Abstract
For quality control of components in Korean red ginseng powder and extract, a new method for simultaneous quantification of 12 ginsenosides (Rg1, Re, Rf, Rh1, Rg2[S], Rg2[R], Rb1, Rc, Rb2, Rd, Rg3[S], and Rg3[R]) was studied. Compared to the official method for quantification of marker substances (ginsenosides Rg1 and Rb1), the proposed methods were guaranteed by in-house method validation. Several criteria such as linearity, specificity, precision and accuracy were evaluated. For red ginseng powder, recovery (averaging 95% to 105%) was calculated, and analysis of variance was carried out to estimate the relative standard deviation (0.20% to 2.12%). For red ginseng extract, the average recovery rate was 90% to 99% and the relative standard deviation was 0.39% to 2.40%. These results indicate that the proposed method could be used in the laboratory for determination of 12 ginsenosides in red ginseng powder and extract. In addition, this method was found to be suitable for quality control of ginseng products and potentially offer time and cost benefits.
Keywords: Panax ginseng, Red ginseng, Ginsenosides, Method validation, Analysis, HPLC-photo diode array detector
INTRODUCTION
Ginseng (Panax ginseng Meyer) has long been considered as one of the most valuable medicinal herbs and is now used as a functional food and a raw material for pharmaceuticals. The efficacy of ginseng has been studied by many researchers [1]. For this reason, the market of “ginseng” especially “red ginseng” has been emerged continuously, more so than other functional food compounds [2]. The Korean government made a public announcement about a standard analysis method for determining the content of marker substances (ginsenosides Rg1 and Rb1) in ginseng product [3]. Additionally the Korea Food & Drug administration announced that the standards and specification for ginseng content was changed from evaluating crude saponin contents to determining the sum of ginsenosides Rg1 and Rb1 [4].
For the analysis of ginsenosides, various analytical methods, such as TLC, GC, HPLC, capillary electrophoresis (CE), near infra-red spectroscopy (NIRS) were performed [5]. For NIRS, statistical algorithms such as principal component analysis and soft independent modeling of class analogy were applied to the data for quality control and distinction of ginseng and ginseng product [6]. CE is highly sensitive and fast, but there have been very few applications in which it was used for the analysis of ginsenosides due to the absence of a charge in its analytes [5]. In the beginning of 1980 when the analysis of ginsenosides by GC was developed, the analysis of ginsenosides by GC was usually performed directly on the trimethylsillyl derivatives of ginsenosides [5].
In 1978 HPLC was commercialized, and it is the most commonly used method for the analysis of ginsenosides in most studies on ginseng plant material and preparations, because of its speed, sensitivity and ability to analyze polar compounds. Different techniques have been used for the detection of ginsenosides such as UV, evaporative light scattering detection (ELSD), fluorescence fluorescence, and MS [1,5].
Fluorescence is one of the most sensitive detection methods in HPLC analysis. However, because ginsenosides do not contain suitable fluorescence chromophore they must be derivatized before detection, thus, fluorescence detection has been limited [5]. With the development of sophisticated ionization techniques, LC-MS techniques have been successfully applied for identification of trace amounts of ginsenosides and its metabolite analysis. This method was used as one of the most powerful verification tools in trace analysis and biochemistry fields [7,8].
HPLC-UV and HPLC-ELSD methods have been widely used for ginsenosides analysis and some modification was performed by many researchers [9,10]. Although both methods have advantages and disadvantages for ginsenosides analysis, HPLC-UV is more widely employed of the two because the detection method is more common. HPLC-UV was employed as a standard method for testing ginseng and ginseng products.
In this paper, a rapid and simple pretreatment method for simultaneous determination of 12 ginsenosides including marker substances of ginseng product (ginsenosides Rg1 and Rb1) was studied. Method validation was carried out by comparison of some parameters such as precision and recovery. As a result, the proposed method may be used for quality control of ginseng products.
