Abstract
The abnormal maturation and ossification of articular chondrocytes play a central role in the pathogenesis of osteoarthritis (OA). Inhibiting the enzymatic degradation of the extracellular matrix and maintaining the cellular phenotype are two of the major goals of interest in managing OA. Ginseng is frequently taken orally, as a crude substance, as a traditional medicine in Asian countries. Ginsenoside Rb1, a major component of ginseng that contains an aglycone with a dammarane skeleton, has been reported to exhibit various biological activities, including anti-inflammatory and anti-tumor effects. However, a chondroprotective effect of ginsenoside Rb1 related to OA has not yet been reported. The purpose of this study was to demonstrate the chondroprotective effect of ginsenoside Rb1 on the regulation of pro-inflammatory factors and chondrogenic genes. Cultured rat articular chondrocytes were treated with 100 μM ginsenoside Rb1 and/or 500 μM hydrogen peroxide (H2O2) and assessed for viability, reactive oxygen species production, nitric oxide (NO) release, and chondrogenic gene expression. Ginsenoside Rb1 treatment resulted in reductions in the levels of pro-inflammatory cytokine and NO in H2O2-treated chondrocytes. The expression levels of chondrogenic genes, such as type II collagen and SOX9, were increased in the presence of ginsenoside Rb1, whereas the expression levels of inflammatory genes related to chondrocytes, such as MMP1 and MMP13, were reduced by approximately 50%. These results suggest that ginsenoside Rb1 has potential for use as a therapeutic agent in OA patients.
Keywords: Panax ginseng, Ginsenoside Rb1, Inflammation, Hydrogen peroxide, Chondrocytes, Osteoarthritis
INTRODUCTION
Osteoarthritis (OA) is one of the most prevalent chronic diseases affecting the elderly and is a major degenerative disease affecting millions of individuals. The most prominent feature of OA is the progressive destruction of articular cartilage, and it is now accepted that OA is a global disease involving synovial membranes, subchondral bone, and periarticular soft tissues [1]. Today, in the search for a cure for OA, the inhibition of the enzymatic degradation of the extracellular matrix (ECM) and the maintenance of the cellular phenotype are two of the major goals in OA research [2,3].
Hydrogen peroxide (H2O2) has a variety of biological effects on various cell types. In chondrocytes, H2O2 inhibits proteoglycan synthesis and induces the degradation of the ECM [4]. H2O2 is also related to aggrecan degradation in bovine articular chondrocytes [5]. H2O2 induces the expression of the inflammatory cytokines interleukin (IL)-1β and tumor necrosis factor (TNF)-α, which exert negative effects on the expression of matrix metalloproteinases (MMPs) and transforming growth factor (TGF)-β1 [6]. Furthermore, H2O2 may produce reactive oxygen species (ROS) and nitric oxygen (NO) species in addition to inducing the apoptosis of chondrocytes [7]. Thus, H2O2 may be an important factor involved in the pathology of OA. Free radicals are related to many chronic inflammatory diseases [4]. Many studies have shown that free radicals play an important role in the pathology of OA [5-8]. Among the various ROS and NO that exist, H2O2 is an important intermediate in superoxide anion metabolism. H2O2 is formed from the superoxide anion by superoxide dismutase and is removed by glutathione peroxidase and catalase [9]. Recent studies show that the activity of catalase in OA joints is decreased [10] and that the accumulation H2O2 in arthritic joints is expected [11]. Therefore, antioxidants that protect against free radicals such as H2O2 are valuable for the protection of chondrocytes by blocking the catabolic signaling cascades that are triggered by inflammatory cytokines and free radical stimulation.
Ginseng is a rich source of ginsenosides, and several epidemiological and animal studies have shown that ginseng consumption is associated with health benefits, including the inhibition of inflammation [12]. Most of the beneficial health effects of ginseng have been shown to be mediated by most prevalent component, ginsenoside. Ginsenoside influences a number of cellular mechanisms and has been shown to inhibit the activities of IL-1β and TNF-α [13]. In addition, ginsenoside Rb1 is also an inhibitor of ROS and NO activity and downregulates the levels of several markers of oxidative stress [13]. Despite several studies in the literature detailing the antioxidant effects of ginsenoside Rb1, the mechanisms underlying protective effects at the chondrocyte cellular level have not been reported. In this study, we evaluated the protective effects of ginsenoside Rb1 on rat articular chondrocytes and the molecular mechanisms involved in the protection.
