Abstract
The metabolic profiles of Panax quinquefolius and its associated therapeutic values are critically affected by the repetitious steaming times. The times-dependent steaming effect of P. quinquefolius is not well-characterized and there is also no official guideline on its times of steaming. In this paper, a UPLC-Q-TOF-MS/MS method was developed for the qualitative profiling of multi-parametric metabolic changes of raw P. quinquefolius during the repetitious steaming process. Our method was successful in discriminating the differentially multi-steamed herbs. Meantime, the repetitious steaming-inducing chemical transformations in the preparation of black American ginseng (American ginseng that was subjected to 9 cycles of steaming treatment) were evaluated by this UPLC-Q-TOF-MS/MS based chemical profiling method. Under the optimized UPLC-Q-TOF-MS/MS conditions, 29 major ginsenosides were unambiguously identified and/or tentatively assigned in both raw and multi-steamed P. quinquefolius within 19 min, among them 18 ginsenosides were detected to be newly generated during the preparatory process of black American ginseng. The mechanisms involved were further deduced to be hydrolysis, dehydration, decarboxylation and addition reactions of the original ginsenosides in raw P. quinquefolius through analyzing mimic 9 cycles of steaming extracts of 14 pure reference ginsenosides. Our novel steaming times-dependent metabolic profiling approach represents the paradigm shift in the global quality control of multi-steamed P. quinquefolius products.
Keywords: Panax ginseng, Black American ginseng, Ginsenosides, UPLC-Q-TOF-MS/MS, Multi-steamed Panax quinquefolius
INTRODUCTION
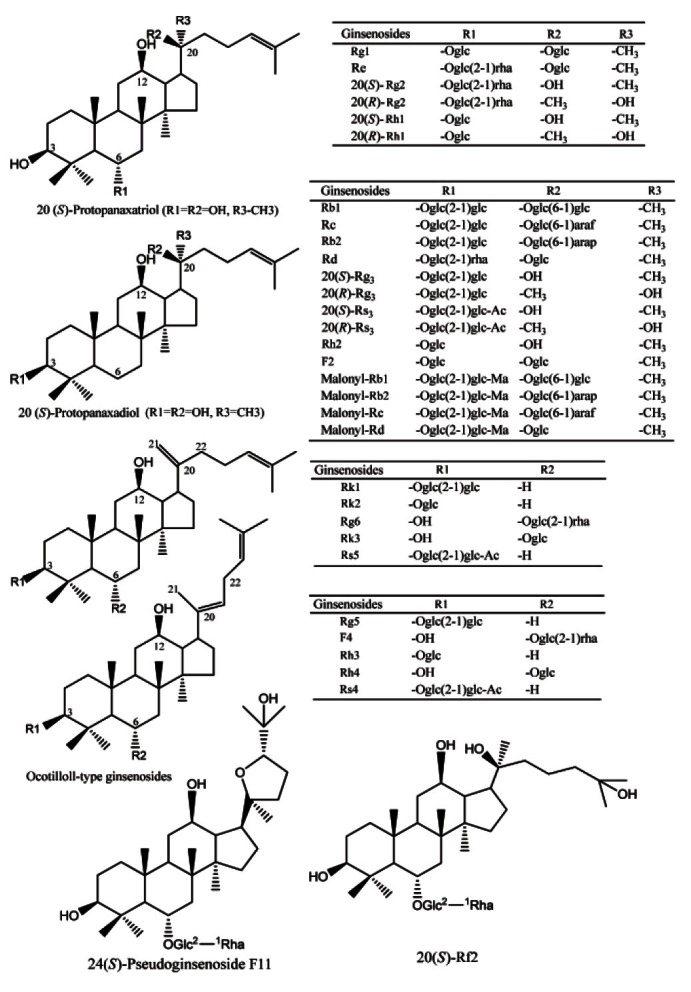
American ginseng, the dried root of Panax quinquefolius L., is “cool” and mainly used to tonify “qi” and nourish “yin” according to the description in Chinese pharmacopoeia [1], and has gained great popularity in the East in the last two decades as a medicinal herb. Undoubtedly, ginsenosides are known to be the major bioactive components of ginseng (or Korean ginseng) and American ginseng, having various bioactive and pharmacological activities, including anti-diabetic, anti-inflammatory and anti-tumor properties [2-4]. To date, more than 80 different ginsenosides have been isolated and characterized, and shown many bioactivities responsible for the pharmacological effects. As summarized in many review articles [5-11], ginsenosides were generally classified into four groups, namely protopanaxadiol (ginsenosides Rb1, Rc, Rb2, Rd, Rg3, Rh2, and Rs3), protopanaxatriol (ginsenosides Rg1, Re, Rg2, and Rh1), ocotillol (24 (S)- pseudoginsenoside F11) (Fig. 1) and oleanolic acid (ginsenosides Ro) types.
Fig. 1. Major goinsenosides identified in the preparation of black American ginseng.
Recently, American ginseng and ginseng, two kinds of the most widely-used traditional Chinese medicines, have become popular in oriental countries as dietary health supplements and additives to foods and beverages. As we all know, there are a variety of commercial ginseng products, including raw ginseng (unsteamed ginseng), red ginseng (two- or three-time steamed ginseng), and black ginseng (nine-time steamed ginseng). Among them, black ginseng is developed from raw P. ginseng by nine-time steaming at 98℃ for 3 h and nine-time drying [12,13], at which point it becomes black in color [14,15], while black American ginseng (BAG, American ginseng that was subjected to 9 cycles of steaming treatment) is still unreported to date. Nine-time steaming and nine-time drying is frequently used for preparing modified or processed Chinese herbal medicines (e.g., Rehmanniae radix Preparata, Polygonatum sibiricum Red., P. ginseng Meyer and Arisaema heterophyllum Blume) so as to reinforce its pharmacological activity, to attenuate its adverse response, to improve the stability or to change its medicinal effects, etc., but studies on the holistic chemical profiles of multi-steamed Chinese herbal medicines were attempted only in recently years [16-18].
As known to all, the pharmacological and biological activities of steam-activated ginseng are greater than non-steamed ginseng. For example, black ginseng exhibits more potent biological activities than raw P. ginseng and red P. ginseng [14,19] and some new ginsenosides (Rg3, Rg5, F4, Rg6, Rk3, Rs3, Rs4, etc.) absent in raw P. ginseng are generated in black ginseng after 9 cycles of steaming treatment. Similarly, steamed P. quinquefolius exhibits stronger cancer chemoprevention than raw P. quinquefolius which is related to the generation of less polar ginsenosides (20(S)/20(R)-Rg3, Rk3, Rh4, Rk1, Rg5, etc.) in steamed ginseng [20]. Although analysis of ginsenosides in steamed P. quinquefolius and the pharmacological effects of steamed P. quinquefolius have been strongly explored, steaming times-dependent chemical profiling of steamed P. quinquefolius and the mechanisms of the ginsenosides transformation involved in the preparatory process of BAG are not completely clear to date. In order to systematically evaluate the bioactivity, safety and efficacy as well as standardization and quality control of BAG, the chemical profiles of BAG should be investigated first.
