Abstract
Panax ginseng (PG) is a globally utilized medicinal herb. The medicinal effects of PG are primarily attributable to ginsenosides located in the root and leaf. The leaves of PG are known to be rich in various bioactive ginsenosides, and the therapeutic effects of ginseng extract and ginsenosides have been associated with immunomodulatory and anti-inflammatory activities. We examined the effect of PG leaf extract and the isolated ginsenosides, on nuclear factor (NF)-κB transcriptional activity and target gene expression by applying a luciferase assay and reverse transcription polymerase chain reaction in tumor necrosis factor (TNF)-α-treated hepatocarcinoma HepG2 cells. Air-dried PG leaf extract inhibited TNF-α-induced NF-κB transcription activity and NF-κB-dependent cyclooxygenase (COX)-2 and inducible nitric oxide synthase (iNOS) gene expression more efficiently than the steamed extract. Of the 10 ginsenosides isolated from PG leaves, Rd and Km most significantly inhibited activity in a dose-dependent manner, with IC50 values of 12.05±0.82 and 8.84±0.99 μM, respectively. Furthermore, the ginsenosides Rd and Km inhibited the TNF-α-induced expression levels of the COX-2 and iNOS gene in HepG2 cells. Air-dried leaf extracts and their chemical components, ginsenoside Rd and Km, are involved in the suppression of TNF-α-induced NF-κB activation and NF-κB-dependent iNOS and COX-2 gene expression. Consequently, air-dried leaf extract from PG, and the purified ginsenosides, have therapeutic potential as anti-inflammatory.
Keywords: Panax ginseng, Anti-inflammation, Nuclear factor-κB, Panax ginseng leaves, Inducible nitric oxide synthase, Cyclooxygenase-2
INTRODUCTION
Although inflammation is a basic response to injury or infectious agents, the harmful effects of inflammation cause various chronic diseases including arthritis, fibrosis, and even cancer. Inflammatory responses play important roles during liver diseases in humans and animals. Hepatitis results from inflammation of the liver, characterized by inflammatory cells present in the organ tissue. Hepatitis can be acute (lasting less than 6 months) or chronic, when it persists longer. Chronic hepatitis is associated with a high risk of liver cancer [1]. At the molecular level, free radicals and aldehydes produced during chronic hepatitis can induce deleterious gene mutations and posttranslational modifications in cancer-associated genes [2,3]. Other inflammatory products, including cytokines, growth factors, and transcription factors such as nuclear factor (NF)-κB, control the expression of cancer genes (e.g., suppressor genes and oncogenes) and key inflammatory enzymes, such as inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 [4-7].
NF-κB is known to play an important role in immune and inflammatory responses by regulating genes encoding pro-inflammatory cytokines, adhesion molecules, chemokines, growth factors, and inducible enzymes such as COX-2 and iNOS [8,9]. Therefore, NF-κB signaling pathway inhibitors could be applied as novel anti-inflammatory compounds [10,11].
Panax ginseng (PG) Meyer (Araliaceae), a herbal drug applied in Oriental traditional medicine, has been used as a tonic for the treatment of various diseases [12-14]. Ginsenosides are the major active components of the PG. Despite the fact that PG root is considered the most valuable, the leaves contain the highest concentrations of ginsenosides [15-17]. Thus, ginseng leaves could function as a supplementary source of pharmacologically active ginsenosides [18,19].
Traditionally, PG root has been air-dried, yielding white ginseng, or steamed at 100℃ to yield red ginseng. Steamed ginseng is believed to be more pharmacologically effective than air-dried ginseng. Differences in the biological effects of air-dried and steamed ginseng are attributed to significant ginsenoside changes during the steaming treatment [20]. However, the anti-inflammatory effects of air-dried and steamed leaves of PG have not been studied.
In our study, the MeOH extract of PG air-dried leaves significantly inhibited NF-κB activity, with an IC50 value of 39.12±2.79 μg/mL. Here, we characterized the effects of 10 dammarane-type saponins from PG air-dried leaves on tumor necrosis factor (TNF)-α-induced NF-κB activation, as well as iNOS and COX-2 gene expression, in HepG2 cells.
