Abstract
The glioblastoma multiforme (GBM) is the most common malignant brain tumor in adults. Despite combination treatments of radiation and chemotherapy, the survival periods are very short. Therefore, this study was conducted to assess the potential of ginsenoside F2 (F2) to treat GBM. In in vitro experiments with glioblastoma cells U373MG, F2 showed the cytotoxic effect with IC50 of 50 μg/mL through apoptosis, confirmed by DNA condensation and fragmentation. The cell population of cell cycle sub-G1 as indicative of apoptosis was also increased. In xenograft model in SD rats, F2 at dosage of 35 mg/kg weight was intravenously injected every two days. This reduced the tumor growth in magnetic resonance imaging images. The immunohistochemistry revealed that the anticancer activity might be mediated through inhibition of proliferation judged by Ki67 and apoptosis induced by activation of caspase-3 and -8. And the lowered expression of CD31 showed the reduction in blood vessel densities. The expression of matrix metalloproteinase-9 for invasion of cancer was also inhibited. The cell populations with cancer stem cell markers of CD133 and nestin were reduced. The results of this study suggested that F2 could be a new potential chemotherapeutic drug for GBM treatment by inhibiting the growth and invasion of cancer.
Keywords: Panax ginseng, Apoptosis, Ginsenoside F2, Glioblastoma multiforme
INTRODUCTION
Glioblastoma multiforme (GBM) is the most common primary malignant brain tumor in adults and is a challenging disease to be treated. The GBM is most aggressive and tumor with grades IV according to the classification of World Health Organization based on cellular origin and histologic appearance. The survival periods of patients with GBM are only 12 mo to 15 mo after diagnosis, despite numerous advances of current conventional therapies [1]. The hallmark features of GBM are rapid proliferation, necrosis in central lesion and angiogenesis [2]. The current standard of care includes maximal safe surgical resection, followed by a combination of radiation and chemotherapy with temozolomide (TMZ). Despite combination therapy of radiation and chemotherapy against GBM, its effect is still pessimistic due to its several characteristics of GBM. The first characteristic of GBM is difficult to control cell cycle with rapid growth of cell proliferation. The second is that GBM cells have endogenous resistance to apoptosis, which made recurrence after chemotherapy and radiation therapy [3]. The third is that GBM diffuses through white matter tract and blood vessel basement, and invades brain tissue by the secretion of protease, which makes it difficult to be operated. The last is that the recurrence of GBM is quite common in a year, and the surviving period is not over 2 yr. With the unsolved problems of GBM, more effective treatments are yet to be investigated. The strategies are being investigated to raise the sensitivity of resistant GBM tumor cells to TMZ and radiation [4].
Panax ginseng Meyer has been used as a general tonic or adaptogen for cancer patients in traditional medicine prescriptions from China, Japan, and Korea. The active compounds in ginseng are the ginsenosides, of which more than four dozens exist both in P. ginseng and other Panax species. There are numbers of investigations focusing on antitumor properties and other pharmacological activities related to cancer treatments. Among ginsenosides, the minor ginsenosides (F1, F2, Rg3, Rh1, Rh2, compound Y, compound Mc, and compound K) are produced by hydrolyzing the sugar moieties of the major ginsenosides [5] and have been known to represent the pharmacological activities of P. ginseng. Rh2 [6,7] and compound K [8] were reported to have the effect to suppress expression of matrix metalloproteinase (MMP)-9 in human astroglioma cells, resulting in inhibiting tumor cell invasion and metastasis which might be therapeutic potential for controlling the growth and invasiveness of GBM. Ginsenoside F2 (F2) is also a member of metabolites produced by hydrolysis of moiety of glucose in protopanaxadiol (PPD). And F2 is further metabolized into Rh2 or compound K. These two metabolites are different only in the position of carbon with glycosidic linkage in PPD aglycone. Rh2 and compound K have one at C-3 and C-20, respectively.
Therefore, this study was conducted to assess a potential of F2 as a new chemotherapeutic drug on GBM treatment in xenograft in vivo model.