MATERIALS AND METHODS
Harvesting chondrocytes and establishing cell cultures
A modified method for harvesting chondrocytes was used as previously described [14]. Briefly, chondrocytes were isolated from the articular cartilage of three-week-old male Sprague Dawley rats. Cartilage was removed from animals that were subsequently euthanized by an overdose of anesthesia. The cartilage was cut into thin slices, washed with sterilized phosphate-buffered saline (PBS), and soaked in 5% penicillin–streptomycin– neomycin (Sigma, St. Louis, MO, USA) for 15 min. The cartilage slices were washed with PBS to remove residual antibiotic solution and digested with 0.02% type II collagenase (Sigma) in Dulbecco’s Modified Eagle’s Medium (DMEM; HyClone, Logan, UT, USA) for 3 h in a 37℃ water bath. The digested cartilage was collected and centrifuged. The pellet was resuspended in DMEM and filtered through 70-μm nylon mesh. The resultant chondrocytes were cultured in DMEM supplement with 10% fetal bovine serum and 1% penicillin–streptomycin– neomycin in a 5% CO2 incubator at 37℃. All experiments were performed when cells reached confluence within the first passage. Ginsenoside Rb1 was purchased from Ambo Institute (Daejeon, Korea).
Cell viability assay
The water soluble tetrazolium cell proliferation assay was used to measure cell viability by calculating the quantitative absorbance of formazan using an EZ-Cytox Cell Viability Assay Kit (DAEIL lab, Seoul, Korea). The EZ-Cytox agent is converted into orange formazan by the mitochondria of active cells. The amount of orange product increases with an increase in cell activity and can be quantified using a spectrophotometer. Cells were plated in 96-well plates at a density of 2.0×104 cells per well, incubated for 24 h and treated with various concentrations (25 to 400 μM) of ginsenoside Rb1. We then determined whether a 1-hour pretreatment with ginsenoside Rb1 (50 or 100 μM) influenced the viability of chondrocytes treated with 500 μM H2O2 for 24 h. After the incubation period, 10 μL of the kit solution was added to each well, and the cells were incubated for 3 h at 37℃ in 5% CO2. The index of cell viability was determined by measuring formazan production with a microplate reader at an absorbance of 480 nm and a reference wavelength of 650 nm.
Enzyme-linked immunosorbent assay
The concentrations of IL-1β and TNF-α in the supernatants were determined by enzyme-linked immunosorbent assay (ELISA) using a kit (Invitrogen, Carlsbad, USA) according to the manufacturer’s instructions. The detection limit of the assay was 1.6 pg/mL.
Intracellular reactive oxygen species assay
The level of intracellular ROS was quantified by fluorescence using dichlorofluorescein diacetate (DCFDA, Invitrogen). Chondrocytes plated in a 48-well plate were left untreated or were pretreated for 1 h with 50 or 100 μM ginsenoside Rb1 in the absence or presence of H2O2, and then the cells were incubated for 0.5 h. After the incubation period, the cells were washed with PBS and stained with 10 μM DCF-DA in PBS for 30 min in the dark. The cells were then washed twice with PBS and extracted with 0.1% Tween-20 in PBS for 10 min at 37℃. Fluorescence was recorded with an excitation wavelength of 490 nm and an emission wavelength of 525 nm.
Nitric oxygen production and activity of inducible nitric oxide synthase
The concentration of NO was measured by the Griess reaction. A 20 μL sample of culture medium supernatant was mixed with 180 μL of Griess reagent at room temperature for 10 min, and then the absorbance was measured in a microplate reader at 570 nm. The activity of inducible nitric oxide synthase (iNOS) was analyzed by immunoblotting. Briefly, total cell extracts were harvested in radioimmunoprecipitation assay buffer and then centrifuged at 15,000 rpm for 15 min at 4℃. Quantification of the total protein was performed with bicinchoninic acid protein assay reagent (Bio-Rad Laboratories, Hercules, CA, USA). Proteins were resolved with sodium dodecyl sulfate polyacrylamide gel electrophoresis at 10% and transferred to a polyvinylidene difluoride membrane. After blocking in 5% skim milk in PBS with 0.1% Tween-20 (PBS-T), the membrane was incubated with specific primary antibodies for iNOS and β-actin (Cell Signaling, Danvers, MA, USA) diluted 1:1,000 in 1% skim milk in PBS-T overnight at 4℃. After washing, the blots were incubated at room temperature for 1 h with a peroxidase-conjugated goat anti-rabbit IgG secondary antibody (Millipore, Bedford, MA, USA) diluted 1:10,000 in PBS-T. The signals were detected with a SuperSignal West Dura Extended Duration Substrate (Thermo Scientific, San Jose, CA, USA), according to the manufacturer’s instructions. Densitometric analysis was conducted directly using the blotted membrane utilizing a ChemiImager analyzer system (Alpha Innotech, San Leandro, CA, USA).