MATERIALS AND METHODS
Chemicals and sample preparation
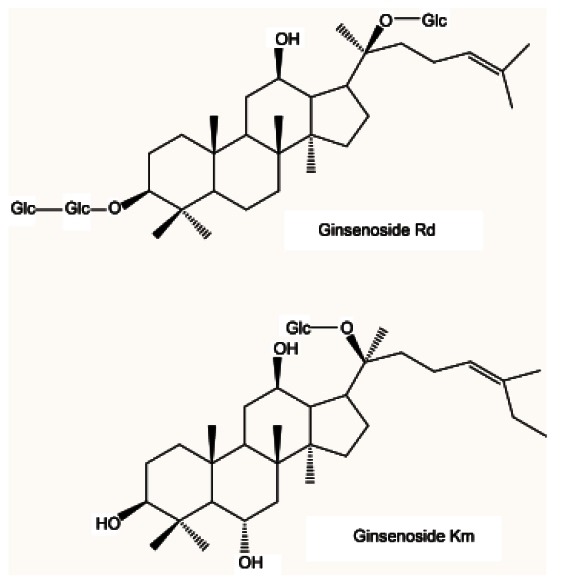
In August 2008, PG leaves were collected in Geumsan, Korea, which is well-known for PG cultivation, and were taxonomically identified. A voucher specimen (CNU 08201) was deposited at the College of Pharmacy, Chungnam National University, Korea. PG air-dried leaves were extracted in MeOH (6.0 L×3, 50℃) and the combined extracts were lyophilized in vacuo. Ten ginsenosides, identified in our previous study, were isolated from PG air-dried leaves, i.e., ginsenosides F1, Rb1, Rb2, Rc, Rd, Re, Rg1, Ki, Km, and vinaginsenoside R4 [16] (Fig. 1).
Fig. 1. Chemical structures of the ginsenosides Rd and Km.
To compare the anti-inflammatory activity of air-dried and steamed leaves, ginseng leaves were steamed at 120℃ for 4 h under 0.15 MPa pressure, without mixing, to yield the steamed-leaves sample, which was used for extraction. Pyrrolidine dithiocarbamate (PDTC), a NF-κB inhibitor, was obtained from Sigma-Aldrich (St. Louis, MO, USA).
Cell lines and culture
HepG2 cells were maintained in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA, USA) containing 10% heat-inactivated fetal bovine serum, 100 units/mL penicillin, and 10 μg/mL streptomycin at 37℃ and 5% CO2. Human TNF-α was purchased from ATgen (Seoul, Korea).
Luciferase assay
Cells were seeded at 1×105 cells per well in a 12-well plate and grown for 24 h. All cells were transfected using lipofectamine LTX (Invitrogen), as described by the manufacturer. Luciferase activity was assayed using an LB 953 Autolumat (EG&G Berthold, Nashua, NH, USA), as described previously [21] and was normalized based on the expression of RSV-β-galatosidase. β-galactosidase activity was assayed colorimetrically. NF-κB-Luc and iNOS-Luc plasmid were kindly provided by Dr. Kyoon E. Kim (Chungnam National University, Daejeon, Korea).
Cytotoxicity assay
An MTS assay (Promega Celltiter 96-Aqueous One Solution Assay; Promega, Madison, WI, USA) was used to analyze the effects of the compounds on cell viability. Cells were cultured overnight in a 96-well plate (~1×104 cells/well). Cell viability was assessed after adding the compounds at 10 μM for 24 h. The number of viable cells was determined by the A490nm of the dissolved formazan product, after addition of MTS for 30 min, as described by the manufacturer (Promega).
Reverse transcription polymerase chain reaction
Total RNA was extracted with Easy-blue reagent (iNtRON Biotechnology, Seoul, Korea). Approximately 2 μg of total RNA was reverse-transcribed using moloney murine leukemia virus reverse transcriptase and oligo-dT primers (Promega) for 1 h at 42℃. Polymerase chain reaction (PCR) of synthetic cDNA was performed using Taq polymerase pre-mixture (Takara, Shiga, Japan). PCR products were subjected to electrophoresis on 1% agarose gels, and stained with EtBr. PCR was conducted with the following primer pairs: iNOS sense 5’-TCATCCGCTATGCTGGCTAC-3’, iNOS antisense 5’-CTCAGGGTCACGGCCATTG-3’, COX-2 sense 5’-GCCCAGCACTTCACGCATCAG-3’, COX-2 an-
tisense 5’-GACCAGGCACCAGACCAAAGACC-3’, GAPDH sense 5′-TGTTGCCATCAATGACCCCTT-3′, and GAPDH antisense 5′-CTCCACGACGTACTCAGCG- 3′. Quantification of PCR products was performed using the Quantity One Program (Bio-Rad, Hercules, CA, USA).
Statistical analysis
All results are expressed as the mean±SEM. Data was analyzed by one-factor ANOVA. Upon observation of a statistically significant effect, the Newman-Keuls test was performed to determine the difference between the groups. A p-value of less than 0.05 was considered to be significant.
