Abstract
Chondrocyte apoptosis has been recognized as an important factor in the pathogenesis of osteoarthritis (OA). Hydrogen peroxide (H2O2), which produces reactive oxygen species, reportedly induces apoptosis in chondrocytes. The ginsenoside Rb1 (GRb1) is the principal component in ginseng and has been shown to have a variety of biological activities, such as anti-arthritis, anti-inflammation, and anti-tumor activities. In this study, we evaluated the effects of G-Rb1 on the mitochondrial permeability transition (MPT) and caspase-3 activity of chondrocyte apoptosis induced by H2O2. Cultured rat articular chondrocytes were exposed to H2O2 with or without G-Rb1 and assessed for viability, MPT, Bcl-xL/Bax expression, caspase-3 activity, and apoptosis. The co-treatment with G-Rb1 showed an inhibition of MPT, caspase-3 activity, and cell death. Additionally, the levels of the apoptotic protein Bax were significantly lower and the levels of the anti-apoptotic protein Bcl-xL were higher compared with H2O2 treatment alone. The results of this study demonstrate that G-Rb1 protects chondrocytes against H2O2-induced apoptosis, at least in part via the inhibition of MPT and caspase-3 activity. These results demonstrate that G-Rb1 is a potentially useful drug for the treatment of OA patients.
Keywords: Ginsenoside Rb1, Hydrogen peroxide, Chondrocytes, Apoptosis, Caspase-3
INTRODUCTION
Pharmacological studies of ginseng have identified the ginsenosides as the plant’s principal active components [1]. Over 40 ginsenosides have been identified; each ginsenoside shows different biological activities based on structural differences. The ginsenosides Rb1 (G-Rb1), one of the most extensively studied of the ginsenosides, and they have been shown to have a variety of biological ac tivities, including anti-inflammatory, and anti-allergic activities anti-allergic activities [2]. However, the anti-arthritic and anti-apoptotic effects of G-Rb1 have not been reported.
Osteoarthritis (OA) is a degenerative joint disease that is principally characterized by the erosion of articular cartilage, meniscus damage, low-grade synovitis, bone remodeling, sclerosis of the subchondral bone and osteophyte formation in some cases [3]. OA occurs as the result of a variety of factors, such as aging, mechanical stress and obesity, and alters the physiological and biomechanical environment of the knee [4,5]. It has been relatively well demonstrated that cartilage matrix degradation and chondrocyte apoptosis induced by certain proteases (including matrix metalloproteinases, collagenases, and aggrecanases) and apoptotic factors (such as cytochome c, Bax and caspases) are the two primary pathogenic events occurring in OA [4,5].
Hydrogen peroxide (H2O2) is formed from a superoxide anion through superoxide dismutase. H2O2 has a variety of biological effects on various cell types. H2O2, which produces reactive oxygen species (ROS), is closely associated with the induction of chondrocyte apoptosis in vivo [6] and in vitro [7]. The prevention of chondrocyte apoptosis can therefore be considered to contribute to the control of the progression of OA. H2O2 alters mitochondrial membrane permeability, which allows for the release of cytochome c into the cytoplasm [8]. Additionally, caspase-3 is one of the key mediators in apoptotic signaling pathways, and the activation of caspase-3 ultimately results in cellular apoptosis [8].
G-Rb1 has garnered a great deal of research interest, and it has been shown to exert protection effects with anti-inflammatory agents [9,10]. The results of previous studies have also demonstrated that G-Rb1 may function as a scavenger of toxic species, such as H2O2 [6,11]. The principal objective of the present study, therefore, was to determine whether G-Rb1 treatment results in the inhibition of both the mitochondrial permeability transition (MPT) and caspase-3 in H2O2-treated chondrocytes.