RNA preparation and real-time reverse transcription polymerase chain reaction
Total cellular RNA was precipitated with Ribo EX (GeneAll, Daejeon, Korea) and dissolved in 0.1% diethylpyrocarbonate-treated distilled water. Total RNA (2 μg) was treated with RNase-free DNase (Invitrogen), and first-strand cDNA was generated using the oligo primers provided in a first-strand cDNA synthesis kit, (Maxime RT PreMix; Intron, Seongnam, Korea), according the manufacturer’s instructions. Specific primers for each gene (Table 1) were designed using Primer Express Software (Applied Biosystems, Carlsbad, CA, USA). The real-time reverse transcription polymerase chain reaction mixture consisted of 10 ng of reverse-transcribed total RNA, 167 nM forward and reverse primers, and PCR Master Mix (2X) in a final volume of 20 μL. Polymerase chain reaction was carried out in 48-well plates using the ABI Step One Plus Sequence Detection System (Applied Biosystems). All experiments were performed in triplicate.
Table 1.
Gene sequences for real-time reverse transcription polymerase chain reaction
| Gene name | Sequences | |
|---|---|---|
|
| ||
| Collagen type II | FOR: GAGTGGAAGAGCGGAGACTACTG | Chondrogenic genes |
| REV: CTCCATGTTGCAGAAGACTTTCA | ||
| SOX9 | FOR: AGAGCGTTGCTCGGAACTGT | |
| REV: TCCTGGACCGAAACTGGTAAA | ||
| MMP1 | FOR: GCCATTACTCACAACAATCCTC | Chondrogenic inflammatory genes |
| REV: ACACAATATCACCTTCCTCCTC | ||
| MMP13 | FOR: AGGCCT TCAGAAAAGCCT TC | |
| REV: GAGCTGCTTGTCCAGGTTTC | ||
| GAPDH | FOR: TGAACGGGAAGCTCACTGG | |
| REV: TCCACCACCCTGTTGCTGTA | ||
RESULTS
Ginsenoside Rb1 increased cell viability
To examine effect of Rb1 on primary chondrocytes, the cell viability of the chondrocytes in the presence of Rb1 or H2O2 was determined. As shown in Fig. 1A, the chondrocytes exposed to ginsenoside Rb1 at various concentrations (0 to 400 μM) did not exhibit significant toxicity over a period of 24 h. The percentage of viable cells was approximately 60% after a 24 h treatment with 500 μM H2O2. Thus, this treatment caused an approximately 40% loss of cell viability (Fig. 1B).
Fig. 1. Cell viability of ginsenoside Rb1 in native chondrocytes and (H2O2)-treated chondrocytes. (A) Ginsenoside Rb1 (25 to 400 μM) was added to culture medium and rat articular chondrocytes were incubated for 24 h. (B) Culture medium pretreated for 1 h with ginsenoside Rb1 (50 and 100 μM) and incubated with 500 μM H2O2 for 24 h. Cell viability was assessed by the level of WST reduction and expressed as a percentage of viable untreated control cells grown in a defined medium. Data are expressed as mean±SEM of three independent experiments, *p<0.05.