Up to now, so many modern hyphenated techniques including HPLC-UV (ELSD), GC-MS, LC-(IT) MSn, HPLC-ESI-MS/MS, HPLC-TSP-MS and UPLC-(Q) TOF-MS (/MS), etc. have been applied for the ginseng analysis [10,21-28]. Among these techniques, UPLC-QTOF- MS (/MS) is considered as the most sophisticated technique for rapidly revealing the chemical profiling of Chinese medicinal herbs. Using sub-2μm particle short columns, UPLC could enhance retention time reproducibility, increase chromatographic resolution, improve sensitivity and shorten operation time far more. What’s more, Q-TOF-MS could provide the low energy collision-induced dissociation (CID) and accurate mass values of the analyte, which made UPLC-Q-TOF-MS (/MS) to be a powerful tool for revealing the differentiation of Panax species (P. ginseng, P. quinquefolius and P. notoginseng) [22,23], identifying and quantifying the ginsenosides of P. notoginseng [24], comparing different parts of P. notoginseng [25], and analyzing the metabolites in vivo [26].
In this paper, a rapid and reliable UPLC-Q-TOF-MS/ MS method was developed for revealing the steaming times-dependent chemical profiles of multi-steamed P. quinquefolius. The chemical changes and its possible mechanisms involved in the preparatory process of BAG were preliminarily investigated and evaluated.
MATERIALS AND METHODS
Materials
Fourteen ginsenosides Rg1 (2), Rb1 (4), malonyl-Rb1 (5), Rc (6), Rb2 (8), malonyl-Rb2 (9), Rd (10), 20(S)- Rg2 (12), 20(S)-Rh1 (13), 20(S)-Rg3 (14), 20(R)-Rg3 (15), 20(R)-Rh1 (18), Rh2 (19) and 20(R)-Rg2 (31) standards (Fig. 1) were purchased from the Hongjiu Biotech Co., Ltd. (Jilin, China). The purity of all these standards was over 97% as indicated by the manufacturer. HPLCMS grade acetonitrile was purchased from Merck Co. (Merck, Darmstadt, Germany). Deionized water was purified by Milli-Q system (Millipore, Bedford, MA, USA). Other chemicals were of reagent grade.
The commercial raw American ginsengs were purchased from different herbal shops in China, and were authenticated according to the standard documented in Chinese pharmacopoeia [1]. Subsequently, the raw P. quinquefolius was repeatedly steamed at 98℃ for 3 h in pottery apparatus and dried at 60℃ for 18 h for 3, 6 and 9 times based on the method of Sun et al. [16], respectively. BAG (raw P. quinquefolius that was subjected to 9 cycles of steaming treatment) became easily noticeable since the brown color of the steamed P. quinquefolius has grown dark with increase of steaming times.
Chromatographic conditions
All analyses were performed on a Waters ACQUITY UPLC system (Waters Corp., Milford, MA, USA), including binary solvent delivery system, auto-sampler, and photo diode array detector. An ACQUITY HSS T3 column (100 mm×2.1 mm, i.d., 1.8 μm) from Waters was used. The column and auto-sampler were maintained at 35℃ and 10℃, respectively. The standards and samples were separated using a gradient mobile phase consisting of (A) 0.1% formic acid in water and (B) ACN containing 0.1% formic acid. The UPLC elution condition was optimized as follows: linear gradient from 10% to 32% B (0-10 min), 32% to 80% B (10-20 min) and isocratic at 80% B (20-21 min). The flow rate was at 0.5 mL/min. Each wash cycle consisted of 300 μL of strong solvent (80% ACN) and 500 μL of weak solvent (30% ACN). The injection volume of sample was 2 μL. Mass spectrometry was carried out on a Waters Q-TOF Premier (Micromass MS Technologies, Manchester, UK) equipped with electro-spray ionization (ESI) source operating in negative mode. The temperatures of electro-spray source and desolvation gas were 100℃ and 400℃, respectively. The flow rate of desolvation gas was set to 600 L/h. The ESI capillary and cone voltages were set at 3,500 and 45 V, respectively. The Q-TOF acquisition rate was 0.2 s and the inter-scan delay was 0.02 s. Argon was used as the collision gas at a pressure of 7.066×10−3 Pa. The energies for CID were set at 5 V for precursor ion and 45 V for product ion information, respectively.
Sample preparation
Reference standards solutions
Stock solutions: a particular amount of ginsenosides Rg1, Rb1, malonyl-Rb1, Rc, Rb2, malonyl-Rb2, Rd, 20(S)-Rg2, 20(S)-Rh1, 20(S)-Rg3, 20(R)-Rg3, 20(R)- Rh1, Rh2 and 20(R)-Rg2 were dissolved with methanol respectively to get fourteen reference standards store solutions (0.2-1.0 mg/mL), which were stored under 4℃.
Mimic 9 cycles of steaming extracts of reference standards: 0.5 mL of each reference standard stock solution was rotary evaporated to dryness, the residue was subjected to 9 cycles of steaming at 98℃ for 3 h and drying at 60℃ for 18 h. Then the residues were dissolved with 1.0 mL of 70% methanol, the solution was filtered by a 0.2 μm PTFE syringe filter before UPLC-Q-TOF-MS/MS analysis.
Extracts of finished ginseng products
Methanol extracts: each raw and steamed P. quinquefolius (0, 3, 6 and 9 times) samples was accurately weighed (approximately 0.2 g) and ultrasonic-extracted with 8.0 mL of methanol for 60 min at room temperature. The extract was then filtered by a 0.2 μm PTFE syringe filter before UPLC–Q-TOF-MS/MS analysis.
Seventy percent methanol extracts: each raw and steamed P. quinquefolius (0, 3, 6, and 9 times) samples was accurately weighed (approximately 0.2 g) and ultrasonic- extracted with 8.0 mL of 70% aqueous methanol for 60 min at room temperature. The extract was then filtered by a 0.2-μm PTFE syringe filter before UPLC-Q-TOF- MS/MS analysis.