MATERIALS AND METHODS
Preparation of ginsenoside F2
The PPD of ginsenosides was transformed to F2 by the incubation with Aspergillus niger. A. niger was cultured in tryptic soy broth supplemented with PPD for 7 d at 37℃ with agitation. The profile of transformation was examined with TLC once a day. After completion of expected performance, the medium was extracted with butanol. Supernatant was fractionated and concentrated with vacuum rotary evaporator. F2 was further purified by using silica gel column chromatography with CHCl3- MeOH-H2O (65:35:10, v/v). Isolated F2 above purity of 96% was characterized by mass spectroscopy and 1Hand 13C-NMR spectrometry. F2 was freshly dissolved in dimethyl sulfoxide (DMSO), of which the final concentration did not exceed 0.2% when added to cell culture.
Cell culture
U373MG glioblastoma cells (ATCC, Manassas, VA, USA) were grown in Dulbecco’s modified Eagle’s medium (DMEM; PAA Laboatories, Linz, Austria) containing 10% heat-inactivated fetal bovine serum (FBS) and penicillin-streptomycin from Gibco Life Technologies (Gaithersburg, MD, USA). Cells were maintained in humidified atmosphere containing 5% CO2 at 37℃.
Cytotoxicity
U373MG cells (5×103 cells/well) were plated in 96- well plates in 100 μL of the medium containing 10% heat-inactivated FBS. After 24 h, medium was replaced by the same volume of medium containing 2% heat-inactivated FBS, and F2 or DMSO. Cells were incubated for 24 h and cytotoxicity was compared as cell viability with colorimetric assays using EZ-Cytox Cell viability assay kit using 3-(4,5-Dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT; Daeil Lab Service Co., Seoul, Korea). All experiments were conducted in quadruplicate. Viability was determined by absorbance at 570 nm using a multiwell scanning spectrophotometer (VersaMax; Molecular Devices, Sunnyvale, CA, USA). Cell viability was expressed as the mean±standard deviation in percentage of the control viability.
DNA fragmentation assays
Cells were treated without or with F2 for 24 h, of which DNA was prepared as following. The cell pellet was suspended in working solution (EDTA 10 mM, 0.5% SDS, 50 mM Tris-HCl, proteinase K 0.5 μg/mL), kept at 50℃ for 1 h, and incubated at 70℃ for 10 min. The supernatant was incubated with RNase A (10 μg/mL) at 37℃ for 60 min, and then separated with 1.8% agarose gel electrophoresis.
Nuclear morphology
Two mL of U373MG was placed in 6-well plates at 105 cells/mL and treated with drugs 5 h after plating. After 24 h, 10 mM of Hoechst 33342 (Molecular Probes, Eugene, OR, USA), a DNA-specific fluorescent dye, was added to the solution in each well, and the plates were incubated for 10 min at 37℃. The stained cells were then observed under a fluorescence microscope (Olympus, London, UK).
Flow cytometric analysis
For the cell-cycle distribution analysis, 2 mL of cells (1×105 cells/mL) were plated in 6-well plates and treated with F2 at a range from 10 to 60 μM for 24 h. After treatment, the cells were collected, fixed in 70% ethanol, and then washed in phosphate buffered saline (PBS) containing 2 mM EDTA. These fixed cells were resuspended in 1 mL PBS containing 1 mg/mL RNase and 50 mg/ mL propidium iodide (Sigma Aldrich, St. Louis, MO, USA), incubated in the dark for 30 min at 37℃, and then analyzed via FACScaliber flow cytometry (Becton Dickinson, San Jose, CA, USA). Data from 10,000 cells were collected for each data file. All histograms were analyzed using Cell Quest (Becton Dickson) to determine the percentage of nuclei with hypodiploid content indicative of apoptosis.