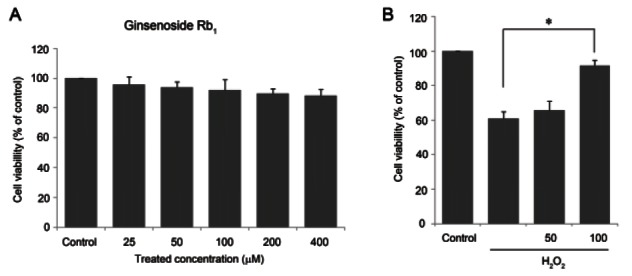
As shown in Fig. 1B, a 1-hour pretreatment with ginsenoside Rb1 (100 μM) before the 24-hour incubation with H2O2 (500 μM) resulted in a higher cell viability (approximately 92%, p<0.05) than H2O2 alone, indicating that 100 μM ginsenoside Rb1 may improve cell viability in the presence of H2O2. Therefore, all subsequent experiments were carried out using chondrocytes that had been pretreated for 1 h with 50 or 100 μM ginsenoside Rb1 and then exposed to 500 μM H2O2 for 24 h.
Ginsenoside Rb1 decreased the release of interleukin- 1β and tumor necrosis factor-α
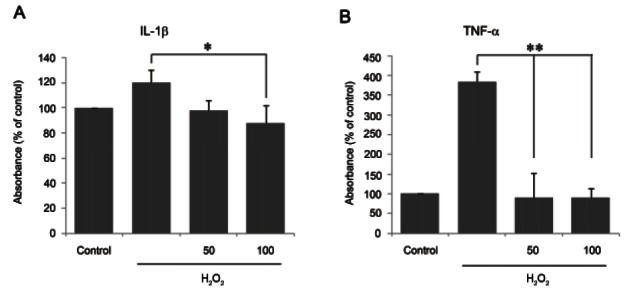
IL-1β and TNF-α are pro-inflammatory cytokines that are induced by various cell stimulators. The absorbances measured by IL-1β and TNF-α ELISA kits were converted into percentages of the control absorbances.
As shown in Fig. 2A and 2B, the absorbances of IL-1β and TNF-α in 500 μM H2O2-treated chondrocytes over 24 h increased approximately 130% and 364%, respectively, compared with non-treated chondrocytes. Pretreatment with ginsenoside Rb1 prior to incubation with 500 μM H2O2 for 24 h attenuated IL-1β (p<0.05) and TNF-α (p<0.01) production by approximately 100%, indicating that ginsenoside Rb1 attenuated the expression of the pro-inflammatory cytokines IL-1β and TNF-α in H2O2- treated chondrocytes.
Fig. 2. Inhibition of interleukin (IL)-1β and tumor necrosis factor (TNF)-α by ginsenoside Rb1 in hydrogen peroxide (H2O2)-treated chondrocytes. Culture medium was pretreated for 1 h with ginsenoside Rb1 (50 and 100 μM) and incubated with 500 μM H2O2 for 24 h. The release of IL-1β (A) and TNF-α (B) was measured in the culture supernatant using enzyme-linked immunosorbent assay. Data are expressed as mean±SEM of three independent experiments, *p<0.05, **p<0.01.
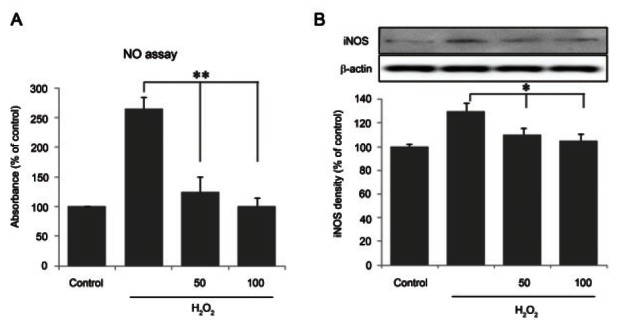
Ginsenoside Rb1 suppressed nitric oxide production and inducible nitric oxide synthase activity
To further analyze the effect of Rb1 on nitric oxygen production and iNOS expression, cells were treated with Rb1 prior to H2O2 treatment. As shown in Fig. 3A, ginsenoside Rb1 dose-dependently suppressed the NO production induced by H2O2 to approximately 250% at the highest concentration. The activity of iNOS was determined by immunoblotting (Fig. 3B), which revealed the dose-dependent suppression of iNOS protein expression. These results indicate that ginsenoside Rb1 suppressed the production of NO (p<0.01) and iNOS expression (p<0.05) in H2O2-treated chondrocytes.
