Abstract
An light-emitting diode (LED)-based light source was used as a monochromatic light source to determine the responses of raw ginseng roots (Panax ginseng Meyer) to specific emission spectra with respect to the production of ginsenosides. The ginsenoside content in the ginseng roots changed in response to the LED light treatments at 25℃ relative to the levels in the control roots that were treated in the dark or at 4℃ for 7 d. Ginseng roots were exposed to LEDs with four different peak emission wavelengths, 380, 450, 470, and 660 nm, in closed compartments. Compared with the control 4℃-treated roots, roots that were treated with 450 and 470 nm light showed a significantly increased production of ginsenosides (p<0.05), with increases of 64.9% and 74.1%, respectively. The contents of the ginsenosides Rb2, Rc, and Rg1 were significantly higher (p<0.05) in the 450 and 470 nm-treated root samples. The ratio of protopanaxadiol ginsenosides (Rb1, Rb2, Rc, and Rd) to protopanaxatriol ginsenosides (Rg1, Rg2, Re, and Rf) was significantly higher (p<0.05) in the 450 and 470 nm-treated root samples than in the control 4℃-treated roots. This is the first report that demonstrates the increase and conversion of ginsenosides in raw ginseng roots in response to exposure to LED light.
Keywords: Panax ginseng, Ginsenosides, Light-emitting diode
INTRODUCTION
Ginseng (Panax ginseng Meyer) is a perennial herbaceous plant, and its roots have been used as herbal medicines for thousands of years [1]. Ginseng has well-known pharmacological activities, such as anti-cancer, anti-aging, anti-diabetic, anti-stress, and neuroprotective effects [2-7]. Ginseng extracts contain various compounds, such as ginsenosides, polysaccharides, flavonoids, peptides, polyacetylene alcohols, and fatty acids [7]. Among these compounds, ginsenosides are considered the most important bioactive ingredients with respect to the pharmacological activities of ginseng. The production of marketable ginseng is very difficult due to its long cultivation (4 to 6 yr) and susceptibility to diseases [8,9]. Recently, there have been several attempts to produce ginsenosides in vitro, but the productivity has been low [10-12].
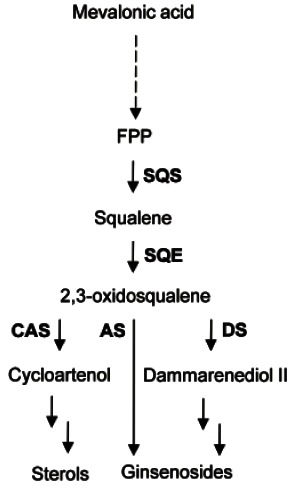
Ginsenosides are triterpenoids that are synthesized through the isoprenoid pathway and consist of a nonsugar component (aglycone) and a sugar component (glycone) of 1-4 molecules such as D-glucose, L-arabinopyranoside, L-arabinofuranoside, D-xylose, and L-rhamnose [13]. Sequential cyclization, hydroxylation and glycosylation generate various ginsenosides from 2,3-oxidosqualene [14]. Oxidosqualene cyclases are located at the branching point in the biosynthesis of phytosterols and triterpenoids. Although the downstream pathway of cyclization is still unclear, enzymes such as cytochrome P450s and UDP-glycosyltransferases may be involved in the conversion of damarenediol-II or β-amyrin into various ginsenosides (Fig. 1) [14].
Fig. 1. Triterpene biosynthetic pathway in ginseng. FPS, farnesyl diphosphate synthase; SQS, squalene synthase; SQE, squalene epoxidase; CAS, cycloartenol synthase; AS, β-amyrin synthase; DS, dammarenediol-II synthase.