Xenograft model
After anesthesia, rats received stereotaxic inoculation of U373MG human glioblastoma cells (1×106 cells in 3 μL of PBS) into the right forebrain at the following coordinates: 3.1 mm lateral and 0.4 mm anterior to bregma at a 4.0 mm depth from the skull surface. Since 7 d after the implantation, F2 (35 mg/kg) and 5-fluorouracil (FU) (15 mg/kg) intravenously injected every two days for 14 d. Lipision (Choongwae Pharma Coporation, Seoul, Korea), purified soybean oil, was used as carrier and negative control. Tumor size was determined by magnetic resonance imaging (MRI) scans (T1- weighted image; Magnum 3.0 T, Medinus Co., Yongin, Korea) 8, 15 and 22 d after transplantation of U373MG cells. Rat (n=6/group) from all experimental groups were anesthetized with 1% isoflurane in an oxygen/ air mixture. Tumor sizes were demonstrated as a pixel number by Image J. Two days after the last injection, glioblastoma-bearing rats were sacrificed, and rat brains were collected, fixed in 4% paraformaldehyde for 24 h, and immersed in 30% sucrose solution for cryoprotection. After 3 d, rat brains were embedded in optimal cutting temperature (OCT) compound (Miles, Elkhart, IN, USA). This study has been reviewed and approved by the Kyung Hee University, Institutional Animal Care and Use Committee.
Immunohistochemistry
For immunohistochemistry, the brain embedded in OCT was cut into 10-μm coronal sections using a cryostat. The sections were attached to slide glass and incubated overnight at 4℃ with primary antibodies against MMP-9 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), Ki67 nuclear antigen (Dako,Glostrup, Denmark), cleaved caspase-3 and -8 (Cell Signaling Technology, Beverly, MA, USA), CD31 (Dako), CD133 (Abcam, Cambridge, UK), and nestin (Millipore, Bedford, MA, USA). Dako REAL EnVision Detection System (Dako) was used to detect according to manufacturer’s instruction. The sections were counterstained with hematoxylin. Tumor cells with red staining were considered positive. The percentage of positive cells was measured in the form of an area percent inside a standard measuring frame per 10 fields using × 50 magnifications with image analysis software (Leica Application Suite, Leica, Switzerland).
Statistics
Statistical comparisons of groups were performed using ANOVA followed by turkey’s test as post hoc. A p-value of <0.05 was considered statistically significant.
RESULTS
Cytotoxicity on human glioblastoma cell line U373MG
At first, we confirmed cytotoxic effect of F2 against human glioblastoma cell line U373MG. Survival of U373MG was significantly decreased after 24 h exposure to F2 in a concentration-dependent manner with IC50 of 50 μg/mL (Fig. 1A).
Fig. 1. Cytotoxicity of ginsenoside F2 (F2) against human glioblastoma cell line U373MG. (A) Cytotoxic effects were quantified by cell viability with 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. The values are expressed as the means±standard deviation (n=3). **, p<0.01, significantly different compared with control (no treatment by ginsenoside F2) by student’s test. (B) Cells were treated with the F2 for 24 h followed by staining with Hoechst 33342. The cell showing DNA condensation was shown. (C) DNA from cells treated by F2 was separated by 1.8% agarose gel electrophoresis of DNA. This was done at least twice using independently prepared DNA.
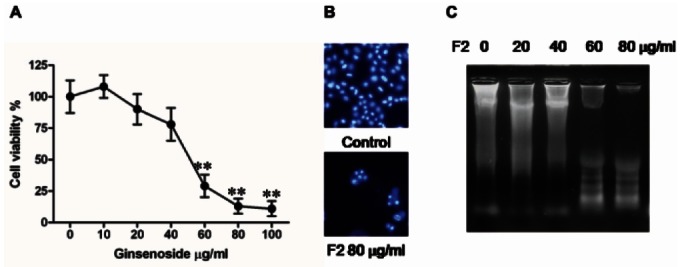
Induction of apoptosis by ginsenoside F2
To elucidate whether the F2-induced decrease in viability was attributable to apoptosis, we performed Hoechst 33342 nuclear staining to observe condensation of DNA as indicative of apoptosis. It was shown in the microscopic observation that the treatment at concentrations of 80 μg/mL of F2 caused the condensation of DNA nuclei compared to control (Fig. 1B). And apoptotic cell death is represented by DNA fragmentation, which could be observed in agarose gel electrophoresis 24 h after treatment of F2 (Fig. 1C). The DNA fragmentation was clear at the concentration of 60 μg/mL of F2, indicating the apoptosis of cells. Apoptosis increases the population of sub-G1 in cell cycle. Therefore, sub-G1 population after F2 treatment was quantified to use it as apoptosis index. Treatment with F2 resulted in a concentrationdependent increase in the sub-G1 cell population (Table 1). This treatment increased the percentage of cells in the sub-G1 phase from 0.99% (0 μg/mL) to 10.58% (60 μg/mL) after 24 h treatment of F2. Taken together, F2 induced cell death by apoptosis in glioblastoma cells U373MG.