MATERIALS AND METHODS
Harvesting chondrocytes and establishing cell cultures
A modified method for harvesting chondrocytes was conducted as described previously [5]. In brief, chondrocytes were isolated from the articular cartilage of three week-old male Sprague-Dawley rats. The cartilage was removed from animals that were subsequently euthanized via an overdose of anesthesia. The cartilage was cut into thin slices, washed with sterile phosphate-buffered saline (PBS) and soaked for 15 min in 5% penicillin–streptomycin– neomycin (Sigma, St. Louis, MO, USA). The cartilage slices were washed in PBS to remove residual antibiotic solution and then digested with 0.02% collagenase type II (Sigma) in Dulbecco’s modified Eagle’s medium (DMEM; HyClone, Logan, UT, USA) for 3 h in a water bath at 37℃. The digested cartilage was collected and centrifuged. The pellet was resuspended in DMEM and filtered through a 70-μM nylon mesh. The resulting chondrocytes were cultured in DMEM supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin– neomycin in a 5% CO2 incubator at 37℃. All of the experiments were conducted when the cells reached confluence within the first passage with G-Rb1 purchased from the Ambo Institute (Daejeon, Korea)
Cell viability assay
Cell survival was determined using a 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kit from Sigma. The chondrocytes were replated in 48-well plates at a density of 1.0×104 cells per well, incubated for 24 h, and treated with various concentrations (25 to 400 μM) of G-Rb1 (Ambo Institute) for 24 h. We then determined whether 1 h of pretreatment with G-Rb1 (50 and 100 μM) affected the cell viability of chondrocytes treated with 500 μM H2O2 for 24 h. After the incubation period, 10 μL of the kit solution was added to each well and incubated for 3 h at 37℃ in 5% CO2. After this treatment, the chondrocytes were incubated with MTT for 2 h at 37℃. The resulting formazan crystals were subsequently dissolved in MTT solubilization solution. The absorbance was determined at 540 nm using a microplate reader.
Mitochondrial permeability transition analysis
Chondrocytes were replated at 2×104 cells in 4-well chamber slides (Nunc, New York, NY, USA). After an overnight incubation under standard tissue culture conditions, the chondrocytes were exposed to 500 μM H2O2 with or without G-Rb1 (100 μM) and C3 (caspase-3 inhibitor). After 2 h, the cells were labeled with Mitotracker Red CMXRos (Invitrogen, Eugene, OR, USA) for 15 min. The cells were washed with PBS, and fluorescence microscopy was conducted using the appropriate filters.
Western blotting
Total proteins from chondrocyte lysates were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis using 12% to 15% gels and electrophoretically transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA, USA). The membranes were blocked in 5% skim milk in PBS and then incubated with primary antibodies against Bcl-xL (Cell Signaling, Danvers, MA, USA), Bax (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and β-actin (Cell Signaling) diluted 1:500 in 1% skim milk in PBS overnight at 4℃. The blots were then incubated with peroxidase-conjugated goat anti-rabbit IgG (1:5,000; Millipore, Bedford, MA, USA) for 1 h. The immunoreactions were visualized with SuperSignal West Dura Extended Duration Substrate (Thermo Scientific, San Jose, CA, USA) and analyzed using a chemiImager analyzer system (Alpha Innotech, San Leandro, CA, USA).
Caspase-3 activity analysis
Caspase-3 activation was assessed using Caspase- Glo3/7 Assays (Promega, Madison, WI, USA) as an index of apoptosis. The chondrocytes were replated at 1.0×104 onto 48-well plates. After 24 h of incubation, the chondrocytes were exposed to 500 μM H2O2 with or without G-Rb1 (100 μM) and C3 (caspase-3 inhibitor). After this treatment, 60 μL of supernatant was transferred from each well to a new 96-well plate. Equal volumes of Caspase-Glo 3/7 reagents were added, and the plate was incubated for 1 h at room temperature before the luminescence was measured.
Cell apoptosis analysis with immunocytochemistry
The chondrocytes were replated at 5×104 in 4-well chamber slides (Nunc). After 24 h of incubation, the chondrocytes were exposed to 500 μM H2O2 with or without G-Rb1 (100 μM) and C3 (caspase-3 inhibitor). After 12 h, the cells were labeled with Hoechst (Invitrogen) and propidium iodine for 15 min. The cells were washed with PBS and then analyzed with fluorescence microscopy using the appropriate filters.
Statistical analysis
The data are expressed as the means±SD. The data were analyzed via Student’s t-test and a repeated measures ANOVA followed by a Bonferroni test. A p-value of less than 0.05 was considered significant.