RESULTS
Nuclear factor-κB inhibitory effect of Panax ginseng leaf extract in HepG2 cells
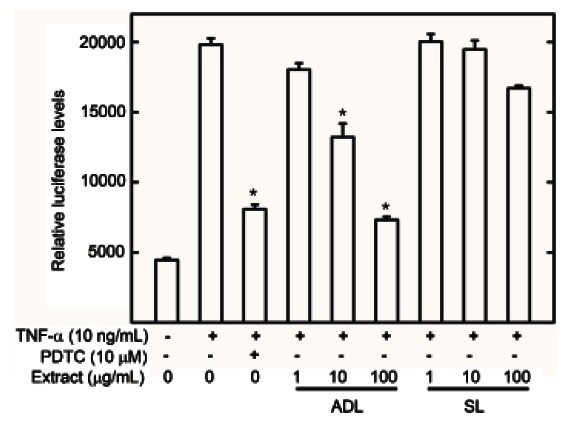
To evaluate and compare the anti-inflammatory activity of ginseng leaves, the nuclear transcription NF-κB cell-reporter system was applied. The anti-inflammatory activities of air-dried and steamed leaf MeOH extracts were measured using a luciferase assay in HepG2 cells. Extracts of air-dried leaves reduced NF-κB activation by 81% at 100 μg/mL and 43% at 10 μg/mL in a dose-dependent manner. Alternatively the steamed leaf extracts reduced NF-κB activation by 20% at 100 μg/mL (Fig. 2). This suggests that air-dried leaf extracts possess more anti-inflammatory effects than steamed leaves by inhibiting NF-κB in HepG2 cells.
Fig. 2. Effect of air-dried (ADL) and steamed (SL) Panax ginseng leaves on tumor necrosis factor (TNF)-α stimulated nuclear factor (NF)-κB transcriptional activity. HepG2 cells transiently transfected with pNF-κB-Luc were pretreated for 1 h with either vehicle (dimethyl sulfoxide) or extracts, then exposed to TNF-α (10 ng/mL) for 1 h. Unstimulated HepG2 cells acted as a negative control. Cells were then harvested and assayed for luciferase activity. Results are expressed as the mean luciferase activity±SEM (n=3). PDTC, pyrrolidine dithiocarbamate. *Significantly different from TNF-α stimulated group (p<0.05).
Effect of Panax ginseng leaf extracts on inducible nitric oxide synthase and cyclooxygenase-2 gene expression in HepG2 cells
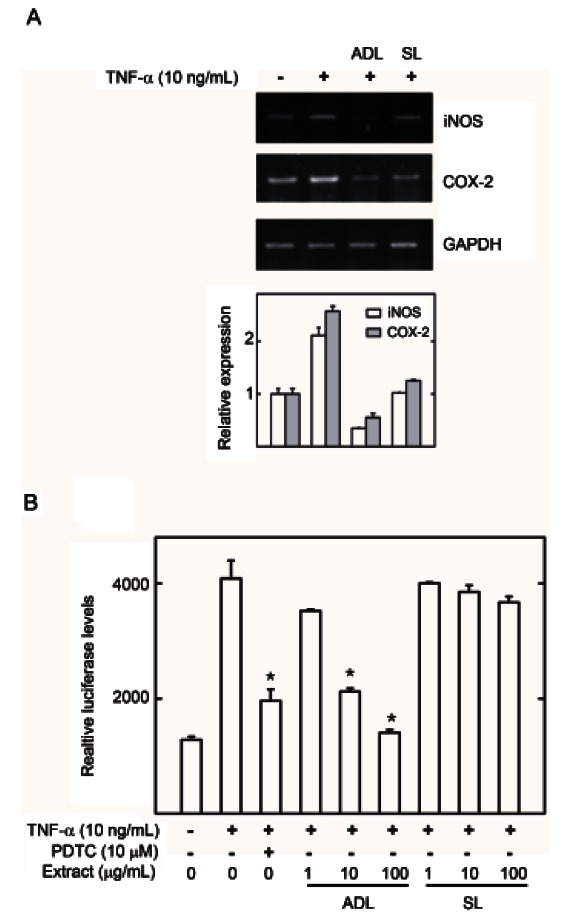
To evaluate the anti-inflammatory activity of ginseng leaves which inhibited NF-κB activation in the luciferase assay, the effects of air-dried leaves and steamed leaf extracts on NF-κB target gene expression were evaluated by reverse transcription (RT)-PCR and iNOS promoter luciferase assay. HepG2 cells treated with TNF-α (10 ng/mL) had significantly upregulated iNOS and COX- 2 mRNA expression (Fig. 3A) and iNOS promoter regulation (Fig. 3B). Air-dried leaf extracts significantly decreased iNOS and COX-2 mRNA and iNOS promoter activity compared to TNF-α-treated HepG2 cells (Fig. 3). Alternatively the steamed leaf extracts did not significantly inhibit gene expression (Fig. 3A). Therefore, air-dried leaves showed potent anti-inflammatory activity by inhibiting NF-κB activation and iNOS and COX-2 gene expression.