Fig. 3. Inhibition of nitric oxide (NO) production and inducible nitric oxide synthase (iNOS) protein analysis by ginsenoside Rb1 in hydrogen peroxide (H2O2)-treated chondrocytes. Culture medium was pretreated for 1 h with ginsenoside Rb1 (50 and 100 μM) and incubated with 500 μM H2O2 for 24 h. (A) The release of NO was measured in the culture supernatant using Griess reagent. (B) Immunoblotting for analysis of iNOS protein expression was performed as described in Materials and Methods. Data are expressed as mean±SEM of three independent experiments, *p<0.05, **p<0.01.
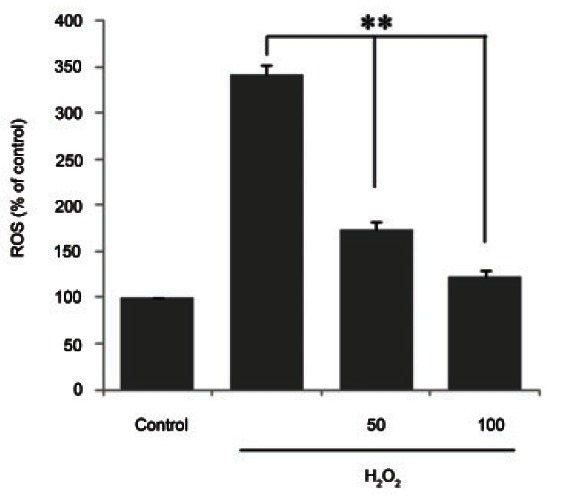
Ginsenoside Rb1 suppressed cellular reactive oxygen species production
The level of ROS in ginsenoside Rb1- and H2O2- treated chondrocytes was measured (Fig. 4). The H2O2- treated cells showed the greatest increase in cellular ROS production (up to threefold) compared with the control. The cells exposed to the 1-hour pretreatment with 100 μM ginsenoside Rb1 and to H2O2 exhibited significantly suppressed production of ROS (down to threefold, p<0.01) compared with the H2O2-only group. This result indicates that ginsenoside Rb1 significantly suppressed ROS production in H2O2-treated chondrocytes.
Fig. 4. Inhibition of reactive oxygen species (ROS) production by ginsenoside Rb1 in hydrogen peroxide (H2O2)-induced in chondrocytes. Chondrocytes were untreated or pretreated for 1 h with 50 and 100 μM ginsenoside Rb1 in the presence of 500 μM H2O2, and incubated for 0.5 h. Measurement of reactive oxygen species (ROS) production was performed as described in Materials and Methods section. Data are expressed as mean±SEM of three independent experiments, **p<0.01.
Ginsenoside Rb1 improved chondrogenic genes and suppressed chondrogenic inflammatory genes
H2O2 induced the expression of the chondrogenic inflammation factors MMP1 and MMP13 in chondrocytes. Additionally, H2O2 reduced the expression of type II collagen and SOX9, proteoglycans produced by chondrocytes with normal phenotypes. Thus, while H2O2 increased MMP1 and MMP13 expression and decreased type II collagen and SOX9 expression, chondrocytes pretreated with 100 μM ginsenoside Rb1 for 1 h prior to exposure to H2O2 showed a different gene expression pattern (Fig. 5).
Fig. 5. Up-regulation of chondrogenic genes and down-regulation of inflammatory genes by ginsenoside in hydrogen peroxide (H2O2)-treated chondrocytes. Total RNA was obtained after 1 h pretreatment with 50 and 100 μM ginsenoside Rb1 in the presence of 500 μM H2O2-treated chondrocytes for 12 h. Real-time reverse transcription polymerase chain reaction analysis for chondrogenic genes (collagen type II and SOX9) and chondrogenic inflammatory genes (MMP1 and MMP13) was performed as described in Materials and Methods section. Results revealed that ginsenoside Rb1 increased chondrogenic gene expression and reduced chondrogenic inflammatory gene expression. Values are expressed as mean±SEM of three independent experiments, *p<0.05.