Currently, more than 40 ginsenosides have been isolated from white and red ginseng [14,15]. Ginsenosides belong to the triterpene saponin family and were designated by Rx (x=0, a, b, c, d, e, f, 20-gluco-f, g, h, etc.) based on the value of the retention factor of spots on TLC plates from the bottom to the top [16]. Ginsenosides can be classified into two types based on the structures of aglycones: 1) dammarane-type ginsenosides, which include Rb, Rc, Rd, Re, Rf, and Rg, and 2) oleanane-type ginsenosides, of which there is only one known, Ro [17]. Dammarane-type ginsenosides are further classified into protopanaxadiol (Rb, Rc, and Rd) and protopanaxatriol (Re, Rf, and Rg) ginsenosides according to the positions of the sugar rings at carbons -3, -6, and -20 [17]. The major ginsenosides (Rb1, Rb2, Rc, Rd, Re, and Rg1) account for more than 80% of the total ginsenoside content in a raw ginseng root, and the minor ginsenosides (F1, F2, Rg3, Rh1, Rh2, compound Y, Mc, and K) can be produced by hydrolyzing the sugar molecules of the major ginsenosides [18]. The minor ginsenosides are pharmaceutically more active due to their easy absorption into the blood stream [19]. There are several methods by which major ginsenosides can be transformed into minor ginsenosides, such as acid hydrolysis, heating, microbial transformation, and enzymatic transformation [13].
In plants, the synthesis of secondary metabolites (alkaloids, terpenoids, flavonoids, phenolic compounds, and phytoalexins) usually fluctuates in response to various stresses [20]. Because ginsenosides are secondary metabolites, the accumulation of these compounds is controlled by the treatment of elicitors such as methyl jasmonate (MJ) and salicylic acid (SA) [10]. The total ginsenoside content increases about fourfold following treatment with MJ and SA in suspension cultures of ginseng roots in bioreactors. MJ and SA are key signaling compounds involved in the elicitation process, which leads to the accumulation of secondary metabolites [21]. Oligogalacturonic acid also induces the synthesis of ginsenosides in ginseng root cells [22]. Irradiation is also an important factor for the accumulation of secondary metabolites. For example, irradiation has been shown to induce the accumulation of anthocyanin pigments in many plants [23,24]. Induction of the synthesis of betalain pigments was also reported in the hairy roots of red beets in response to treatment with blue plus far red light [25]. Recent advances in the semiconductor field have made it possible to produce light-emitting diodes (LEDs) with high-fluence-rate light at specific wavelengths. Therefore, LEDs have many advantages over colored filters used in conjunction with standard bulbs, including the following: 1) the ease of controlling the output with simple electronics, 2) the precision of the emission spectra, which have narrow band widths, 3) the low energy requirements, and 4) the lack of heat generation.
Because ginsenosides are the major active components of ginseng roots, increased ginsenoside contents can increase the market value of ginseng roots. This study was conducted to investigate the effect of the specific emission spectra from various LED light sources on ginsenoside production in raw ginseng roots.
MATERIALS AND METHODS
Ginseng root materials and treatment of ginseng roots with light-emitting diode light
Fresh and healthy-looking raw ginseng roots (3 years old) were purchased from E-mart (Gyeongsan, Korea), which were stored at 4℃ after harvesting from Ganghwa, Korea for less than 1 mo. Three ginseng roots were put into a plastic zipper bag, which was then placed in one of the closed LED boxes (24×50×60 cm, width×height×depth) at 25℃. A PGL-E06-6W device (6 watt) was used for the LED light source, which was manufactured by PARUSkorea Inc. (Yeongam, Korea). Five LED chips were included in each device. Each LED box was separated by a black acryl panel and equipped with two LED light devices with different emission wavelengths, such as 380, 450, 470, and 660 nm. The top of the LED frame was placed at 50 cm below the top of the root samples. For the control treatment, the roots were stored at 4℃ in the dark at 25℃ for 7 d.
HPLC analysis of ginsenoside composition
The extraction and determination of the ginsenoside concentrations were performed according to a previously established method [26]. Ginsenosides were analyzed using HPLC with a Capcell Pak C18 MG (4.6×250 mm) column (Shiseido Inc., Tokyo, Japan). The HPLC analysis conditions were as follows: gradient elution, with the eluents being 0-10 min, 18%-18% acetonitrile; 10-24 min, 18%-19%; 24-35 min, 19%-25%; 35-54 min, 25%- 25%; 54-71 min, 25%-38%; 71-100 min, 38%-100%; and 100-105 min, 100%-100%. Ginsenoside standards (Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, and Rg2) were purchased from ChromaDex Inc. (Irvine, CA, USA). Experiments were conducted in triplicate, and the results were expressed as the mean values.
Statistical analyses were performed using SASS (SASS Inc., Cary, NC, USA). Data were expressed as the means with standard errors. The statistical significance of the differences between the mean values was assessed at the 5% level using Duncan’s multiple range test.