Fig. 3. Effect of air-dried (ADL) and steamed (SL) Panax ginseng leaves on inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 gene expression in HepG2 cell lines. (A) HepG2 cells were pretreated with extract or vehicle (dimethyl sulfoxide, DMSO) for 1 h, then exposed to tumor necrosis factor (TNF)-α (10 ng/mL) for 6 h. Total mRNA was extracted from the cell pellets using Easy-Blue reagent. The relative mRNA levels were assessed by reverse transcription polymerase chain reaction. Expression levels are displayed as the ratio of iNOS and COX-2 signal strength to a reference gene (GAPDH) to compensate for varying concentrations of RNA. (B) HepG2 cells transiently transfected with piNOS-Luc were pretreated for 1 h with either vehicle (DMSO) or extracts, then exposed to TNF- α (10 ng/mL) for 1 h. Unstimulated HepG2 cells acted as a negative control. Cells were then harvested and assayed for luciferase activity. Results are expressed as the mean luciferase activity±SEM (n=3). PDTC, pyrrolidine dithiocarbamate. *Significantly different from TNF- α stimulated group (p<0.05).
Effect of ginsenosides Rd and Km on inducible nitric oxide synthase and cyclooxygenase-2 gene expression through the nuclear factor-κB pathway
To identify novel NF-κB inhibitors from PG air-dried leaf extracts, 10 dammarane-type ginsenosides were evaluated using the lucifease assay and RT-PCR. Initially, to determine nontoxic concentrations, HepG2 cells were treated with various concentrations (0.1, 1, and 10 μM) of the compounds and cell viability was measured using an MTS assay. No compounds were significantly cytotoxic at up to 10 μM, indicating that NF-κB inhibition was not due to cell toxicity (Fig. 4A).
Fig. 4. Effects of 10 gensenosides on cell viability and nuclear factor (NF)-κB transcriptional activity. (A) Cell viability was determined using the Promega Celltiter 96-Aqueous One Solution Assay. The results are expressed in terms of percentage relative cell viability. All values are means±SEM (n=3) vs. control. (B) Effect of Rd and Km on tumor necrosis factor (TNF)-α stimulated NF-κB transcriptional activity. HepG2 cells transiently transfected with pNF-κB-Luc were pretreated for 1 h with either vehicle (dimethyl sulfoxide, DMSO) or compounds, then treated with TNF-α (10 ng/mL). Unstimulated HepG2 cells acted as a negative control. Cells were harvested and assayed for luciferase activity. Results are expressed as the mean luciferase activity±SEM (n=3). PDTC, pyrrolidine dithiocarbamate. *Significantly different from TNF-α stimulated group (p<0.05).