MATERIALS AND METHODS
Materials and equipments
All distilled water used in the experiment were purified by Milli-Q water system (Millipore, Bedford, MA, USA) and the resistance value was measured as 18 MΩ prior to use. HPLC analysis was carried out by using an Alliance 2695 HPLC system (Waters, Milford, MA, USA) and 2996 photo diode array detector (Waters) as shown in Table 1.
Table 1.
Instrumental conditions for ginsenosides analysis
| Time (min) | Flow (mL/min) | A%, ACN | B%, DW |
|---|---|---|---|
|
| |||
| Initial | 1.6 | 80 | 20 |
| 40 | 1.6 | 68 | 32 |
| 55 | 1.6 | 50 | 50 |
| 70 | 1.6 | 35 | 65 |
| 71 | 1.6 | 10 | 90 |
| 81 | 1.6 | 80 | 20 |
| 90 | 1.6 | 80 | 20 |
Column: Supelco Discovery C18, 5 μm, 250×4.6 mm; injection volume: 20 μL; column temperature: 40±2℃ ; detector: Waters 2998 photo diode array detector, 203±0.2 nm.
ACN, acetonitrile; DW, deionized water.
Ginsenoside Rg1, Re, Rf, Rh1, Rb1, Rc, Rb2, Rd, Rg3(S), and Rg3(R) were purchased from Chromadex Co. (Irvine, CA, USA) and ginsenoside Rg2(S) and Rg2(R) were obtained from Embo Lab. (Seoul, Korea). All reagents used in the experiment were guaranteed reagent grade, and HPLC-grade acetonitrile and methanol were purchased from Merck (Darmstadt, Germany). Red ginseng powder (lot no. 1019008) and red ginseng extract (lot no. 2019119) was purchased from the local health food shop (Korea Ginseng Corporation, Daejeon, Korea).
Method validation
To obtain validity and similarity between the proposed method and the official method, the analytical results of ginsenosides were compared to one other in accordance with International Conference on Harmonisation (ICH) guideline [11,12]. The precision and accuracy of the analytical methods were determined by comparison of 3 replicates at 3 different concentrations using the same sample and conditions. More details are provided in the section that follows.
Analytical procedure for red ginseng powder
Official method
One gram of red ginseng powder was weighed in a round bottom flask, and 50 mL of 50% MeOH was added. The solution was then refluxed for 1 h. After cooling, the sample was filtered (Whatman no. 4; Whatman, Clifton, NJ, USA). The supernatant solution was collected, and the residue was treated as the same procedure. The collected liquids were combined and evaporated (Butchi, Flawil, Switzerland) under reduced pressure at a temperature not exceeding 60℃ and the residue was dissolved in 25 mL of solvent (deionized water:acetonitrile=8:2). Then, this solution was filtered (0.2 μm; Acrodisk, Port Washington, NY, USA) and injected into the HPLC system.
Proposed method
A half of gram of red ginseng powder was weighed in a centrifugal tube (15 mL, polypropylene-single use; BioLogix, USA) and shaken vigorously after the addition of 10 mL of 50% MeOH. Extraction was performed in an ultrasonic cleaner (60 Hz; Wiseclean, Seoul, Korea) for 30 min. After ultrasonic extraction, centrifugal separation (Legend Mach 1.6R; Thermo, Frankfurt, Germany) was performed for 10 min, at 3,000 rpm. The resulting supernatant solution was filtered (0.2 μm, Acrodisk) and injected into the HPLC system.
Analytical procedure for red ginseng extract
Official method
Two gram of red ginseng extract was weighed in a beaker, and 20 mL of deionized water was added. After sitting at room temperature for 1 h, the diluted sample was dissolved in 50 mL of deionized water. The solution was filtered (0.2 μm, Acrodisk) and injected into the HPLC system.
Proposed method
Two gram of red ginseng extract were weighed in a beaker, and 25 mL of deionized water was added. After sitting at room temperature for 1 h, the diluted sample was transferred in a 50 mL volumetric flask where the volume was brought up to 50 mL by adding MeOH. Extraction was performed in an ultrasonic cleaner (60 Hz, Wiseclean) for 30 min. Then, the solution was filtered (0.2 μm, Acrodisk) and injected into the HPLC system.