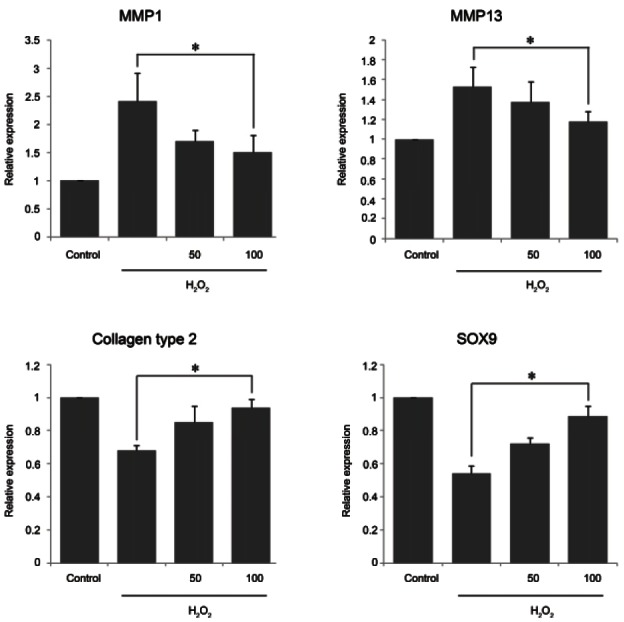
After the addition of H2O2 for 12 h, cultures that had been pretreated with ginsenoside Rb1 exhibited decreased MMP1 and MMP13 expression levels (from 2.5 to 1.4 fold, p<0.05, and 1.5 to 1.2 fold, p<0.05, respectively) compared with cultures treated with H2O2 alone. The reductions in the expression levels of type II collagen and SOX9 were restored by pretreatment with 100 μM ginsenoside Rb1 compared with H2O2-only treated cells. These results indicate that ginsenoside Rb1 attenuated chondrogenic inflammation and improved the expression of proteoglycans that protect against H2O2- induced inflammation in rat chondrocytes.
DISCUSSION
The abnormal maturation and ossification of articular chondrocytes play a central role in the pathogenesis of OA [15]. The pathology of OA involves various risk factors. Among these risk factors, oxidative stress and free radicals have been suggested to be important factors involved in OA [16].
Previously published studies have demonstrated that OA chondrocytes have a high lipoxidative activity [17]. Furthermore, the antioxidant capacity is diminished by aging, and increased lipid peroxidation has been observed in OA patients. These studies showed that oxidative stress plays an important role in the pathology of OA [18].
Excessive oxidative stress causes damage to DNA, lipids, and proteins and induces concomitant cellular damage [19]. H2O2 is a key intermediate in superoxide anion metabolism [3]. Several studies have shown that the activity of catalase (a free radical scavenger) is decreased in arthritic joints, including joints affected by OA [4]. Moreover, the major inflammatory cytokines IL-1β and TNF-α are highly expressed in OA. These cytokines downregulate the enzymatic antioxidant defenses in chondrocytes, resulting in the transient accumulation of H2O2 [5,20]. Therefore, using antioxidants to protect against free radicals may be important the regulation of OA. In this study, H2O2 was employed to create an oxidative stress environment, and the effect of ginsenoside Rb1 on the regulation of various genes in H2O2-stimulated chondrocytes was evaluated. A previous study demonstrated the anti-oxidative and anti-inflammatory effects of ginsenoside Rb1 on 6-hydroxydopamine-treated human dopaminergic cells [21] and lipopolysaccharidetreated RAW 264.7 cells [22]. Our results revealed that ginsenoside Rb1 exhibits strong cytoprotective effects in a dose-dependent manner (Fig. 1B). The levels of the H2O2-elicited proinflammatory cytokines IL-1β and TNF-α were significantly inhibited by ginsenoside Rb1 (Fig. 2). Chondrocytes in the articular cavity might be the primary source of NO because they can generate more than any other cell type. Compared with the control, the chondrocytes in our in vitro model of OA produced significantly greater levels of NO (Fig. 3A). NO is generated by activated iNOS during inflammatory processes [23]. Our results showed that H2O2-elicited NO, iNOS, and ROS production were significantly suppressed by ginsenoside Rb1 (Figs. 3 and 4). NO can induce intracellular signal transduction and inflammatory gene activation. Furthermore, NO can inhibit the synthesis of collagens and proteoglycans and can increase MMP activity in chondrocytes [24]. Pathologic changes include the decreased synthesis of ECM proteins, and the local accumulation of destructive enzymes was observed in H2O2-stimulated chondrocytes [25]. Drugs for the treatment of degenerative diseases of articular joints may be developed by increasing the synthesis of ECM molecules such as type II collagen and SOX9 and by inhibiting destructive enzymes such as MMPs [26]. Among members of the MMP superfamily, MMP1 and MMP13 have been shown to degrade type II collagen in OA. Our results revealed that ginsenoside Rb1 antagonized chondrogenic inflammatory genes (MMP1 and MMP13) and agonized chondrogenic genes (type II collagen and SOX8) to protect against H2O2-induced oxidative stress (Fig. 5). These results suggest that ginsenoside Rb1 has potential for use as a therapeutic agent for OA due to this compound’s anti-inflammatory effects.