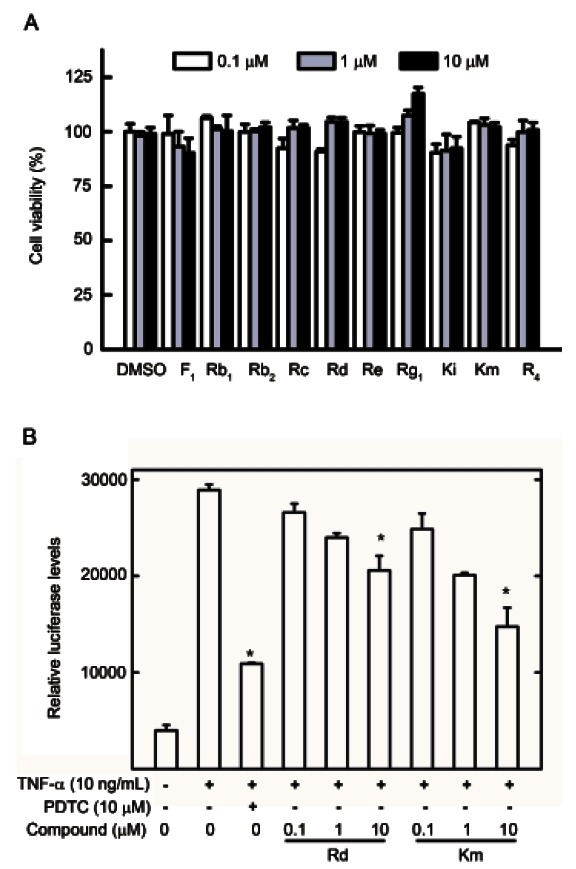
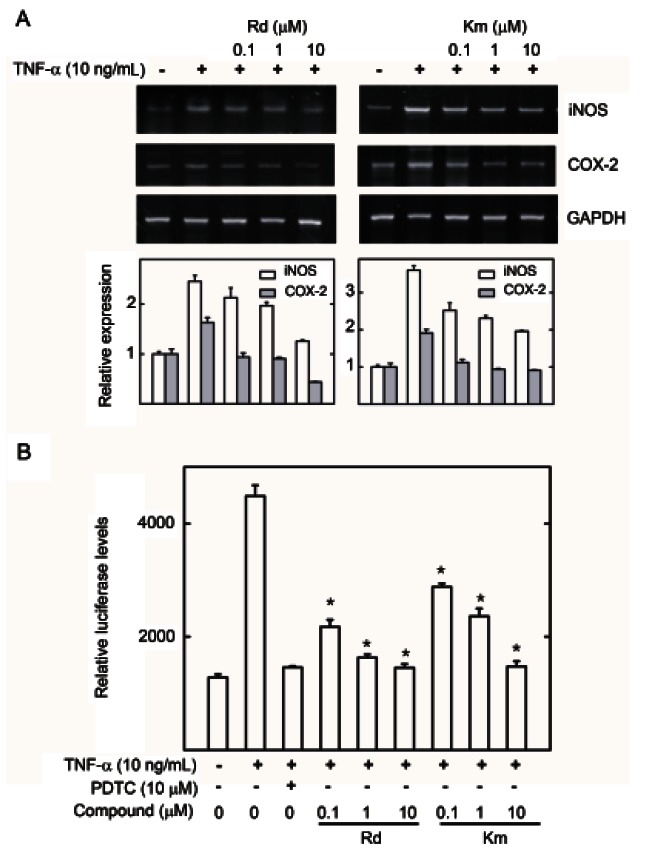
HepG2 cells were pretreated with various ginsenosides at concentrations ranging from 0.1 to 10 μM for 1 h and then induced with TNF-α for 20 h. Of these ginsenosides, Rd and Km significantly inhibited TNF-α-induced NF-κB transcriptional activity with IC50 values of 12.05±0.82 and 8.84±0.99 μM, respectively (Table 1 and Fig. 4B), while ginsenosides Rb1, Rg1, and F1 reduced NF-κB transcriptional activity with IC50 values of 27.45±3.69, 28.14±0.28, and 22.36±2.20 μM, respectively. Ginsenoside Rb2 and vinaginsenoside R4 weakly inhibited NF-κB transcriptional activity (Table 1). Furthermore, ginsenosides Rc, Re, and Ki had no effects (Table 1). PDTC, as a positive control, potently inhibited NF-κB transcriptional activity with an IC50 value of 3.35±0.61 μM. Ginsenoside Rd and Km also inhibited expression of COX-2 and iNOS mRNA (Fig. 5A) and iNOS promoter activity in a dose-dependent manner (Fig. 5B). Therefore, ginsenoside Rd and Km showed potent anti-inflammatory activity by inhibiting NF-κB activation and iNOS and COX-2 gene expression.
Table 1.
Inhibitory activity (IC50) of 10 ginsenosides isolated from air-dried leaves of PG on TNF-α induced NF-κB activation in HepG2 cells
| Compound | IC50 (μM)1) |
|---|---|
|
| |
| Ginsenoside F1 | 22.36±2.20 |
| Ginsenoside Rb1 | 27.45±3.69 |
| Ginsenoside Rb2 | 67.18±3.57 |
| Ginsenoside Rc | ND |
| Ginsenoside Rd | 12.05±0.82 |
| Ginsenoside Re | ND |
| Ginsenoside Rg1 | 28.14±0.28 |
| Ginsenoside Ki | 21.45±3.69 |
| Ginsenoside Km | 8.84±0.99 |
| Vinaginsenoside R4 | 47.21±3.69 |
| PDTC 2) | 3.35±0.61 |
p˂0.05, compared to normal or control group.
PG, Panax ginseng; TNF, tumor necrosis factor; NF, nuclear factor; ND, not detected; PDTC, pyrrolidine dithiocarbamate.
1)Results are the means±SEM of three independent experiments performed in triplicate.
2)Positive control.