Table 1.
The percentage of U373MG cells in the cell cycles 24 h after treatment of ginsenoside F2
| F2 (μg/mL) | Sub-G1 | G1 | S | G2/M |
|---|---|---|---|---|
|
| ||||
| Control | 0.99±0.45a | 54.82±7.89a | 19.79±3.45a | 24.51±4.78a |
| 10 | 1.74±0.65a | 54.82±6.29a | 19.20±2.45a | 24.44±3.56a |
| 20 | 1.52±0.75a | 55.07±11.32a | 18.64±2.09a | 24.57±5.78a |
| 40 | 3.95±0.98a | 54.87±6.90a | 18.18±3.12a | 23.02±3.56a |
| 60 | 10.58±2.34b | 52.88±9.01a | 16.19±2.38a | 20.48±2.38a |
Data are presented as mean±standard deviation (n=3). p˂0.05, significantly different compared with control. The different letters in each column mean difference between groups.
G1, gap 1; S, synthesis; G2, gap 2; M, mitosis.
Anti-cancer effect of ginsenoside F2 in xenograft model in SD rats
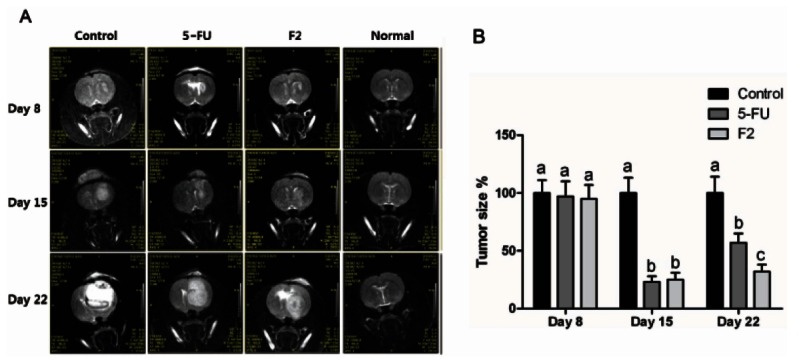
For in vivo assessment of therapeutic efficacy, human glioblastoma cells U373MG were implanted into right striatum of SD rats. Implantation of U373MG developed corresponding tumor, of which sizes were measured by MRI scans 8 d after implantation of U373 cells. SD rats were divided into groups, of which mean tumor size was not statistically significant (Fig. 2B). Since day 8, tumorbearing rat were intravenously injected with F2, 5-FU as a positive control or Lipision as a negative control every other day. In control group at day 15, tumor was shown to have excessive growth with respect to mass and density, which was accompanied by edema and necrosis around tumor. At day 22, the rapid growth of tumor on the implanted sites expelled the normal brain tissue and occupied the space, which formed the difference of density and border. The injection of 5-FU made the density of tumor lower than negative control at day 15, which indicated the inhibition of tumor proliferation. However, tumor mass and density at day 22 rapidly became more than at day 15, indicating the resistance of glioblastoma to chemotherapy. In respect to edema and expulsion of normal tissue, the extent was less than negative control. At day 15, the intravenous injection of F2 made the formation of tumor in the size and density less than negative control. Similar to 5-FU, the size of tumor and density was more at day 22 than at day 15.