Our study evaluated the cytoprotective effects of ginsenoside Rb1 on H2O2-stimulated chondrocytes. By detecting major proteins and inflammatory genes related to chondrogenic enzymes, we determined that ginsenoside Rb1 plays a protective role in articular chondrocytes. In conclusion, the present study demonstrated the protective effect of ginsenoside Rb1 on chondrocytes and provided new insight into the medicinal qualities of ginseng extracts. Our results suggest the possibility of including ginsenoside Rb1 in therapeutic interventions for OA.
References
- 1.Drissi H, Zuscik M, Rosier R, O’Keefe R. Transcriptional regulation of chondrocyte maturation: potential involvement of transcription factors in OA pathogenesis. Mol Aspects Med. 2005;26:169–179. doi: 10.1016/j.mam.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Matsumoto T, Cooper GM, Gharaibeh B, Meszaros LB, Li G, Usas A, Fu FH, Huard J. Cartilage repair in a rat model of osteoarthritis through intraarticular transplantation of muscle-derived stem cells expressing bone morphogenetic protein 4 and soluble Flt-1. Arthritis Rheum. 2009;60:1390–1405. doi: 10.1002/art.24443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathy-Hartert M, Martin G, Devel P, Deby-Dupont G, Pujol JP, Reginster JY, Henrotin Y. Reactive oxygen species downregulate the expression of pro-inflammatory genes by human chondrocytes. Inflamm Res. 2003;52:111–118. doi: 10.1007/s000110300023. [DOI] [PubMed] [Google Scholar]
- 4.Asada S, Fukuda K, Oh M, Hamanishi C, Tanaka S. Effect of hydrogen peroxide on the metabolism of articular chondrocytes. Inflamm Res. 1999;48:399–403. doi: 10.1007/s000110050478. [DOI] [PubMed] [Google Scholar]
- 5.Jallali N, Ridha H, Thrasivoulou C, Underwood C, Butler PE, Cowen T. Vulnerability to ROS-induced cell death in ageing articular cartilage: the role of antioxidant enzyme activity. Osteoarthritis Cartilage. 2005;13:614–622. doi: 10.1016/j.joca.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Martin G, Andriamanalijaona R, Mathy-Hartert M, Henrotin Y, Pujol JP. Comparative effects of IL-1beta and hydrogen peroxide (H2O2) on catabolic and anabolic gene expression in juvenile bovine chondrocytes. Osteoarthritis Cartilage. 2005;13:915–924. doi: 10.1016/j.joca.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Asada S, Fukuda K, Nishisaka F, Matsukawa M, Hamanisi C. Hydrogen peroxide induces apoptosis of chondrocytes; involvement of calcium ion and extracellular signal-regulated protein kinase. Inflamm Res. 2001;50:19–23. doi: 10.1007/s000110050719. [DOI] [PubMed] [Google Scholar]
- 8.Henrotin Y, Deberg M, Christgau S, Henriksen D, Seidel L, Reginster JY. Type II collagen derived fragment (COLL2- 1) is a new marker predictive of osteoarthritic progression. Osteoporos Int. 2002;13(Suppl 3):S17. [Google Scholar]
- 9.Afonso V, Champy R, Mitrovic D, Collin P, Lomri A. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine. 2007;74:324–329. doi: 10.1016/j.jbspin.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Altindag O, Erel O, Aksoy N, Selek S, Celik H, Karaoglanoglu M. Increased oxidative stress and its relation with collagen metabolism in knee osteoarthritis. Rheumatol Int. 2007;27:339–344. doi: 10.1007/s00296-006-0247-8. [DOI] [PubMed] [Google Scholar]