RESULTS AND DISCUSSION
Optimization of chromatographic conditions and Q-TOF-MS/MS method development
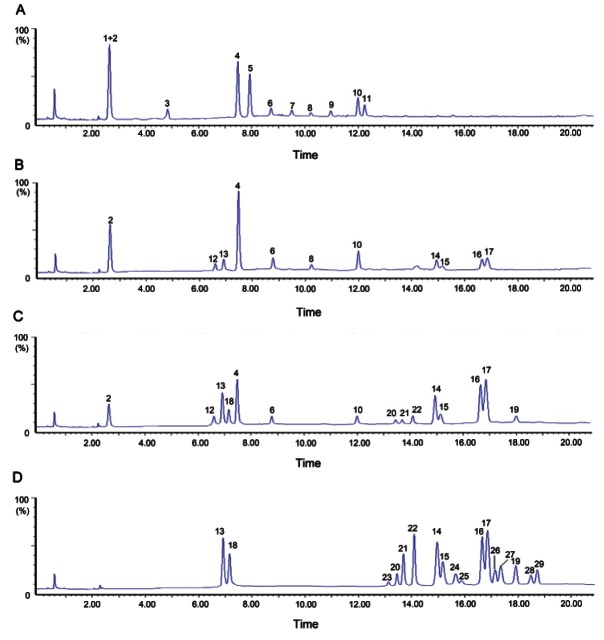
Obviously, peak capacity is critical for revealing the chemical profiling of Chinese medicinal herbs using LCMS(/ MS) approach. As shown in some published articles [22-25], An ACQUITY BEH C18 column has been frequently employed for separating ginsenosides. However, the ACQUITY BEH C18 column has lower peak capacity and weaker retention ability for hydrophilic compounds compared to the ACQUITY HSS T3 column [29]. By comparing the effectiveness of the two columns for ginsenosides analysis in the raw and steamed P. quinquefolius (0, 3, 6, and 9 times), the ACQUITY HSS T3 (100 mm × 2.1 mm, i.d., 1.8 μm) is considered as the preferred column for the analysis of raw and multi-steamed P. quinquefolius because more hydrophilic ginsenosides are retained and separated, and over 29 peaks are detected within 19 min under the optimal chromatographic condition (Fig. 2). Both positive and negative ion modes were tested. By comparison with the positive ion mode, ginsenosides could acquire higher sensitivity and accurate mass spectra in the negative ion mode, which made it easier to detect the lower abundance ginsenosides in BAG, and to confirm molecular ions or quasi-molecular ions in the identification of each peak. All data acquired in negative ion mode was further used for the ginsenosides analysis and characterization.
Fig. 2. Representative chromatograms of raw (A) and multi-steamed Panax quinquefolius extracted samples (3 [B], 6 [C], and 9 times [D]).
Identity assignment and confirmation of the ginsenosides detected in raw and multi-steamed Panax quinquefolius
Under the optimal chromatographic and MS conditions, over 29 major ginsenosides were unambiguously identified from both raw and steamed P. quinquefolius (0, 3, 6, and 9 times) (Figs. 1 and 2), 14 of which (Rg1, Rb1, malonyl-Rb1, Rc, Rb2, malonyl-Rb2, Rd, 20(S)-Rg2, 20(S)-Rh1, 20(S)-Rg3, 20(R)-Rg3, 20(R)-Rh1, Rh2 and 20(R)-Rg2) were identified by comparing the retention time and mass spectra with that of reference standards. The others without reference standards were tentatively assigned by matching the empirical molecular formula with that of the reported known ginsenosides, and further confirmed by elucidating the low energy CID fragmentions, especially for those isomeric ginsenosides. Moreover, the chromatographic behaviors of ginsenosides in the literatures provided the complementary information for the identity confirmation of isomers. All details of ginsenosides identified from both raw and multi-steamed P. quinquefolius were summarized in Table 1.
Table 1.
Ginsenosides identified from raw and multi-steamed Panax quinquefolius
| Peak no. | tR (min) | Identity | Molecular formula | [M-H]― (m/z) | [M-H+HCOOH]― (m/z) | Fragmentions of [M-H]― (m/z) | References |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | 2.62 | Rg1 | C42H72O14 | 799.48 | 845.49 | 637.43 [M−H-(Glc-H2O)]−; 475.38 Agl | [18,37] |
| 2 | 2.64 | Re | C48H82O18 | 945.54 | 991.55 | 799.48 [M−H-(Rha-H2O)]− ; 783.49 [M−H-(Glc-H2O)]− ; | [10,18,37] |
| 637.43 [M−H-(Rha-H2O)-(Glc-H2O)]− ; 475.38 Agl | |||||||
| 3 | 4.82 | 24(S)-Pseudo F11 | C42H72O14 | 799.49 | 845.49 | 653.43 [M−Rha-H2O]−; 635.41 [M−H-(Rha-H2O)-H2O]−; | [10,37] |
| 491.37 [M−H-(Rha-H2O)-(Glc-H2O)]− | |||||||
| 4 | 7.52 | Rb1 | C54H92O23 | 1107.59 | 1153.59 | 945.54 [M−H-(Glc-H2O)]− ; 783.49 [M−H-2(Glc-H2O)]− ; | [18,37] |
| 621.43 [M−H-3(Glc-H2O)]−; | |||||||
| 5 | 7.96 | Ma-Rb1 | C57H94O26 | 1193.60 | — | 1149.61 [M−H-CO2]− | [18,30,37] |
| 6 | 8.76 | Rc | C53H90O22 | 1077.59 | 1123.59 | 945.54 [M−H-(Ara(f)-H2O)]−; 621.44 [M−H-(Ara(f)-H2O-2(Glc-H2O)]−; | [10,18,37] |
| 783.48 [M−H-(Ara(f)-H2O-(Glc-H2O)]−; 915.53 [M−H-(Glc-H2O)]− | |||||||
| 7 | 9.48 | Ma-Rc | C56H92O25 | 1163.58 | — | 1119.60 [M−H-CO2]− | [18,30,37] |
| 8 | 10.24 | Rb2 | C53H90O22 | 1077.59 | 1123.59 | 945.54 [M−H-(Ara(f)-H2O)]−; 915.53 [M−H-(Glc-H2O)]−; | [16,18,37] |
| 783.48 [M−H-(Ara(f)-H2O-(Glc-H2O)]−; | |||||||
| 9 | 10.98 | Ma-Rb2 | C56H92O25 | 1163.58 | — | 1119.60 [M−H-CO2]− | [18,30,37] |
| 10 | 11.98 | Rd | C48H82O18 | 945.55 | 991.56 | 783.49 [M−H-(Glc-H2O)]−; 621.43 [M−H-2(Glc-H2O]− | [18,30,37] |
| 11 | 12.24 | Ma-Rd | C51H84O21 | 1031.54 | — | 987.56 [M−H-CO2]− | [18,30,37] |
| 12 | 6.61 | 20(S)-Rg2 | C42H72O13 | 783.48 | 829.50 | 621.44 [M−H-(Glc-H2O)]−; 459.38 [M−H-2(Glc-H2O)]− | [16,18,37] |
| 13 | 6.94 | 20(S)-Rh1 | C36H62O9 | 637.43 | 683.44 | 475.38 Agl | [18,37] |
| 14 | 14.98 | 20(S)-Rg3 | C42H72O13 | 783.49 | 829.50 | 621.44 [M−H-(Glc-H2O)]−; 459.38 [M−H-2(Glc-H2O)]− | [16,18,37] |
| 15 | 15.18 | 20(R)-Rg3 | C42H72O13 | 783.49 | 829.50 | 621.44 [M−H-(Glc-H2O)]−; 459.38 [M−H-2(Glc-H2O)]− | [16,18,37] |
| 16 | 16.66 | Rk1 | C42H70O12 | 765.48 | 811.49 | 603.43 [M−H-(Glc-H2O)]− | [16,18,30,37] |
| 17 | 16.84 | Rg5 | C42H70O12 | 765.48 | 811.48 | 603.43 [M−H-(Glc-H2O)]− | [16,18,37] |
| 18 | 7.18 | 20(R)-Rh1 | C36H62O9 | 637.43 | 683.44 | 475.38 Agl | [36,42] |
| 19 | 17.92 | Rh2 | C36H62O8 | 621.44 | 667.44 | 459.38 Agl | [16,18,37] |
| 20 | 13.42 | F4 | C42H70O12 | 765.48 | 811.49 | 619.43 [M−H-(Rha-H2O)]− | [16,18,37] |
| 21 | 13.72 | Rk3 | C36H60O8 | 619.42 | 665.42 | 457.36 [M−H-(Glc-H2O)]− | [16,18,37] |