RESULTS AND DISCUSSION
The ginsenoside contents of the ginseng roots were evaluated after exposure of raw ginseng roots to LED light for 7 d. Compared with the untreated (4℃) ginseng roots, roots exposed to various emission wavelengths LED light sources had total ginsenoside concentrations that were 2% to 74% higher (Table 1).
Table 1.
The changes in ginsenoside content in ginseng root following light-emitting diodes treatment compared to untreated (4℃) ginseng roots
| Treatment | Ginsenoside(mg/100 mg DW) | Changes(%) | PPD/PPT |
|---|---|---|---|
|
| |||
| 4℃ | 2.348±0.303c | 0.0 | 0.778±0.085b |
| Dark | 2.200±0.251c | -6.3 | 0.852±0.037b |
| 380 nm | 2.480±0.549bc | +5.6 | 1.057±0.140ab |
| 450 nm | 3.871±0.133ab | +64.9 | 1.448±0.098a |
| 470 nm | 4.087±0.477a | +74.1 | 1.142±0.081ab |
| 660 nm | 2.395±0.115bc | +2.0 | 0.836±0.098b |
Values are presented as mean±SE of 3 replications. Different letters within a column are significantly different at a 5% level by Duncan’s multiple range test.
DW, dry weight; PPD, protopanaxadiol (Rb1, Rb2, Rc, and Rd); PPT, protopanaxatriol (Rg1, Rg2, Re, and Rf).
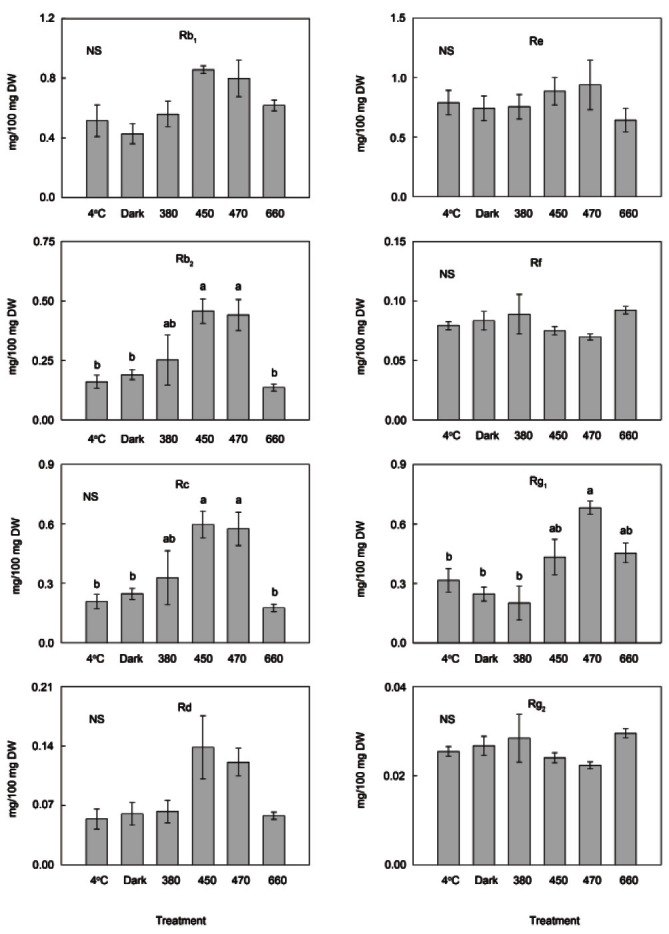
Although the other treatments (dark, 380 and 660 nm) did not significantly affect the ginsenoside concentrations, the exposure to both 450 and 470 nm light significantly increased the concentrations of ginsenosides (p<0.05) by 64.9% and 74.1%, respectively. These two treatments significantly increased the levels of Rb2 and Rc (p<0.05) by more than 100%, and the level of Rg1 significantly increased in response to exposure to 470 nm light (116%) (Fig. 2).
Fig. 2. Comparison of ginsenoside contents. Ginsenoside contents of ginseng roots were compared after treatments with 4℃ and dark as well as light-emitting diodes with 4 different light emission spectra (380, 450, 470, and 670 nm) at 25℃ for 7 d. The content of individual ginsenosides changed after treatments. Values are presented as mean±SE of 3 replications. Significant differences at a 5% level among groups are indicated by different letters above each bar. NS, not significant; DW, dry weight.