Fig. 2. Human glioblastoma cells (U373MG) were implanted into the right forebrain (n=6/group). (A) Since day 8 after implantation, drugs are intravenously injected every two days at dosages of 35 and 15 mg/kg weight of ginsenoside F2 (F2) and 5-fluorouracil (FU), respectively. T1 weighted magnetic resonance imaging were carried out at 8, 15 and 22 days after implantation of U373MG cells. (B) Tumor sizes were demonstrated as a pixel number by Image J and represented relatively to control. p<0.05, significantly different, compared with control by one-way ANOVA followed by Turkey’s test. The different letters in each day mean difference between groups.
Immunohistological analysis
Our immunohistological data (Fig. 3) showed that F2 enhanced expression of caspase-3 and caspase-8 in tumor, which meant, like in vitro results, to induce apoptotic activity. A protein, Ki67, existing in nucleus, is expressed on the phases of cell cycle except G0 and G1, and hallmark of cell proliferative capacity [9].
Fig. 3. Immunohistochemistry in human glioblastoma (U373MG) xenograft intravenously treated with ginsenoside F2 (F2, 35 mg/kg weight) (n=6/ group) against the markers related to severeness of glioblastoma: caspase-3 and -8 were used for apoptosis; Ki67, proliferation; CD31, blood vessel density; matrix metalloproteinase (MMP)-9, invasion; CD133 and nestin, cancer stem cell. The percentage of positive cells was measured in the form of an area percent inside a standard measuring frame of area 11434.9 μm2 per 10 fields using a ×50 magnifications with image analysis software. Representative area indicated by arrow was shown using ×100 magnifications in inset. p<0.05, significantly different compared with control by one-way ANOVA. The different letters in each marker mean difference between groups.
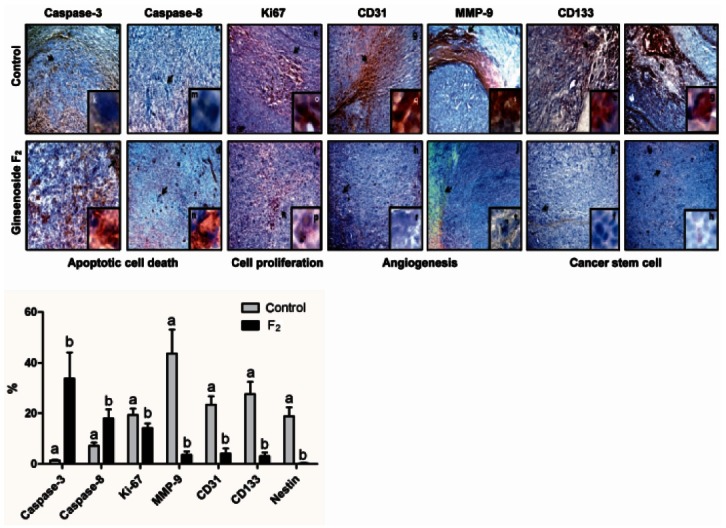
The present data showed the decreased expression of Ki67 in F2-treated groups compared to the control groups, which indicated the retarded cell proliferation rates in tumor region. The reduction in tumor cell proliferation following the intravenous injection of F2 paralleled with reduced reactivity for a marker of blood vessel density, CD31. Additionally, immunohistology revealed that the expression of MMP-9 in the border between normal brain tissues and glioblastoma was reduced by F2. This result indicated that F2 suppressed the signaling pathway to induce the expression of MMP-9, which has been known to play key roles in malignant tumor invasion. Collectively, these results demonstrated that intravenous injection of F2 evokes the mechanisms to kill glial tumor including the apoptosis, the inhibition of proliferation of tumor cells, angiogenesis and invasion.
Tumor stem cells including glioma stem cells are believed to be a significant component of persistent and recurrent disease. Immunoreactivities against cancer stem cell markers, CD133 and nestin were reduced by F2. This implied that F2 could reduce tumor cell proliferation by killing both CD133+ and CD133- cells.