| 22 | 14.12 | Rh4 | C36H60O8 | 619.42 | 665.43 | 457.36 [M−H-(Glc-H2O)]− | [16,18,37] |
| 23 | 13.18 | Rg6 | C42H70O12 | 765.48 | 811.49 | 619.43 [M−H-(Rha-H2O)]− | [16,18,37] |
| 24 | 15.64 | 20(S)-Rs3 | C44H74O14 | 825.50 | 871.51 | 783.49 [M−H-Ac]− ; 621.44 [M−H-Ac-(Glc-H2O)]− | [16,18,37] |
| 25 | 15.82 | 20(R)-Rs3 | C44H74O14 | 825.50 | 871.51 | 783.49 [M−H-Ac]− ; 621.44 [M−H-Ac-(Glc-H2O)]− | [16,18,37] |
| 26 | 17.12 | Rs5 | C44H72O13 | 807.49 | — | 765.48 [M−H-Ac]−; 603.42 [M−H-Ac-(Glc-H2O)]−; | [16,18,30,37] |
| 27 | 17.36 | Rs4 | C44H72O13 | 807.49 | — | 765.48 [M−H-Ac]−; 603.42 [M−H-Ac-(Glc-H2O)]−; | [16,18,37] |
| 28 | 18.48 | Rk2 | C36H60O7 | 603.40 | 649.42 | 441.34 [M−H-(Glc-H2O)]− | [43] |
| 29 | 18.78 | Rh3 | C36H60O7 | 603.40 | 649.42 | 441.34 [M−H-(Glc-H2O)]− | [43] |
| 30 | 2.32 | 20(S)-Rf2 | C42H74O14 | 801.50 | 847.51 | 655.44 [M−H-(Rha-H2O)]−; 637.43 [M−H-(Rha-H2O)-H2O]−; | [37,41] |
| 493.39 [M−H-(Rha-H2O)-(Glc-H2O)] − | |||||||
| 31 | 7.36 | 20(R)-Rg2 | C42H72O13 | 783.48 | 829.50 | 621.44 [M−H-(Glc-H2O)]−; 459.38 [M−H-2(Glc-H2O)]− | [18,37] |
| 32 | 13.28 | F2 | C42H72O13 | 783.49 | 829.50 | 621.44 [M−H-(Glc-H2O)]− | [10,37] |
The adduction [M-H+HCOOH]− would be generated when formic acid was added to the mobile phase as a chromatographic modifier, which was helpful for the confirmation of deprotonated molecular ions [M-H]−, and for the farther distinguishment of the ginsenoside type. For instance, protopanaxadiol and protopanaxatriol-type ginsenosides could generate adductions [M-H+ HCOOH]−, while malonyl- and acetyl-ginsenosides did not under the optimal conditions (Table 1).
Although two not easily separated ginsenosides Re and Rg1 were also co-eluted under the present chromatographic conditions as described in the most of the published literatures [30-32], these two ginsenosides existed in raw P. quinquefolius were confirmed by their deprotonated ions [M-H]−, adductions [M-H+HCOOH]− and low energy CID fragment ions m/z 783.49 of Re (Table 1). The retention sequence of isomeric malonyl-ginsenosides Rc and Rb2 was deduced from the chromatographic behaviors of their corresponding demalonylated ginsenosides Rc and Rb2, while other isomers Rg6/F4/Rk1/ Rg5, Rk3/Rh4, 20(S)-Rg3/20(R)-Rg3, 20(S)-Rs3/20(R)- Rs3, Rk2/Rh3, and Rs5/Rs4 were distinguish based on their chromatographic features published in previous studies [16,32] owing to unavailable reference standards.
Nine cycles of steaming-induced chemical transformations in black American ginseng
The chemical profiles of methanol extracts and 70% methanol extracts of two batches of raw and multi-steamed P. quinquefolius were compared using the UPLC-Q-TOF-MS/MS approach presented in Chromatographic Conditions section. It was found that the chemical profiles of methanol and 70% methanol extracts of the same batch of sample are similar to each other, thus 70% aqueous methanol was used as solvent to extract samples for further study. Interestingly, the chemical profiles of 70% methanol extracts from both raw and 3 times-steamed American ginsengs showed significant difference as demonstrated in Fig. 2A and 2B. The peaks 1, 3, 5, 7, 9, and 11 were detected only in raw P. quinquefolius, and the peak areas of peaks 4, 6, and 8 were greatly lower than that in three times-steamed American ginsengs (Fig. 2A, B). As shown in Table 1, peaks 5, 7, 9, and 11 were assigned as malonyl-ginsenosides, which was in accordance with the previous findings that the content of malonyl-ginsenosides decreased greatly after steaming [33], the mechanisms of which were assumed to be degradation of the thermally unstable malonyl-ginsenosides into their corresponding neutral ginsenosides [34]. By comparison with the 70% methanol extracts (Fig. 2A) of raw P. quinquefolius, new generated peaks (peaks 12-29) were gradually detected in multi-steamed P. quinquefolius (Fig. 2B-D) and peaks (1-11) were completely undetected in BAG (raw P. quinquefolius undergone 9 cycles of steaming and drying) (Fig. 2D), which indicated that chemical conversions were occurred during the 9 cycles of steaming process of BAG. What is more, it was interesting to note that there is also significant difference among the steaming times-dependent chemical profiles of multi-steamed P. quinquefolius (Fig. 2B-D). Peaks 24, 25, 26, and 27 were only detected in the BAG derived from raw P. quinquefolius and were identified as acetyl-ginsenosides (Table 1). All above-mentioned results suggested that steaming times be one of factors that can greatly influence the quality of steamed P. quinquefolius, although many other factors i.e. steaming temperature, steaming time, drying time, etc. may also affect the chemical profiles of ginseng. With the comparison of ginsengs steamed for 2 to 3 times, dried by sunlight [16-18,35] or puffed by high pressure [36], ginsengs undergone 9 cycles of steaming undoubtedly possessed more ginsenosides of low polarity. What’s more, the starch molecules in the ginseng tissue were gradually changed from sol state to gel state in the BAG preparatory process, which made BAG easier to be absorbed by human body. Meanwhile, the color of nine-time steamed ginsengs became much darker than that of raw ginsengs and two- or three-time steamed ginsengs, which was in accord with the findings reported by Lee et al. [12] and Song et al. [13].