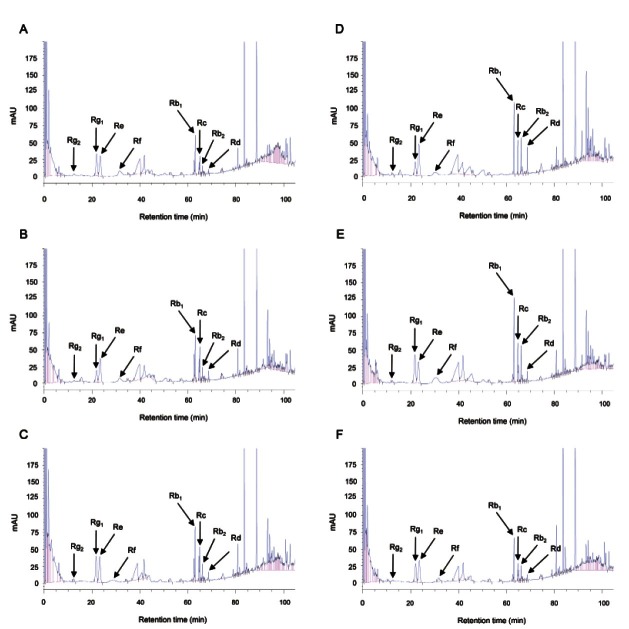
The concentrations of Rb2 and Rc were moderately increased in response to 380 nm emission spectrum (57%). The ratio of protopanaxadiol (PPD)-type ginsenosides (Rb1, Rb2, Rc, and Rd) to protopanaxatriol (PPT)-type ginsenosides (Rg1, Rg2, Re, and Rf) was also changed by the LED light treatment. Exposure to three emission wavelengths (380, 450, and 470 nm) significantly increased the PPD/PPT ratio (p<0.05), resulting in ratios that were higher than 1.0, whereas the three other treatments (4℃, darkness, 660 nm) resulted in ratios less than 1.0 (Table 1). The identity of each peak was confirmed by comparison of retention times and HPLC spectra of each peak with those of the 8 major ginsenosides (Fig. 3).
Fig. 3. HPLC chromatograms of ginseng root extracts. Control raw ginseng roots were incubated at 4℃ (A) for 7 d. Raw ginseng roots were treated in the dark (B), 380 nm (C), 450 nm (D), 470 nm (E), and 670 nm (F) for 7 d at 25℃.
The production of various secondary metabolites including ginsenosides is usually associated with defense responses to stresses [20]. In many studies, the ginsenoside contents were significantly increased by treatment with elicitors such as MJ, SA, oligogalacturonic acid, ethephon, and organic germanium [22,27-30]. Elicitor treatments may activate key regulatory enzymes in the isoprenoid pathway (e.g., squalene synthase), resulting in the induction of ginsenoside synthesis. Elicitor treatments usually induce the accumulation of reactive oxygen species (ROS) such as H2O2 and O2 -, which may increase the activity of defense genes, resulting in the accumulation of ginsenosides [27,31,32]. Various stresses, including treatment with MJ and SA, may damage plant cell membranes as the result of the generation of ROS. ROS cause the peroxidation of membrane lipids, which leads to plant membrane damage [27,33]. The induction of the synthesis of terpenoid compound taxoids (10-DAB III and paclitaxel) was observed in excised twigs from a yew tree (Taxus baccata) in response to irradiation with ultraviolet (UV-A and UV-C) light [34]. The level of paclitaxel synthesis was twofold higher in response to UV-C than in response to UV-A treatment. The synthesis of the sesquiterpene lactone parthenolide in feverfew (Tanacetum parthenium) was also increased by approximately threefold in response to UV (UV-A + UV-B) irradiation [35]. The induction of secondary metabolite production in response to light, including UV light, could be due to a general stress response to protect the plant and/or to a sun-screening effect to protect the plant from radiation. For example, it is well known that flavonoids function as sunscreens and protectants against ROS that are induced by light. Melatonin accumulated in Glycyrrhiza roots when the shoot was treated with UV light; this melatonin accumulation represents a general stress response. However, it is not clear whether the mechanism by which light from LEDs induces ginsenosides in ginseng roots is the same as that for other treatments. Further study is required to determine the induction mechanism.