RESULTS AND DISCUSSION
Specifi city, linearity, and limit of detection
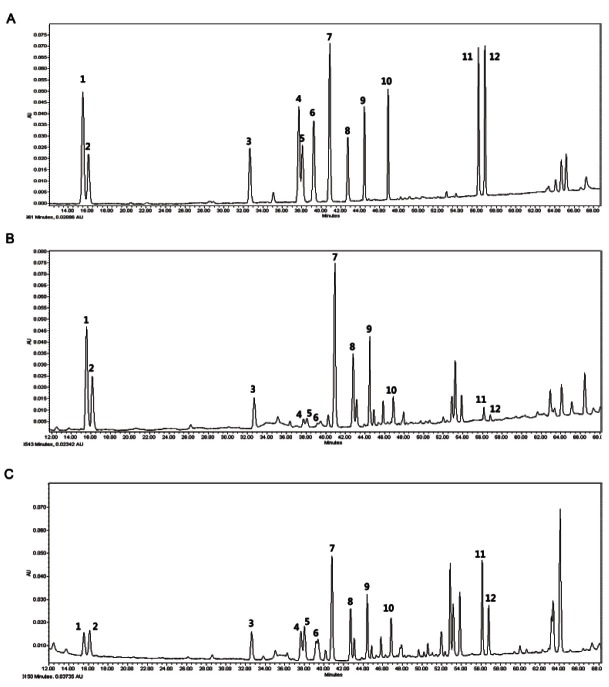
The specificity of individual ginsenosides was confirmed by demonstrating a sufficient separation of the substance present in the sample matrix. As shown in Fig. 1, sample chromatogram compared with that of the standard solution was sufficient to confi rm the specifi city of ginsenosides. In other words appropriate separation means that there is adequate resolution between the analyte peaks and the impurity and placebo peaks that need not be separated from each other [12].
Fig. 1. Representative HPLC chromatogram of mixed standards (A), red ginseng powder (B), and red ginseng extract (C). 1, Rg1 (15.53 min); 2, Re (16.12 min); 3, Rf (32.66 min); 4, Rh1 (37.67 min); 5, Rg2(S) (38.04 min); 6, Rg2(R) (39.22 min); 7, Rb1 (40.89 min); 8, Rc (42.74 min); 9, Rb2 (44.44 min); 10, Rd (46.86 min); 11, Rg3(S) (56.15 min); 12, Rg3(R) (56.80 min).
A linear dependence of the signal and the analyte concentration is the most convenient indicator of sample quality or purity and is widely used in pharmaceutical analysis [12]. The standard solution containing 20 to 150 μg/mL of each ginsenosides were injected into the HPLC. Calibration curves were plotted as the peak area versus the amount of each analyte. The linearity was evaluated by linear regression analysis, which is calculated by the least squares regression method. The limit of quantification under the present chromatographic conditions was determined on the basis of the response at a signal to noise ratio of 10. The linearity of calibration curve for 12 ginsenosides was demonstrated in Table 2.
Table 2.
Linearity of calibration curve for ginsenosides
| Ginsenoside | Calibration curve1) | R2 | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|
|
| ||||
| Rg1 | Y = 4.52*103 X – 4.25*103 | 0.999933 | 1.74 | 5.80 |
| Re | Y = 3.82*103 X + 8.88*102 | 0.999961 | 1.71 | 5.70 |
| Rf | Y = 3.92*103 X – 9.62*102 | 0.999958 | 1.08 | 3.59 |
| Rh1 | Y = 6.45*103 X – 7.48*103 | 0.999970 | 0.84 | 2.82 |
| Rg2 (S) | Y = 5.03*103 X – 1.68*104 | 0.999932 | 0.36 | 1.21 |
| Rg2 (R) | Y = 5.30*103 X + 1.16*104 | 0.999978 | 0.48 | 1.58 |
| Rb1 | Y = 3.33*103 X – 4.70*103 | 0.999937 | 0.90 | 2.97 |
| Rc | Y = 3.69*103 X – 1.12*104 | 0.999937 | 1.92 | 6.37 |
| Rb2 | Y = 3.55*103 X – 5.54*103 | 0.999937 | 1.77 | 5.86 |
| Rd | Y = 4.12*103 X – 5.72*103 | 0.999924 | 1.47 | 4.85 |
| Rg3 (S) | Y = 4.18*103 X – 3.41*103 | 0.999910 | 0.33 | 1.12 |
| Rg3 (R) | Y = 5.71*103 X – 6.03*103 | 0.999919 | 0.20 | 0.66 |
LOD, limit of detection; LOQ, limit of quantification.