RESULTS
Cell viability
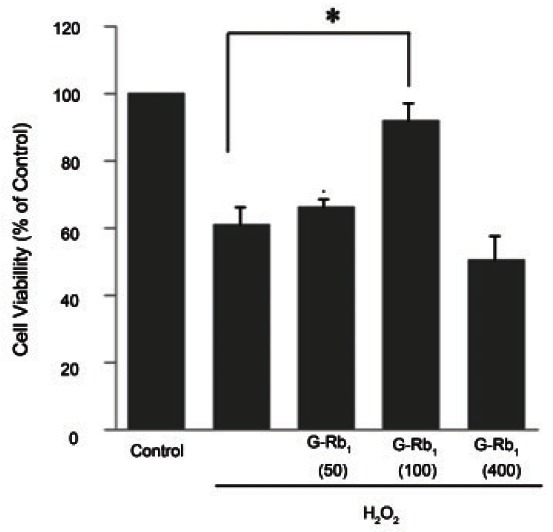
MTT assays were conducted to determine the effects of G-Rb1 on cell viability in relation to the mitochondrial activity of H2O2-treated chondrocytes. The chondrocytes exposed to G-Rb1 at various concentrations (0 to 400 μM) showed no significant toxicity for 24 h as previously reported [12]. The percentage of cell viability was approximately 60% after 24 h of treatment with 500 μM H2O2. As shown in Fig. 1, 1 h of pretreatment with G-Rb1 (100 μM) prior to 24 h of incubation with H2O2 (500 μM) produced higher cell viability (approximately 93%, p<0.05) than H2O2 alone. Therefore, all of the subsequent experiments were conducted in chondrocytes that were pretreated for 1 h with 50 or 100 μM G-Rb1 followed by 24 h of treatment with 500 μM H2O2.
Fig. 1. Cell viability of ginsenosides Rb1 (G-Rb1) in native chondrocytes and hydrogen peroxide (H2O2)-treated chondrocytes. Culture medium pretreated for 1 h with G-Rb1 (50 to 400 μM) and incubated with 500 μM H2O2 for 24 h. After incubation, cell viability was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide analysis and expressed as a percentage of viable untreated control cells grown in a defined medium. Data are expressed as the means ± SD of four to six independent experiments. Statistical analysis: *p<0.05 vs. positive control (H2O2 alone treatment).
Mitochondrial permeability transition analysis
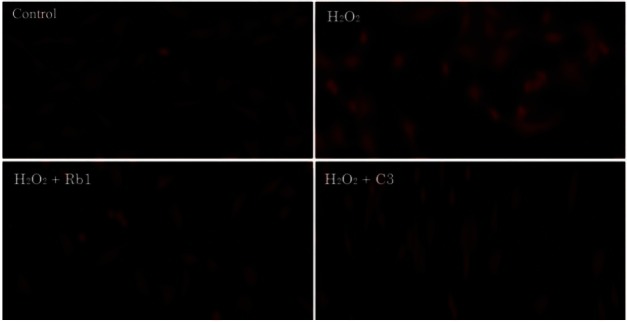
MPT leads to apoptosis in various cells after oxidative stress. To determine whether MPT was a requirement for H2O2-induced chondrocyte apoptosis, we conducted MPT analyses. Chondrocytes pretreated with G-Rb1 (100 μM) and C3 (50 μM) prior to treatment with H2O2 (500 μM) were stained with Mitotracker Red CMXRos. The results showed that MPT was inhibited, and the mitochondrial membrane potential was maintained (Fig. 2).
Fig. 2. Mitochondrial permeability transition analysis of chondrocytes exposed to 500 μM hydrogen peroxide (H2O2) with and without ginsenosides Rb1 (G-Rb1) and C3. Chondrocytes were treated with media alone (negative control), H2O2 (500 μM, positive control) and H2O2 (500 μM) after pretreatment with G-Rb1 (100 μM) and C3 (50 μM, caspase-3 inhibitor). The chondrocytes were then labeled with the potential sensitive dye Mitotracker Red CMXRos. Cells with active mitochondria evidence bright red cytoplasmic staining.