- 11.Mathy-Hartert M, Hogge L, Sanchez C, Deby-Dupont G, Crielaard JM, Henrotin Y. Interleukin-1beta and interleukin- 6 disturb the antioxidant enzyme system in bovine chondrocytes: a possible explanation for oxidative stress generation. Osteoarthritis Cartilage. 2008;16:756–763. doi: 10.1016/j.joca.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Wu JY, Gardner BH, Murphy CI, Seals JR, Kensil CR, Recchia J, Beltz GA, Newman GW, Newman MJ. Saponin adjuvant enhancement of antigen-specific immune responses to an experimental HIV-1 vaccine. J Immunol. 1992;148:1519–1525. [PubMed] [Google Scholar]
- 13.Mochizuki M, Yoo YC, Matsuzawa K, Sato K, Saiki I, Tono-oka S, Samukawa K, Azuma I. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3, of red ginseng. Biol Pharm Bull. 1995;18:1197–1202. doi: 10.1248/bpb.18.1197. [DOI] [PubMed] [Google Scholar]
- 14.Goldring MB. Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best Pract Res Clin Rheumatol. 2006;20:1003–1025. doi: 10.1016/j.berh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64:460–466. [PubMed] [Google Scholar]
- 16.Bubici C, Papa S, Dean K, Franzoso G. Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: molecular basis and biological significance. Oncogene. 2006;25:6731–6748. doi: 10.1038/sj.onc.1209936. [DOI] [PubMed] [Google Scholar]
- 17.Tiku ML, Shah R, Allison GT. Evidence linking chondrocyte lipid peroxidation to cartilage matrix protein degradation. Possible role in cartilage aging and the pathogenesis of osteoarthritis. J Biol Chem. 2000;275:20069–20076. doi: 10.1074/jbc.M907604199. [DOI] [PubMed] [Google Scholar]
- 18.Aigner T, Sachse A, Gebhard PM, Roach HI. Osteoarthritis: pathobiology-targets and ways for therapeutic intervention. Adv Drug Deliv Rev. 2006;58:128–149. doi: 10.1016/j.addr.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 20.Situnayake RD, Thurnham DI, Kootathep S, Chirico S, Lunec J, Davis M, McConkey B. Chain breaking antioxidant status in rheumatoid arthritis: clinical and laboratory correlates. Ann Rheum Dis. 1991;50:81–86. doi: 10.1136/ard.50.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang YP, Jeong HG. Ginsenoside Rb1 protects against 6-hydroxydopamine-induced oxidative stress by increasing heme oxygenase-1 expression through an estrogen receptor-related PI3K/Akt/Nrf2-dependent pathway in human dopaminergic cells. Toxicol Appl Pharmacol. 2010;242:18–28. doi: 10.1016/j.taap.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Park EK, Shin YW, Lee HU, Kim SS, Lee YC, Lee BY, Kim DH. Inhibitory effect of ginsenoside Rb1 and compound K on NO and prostaglandin E2 biosyntheses of RAW264.7 cells induced by lipopolysaccharide. Biol Pharm Bull. 2005;28:652–656. doi: 10.1248/bpb.28.652. [DOI] [PubMed] [Google Scholar]
- 23.Zhou PH, Liu SQ, Peng H. The effect of hyaluronic acid on IL-1beta-induced chondrocyte apoptosis in a rat model of osteoarthritis. J Orthop Res. 2008;26:1643–1648. doi: 10.1002/jor.20683. [DOI] [PubMed] [Google Scholar]
- 24.Mendes AF, Caramona MM, Carvalho AP, Lopes MC. Hydrogen peroxide mediates interleukin-1beta-induced AP-1 activation in articular chondrocytes: implications for the regulation of iNOS expression. Cell Biol Toxicol. 2003;19:203–214. doi: 10.1023/B:CBTO.0000003730.21261.fa. [DOI] [PubMed] [Google Scholar]
- 25.Chen MP, Yang SH, Chou CH, Yang KC, Wu CC, Cheng YH, Lin FH. The chondroprotective effects of ferulic acid on hydrogen peroxide-stimulated chondrocytes: inhibition of hydrogen peroxide-induced pro-inflammatory cytokines and metalloproteinase gene expression at the mRNA level. Inflamm Res. 2010;59:587–595. doi: 10.1007/s00011-010-0165-9. [DOI] [PubMed] [Google Scholar]
- 26.Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4:157–164. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]