Light has subsequent effects on the participants in complex signal transduction, such as enzymes, metabolites and messengers. Therefore, light could be used as a tool to control the nutritional quality of medicinal plants such as ginseng. However, it is difficult to obtain positive effects because different metabolic pathways react differently to light. In this study, we observed the accumulation of ginsenosides in raw ginseng roots in response to 450 and 470 nm light from LEDs. This report is the first to describe the increase in the concentrations of ginsenosides in response to light from LEDs. Typically, raw ginseng root is processed into white ginseng by an air-drying method and into red ginseng by steaming at 100℃ to enhance the shelf life and efficacy [36]. During the steaming process, the concentrations of pharmacologically effective components (e.g., Rg3 and Rh2) increase and the total amount of ginsenosides decreases as the result of non-enzymatic reactions [37-39]. Therefore, LED light treatment before the processing required to produce red ginseng would compensate for the decrease in the total amount of ginsenosides that occurs during the steaming process; Thus, exposure to LED light may allow the production of red ginseng with higher overall levels of pharmacological components and thus a greater commercial value. Further research is needed to elucidate the mechanism by which LED light affects ginsenoside accumulation.
Acknowledgments
This research was conducted as part of the industrial infrastructure program for fundamental technologies (no. 10033630), which is funded by the Ministry of Knowledge Economy (Korea).
References
- 1.Chang YS, Chang YH, Sung JH. The effect of ginseng and caffeine products on the antioxidative activities of mouse kidney. J Ginseng Res. 2006;30:15–21. doi: 10.5142/JGR.2006.30.1.015. [DOI] [Google Scholar]
- 2.Ernst E. Panax ginseng: an overview of the clinical evidence. J Ginseng Res. 2010;34:259–263. doi: 10.5142/jgr.2010.34.4.259. [DOI] [Google Scholar]
- 3.Lee ST. Cognitive improvement by ginseng in Alzheimer’s disease. J Ginseng Res. 2007;31:51–53. doi: 10.5142/JGR.2007.31.1.051. [DOI] [Google Scholar]
- 4.Wolf-Dieter R, Lin WM, Gabriele G, Radad K. Perspectives for ginsenosides in models of Parkinson’s disease. J Ginseng Res. 2007;31:127–136. doi: 10.5142/JGR.2007.31.3.127. [DOI] [Google Scholar]
- 5.Nam KY. Clinical applications and efficacy of Korean ginseng (Panax ginseng C.A. Meyer). J Ginseng Res. 2002;26:111–131. doi: 10.5142/JGR.2002.26.3.111. [DOI] [Google Scholar]
- 6.Vuksan V, Sievenpipper J, Jovanovski E, Jenkins AL. Current clinical evidence for Korean red ginseng in management of diabetes and vascular disease: a Toronto’s ginseng clinical testing program. J Ginseng Res. 2010;34:264–273. doi: 10.5142/jgr.2010.34.4.264. [DOI] [Google Scholar]
- 7.Lee SY, Kim YK, Park NI, Kim CS, Lee CY, Park SU. Chemical constituents and biological activities of the berry of Panax ginseng. J Med Plants Res. 2010;4:349–353. [Google Scholar]
- 8.Cho DH, Park KJ, Yu YH, Ohh SH, Lee HS. Root-rot development of 2-year old ginseng (Panax ginseng C.A. Meyer) caused by Cylindrocarpon destructans (Zinssm.) Scholten in the continuous cultivation filed. Korean J Ginseng Sci. 1995;19:175–180. [Google Scholar]
- 9.Ginseng production guide for commercial growers. Victoria; British Columbia: 2003. Ministry of Agriculture, Food and Fisheries. [Google Scholar]
- 10.Ali MB, Hahn EJ, Paek KY. Copper-induced changes in the growth, oxidative metabolism, and saponin production in suspension culture roots of Panax ginseng in bioreactors. Plant Cell Rep. 2006;25:1122–1132. doi: 10.1007/s00299-006-0174-x. [DOI] [PubMed] [Google Scholar]