1)Y: peak area; X: amount of ginsenoside (μg/mL).
Precision and accuracy for analysis of red ginseng powder
The precision of the proposed method was tested by comparing results from this methods with results obtained from using the official method. Thus 9 data points spanning 3 concentration levels were obtained by both methods. As shown in Table 3, the relative standard deviation of 3 results was below 3%, which was sufficient to prove the validity of the proposed method. However, some trends in these variations were observed between the proposed and official analytical results. The analytical results of the official method for major ginsenosides such as Rg1, Re, Rf, Rb1, Rc, and Rb2 were below that of the proposed method. This is because thermal degradation occurs in the glycosidic linkage at carbon 20 in the ginsenoside backbone. In other words, major ginsenosides were transformed to other ginsenosides by thermal energy during refl ux. Minor ginsenosides such as Rh1, Rg2, and Rg3 showed a wider range of variation (17% to 45%) because of the random errors that more seriously affected the trace amount analysis.
Table 3.
Ginsenosides amount (mg/g) and precision result using red ginseng powder (means of 9 determinations over 3 concentration levels)
| Ginsenoside | Official method(mg/g) | Proposed method(mg/g) | Bias(%) |
|---|---|---|---|
|
| |||
| Rg1 | 2.966±0.012 | 3.260±0.009 | 9.0 |
| Re | 1.709±0.011 | 1.907±0.012 | 10.4 |
| Rf | 0.955±0.014 | 1.052±0.005 | 9.2 |
| Rh1 | 0.095±0.002 | 0.135±0.002 | 29.6 |
| Rg2 (S) | 0.138±0.001 | 0.188±0.001 | 26.6 |
| Rg2 (R) | 0.121±0.001 | 0.110±0.001 | -10.0 |
| Rb1 | 5.022±0.007 | 5.301±0.022 | 5.3 |
| Rc | 1.958±0.024 | 2.042±0.018 | 4.1 |
| Rb2 | 1.832±0.023 | 1.937±0.006 | 5.4 |
| Rd | 0.479±0.003 | 0.579±0.002 | 17.3 |
| Rg3 (S) | 0.214±0.001 | 0.208±0.001 | -2.9 |
| Rg3 (R) | 0.084±0.001 | 0.058±0.001 | -44.8 |
The accuracy of the proposed method was tested by spiking experiments for recovery investigations. Thus crude saponin fractions were spiked into the analytical samples for recovery tests of the 12 ginsenosides. As shown in Table 4, the statistical values for both methods were good. However, with regards to the precision of these experiments, the results from the official method demonstrated poorer recovery values than proposed method. It should be confirmed that the proposed method was more suitable for simultaneous determination of ginsenosides than the official method because the official method focuses primarily on quantitative analysis of ginsenosides Rg1 and Rb1.
Table 4.