Fig. 5. Effect of ginsenoside Rd and Km on inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 gene expression in HepG2 cell lines. (A) HepG2 cells were pretreated with the ginsenosides Rd and Km, or the vehicle (dimethyl sulfoxide, DMSO) for 1 h, then treated with tumor necrosis factor (TNF)-α (10 ng/mL) for 6 h. Total mRNA was extracted from the cell pellets using Easy-Blue reagent. Relative mRNA levels were assessed by reverse transcription polymerase chain reaction. Expression levels are displayed as the ratio of iNOS and COX-2 signal strength to a reference gene (GAPDH), compensating for variations in the RNA concentrations. (B) HepG2 cells transiently transfected with piNOS-Luc were pretreated for 1 h with either vehicle (DMSO) or compounds, then treated with TNF-α (10 ng/mL). Unstimulated HepG2 cells acted as a negative control. Cells were harvested and assayed for luciferase activity. Results are expressed as the mean luciferase activity±SEM (n=3). PDTC, pyrrolidine dithiocarbamate. *Significantly different from TNF- α stimulated group (p<0.05).
DISCUSSION
This study demonstrated that PG air-dried leaf extract, and its components, Rd and Km, inhibit TNF-α-induced NF-κB promoter activity in HepG2 cells in a dose-dependent manner by interfering with gene transcription. Air-dried leaf extract, Rd and Km also inhibited the expression of two inducible enzymes, COX-2 and iNOS, at the transcriptional level, as demonstrated by reduced mRNA levels in TNF-α-treated HepG2 cells. Ginsenoside Rd was recently reported to suppress the inflammatory response by inhibiting expression of iNOS and COX-2 in rats, unexplained the regulatory mechanism [22]. In our study, we report the NF-κB pathway, as a potential mechanism, by ginsenoside Rd in inflammation. To our knowledge, we are the first to report that air-dried leaf extracts and ginsenoside Km have an inhibitory effect on TNF-α-induced NF-κB activation as well as iNOS and COX-2 expression in HepG2 cells.
Ginseng extracts and ginsenosides were previously shown to inhibit the NF-κB pathway [23]. Saponins and n-butanol extracts of P. notoginseng (PNG) also inhibit the NF-κB pathway in zymosan A- and collagen-induced mice [24]. Flower extracts from PNG attenuate the infarct volume in rat ischemic brain and the inflammatory response of microglia cells [25]. Also, the PG acid polysaccharide extract, ginsan, inhibits the production of interleukin (IL)-1β, IL-6, IL-12, IL-18, and interferon- γ and the activation of MAPK, c-Jun N-terminal kinase, and NF-κB pathways in Staphylococcus aureus-infected mice [26,27]. However, the NF-κB inhibitory effect of PG leaf extracts had not been evaluated.
In this study, ginsenosides Rb1, Rg1, and F1 reduced NF-κB transcriptional activity and ginsenoside Rb2 and vinaginsenoside R4 showed very low inhibitory activities (Table 1). Furthermore, ginsenosides Rc, Re, and Ki had no effects. In corroboration of our results, a study reported that ginsenoside Rb1 inhibits the expression vascular cell adhesion molecule (VCAM)-1 by inhibiting the TNF-α-induced NF-κB pathway in human umbilical vein endothelial cells [28]. Also, ginsenoside Rg1 inhibits iNOS production in mice injected with LPS and VCAM- 1 as well as ICAM-1 production in ECV304 human endothelial cells induced by TNF-α [29]. Rb2 potently inhibited TNF-α production in LPS-induced N9 microglia [30]. Vinaginsenoside R4, identified from P. vietnamensis [31], had not previously been characterized. Ginsenoside F1 protects human HaCat keratinocytes from ultraviolet- B-induced apoptosis [32]. However, the anti-inflammatory and NF-κB inhibitory effects of ginsenoside F1 had not been described. This is the first report of vinaginsenoside R4 and ginsenoside F1 inhibiting TNF-α-induced NF- κB activation as well as iNOS and COX-2 gene expression in HepG2 cells. Although vinaginsenoside R4 and ginsenoside F1 may play the potential anti-inflammatory compound, further studies are necessary to determine the precise mechanism on TNF-α-mediated NF-κB activation and inflammation.
In conclusion, we demonstrated that air-dried leaf extracts and their chemical components, ginsenoside Rd and Km, are involved in the suppression of TNF- α-induced NF-κB activation; this was shown in a gene reporter assay by a reduction in luciferase activity, which subsequently inhibited iNOS and COX-2 gene expression. Our data demonstrated that air-dried leaf extract from PG and the purified ginsenosides, have therapeutic potential as anti-inflammatory, anti-atherosclerotic, antiarthritic, anti-oxidative and antiallergic substances. However, detailed mechanisms of TNF-α-induced NF-κB pathway inhibition and subsequent decreases in iNOS and COX-2 gene expression with air-dried leaf extracts from PG and ginsenosides require further investigation.