- 11.Thanh NT, Murthy HN, Yu KW, Seung Jeong C, Hahn EJ, Paek KY. Effect of oxygen supply on cell growth and saponin production in bioreactor cultures of Panax ginseng. J Plant Physiol. 2006;163:1337–1341. doi: 10.1016/j.jplph.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Woo SS, Song JS, Lee JY, In DS, Chung HJ, Liu JR, Choi DW. Selection of high ginsenoside producing ginseng hairy root lines using targeted metabolic analysis. Phytochemistry. 2004;65:2751–2761. doi: 10.1016/j.phytochem.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 13.Park CS, Yoo MH, Noh KH, Oh DK. Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases. Appl Microbiol Biotechnol. 2010;87:9–19. doi: 10.1007/s00253-010-2567-6. [DOI] [PubMed] [Google Scholar]
- 14.Liang Y, Zhao S. Progress in understanding of ginsenoside biosynthesis. Plant Biol (Stuttg) 2008;10:415–421. doi: 10.1111/j.1438-8677.2008.00064.x. [DOI] [PubMed] [Google Scholar]
- 15.Cheng LQ, Na JR, Kim MK, Bang MH, Yang DC. Microbial conversion of ginsenoside Rb1 to minor ginsenoside F2 and gypenoside XVII by Intrasporangium sp. GS603 isolated from soil. J Microbiol Biotechnol. 2007;17:1937–1943. [PubMed] [Google Scholar]
- 16.Okazaki H, Tazoe F, Okazaki S, Isoo N, Tsukamoto K, Sekiya M, Yahagi N, Iizuka Y, Ohashi K, Kitamine T, et al. Increased cholesterol biosynthesis and hypercholesterolemia in mice overexpressing squalene synthase in the liver. J Lipid Res. 2006;47:1950–1958. doi: 10.1194/jlr.M600224-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Kim MK, Lee BS, In JG, Sun H, Yoon JH, Yang DC. Comparative analysis of expressed sequence tags (ESTs) of ginseng leaf. Plant Cell Rep. 2006;25:599–606. doi: 10.1007/s00299-005-0095-0. [DOI] [PubMed] [Google Scholar]
- 18.Noh KH, Oh DK. Production of the rare ginsenosides compound K, compound Y, and compound Mc by a thermostable beta-glycosidase from Sulfolobus acidocaldarius. Biol Pharm Bull. 2009;32:1830–1835. doi: 10.1248/bpb.32.1830. [DOI] [PubMed] [Google Scholar]
- 19.Karikura M, Miyase T, Tanizawa H, Taniyama T, Takino Y. Studies on absorption, distribution, excretion and metabolism of ginseng saponins. VII. Comparison of the decomposition modes of ginsenoside-Rb1 and -Rb2 in the digestive tract of rats. Chem Pharm Bull (Tokyo) 1991;39:2357–2361. doi: 10.1248/cpb.39.2357. [DOI] [PubMed] [Google Scholar]
- 20.Zhao J, Davis LC, Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv. 2005;23:283–333. doi: 10.1016/j.biotechadv.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Ebel J, Mithofer A. Early events in the elicitation of plant defence. Planta. 1998;206:335–348. doi: 10.1007/s004250050409. [DOI] [Google Scholar]
- 22.Hu X, Neill S, Cai W, Tang Z. Hydrogen peroxide and jasmonic acid mediate oligogalacturonic acid-induced saponin accumulation in suspension-cultured cells of Panax ginseng. Physiol Plant. 2003;118:414–421. doi: 10.1034/j.1399-3054.2003.00124.x. [DOI] [Google Scholar]
- 23.Kim BG, Kim JH, Kim J, Lee C, Ahn JH. Accumulation of flavonols in response to ultraviolet-B irradiation in soybean is related to induction of flavanone 3-beta-hydroxylase and flavonol synthase. Mol Cell. 2008;25:247–252. [PubMed] [Google Scholar]
- 24.Zhou B, Li Y, Xu Z, Yan H, Homma S, Kawabata S. Ultraviolet A-specific induction of anthocyanin biosynthesis in the swollen hypocotyls of turnip (Brassica rapa). J Exp Bot. 2007;58:1771–1781. doi: 10.1093/jxb/erm036. [DOI] [PubMed] [Google Scholar]
- 25.Shin KS, Murthy HN, Heo JW, Paek KY. Induction of betalain pigmentation in hairy roots of red beet under different radiation sources. Biol Plant. 2003;47:149–152. [Google Scholar]