Recovery and RSD values of red ginseng powder (9 determinations over 3 concentration levels)
| Component | Official method | Proposed method | Bias (%) | ||
|---|---|---|---|---|---|
|
|
|
||||
| Recovery (%)1) | RSD | Recovery (%)1) | RSD | ||
|
| |||||
| Rg1 | 102.8 | 2.82 | 102.8 | 5.71 | 0.0 |
| Re | 98.1 | 3.17 | 101.6 | 5.26 | 3.4 |
| Rf | 101.0 | 5.66 | 103.6 | 5.07 | 2.5 |
| Rh1 | 82.5 | 9.92 | 103.6 | 2.87 | 20.4 |
| Rg2 (S) | 78.1 | 5.83 | 97.2 | 5.10 | 19.6 |
| Rg2 (R) | 101.1 | 5.79 | 105.2 | 2.60 | 3.9 |
| Rb1 | 95.5 | 6.83 | 101.9 | 4.48 | 6.2 |
| Rc | 98.0 | 6.67 | 101.9 | 3.46 | 3.8 |
| Rb2 | 99.4 | 6.82 | 102.4 | 4.85 | 2.9 |
| Rd | 98.3 | 8.10 | 99.5 | 4.61 | 1.2 |
| Rg3 (S) | 97.3 | 5.64 | 95.0 | 4.90 | 2.5 |
| Rg3 (R) | 96.2 | 6.21 | 96.8 | 4.49 | 0.7 |
RSD, relative standard deviation.
1)Mean value of 9 determinations over 3 concentration levels.
Precision and accuracy data for red ginseng extract
The validation of analytical methods for the red ginseng extract was also performed to evaluate the red ginseng powder. The precision of the 9 data point spanning 3 different concentrations are shown in Table 5, and Table 6 reports the accuracy of these experiments.
Table 5.
Ginsenosides amount (mg/g) and precision result using red ginseng extract (means of 9 determinations over 3 concentration levels)
| Ginsenoside | Official method(mg/g) | Proposed method(mg/g) | Bias (%) |
|---|---|---|---|
|
| |||
| Rg1 | 0.835±0.012 | 0.867±0.011 | 3.7 |
| Re | 1.059±0.014 | 1.110±0.014 | 4.6 |
| Rf | 1.167±0.006 | 1.287±0.013 | 9.3 |
| Rh1 | 0.770±0.004 | 0.877±0.011 | 12.2 |
| Rg2 (S) | 1.134±0.010 | 1.297±0.006 | 11.3 |
| Rg2 (R) | 0.537±0.011 | 0.598±0.012 | 10.2 |
| Rb1 | 4.109±0.058 | 4.278±0.039 | 4.0 |
| Rc | 1.775±0.028 | 1.896±0.045 | 6.4 |
| Rb2 | 1.698±0.025 | 1.790±0.007 | 5.1 |
| Rd | 1.001±0.018 | 1.072±0.005 | 6.6 |
| Rg3 (S) | 1.948±0.028 | 2.215±0.049 | 12.1 |
| Rg3 (R) | 0.797±0.004 | 0.674±0.008 | -18.2 |
Table 6.
Recovery and RSD values of red ginseng extract (9 determinations over 3 concentration levels)
| Component | Official method | Proposed method | Bias (%) | ||
|---|---|---|---|---|---|
|
|
|
||||
| Recovery (%)1) | RSD | Recovery (%)1) | RSD | ||
|
| |||||
| Rg1 | 95.2 | 3.51 | 93.6 | 2.11 | 1.7 |
| Re | 97.5 | 3.84 | 95.0 | 2.43 | 2.6 |
| Rf | 95.8 | 4.21 | 92.3 | 1.53 | 3.9 |
| Rh1 | 98.7 | 8.53 | 96.5 | 2.85 | 2.3 |
| Rg2 (S) | 95.8 | 5.99 | 99.1 | 7.72 | 3.3 |
| Rg2 (R) | 93.2 | 3.40 | 96.4 | 9.41 | 3.2 |
| Rb1 | 91.4 | 2.57 | 91.3 | 1.72 | 0.1 |
| Rc | 97.0 | 5.18 | 92.5 | 1.41 | 4.9 |
| Rb2 | 97.1 | 4.13 | 90.6 | 1.68 | 7.1 |
| Rd | 96.9 | 3.94 | 92.9 | 3.05 | 4.3 |
| Rg3 (S) | 100.9 | 4.42 | 92.2 | 3.15 | 9.5 |
| Rg3 (R) | 105.4 | 3.47 | 96.5 | 4.27 | 9.3 |
RSD, relative standard deviation.
1)Mean value of 9 determinations over 3 concentration levels.