Acknowledgments
This study was supported by the Priority Research Centers Program through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology (2009-0093815).
References
- 1.Berasain C, Castillo J, Perugorria MJ, Latasa MU, Prieto J, Avila MA. Inflammation and liver cancer: new molecular links. Ann N Y Acad Sci. 2009;1155:206–221. doi: 10.1111/j.1749-6632.2009.03704.x. [DOI] [PubMed] [Google Scholar]
- 2.Kawanishi S, Hiraku Y, Pinlaor S, Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol Chem. 2006;387:365–372. doi: 10.1515/BC.2006.049. [DOI] [PubMed] [Google Scholar]
- 3.Yamashita T, Kaneko S. Molecular pathogenesis of hepatocellular carcinoma. Gan To Kagaku Ryoho. 2010;37:14–17. [PubMed] [Google Scholar]
- 4.Elsharkawy AM, Mann DA. Nuclear factor-kappaB and the hepatic inflammation-fibrosis-cancer axis. Hepatology. 2007;46:590–597. doi: 10.1002/hep.21802. [DOI] [PubMed] [Google Scholar]
- 5.Farinati F, Piciocchi M, Lavezzo E, Bortolami M, Cardin R. Oxidative stress and inducible nitric oxide synthase induction in carcinogenesis. Dig Dis. 2010;28:579–584. doi: 10.1159/000320052. [DOI] [PubMed] [Google Scholar]
- 6.Holt AP, Salmon M, Buckley CD, Adams DH. Immune interactions in hepatic fibrosis. Clin Liver Dis. 2008;12:861–882. doi: 10.1016/j.cld.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lentsch AB, Ward PA. The NFkappaBb/ IkappaB system in acute inflammation. Arch Immunol Ther Exp (Warsz) 2000;48:59–63. [PubMed] [Google Scholar]
- 8.Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001;480-481:243–268. doi: 10.1016/S0027-5107(01)00183-X. [DOI] [PubMed] [Google Scholar]
- 9.Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Signalling networks regulating cyclooxygenase-2. Int J Biochem Cell Biol. 2006;38:1654–1661. doi: 10.1016/j.biocel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Luqman S, Pezzuto JM. NFkappaB: a promising target for natural products in cancer chemoprevention. Phytother Res. 2010;24:949–963. doi: 10.1002/ptr.3171. [DOI] [PubMed] [Google Scholar]
- 11.Nam NH. Naturally occurring NF-kappaB inhibitors. Mini Rev Med Chem. 2006;6:945–951. doi: 10.2174/138955706777934937. [DOI] [PubMed] [Google Scholar]
- 12.Ernst E. Panax ginseng: an overview of the clinical evidence. J Ginseng Res. 2010;34:259–263. doi: 10.5142/jgr.2010.34.4.259. [DOI] [Google Scholar]
- 13.Hofseth LJ, Wargovich MJ. Inflammation, cancer, and targets of ginseng. J Nutr. 2007;137(1 Suppl):183S–185S. doi: 10.1093/jn/137.1.183S. [DOI] [PubMed] [Google Scholar]
- 14.Vuksan V, Sievenpipper J, Jovanovski E, Jenkins AL. Current clinical evidence for Korean red ginseng in management of diabetes and vascular disease: a Toronto’ s ginseng clinical testing program. J Ginseng Res. 2010;34:264–273. doi: 10.5142/jgr.2010.34.4.264. [DOI] [Google Scholar]
- 15.Liu GY, Li XW, Wang NB, Zhou HY, Wei W, Gui MY, Yang B, Jin YR. Three new dammarane-type triterpene saponins from the leaves of Panax ginseng C.A. Meyer. J Asian Nat Prod Res. 2010;12:865–873. doi: 10.1080/10286020.2010.508035. [DOI] [PubMed] [Google Scholar]
- 16.Tung NH, Song GY, Park YJ, Kim YH. Two new dammarane- type saponins from the leaves of Panax ginseng. Chem Pharm Bull (Tokyo) 2009;57:1412–1414. doi: 10.1248/cpb.57.1412. [DOI] [PubMed] [Google Scholar]
- 17.Tung NH, Song GY, Minh CV, Kiem PV, Jin LG, Boo HJ, Kang HK, Kim YH. Steamed ginseng-leaf components enhance cytotoxic effects on human leukemia HL-60 cells. Chem Pharm Bull (Tokyo) 2010;58:1111–1115. doi: 10.1248/cpb.58.1111. [DOI] [PubMed] [Google Scholar]