DISCUSSION
This study assessed the chemotherapeutic potential of F2 against GBM, which could be used alone or combination with other therapy such as radiation. Cytotoxicity of F2 against glioblastoma cells U737MG was examined, of which IC50 was 50 μg/mL 24 h after treatment. F2 induced DNA condensation and fragmentation indicative of apoptosis, which was also reflected by the increase in the population of sub-G1 in the cell cycle.
F2 has two glycosides at C-3 and C-20 positions of the aglycon. And ginsenoside Rd (dammar-24(25)-ene- 3β,12β,20(S)-triol-(20-O-β-D-glucopyranosyl)-3-O-β- D-glucopyranosyl-(1→2)-β-D-glucopyranoside) has another glucoside linked to glucose attached to C-3 in F2. Rd is known to be lipophilic and easily diffuse across biological membranes and blood brain barrier [10]. And ginsenoside Rd exhibited remarkable neuroprotection in middle cerebral artery occlusion in rats.
The intravenous injection of F2 suppressed the growth of tumor formed by intracranial injection of U373MG with respect to tumor mass, density, invasion, shifting, edema and central necrosis in MRI images. It has been known that the rapid cell proliferation may lead to central necrosis in the focal lesion of GBM, and around which is called hypercellular zone. In fact, the rate of tumor cell proliferation compared to control was significantly reduced, as judged by immunoreactivity for Ki67. This effect might be mediated through apoptosis, which was proven by increased immunoreactivities against caspase- 3 and -8. Ginsenoside-Rh2 is the further metabolite of F2, which was reported to induce apoptotic cell death by activation of caspases in neuroblastoma cells [11]. In addition, Rh2 suppressed tumor cell proliferation and caused apoptosis in breast cancer xenograft through upregulation of proapoptosis gene such as Bax [6].
Another characteristic is that GBM forms abundant tumor microvessels. This abnormally accumulates interstitial fluid and cause edema. Our results showed that there was very little edema in F2 treated group compared to controls. Consistently, F2 might influence the factors related to angiogenesis such as blood vessel density shown by CD31 and MMP-9. MMP-9 expression levels have been reported to be significantly higher in human glioblastoma tissue samples than in low-grade brain tumors and normal brain tissue [12]. In addition, MMP-9 upregulation plays a role in glioma invasion and angiogenesis in vivo [13]. Therefore, the development of various compounds that could inhibit or suppress MMP- 9 is required to treat brain tumors. In this view, F2 could be one among candidates. In the previous report, the memetabolites of F2, compound K [7] and Rh2 [8], were reported to have the anti-invasive activity by inhibition of MMP-1 expression.
Our results showed that the population expressing cancer stem cell markers, CD133 and nestin, was reduced in the F2 treated group. This observation was important in the following aspects. Tumor stem cells, including glioma stem cells, are believed to be a significant component of persistent and recurrent disease. Although whether tumor-initiating potential was limited to CD133+ cells are controversial [14], it has been shown as a cell-surface marker in a sub-population of tumor cells from glioblastoma to initiate and sustain tumor growth after therapeutic interventions in these diseases [15,16]. Furthermore, CD133+ progenitors may differentiate into endothelial cells and favor vascularization and tumor growth [17].
In conclusion, we reported that F2 induced the glioma cells including CD133+ cells to commit apoptosis, and inhibit angiogenesis, which may provide potential strategies for treating brain tumors alone or combination with other therapy such as radiation.
Acknowledgments
This research was supported by Industrialization Support Program for Bio-technology of Agriculture and Forestry, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea with grant no. 810006-03.