Western blot analysis of Bcl-xL and Bax expression
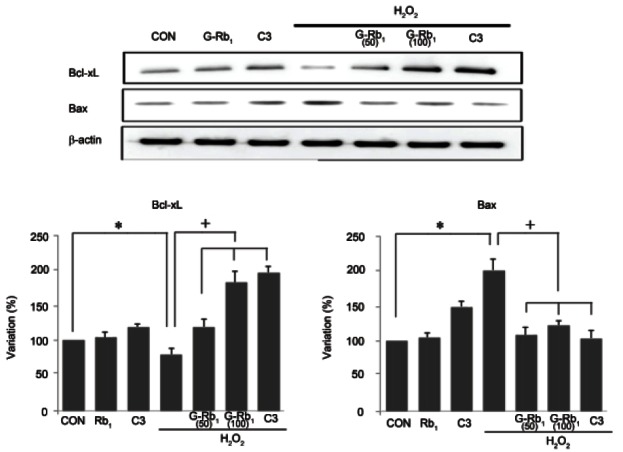
To assess the correlation of apoptotic proteins with MPT, Bcl-xL and Bax expression levels were detected. The expression of the pro-apoptotic protein Bax was higher in the H2O2-treated cells than the control cells (p<0.05), whereas the expression of the anti-apoptotic protein Bcl-xL was significantly lower in the H2O2- treated cells. However, after co-treatment with G-Rb1 and C3, Bax expression was significantly lower and BclxL expression was higher compared with H2O2 treatment alone (p<0.05) (Fig. 3)
Fig. 3. Effect of ginsenosides Rb1 (G-Rb1) on Bcl-2 family protein expression in hydrogen peroxide (H2O2)-treated chondrocytes. Chondrocytes were treated with media alone (negative control), H2O2 (500 μM, positive control) and H2O2 (500 μM) after pretreatment with G-Rb1 (50 μM, 100 μM) and C3 (50 μM, caspase-3 inhibitor). Bax expression was induced significantly by H2O2 and was suppressed by G-Rb1 and C3. By way of contrast, H2O2 significantly reduced the expression of Bcl-xL, whereas G-Rb1 and C3 significantly increased Bcl-xL expression. Data are expressed as the means±SD of four independent experiments. Statistical analysis: *p<0.05 vs. negative control; +p<0.05 vs. positive control (H2O2 alone treatment). CON, negative control.
Caspase-3 activity
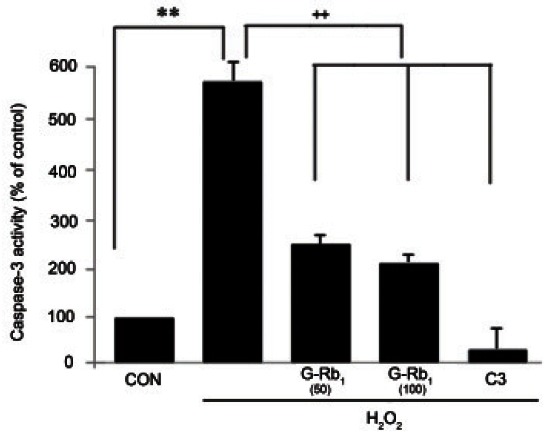
To assess the effect of H2O2 and G-Rb1 on caspase-3 activity, caspase-Glo assays were conducted. Caspase-3 activity was increased by the addition of H2O2. However, G-Rb1 significantly suppressed the H2O2-induced activation of caspase-3 in a dose-dependent manner, similar to C3 (Fig. 4).
Fig. 4. Effect of ginsenosides Rb1 (G-Rb1) on caspase-3 activity on hydrogen peroxide (H2O2)-treated chondrocytes. Chondrocytes were treated with media alone (negative control), H2O2 (500 μM, positive control) and H2O2 (500 μM) after pretreatment with G-Rb1 (50 μM, 100 μM) and C3 (50 μM, caspase-3 inhibitor). The chondrocytes were incubated with Caspase-Glo 3/7 reagents for 1 h, followed by luminescence measurements. The caspase-3 activity was significantly induced by H2O2 and was significantly suppressed by G-Rb1 and C3. Data were expressed as the means±SD of four to four independent experiments. Statistical analysis: **p<0.01 vs. negative control. ++p<0.01 vs. positive control (H2O2 alone treatment). CON, negative control.