The variability of data from these experiments was below 3% relative standard deviation (RSD), suggesting high precision and repeatability. In terms of accuracy, the recovery of ginsenosides was 90% to 105%, with respect to the spiked amount. Therefore, both methods were appropriate based on the accuracy of the data.
The analytical method was recognized as a valid process for ginseng analysis when high precision and repeatability of the data was demonstrated (5.3% to 7.3% RSD) as well as a high degree of accuracy of the data (80% to 107% of recovery). These criteria were set based on analyte content of 10 to 100 ug/mL in samples. Based on these criteria, the official method was inappropriate for analysis of trace amounts of ginsenosides Rh1, Rg2, Rg3 and the proposed method was more suitable for simultaneous determination of ginsenosides.
In this paper, methods to perform simultaneous analysis of multiple ginsensides in red ginseng powder and red ginseng extract were evaluated. Validation of the proposed method was performed with guidance from the ICH guidelines.
Both methods were valid for analysis of ginsenosides Rg1 and Rb1 as a marker substance. However, judging from the low precision and accuracy, the official method was not suitable for determining trace amounts of ginsenosides in red ginseng powder or red ginseng extract. Therefore the proposed method may be applied for determination of ginsenoside content in ginseng products for quality control. The application of this method may have additional benefit of saving time and labor costs.
References
- 1.Christensen LP. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 2.Park CK, Kwak YS, Hwang MS, Kim SC, Do JH. Trends and prospect of ginseng products in market health functional food. Food Sci Ind. 2007;40:30–45. [Google Scholar]
- 3.Korean Agency for Technology and Standards. KSH2153. Ginseng and ginseng products - determination of ginsenoside (Rg1, Rb1) contents - method by high performance liquid chromatography. 2008 Mar 26;
- 4.Korea Food & Drug Administration. Health functional food code. Korea Food & Drug Administration; Seoul: 2010. [Google Scholar]
- 5.Fuzzati N. Analysis methods of ginsenosides. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;812:119–133. doi: 10.1016/j.jchromb.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 6.Yap KY, Chan SY, Lim CS. Infrared-based protocol for the identification and categorization of ginseng and its products. Food Res Int. 2007;40:643–652. doi: 10.1016/j.foodres.2006.11.009. [DOI] [Google Scholar]
- 7.Ji HY, Lee HW, Kim HK, Kim HH, Chang SG, Sohn DH, Kim J, Lee HS. Simultaneous determination of ginsenoside Rb1 and Rg1 in human plasma by liquid chromatography- mass spectrometry. J Pharm Biomed Anal. 2004;35:207–212. doi: 10.1016/j.jpba.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Dan M, Su M, Gao X, Zhao T, Zhao A, Xie G, Qiu Y, Zhou M, Liu Z, Jia W. Metabolite profiling of Panax notoginseng using UPLC-ESI-MS. Phytochemistry. 2008;69:2237–2244. doi: 10.1016/j.phytochem.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, Sun C, Zheng B, Li Y, Wang Y. Simultaneous determination of nine ginsenosides in functional foods by high performance liquid chromatography with diode array detector detection. Food Chem. 2010;123:1322–1327. doi: 10.1016/j.foodchem.2010.06.014. [DOI] [Google Scholar]
- 10.Kim SN, Ha YW, Shin H, Son SH, Wu SJ, Kim YS. Simultaneous quantification of 14 ginsenosides in Panax ginseng C.A. Meyer (Korean red ginseng) by HPLCELSD and its application to quality control. J Pharm Biomed Anal. 2007;45:164–170. doi: 10.1016/j.jpba.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Taverniers I, Loose MD, Bockstaele EV. Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. TrAC Trends Anal Chem. 2004;23:535–552. doi: 10.1016/j.trac.2004.04.001. [DOI] [Google Scholar]
- 12.Ermer J. Validation in pharmaceutical analysis. Part I: an integrated approach. J Pharm Biomed Anal. 2001;24:755–767. doi: 10.1016/S0731-7085(00)00530-6. [DOI] [PubMed] [Google Scholar]