Possible mechanisms involved in the 9 cycles of steaming-induced chemical changes in black American ginseng
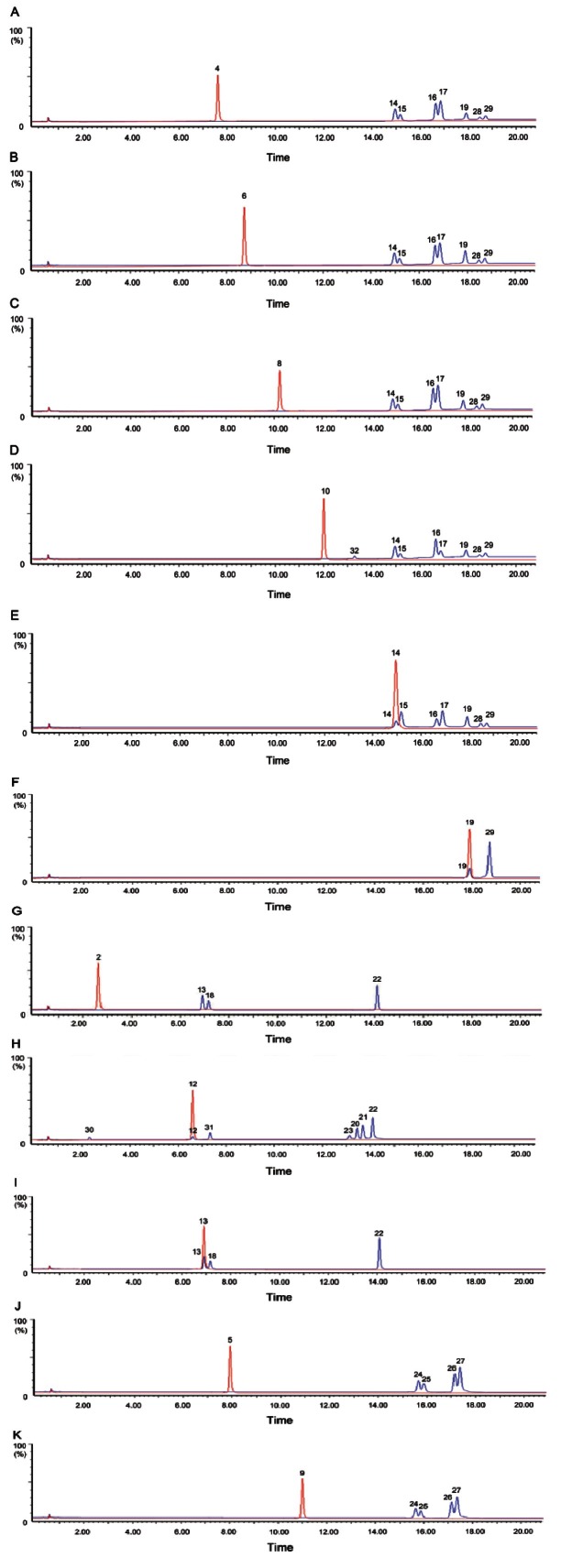
In order to elucidate the mechanisms underlying the 9 cycles of steaming-induced chemical changes in BAG, 14 available pure ginsenosides i.e., Rg1, Rb1, malonyl- Rb1, Rc, Rb2, malonyl-Rb2, Rd, 20(S)/20(R)-Rg2, 20(S)/20(R)-Rh1, 20(S)/20(R)-Rg3, and Rh2 underwent 9 cycles of steaming respectively in the same way as in the preparation of BAG. The mimic 9 cycles of steaming extracts of 14 pure ginsenosides were subjected to UPLC-Q-TOF-MS/MS analysis. It was found that significant chemical changes happen for most ginsenosides tested, except for three ginsenoside 20(R)-Rg2, 20(R)- Rh1 and 20(R)-Rg3 (data not shown). Extra compounds were detected in the mimic 9 cycles of steaming extracts of 11 ginsenosides Rg1, 20(S)-Rg2, Rb1, Rd, malonyl- Rb1, 20(S)-Rh1, 20(S)-Rg3, Rh2, Rb2, Rc, malonyl-Rb2 (Fig. 3).
Fig. 3. Representative chromatograms of 70% methanol aqueous solutions of 11 reference compounds (red color profile) and mimic 9 cycles of steaming extracts (blue color profile) of 11 reference compounds: (A) ginsenoside Rb1, (B) ginsenoside Rc, (C) ginsenoside Rb2, (D) ginsenoside Rd, (E) ginsenoside 20(S)-Rg3, (F) ginsenoside Rh2, (G) ginsenoside Rg1, (H) ginsenoside 20(S)-Rg2, (I) ginsenoside 20(S)-Rh1, (J) malonyl-ginsenoside Rb1, (K) malonyl-ginsenoside Rb2.
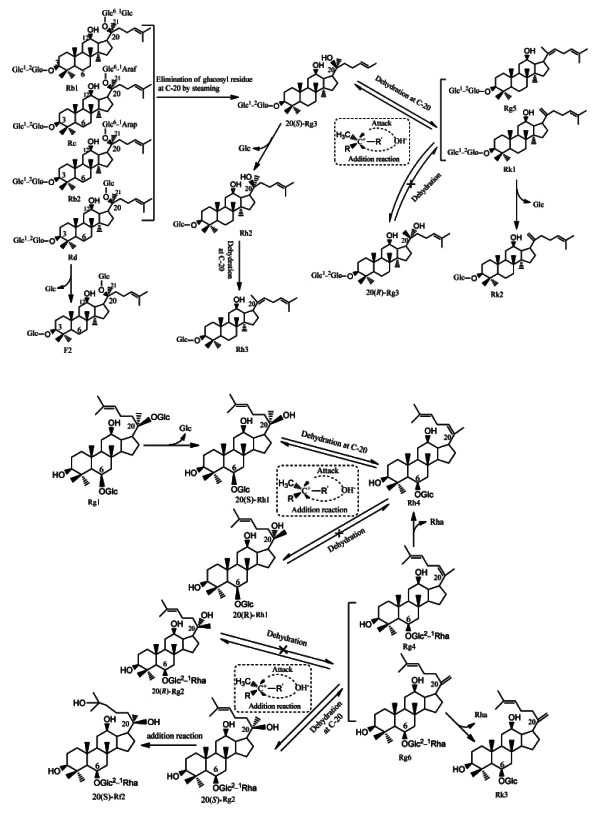
Seven extra peaks (peaks 14 -17, 19, 28, and 29) were detected in the mimic 9 cycles of steaming extracts of ginsenosides Rb1, Rd, Rc, and Rb2 (Fig. 3A-D), and were identified as 20(S)-Rg3, 20(R)-Rg3, Rk1, Rg5, Rh2, Rk2, and Rh3, respectively [18,37]. Similarly, the abovementioned seven peaks (peaks 14-17, 19, 28, and 29) was also detected in the mimic 9 cycles of steaming extracts of ginsenosides 20 (S)-Rg3 (Fig. 3E). Meanwhile, only one extra peak (peak 29) was generated from the mimic 9 cycles of steaming extracts of ginsenosides Rh2 (Fig. 3F) and was identified as Rh3. The mechanisms involved were deduced to be the loss of glycosyl moiety at C-20-OH of Rb1, Rb2, Rc and Rd through hydrolysis to generate 20(S)-Rg3, then 20(S)-Rg3 further underwent dehydration to generate ginsenosides Rk1 and Rg5, or eliminated the terminal glycosyl moiety at C-3 position to generate ginsenoside Rh2, which was consistent with our previous findings [16,18]. Subsequently, ginsenosides Rh3 and Rk2 were further generated through dehydration at C-20 of Rh2 and elimination the terminal glycosyl moiety at C-3 of Rk1 respectively. It should be noted that 20(R)-Rg3 is detected in multi-steamed P. quinquefolius and mimic 9 cycles of steaming extracts of ginsenoside Rb1, Rb2, Rc, and Rd, and its peak area and height always are less than those of 20(S)-Rg3 (Figs. 2B-D and 3A-D), which was similar with the finding reported by the references [18,38]. No naturally occurring 20(R)-ginsenosides have reported so far, therefore 20(R)- Rg3 was assumed to be generated from Rk1 or Rg5 via addition reaction. There might be a chemical equilibrium among 20(S)-Rg3, 20(R)-Rg3 and Rk1 or Rg5 (Fig. 4). Because Rk1 and Rg5 were not detected in the mimic 9 cycles of steaming extrac ts of 20(R)-Rg3 (data not shown), the Rk1 and Rg5 found in multi-steamed P. quinquefolius were thought to be derived from the dehydration of 20(S)-Rg3, which was different from what the previous studies reported that the stereoisomers 20(S)-Rg3 and 20(R)-Rg3 are generated firstly from the ginsenosides Rb2 by the selective attack of OH − after the elimination of the glycosyl residue at C-20 during the steaming process [39,40]. In addition to these four artifacts (20(S)-Rg3, 20(R)-Rg3, Rk1, and Rg5), another compound (peak 32) was also detected in the mimic 9 cycles of steaming extracts of ginsenoside Rd (Fig. 3D). Since only one product ion m/z 621.44 [M-H-(Glc-H2O)]- was found in its low energy CID mass spectrum (Table 1), indicating that only one glycosyl moiety was attached at C-20, thus it was assigned to be ginsenoside F2 [10,37]. It was assumed to be generated from Rd through hydrolysis of a glycosyl moiety at C-3-OH. Similarly, three extra peaks (peaks 13, 18 and 22) were detected in the mimic 9 cycles of steaming extract of ginsenoside Rg1 (Fig. 3G) and were assigned as ginsenosides 