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Claes A, Idema AJ, Wesseling P. Diffuse glioma growth: a guerilla war. Acta Neuropathol. 2007;114:443–458. doi: 10.1007/s00401-007-0293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lefranc F, Brotchi J, Kiss R. Possible future issues in the treatment of glioblastomas: special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J Clin Oncol. 2005;23:2411–2422. doi: 10.1200/JCO.2005.03.089. [DOI] [PubMed] [Google Scholar]
- 4.Cheng L, Bao S, Rich JN. Potential therapeutic implications of cancer stem cells in glioblastoma. Biochem Pharmacol. 2010;80:654–665. doi: 10.1016/j.bcp.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasegawa H, Sung JH, Matsumiya S, Uchiyama M. Main ginseng saponin metabolites formed by intestinal bacteria. Planta Med. 1996;62:453–457. doi: 10.1055/s-2006-957938. [DOI] [PubMed] [Google Scholar]
- 6.Choi S, Oh JY, Kim SJ. Ginsenoside Rh2 induces Bcl-2 family proteins-mediated apoptosis in vitro and in xenografts in vivo models. J Cell Biochem. 2011;112:330–340. doi: 10.1002/jcb.22932. [DOI] [PubMed] [Google Scholar]
- 7.Kim SY, Kim DH, Han SJ, Hyun JW, Kim HS. Repression of matrix metalloproteinase gene expression by ginsenoside Rh2 in human astroglioma cells. Biochem Pharmacol. 2007;74:1642–1651. doi: 10.1016/j.bcp.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Jung SH, Woo MS, Kim SY, Kim WK, Hyun JW, Kim EJ, Kim DH, Kim HS. Ginseng saponin metabolite suppresses phorbol ester-induced matrix metalloproteinase-9 expression through inhibition of activator protein-1 and mitogen-activated protein kinase signaling pathways in human astroglioma cells. Int J Cancer. 2006;118:490–497. doi: 10.1002/ijc.21356. [DOI] [PubMed] [Google Scholar]
- 9.Kruse AJ, Baak JP, Janssen EA, Kjellevold KH, Fiane B, Lovslett K, Bergh J, Robboy S. Ki67 predicts progression in early CIN: validation of a multivariate progression-risk model. Cell Oncol. 2004;26:13–20. doi: 10.1155/2004/108305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye R, Kong X, Yang Q, Zhang Y, Han J, Li P, Xiong L, Zhao G. Ginsenoside rd in experimental stroke: superior neuroprotective efficacy with a wide therapeutic window. Neurotherapeutics. 2011;8:515–525. doi: 10.1007/s13311-011-0051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim YS, Jin SH. Ginsenoside Rh2 induces apoptosis via activation of caspase-1 and -3 and up-regulation of Bax in human neuroblastoma. Arch Pharm Res. 2004;27:834–839. doi: 10.1007/BF02980175. [DOI] [PubMed] [Google Scholar]
- 12.Rao JS, Steck PA, Mohanam S, Stetler-Stevenson WG, Liotta LA, Sawaya R. Elevated levels of M(r) 92,000 type IV collagenase in human brain tumors. Cancer Res. 1993;53(10 Suppl):2208–2211. [PubMed] [Google Scholar]
- 13.Sawaya R, Go Y, Kyritisis AP, Uhm J, Venkaiah B, Mohanam S, Gokaslan ZL, Rao JS. Elevated levels of Mr 92,000 type IV collagenase during tumor growth in vivo. Biochem Biophys Res Commun. 1998;251:632–636. doi: 10.1006/bbrc.1998.9466. [DOI] [PubMed] [Google Scholar]
- 14.Joo KM, Kim SY, Jin X, Song SY, Kong DS, Lee JI, Jeon JW, Kim MH, Kang BG, Jung Y, et al. Clinical and biological implications of CD133-positive and CD133-negative cells in glioblastomas. Lab Invest. 2008;88:808–815. doi: 10.1038/labinvest.2008.57. [DOI] [PubMed] [Google Scholar]
- 15.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2006;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 16.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 17.Bruno S, Bussolati B, Grange C, Collino F, Graziano ME, Ferrando U, Camussi G. CD133+ renal progenitor cells contribute to tumor angiogenesis. Am J Pathol. 2006;169:2223–2235. doi: 10.2353/ajpath.2006.060498. [DOI] [PMC free article] [PubMed] [Google Scholar]