Immunocytochemistry
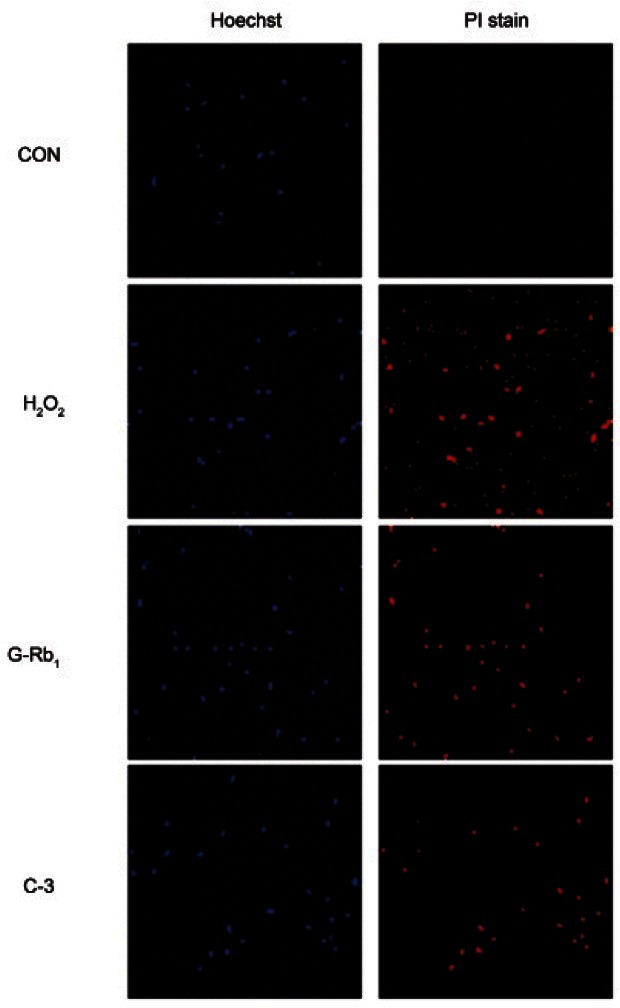
In the chondrocytes treated with H2O2, prominent positively stained cells were observed. In contrast, few positively stained cells were observed in the chondrocytes pretreated with G-Rb1 (100 μM) and C3 (50 μM) prior to treatment with H2O2 (500 μM). G-Rb1 was shown to reduce the H2O2-induced apoptosis of chondrocytes (Fig. 5). The untreated controls showed a similar appearance to cells treated with H2O2 plus G-Rb1 and C3.
Fig. 5. Anti-apoptotic effect of ginsenosides Rb1 (G-Rb1) in hydrogen peroxide (H2O2)-treated chondrocytes. Chondrocytes were treated with media alone (negative control), H2O2 (500 μM, positive control) and H2O2 (500 μM) after pretreatment with G-Rb1 (100 μM) and C3 (50 μM, caspase-3 inhibitor). The chondrocytes were labeled with the potential sensitive dye Hoechst and propidium iodide staining solution. Immunocytochemistry confirmed via fluorescence microscopy (×10). CON, negative control; PI, propidium iodine.
DISCUSSION
Ginsenosides are unique saponins that exist only in ginseng, and they have a variety of pharmacological effects, such as anticancer activities [10]. The results of the present study demonstrated that Rb1, one of the principal active ingredients in ginseng, can protect chondrocytes against H2O2-induced apoptotic insults. Thus, we demonstrated that in vitro treatment with G-Rb1 exerted protective effects against H2O2-induced cytotoxicity in rat articular chondrocytes (Fig. 1).