20(S)/20(R)- Rh1 and Rh4, respectively. Meanwhile, the same peaks (peaks 13, 18- and 22) were also detected in the mimic 9 cycles of steaming extracts of ginsenosides 20(S)-Rh1 (Fig. 3I). The mechanisms involved were deduced to be the loss of glycosyl moiety at C-20-OH of Rg1 through hydrolysis to generate 20(S)-Rh1, then 20(S)-Rh1 further underwent dehydration at C-20 to generate ginsenosides Rh4, which was also demonstrated in the previous studies [18,37]. It should be noted that 20(R)-Rh1 is detected in 6 or 9 times-steamed P. quinquefolius and mimic 9 cycles of steaming extracts of ginsenoside Rg1 and 20(S)-Rh1, and its peak area always seems similar to that of 20(S)-Rh1 (Figs. 2C, D and 3G, I), but no naturally occurring 20(R)-ginsenosides have been reported so far, therefore 20(R)-Rh1 was assumed to be generated from Rh4 via addition reaction.
Fig. 4. Possible mechanisms involved in nine-time steaming-induced chemical conversions of protopanaxdiol-type ginsenosides Rb1, Rc, Rb2,Rd and protopanaxtriol-type ginsenosides Rg1, 20(S)-Rg2.
What’s more, ginsenoside Rh4 was not detected in the mimic 9 cycles of steaming extracts of 20(R)-Rh1 (data not shown), the Rh4 found in 6 or 9 times-steamed P. quinquefolius was thought to be derived from the dehydration of 20(S)-Rh1. The putative chemical conversion schemes were demonstrated in Fig. 4. Interestingly, six extra peaks (peaks 20-23, 30, and 31) were detected in the mimic 9 cycles of steaming extract of ginsenoside 20 (S)-Rg2 (Fig. 3H) and were assigned as ginsenoside F4, Rk3, Rh4, Rg6, 20(S)-Rf2 and 20(R)- Rg2, respectively. Ginsenosides Rg6 and Rg4 (F4) were deduced to be generated through dehydration reaction at C-20 (21) or C-20(22) of 20(S)-Rg2. Subsequently, ginsenosides Rh4 and Rk3 were generated respectively through eliminating the terminal glycosyl moiety at C-6 position of ginsenosides F4 and Rg6 [16], while ginsenoside 20(S)-Rf2 was deduced to be generated through addition reaction of 20(S)-Rg2. Unexpectedly, 20(R)- Rg2 was only detected in the mimic 9 cycles of steaming extracts of ginsenoside 20(S)-Rg2, and its peak area seemed very low (Fig. 3H). Since no naturally occurring 20(R)-ginsenosides had been reported so far, 20(R)-Rg2 was assumed to be generated from Rg6 or Rg4 via addition reaction. In addition, ginsenoside Rg6 or Rg4 was not detected in the mimic 9 cycles of steaming extracts of 20(R)-Rg2 (data not shown), the Rg6 or Rg4 found in 6 and 9 times-steamed P. quinquefolius was thought to be from the dehydration of 20(S)-Rg2 (Fig. 4).
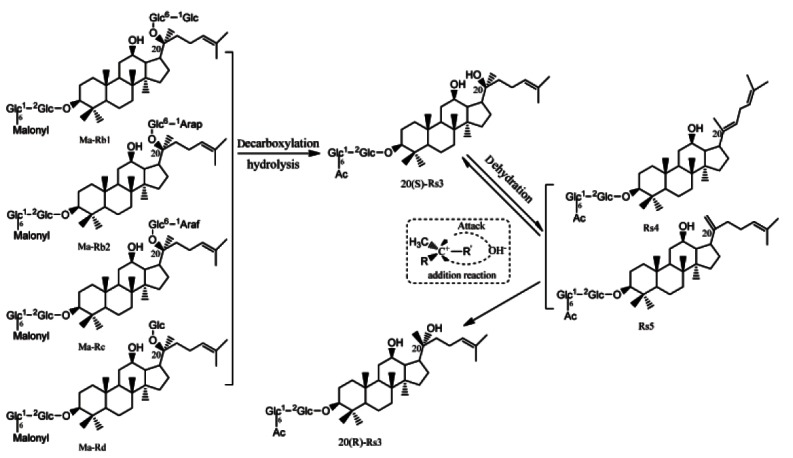
From the analysis of the mimic 9 cycles of steaming extracts of pure ginsenosides described above, it could be concluded that those compounds newly generated in multi-steamed P. quinquefolius, such as 20(S)-Rg3, 20(R)-Rg3, Rk1, Rh2, Rg5, Rk2, and Rh3 are mainly developed from Rb1, Rb2, Rc and Rd, while Rk3, Rh4, Rg6, and F4 are from Rg2 and 20(S)/20(R)-Rh1 are derived from Rg1 via hydrolysis, dehydration, deglycosylation and addition reactions during the preparatory process of BAG. Those acetyl-ginsenosides in BAG such as 20(S)-Rs3, 20(R)-Rs3, Rs4, and Rs5 (Fig. 2D) were assumed to be generated from the malonyl-ginsenosides through decarboxylation, hydrolysis, dehydration and addition reactions, which was consistent with the finding reported by the references [18,37]. Further demonstrated experiments showed that four extra peaks (peaks 24-27) were detected in the mimic 9 cycles of steaming extracts of ginsenosides malonyl-Rb1, malonyl-Rb2 (Fig. 3J, K) and malonyl-Rc, malonyl-Rd (data not shown), and were identified as 20(S)-Rs3, 20(R)-Rs3, Rs5 and Rs4, respectively [16]. The putative chemical conversion schemes were demonstrated in Fig. 5. 20(S)-Rs3 and 20(R)-Rs3 were deduced to be generated from the hydrolysis of glycosyl moiety at C-20-OH and decarboxylation of malonyl moiety attached to glycosyl linkage at C-3-OH of malonyl-Rb1, malonyl-Rb2, malonyl-Rc and malonyl-Rd3 further underwent dehydration to generate Rs4 and Rs5, which was the same as the finding reported by the references [18,37]. Like the conversions among 20(S)-Rg3, 20(R)-Rg3, Rk1 and Rg5, Rs4 and Rs5 may undergo addition reaction to generate 20(S)-Rs3 and 20(R)-Rs3, and chemical equilibrium may also occur among these four components (Fig. 5).