- 18.Christensen LP. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Peng D, Xie J. Ginseng leaf-stem: bioactive constituents and pharmacological functions. Chin Med. 2009;4:20. doi: 10.1186/1749-8546-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baek NI, Kim DS, Lee YH, Park JD, Lee CB, Kim SI. Ginsenoside Rh4, a genuine dammarane glycoside from Korean red ginseng. Planta Med. 1996;62:86–87. doi: 10.1055/s-2006-957816. [DOI] [PubMed] [Google Scholar]
- 21.Kim KK, Park KS, Song SB, Kim KE. Up regulation of GW112 Gene by NF kappaB promotes an antiapoptotic property in gastric cancer cells. Mol Carcinog. 2010;49:259–270. doi: 10.1002/mc.20596. [DOI] [PubMed] [Google Scholar]
- 22.Ye R, Yang Q, Kong X, Han J, Zhang X, Zhang Y, Li P, Liu J, Shi M, Xiong L, Zhao G. Ginsenoside Rd attenuates early oxidative damage and sequential inflammatory response after transient focal ischemia in rats. Neurochem Int. 2011;58:391–398. doi: 10.1016/j.neuint.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Lee DC, Lau AS. Effects of Panax ginseng on tumor necrosis factor-α -mediated inflammation: a mini-review. Molecules. 2011;16:2802–2816. doi: 10.3390/molecules16042802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang SH, Choi Y, Park JA, Jung DS, Shin J, Yang JH, Ko SY, Kim SW, Kim JK. Anti-inflammatory effects of BT-201, an n-butanol extract of Panax notoginseng, observed in vitro and in a collagen-induced arthritis model. Clin Nutr. 2007;26:785–791. doi: 10.1016/j.clnu.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Son HY, Han HS, Jung HW, Park YK. Panax notoginseng attenuates the infarct volume in rat ischemic brain and the inflammatory response of microglia. J Pharmacol Sci. 2009;109:368–379. doi: 10.1254/jphs.08197FP. [DOI] [PubMed] [Google Scholar]
- 26.Ahn JY, Choi IS, Shim JY, Yun EK, Yun YS, Jeong G, Song JY. The immunomodulator ginsan induces resistance to experimental sepsis by inhibiting Toll-like receptor-mediated inflammatory signals. Eur J Immunol. 2006;36:37–45. doi: 10.1002/eji.200535138. [DOI] [PubMed] [Google Scholar]
- 27.Ahn JY, Song JY, Yun YS, Jeong G, Choi IS. Protection of Staphylococcus aureus-infected septic mice by suppression of early acute inflammation and enhanced antimicrobial activity by ginsan. FEMS Immunol Med Microbiol. 2006;46:187–197. doi: 10.1111/j.1574-695X.2005.00021.x. [DOI] [PubMed] [Google Scholar]
- 28.Chai H, Wang Q, Huang L, Xie T, Fu Y. Ginsenoside Rb1 inhibits tumor necrosis factor-alpha-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Biol Pharm Bull. 2008;31:2050–2056. doi: 10.1248/bpb.31.2050. [DOI] [PubMed] [Google Scholar]
- 29.Hien TT, Kim ND, Kim HS, Kang KW. Ginsenoside Rg3 inhibits tumor necrosis factor-alpha-induced expression of cell adhesion molecules in human endothelial cells. Pharmazie. 2010;65:699–701. [PubMed] [Google Scholar]
- 30.Wu CF, Bi XL, Yang JY, Zhan JY, Dong YX, Wang JH, Wang JM, Zhang R, Li X. Differential effects of ginsenosides on NO and TNF-alpha production by LPS-activated N9 microglia. Int Immunopharmacol. 2007;7:313–320. doi: 10.1016/j.intimp.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen MD, Kasai R, Ohtani K, Ito A, Nguyen TN, Yamasaki K, Tanaka O. Saponins from Vietnamese ginseng, Panax vietnamensis HA et Grushv. Collected in central Vietnam. II. Chem Pharm Bull (Tokyo) 1994;42:115–122. doi: 10.1248/cpb.42.115. [DOI] [PubMed] [Google Scholar]
- 32.Lee EH, Cho SY, Kim SJ, Shin ES, Chang HK, Kim DH, Yeom MH, Woe KS, Lee J, Sim YC, et al. Ginsenoside F1 protects human HaCaT keratinocytes from ultraviolet-B-induced apoptosis by maintaining constant levels of Bcl-2. J Invest Dermatol. 2003;121:607–613. doi: 10.1046/j.1523-1747.2003.12425.x. [DOI] [PubMed] [Google Scholar]