ROS are generated during metabolic processes and perform several biological functions. However, excessive oxidative stress causes cellular damage to DNA, lipids, and proteins [13,14]. H2O2 results in direct injury to the cartilage matrix by inhibiting proteoglycan synthesis in the chondrocytes. We analyzed the effects of G-Rb1 on mitochondrial stability and caspase activation. MPT caused by ROS is dependent on non-selective inner membrane permeabilization that may precede actual apoptotic cell death [14,15]. Our results demonstrated that G-Rb1 had strong MPT inhibition effects similar to C3, the caspase-3 inhibitor, in H2O2-treated chondrocytes (Fig. 2). Additionally, the expression of the antiapoptotic protein Bcl-xL was increased in H2O2-treated chondrocytes after G-Rb1 treatment (Fig. 3). Bcl-xL is a potent cell death inhibitor, and its expression prevents cell death. Conversely, the reduced level of the apoptotic protein Bax induced by G-Rb1 treatment is consistent with the inhibition of MPT. The ratio between the two subsets helps to determine, in part, the susceptibility of cells to a death signal. These proteins regulate the apoptotic process principally via the mitochondrial pathway, which is activated via the activation of major apoptotic executioner caspases resulting from the release of cytochome c from the mitochondria [8]. Therefore, the apoptotic cells observed in H2O2-treated chondrocytes (Fig. 5) most likely depend on variations in the balance between Bax and Bcl-xL. Some recent reports have demonstrated the ability of caspase inhibitors to attenuate chondrocyte apoptosis while maintaining cell functionality [11]. Although apoptosis can occur independently of caspase involvement in some cell types [16,17], all of the existing data indicate that caspase activation is a prerequisite for chondrocyte apoptosis. In particular, caspase-3 is a critical mediator of apoptosis in many cell types, including chondrocytes. Caspase-3 activity in cultured chondrocytes was increased after H2O2 exposure (Fig. 4). These findings clearly demonstrate that caspase-3 activation is a necessary step in the cascade of cellular events that leads to chondrocyte apoptosis after exposure to H2O2. Our results showed that H2O2-elicited caspase-3 activity was significantly suppressed by G-Rb1 (Fig. 4). This result is consistent with our observation that MPT inhibition and Bcl-xL are increased by G-Rb1 treatment.
In our assays, the cytoprotective effects of G-Rb1 were evaluated on H2O2-stimulated chondrocytes. By detecting mitochondrial stabilization and the inhibition of caspase-3 activity, we showed the protective function of G-Rb1 in articular chondrocyte death induced by H2O2. This result is consistent with recent studies indicating that Bcl-2 family members interfere with the activation of caspases [18], regulate the mitochondrial membrane potential, and inhibit the accumulation of cytochome c in the cytosol [19]. Bcl-xL has been reported to maintain cell viability after the activation of caspases [20]. In conclusion, the results of this study demonstrated the protective effect of G-Rb1 with anti-apoptotic effects in chondrocytes and suggested that G-Rb1 might prove useful as a novel preventive agent against H2O2-induced cytotoxicity.
References
- 1.Kim SK, Park JH. Trends in Ginseng Research in 2010. J Ginseng Res. 2011;35:389–398. doi: 10.5142/jgr.2011.35.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park HJ, Byeon HE, Choi KW, Rhee DK, Lee KR, Pyo SN. Inhibitory Effects of Ginsenoside Rb1 on Atopic Dermatitis-Like Skin Lesions in Mice. J Ginseng Res. 2010;34:363–368. doi: 10.5142/jgr.2010.34.4.363. [DOI] [Google Scholar]
- 3.Drissi H, Zuscik M, Rosier R, O’Keefe R. Transcriptional regulation of chondrocyte maturation: potential involvement of transcription factors in OA pathogenesis. Mol Aspects Med. 2005;26:169–179. doi: 10.1016/j.mam.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Roach HI, Tiley S. The pathogenesis of osteoarthritis. Springer; London: 2008. [Google Scholar]