Fig. 5. Possible mechanisms involved in nine-time steaming-induced generation of acetyl ginsenosides in the preparation of black American ginseng.
In this study, UPLC-Q-TOF-MS/MS was used for steaming times-dependent chemical profiling of steamed American ginsengs derived from raw P. quinquefolius. Over 29 ginsenosides were unambiguously identified and/or tentatively assigned in both raw and repetitiously steamed P. quinquefolius, 18 of which were detected to be newly generated in BAG preparation. The mechanisms underlying the 9 cycles of steaming-induced chemical changes in BAG were deduced to be hydrolysis, dehydration, decarboxylation and addition reactions of the original ginsenosides in raw P. quinquefolius. In addition, the 70% methanol extracts of raw and multi-steamed P. quinquefolius showed significant difference in their chemical profiles, which suggested that steaming times may greatly influence the quality consistency of multi-steamed P. quinquefolius. It is probably no exaggeration to say that UPLC-Q-TOF-MS/MS based chemical profiling is a rapid and powerful approach for overall quality evaluation of multi-steamed P. quinquefolius, and is promising for the global quality investigation of repetitious steaming Chinese medicinal herbs.
References
- 1.Chinese Pharmacopeia Commission. Pharmacopeia of the People’s Republic of China 2010. Vol. 1. China Medical Science Publisher; Beijing: 2010. [Google Scholar]
- 2.Yuan CS, Wang CZ, Wicks SM, Qi LW. Chemical and Pharmacological Studies of Saponins with a Focus on American Ginseng. J Ginseng Res. 2010;34:160–167. doi: 10.5142/jgr.2010.34.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li B, Wang CZ, He TC, Yuan CS, Du W. Antioxidants potentiate Amellrican ginseng-induced killing of colorectal cancer cells. Cancer Lett. 2010;289:62–70. doi: 10.1016/j.canlet.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuan HD, Quan HY, Jung MS, Kim SJ, Huang B, Kim DY, Chung SH. Anti-Diabetic Effect of Pectinase-Processed Ginseng Radix (GINST) in High Fat Diet-Fed ICR Mice. J Ginseng Res. 2011;35:308–314. doi: 10.5142/jgr.2011.35.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu SF, Zhang JT. New achievements in ginseng research and its future prospects. Chin J Integr Med. 2009;15:403–408. doi: 10.1007/s11655-009-0403-6. [DOI] [PubMed] [Google Scholar]
- 6.Jia L, Zhao Y. Current evaluation of the millennium phytomedicine. Ginseng (I): etymology, pharmacognosy, phytochemistry, market and regulations. Curr Med Chem. 2009;16:2475–2484. doi: 10.2174/092986709788682146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia L, Zhao Y, Liang XJ. Current evaluation of the millennium phytomedicine. Ginseng (II): collected chemical entities, modern pharmacology, and clinical applications emanated from traditional Chinese medicine. Curr Med Chem. 2009;16:2924–2942. doi: 10.2174/092986709788803204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu JM, Yao Q, Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo JZ, Luo L. Ginseng on hyperglycemia: effects and mechanisms. Evid Based Complement Alternat Med. 2009;6:423–427. doi: 10.1093/ecam/nem178. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Christensen LP. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 11.Jang DJ, Lee MS, Shin BC, Lee YC, Ernst E. Red ginseng for treating erectile dysfunction: a systematic review. Br J Clin Pharmacol. 2008;66:444–450. doi: 10.1111/j.1365-2125.2008.03236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JH, Shen GN, Kim EK, Shin HJ, Myung CS, Oh HJ, Kim DH, Roh SS, Cho W, Seo YB, et al. Preparation of black ginseng and its antitumor activity. Korean J Orient Med Physiol Pathol. 2006;20:951–956. [Google Scholar]
- 13.Song GY, Oh HJ, Roh SS, Seo YB, Park YJ, Myung CS. Effect of black ginseng on body weight and lipid profiles in male rats fed normal diets. Yakhak Hoeji. 2006;50:381–385. [Google Scholar]
- 14.Lee SR, Kim MR, Yon JM, Baek IJ, Park CG, Lee BJ, Yun YW, Nam SY. Black ginseng inhibits ethanol-induced teratogenesis in cultured mouse embryos through its effects on antioxidant activity. Toxicol In Vitro. 2009;23:47–52. doi: 10.1016/j.tiv.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Zhu XM, Wang QJ, Zhang DL, Fang ZM, Wang CY, Wang Z, Sun BS, Wu H, Sung CK. Enzymatic preparation of 20(S, R)-protopanaxadiol by transformation of 20(S, R)-Rg3 from black ginseng. Phytochemistry. 2010;71:1514–1520. doi: 10.1016/j.phytochem.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Sun BS, Gu LJ, Fang ZM, Wang CY, Wang Z, Lee MR, Li Z, Li JJ, Sung CK. Simultaneous quantification of 19 ginsenosides in black ginseng developed from Panax ginseng by HPLC-ELSD. J Pharm Biomed Anal. 2009;50:15–22. doi: 10.1016/j.jpba.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Kim SN, Kang SJ. Effects of black ginseng (9 times-steaming ginseng) on hypoglycemic action and changes in the composition of ginsenosides on the steaming process. Korean J Food Sci Technol. 2009;41:77–81. [Google Scholar]
- 18.Sun BS, Pan FY, Sung CK. Repetitious steaming-induced chemical transformations and global quality of black ginseng derived from Panax ginseng by HPLC-ESI-MS/MSn based chemical profiling approach. Biotechnol Bioprocess Eng. 2011;16:956–965. doi: 10.1007/s12257-011-0079-6. [DOI] [Google Scholar]
- 19.Kim AJ, Kang SJ, Lee KH, Lee M, Ha SD, Cha YS, Kim SY. The chemopreventive potential and anti-inflammatory activities of Korean black ginseng in colon 26-M3.1 carcinoma cells and macrophages. J Korean Soc Appl Biol Chem. 2010;53:101–105. [Google Scholar]
