intestinal bacteria and its related antiallergic activity. Biol Pharm Bull. 2002;25:743–747. doi: 10.1248/bpb.25.743. [DOI] [PubMed] [Google Scholar]
- 150.Bae EA, Shin JE, Kim DH. Metabolism of ginsenoside Re by human intestinal microflora and its estrogenic effect. Biol Pharm Bull. 2005;28:1903–1908. doi: 10.1248/bpb.28.1903. [DOI] [PubMed] [Google Scholar]
- 151.Yu H, Gong J, Zhang C, Jin F. Purification and characterization of ginsenoside-alpha-L-rhamnosidase. Chem Pharm Bull (Tokyo) 2002;50:175–178. doi: 10.1248/cpb.50.175. [DOI] [PubMed] [Google Scholar]
- 152.Ruan CC, Zhang H, Zhang LX, Liu Z, Sun GZ, Lei J, Qin YX, Zheng YN, Li X, Pan HY. Biotransformation of ginsenoside Rf to Rh1 by recombinant beta-glucosidase. Molecules. 2009;14:2043–2048. doi: 10.3390/molecules14062043. [DOI] [PMC free article] [PubMed] [Google Scholar]