- 26.Yu KW, Gao W, Hahn EJ, Paek KY. Jasmonic acid improves ginsenoside accumulation in adventitious root culture of Panax ginseng C. A. Meyer. Biochem Eng J. 2002;11:211–215. doi: 10.1016/S1369-703X(02)00029-3. [DOI] [Google Scholar]
- 27.Ali MB, Yu KW, Hahn EJ, Paek KY. Methyl jasmonate and salicylic acid elicitation induces ginsenosides accumulation, enzymatic and non-enzymatic antioxidant in suspension culture Panax ginseng roots in bioreactors. Plant Cell Rep. 2006;25:613–620. doi: 10.1007/s00299-005-0065-6. [DOI] [PubMed] [Google Scholar]
- 28.Bae KH, Choi YE, Shin CG, Kim YY, Kim YS. Enhanced ginsenoside productivity by combination of ethephon and methyl jasmoante in ginseng (Panax ginseng C.A. Meyer) adventitious root cultures. Biotechnol Lett. 2006;28:1163–1166. doi: 10.1007/s10529-006-9071-1. [DOI] [PubMed] [Google Scholar]
- 29.Kim YS, Yeung EC, Hahn EJ, Paek KY. Combined effects of phytohormone, indole-3-butyric acid, and methyl jasmonate on root growth and ginsenoside production in adventitious root cultures of Panax ginseng C.A. Meyer. Biotechnol Lett. 2007;29:1789–1792. doi: 10.1007/s10529-007-9442-2. [DOI] [PubMed] [Google Scholar]
- 30.Yu KW, Murthy HN, Jeong CS, Hahn EJ, Paek KY. Organic germanium stimulates the growth of ginseng adventitious roots and ginsenoside production. Process Biochem. 2005;40:2959–2961. doi: 10.1016/j.procbio.2005.01.015. [DOI] [Google Scholar]
- 31.Chen H, Chen F. Kinetics of cell growth and secondary metabolism of a high-tanshinone-producing line of the Ti transformed Salvia miltiorrhiza cells in suspension culture. Biotechnol Lett. 1999;21:701–705. doi: 10.1023/A:1005562410037. [DOI] [Google Scholar]
- 32.Jabs T, Tschope M, Colling C, Hahlbrock K, Scheel D. Elicitor-stimulated ion fluxes and O2 - from the oxidative burst are essential components in triggering defense gene activation and phytoalexin synthesis in parsley. Proc Natl Acad Sci U S A. 1997;94:4800–4805. doi: 10.1073/pnas.94.9.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chong TM, Abdullah MA, Fadzillah NM, Lai OM, Lajis NH. Jasmonic acid elicitation of anthraquinones with some associated enzymic and non-enzymic antioxidant responses in Morindaelliptica. Enzym Microb Technol. 2005;36:469–477. doi: 10.1016/j.enzmictec.2004.11.002. [DOI] [Google Scholar]
- 34.Hajnos ML, Zobel AM, Głowniak K. The influence of ultraviolet radiation on the content of pharmacologically active taxoids in yew tissues. Phytomedicine. 2001;8:139–143. doi: 10.1078/0944-7113-00010. [DOI] [PubMed] [Google Scholar]
- 35.Fonseca JM, Rushing JW, Rajapakse NC, Thomas RL, Riley MB. Potential implications of medicinal plant production in controlled environments: the case of feverfew (Tanacetum parthenium). HortScience. 2006;41:531–535. [Google Scholar]
- 36.Korea Ginseng & Tobacco Research Institute. Korean Ginseng. Korea Ginseng &Tobacco Research Institute; Daejeon: 1994. pp. 43–62.Chapter 5 Process of ginseng. [Google Scholar]
- 37.Kim WY, Kim JM, Han SB, Lee SK, Kim ND, Park MK, Kim CK, Park JH. Steaming of ginseng at high temperature enhances biological activity. J Nat Prod. 2000;63:1702–1704. doi: 10.1021/np990152b. [DOI] [PubMed] [Google Scholar]
- 38.Takaku T, Kameda K, Matsuura Y, Sekiya K, Okuda H. Studies on insulin-like substances in Korean red ginseng. Planta Med. 1990;56:27–30. doi: 10.1055/s-2006-960877. [DOI] [PubMed] [Google Scholar]
- 39.Yun TK, Lee YS, Lee YH, Kim SI, Yun HY. Anticarcinogenic effect of Panax ginseng C.A. Meyer and identification of active compounds. J Korean Med Sci. 2001;16(Suppl):S6–S18. doi: 10.3346/jkms.2001.16.S.S6. [DOI] [PMC free article] [PubMed] [Google Scholar]