- 20.Qi LW, Wang CZ, Yuan CS. American ginseng: potential structure-function relationship in cancer chemoprevention. Biochem Pharmacol. 2010;80:947–954. doi: 10.1016/j.bcp.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 21.Chan TW, But PP, Cheng SW, Kwok IM, Lau FW, Xu HX. Differentiation and authentication of Panax ginseng, Panax quinqueflium, and ginseng products by using HPLC/MS. Anal Chem. 2000;72:1281–1287. doi: 10.1021/ac990819z. [DOI] [PubMed] [Google Scholar]
- 22.Xie GX, Ni Y, Su MM, Zhang YY, Zhao AH, Gao XF, Liu Z, Xiao PG, Jia W. Application of ultra-performance LC-TOF MS metabolite profiling techniques to the analysis of medicinal Panax herbs. Metabolomics. 2008;4:248–260. [Google Scholar]
- 23.Xie G, Plumb R, Su M, Xu Z, Zhao A, Qiu M, Long X, Liu Z, Jia W. Ultra-performance LC/ TOF MS analysis of medicinal Panax herbs for metabolomic research. J Sep Sci. 2008;31:1015–1026. doi: 10.1002/jssc.200700650. [DOI] [PubMed] [Google Scholar]
- 24.Dan M, Xie G, Gao X, Long X, Su M, Zhao A, Zhao T, Zhou M, Qiu Y, Jia W. A rapid ultra-performance liquid chromatography-electrospray Ionisation mass spectrometric method for the analysis of saponins in the adventitious roots of Panax notoginseng. Phytochem Anal. 2009;20:68–76. doi: 10.1002/pca.1099. [DOI] [PubMed] [Google Scholar]
- 25.Dan M, Su M, Gao X, Zhao T, Zhao A, Xie G, Qiu Y, Zhou M, Liu Z, Jia W. Metabolite profiling of Panax notoginseng using UPLC-ESI-MS. Phytochemistry. 2008;69:2237–2244. doi: 10.1016/j.phytochem.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Zhao T, Gao X, Dan M, Jia W. Simultaneous determination of 17 ginsenosides in rat urine by ultra performance liquid chromatography-mass spectrometry with solid-phase extraction. Anal Chim Acta. 2007;594:265–273. doi: 10.1016/j.aca.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 27.Angelova N, Kong HW, van der Heijden R, Yang SY, Choi YH, Kim HK, Wang M, Hankemeier T, van der Greef J, Xu G. Recent methodology in the phytochemical analysis of ginseng. Phytochem Anal. 2008;19:2–16. doi: 10.1002/pca.1049. [DOI] [PubMed] [Google Scholar]
- 28.Fuzzati N. Analysis methods of ginsenosides. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;812:119–133. doi: 10.1016/j.jchromb.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 29.Li SL, Song JZ, Choi FF, Qiao CF, Zhou Y, Han QB, Xu HX. Chemical profiling of Radix Paeoniae evaluated by ultra-performance liquid chromatography/photo-diode-array/quadrupole time-of-flight mass spectrometry. J Pharm Biomed Anal. 2009;49:253–266. doi: 10.1016/j.jpba.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Fuzzati N, Gabetta B, Jayakar K, Pace R, Peterlongo F. Liquid chromatography-electrospray mass spectrometric identification of ginsenosides in Panax ginseng roots. J Chromatogr A. 1999;854:69–79. doi: 10.1016/s0021-9673(99)00463-x. [DOI] [PubMed] [Google Scholar]
- 31.Ma X, Xiao H, Liang X. Identification of ginsenosides in Panax quinquefolium by LC-MS. Chromatographia. 2006;64:31–36. [Google Scholar]
- 32.Liu JH, Wang X, Cai SQ, Komatsu K, Namba T. Analysis of the constituents in the Chinese drug Notoginseng by liquid chromatography-electrospray mass spectrometry. J Chin Pharm Sci. 2004;13:225–237. [Google Scholar]
- 33.Du XW, Wills RB, Stuart DL. Changes in neutral and malonyl ginsenosides in American ginseng (Panax quinquefolium) during drying, storage and ethanolic extraction. Food Chem. 2004;86:155–159. [Google Scholar]
- 34.Ren G, Chen F. Degradation of ginsenosides in American ginseng (Panax quinquefolium) extracts during microwave and conventional heating. J Agric Food Chem. 1999;47:1501–1505. doi: 10.1021/jf980678m. [DOI] [PubMed] [Google Scholar]
- 35.Lee SA, Jo HK, Im BO, Kim S, Whang WK, Ko SK. Changes in the contents of prosapogenin in the red ginseng (Panax ginseng) depending on steaming batches. J Ginseng Res. 2012;36:102–106. doi: 10.5142/jgr.2012.36.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.An YE, Ahn SC, Yang DC, Park SJ, Kim BY, Baik MY. Chemical conversion of ginsenosides in puffed red ginseng. LWT Food Sci Technol. 2011;44:370–374. [Google Scholar]
- 37.Li SL, Lai SF, Song JZ, Qiao CF, Liu X, Zhou Y, Cai H, Cai BC, Xu HX. Decocting-induced chemical transformations and global quality of Du-Shen-Tang, the decoction of ginseng evaluated by UPLC-Q-TOF-MS/ MS based chemical profiling approach. J Pharm Biomed Anal. 2010;53:946–957. doi: 10.1016/j.jpba.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Wang H, Chen S. Analysis of ginsenosides in Sheng-Mai-Yin decoction by high performance liquid chromatography-diode array detection-electrospray mass spectrometry. Chin J Chromatogr. 2006;24:325–330. [PubMed] [Google Scholar]
- 39.Kang KS, Kim HY, Baek SH, Yoo HH, Park JH, Yokozawa T. Study on the hydroxyl radical scavenging activity changes of ginseng and ginsenoside-Rb2 by heat processing. Biol Pharm Bull. 2007;30:724–728. doi: 10.1248/bpb.30.724. [DOI] [PubMed] [Google Scholar]
- 40.Park JD. Recent studies on the chemical constituents of Korean ginseng (Panax ginseng C. A. Meyer). Korean J Ginseng Sci. 1996;20:389–415. [Google Scholar]
- 41.Park JD, Lee YH, Kim SI. Ginsenoside Rf2, a new dammarane glycoside from Korean red ginseng (Panax ginseng). Arch Pharm Res. 1998;21:615–617. doi: 10.1007/BF02975384. [DOI] [PubMed] [Google Scholar]
- 42.Ma XQ, Liang XM, Xu Q, Zhang XZ, Xiao HB. Identification of ginsenosides in roots of Panax ginseng by HPLC-APCI/ MS. Phytochem Anal. 2005;16:181–187. doi: 10.1002/pca.842. [DOI] [PubMed] [Google Scholar]
- 43.Park IH, Kim NY, Han SB, Kim JM, Kwon SW, Kim HJ, Park MK, Park JH. Three new dammarane glycosides from heat processed ginseng. Arch Pharm Res. 2002;25:428–432. doi: 10.1007/BF02976595. [DOI] [PubMed] [Google Scholar]