- 5.Goldring MB. Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best Pract Res Clin Rheumatol. 2006;20:1003–1025. doi: 10.1016/j.berh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Schalkwijk J, van den Berg WB, van de Putte LB, Joosten LA. An experimental model for hydrogen peroxide-induced tissue damage. Effects of a single inflammatory mediator on (peri)articular tissues. Arthritis Rheum. 1986;29:532–538. doi: 10.1002/art.1780290411. [DOI] [PubMed] [Google Scholar]
- 7.Asada S, Fukuda K, Oh M, Hamanishi C, Tanaka S. Effect of hydrogen peroxide on the metabolism of articular chondrocytes. Inflamm Res. 1999;48:399–403. doi: 10.1007/s000110050478. [DOI] [PubMed] [Google Scholar]
- 8.Nuttall ME, Nadeau DP, Fisher PW, Wang F, Keller PM, DeWolf WE Jr, Goldring MB, Badger AM, Lee D, Levy MA, et al. Inhibition of caspase-3-like activity prevents apoptosis while retaining functionality of human chondrocytes in vitro. J Orthop Res. 2000;18:356–363. doi: 10.1002/jor.1100180306. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda K, Kumano F, Takayama M, Saito M, Otani K, Tanaka S. Zonal differences in nitric oxide synthesis by bovine chondrocytes exposed to interleukin-1. Inflamm Res. 1995;44:434–437. doi: 10.1007/BF01757700. [DOI] [PubMed] [Google Scholar]
- 10.Fujita K, Hakuba N, Hata R, Morizane I, Yoshida T, Shudou M, Sakanaka M, Gyo K. Ginsenoside Rb1 protects against damage to the spiral ganglion cells after cochlear ischemia. Neurosci Lett. 2007;415:113–117. doi: 10.1016/j.neulet.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Kim SW, Kwon HY, Chi DW, Shim JH, Park JD, Lee YH, Pyo S, Rhee DK. Reversal of P-glycoprotein-mediated multidrug resistance by ginsenoside Rg3. Biochem Pharmacol. 2003;65:75–82. doi: 10.1016/s0006-2952(02)01446-6. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Na JY, Song KB, Choi DS, Kim JH, Kwon YB, Kwon J. Protective Effect of Ginsenoside Rb1 on Hydrogen Peroxide-induced Oxidative Stress in Rat Articular Chondrocytes. J Ginseng Res. 2012;36:161–168. doi: 10.5142/jgr.2012.36.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lo MY, Kim HT. Chondrocyte apoptosis induced by hydrogen peroxide requires caspase activation but not mitochondrial pore transition. J Orthop Res. 2004;22:1120–1125. doi: 10.1016/j.orthres.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Asada S, Fukuda K, Nishisaka F, Matsukawa M, Hamanisi C. Hydrogen peroxide induces apoptosis of chondrocytes; involvement of calcium ion and extracellular signal-regulated protein kinase. Inflamm Res. 2001;50:19–23. doi: 10.1007/s000110050719. [DOI] [PubMed] [Google Scholar]
- 15.D’Lima DD, Hashimoto S, Chen PC, Colwell CW Jr, Lotz MK. Human chondrocyte apoptosis in response to mechanical injury. Osteoarthritis Cartilage. 2001;9:712–719. doi: 10.1053/joca.2001.0468. [DOI] [PubMed] [Google Scholar]
- 16.Chinnaiyan AM, Orth K, O’Rourke K, Duan H, Poirier GG, Dixit VM. Molecular ordering of the cell death pathway. Bcl-2 and Bcl-xL function upstream of the CED- 3-like apoptotic proteases. J Biol Chem. 1996;271:4573–4576. doi: 10.1074/jbc.271.9.4573. [DOI] [PubMed] [Google Scholar]
- 17.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 18.Lorenzo HK, Susin SA, Penninger J, Kroemer G. Apoptosis inducing factor (AIF): a phylogenetically old, caspase independent effector of cell death. Cell Death Differ. 1999;6:516–524. doi: 10.1038/sj.cdd.4400527. [DOI] [PubMed] [Google Scholar]
- 19.Slee EA, Adrain C, Martin SJ. Serial killers: ordering caspase activation events in apoptosis. Cell Death Differ. 1999;6:1067–1074. doi: 10.1038/sj.cdd.4400601. [DOI] [PubMed] [Google Scholar]
- 20.Boise LH. Thompson CB. Bcl-x(L) can inhibit apoptosis in cells that have undergone Fas-induced protease activation. Natl Acad Sci U S A. 1997;94:3759–3764. doi: 10.1073/pnas.94.8.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]


