Abstract
The current inquiry was conducted to assess the change in sleep architecture after long periods of administration to determine whether ginseng can be used in the therapy of sleeplessness. Following post-surgical recovery, red ginseng extract (RGE, 200 mg/ kg) was orally administrated to rats for 9 d. Data were gathered on the 1st, 5th, and 9th day, and an electroencephalogram was recorded 24 h after RGE administration. Polygraphic signs of unobstructed sleep-wake activities were simultaneously recorded with sleep-wake recording electrodes from 11:00 a.m. to 5:00 p.m. for 6 h. Rodents were generally tamed to freely moving polygraphic recording conditions. Although the 1st and 5th day of RGE treatment showed no effect on power densities in non-rapid eye movement (NREM) and rapid eye movement (REM) sleep, the 9th day of RGE administration showed augmented α-wave (8.0 to 13.0 Hz) power densities in NREM and REM sleep. RGE increased total sleep and NREM sleep. The total percentage of wakefulness was only decreased on the 9th day, and the number of sleep-wake cycles was reduced after the repeated administration of RGE. Thus, the repeated administration of RGE increased NREM sleep in rats. The α-wave activities in the cortical electroencephalograms were increased in sleep architecture by RGE. Moreover, the levels of both α- and β-subunits of the γ-aminobutyric acid (GABA)A receptor were reduced in the hypothalamus of the RGE-treated groups. The level of glutamic acid decarboxylase was over-expressed in the hypothalamus. These results demonstrate that RGE increases NREM sleep via GABAAergic systems.
Keywords: Panax ginseng, Electroencephalogram, Non-rapid eye movement, Power density, Rapid eye movement
INTRODUCTION
The modulation of sleep is a homeostatic course in which control mechanisms are revitalized to indemnify for deficiency and superabundant sleep [1]. In mammals, sleep is composed of two principal phases, rapid eye movement (REM) sleep and non-REM (NREM) sleep. REM sleep takes turns with cases of NREM sleep, and the voluntary NREM-REM sleep cycle takes sketchy 12 to 20 min in rats [2]. For the past forty years, mainly sleep research places emphasis on identifying referential brain structures, neuronal networks, and transmitters included in the genesis and modulation of NREM and REM sleep [3]. Some is known about the mechanisms and regulation of the rhythmed appearance of NREMREM sleep. Insomnia is a prevalent problem in late life. The reported prevalence of insomnia ranges between 30% and 60%. Pharmacological accesses have been performed in customary medical treatment for sleeplessness. Recently, some herbs have been charming medicines for considerable sufferers with sleep disabilities or insomnia [4,5].
Ginseng has been used as a conventional medicine during thousands of years. People take ginseng to expend vitality and keen the mind. Ginseng has a complicated activity that is occasionally difficult to symmetrize neurochemical reports. Ginseng tranquillizes and equalizes physiology and may help sustentation normal sleep and wakefulness [6]. Ginseng has been clinically used for the remedy of sleeplessness [7]. The greater part of the advantages of ginseng is immediately originated from ginsenosides, the major active components in ginseng root. Ginsenosides influence metabolism, incorporating exploding vigor, encouraging mental efficacy, and furthering larger stamina and patience, through protecting exhaustion and the debilitating efficiencies of old age. Ginsenosides affect as tonics to assist the human organism adapt to biological strain in a natural method. Together with its fitness-increasing effects, ginseng improves brain activity and favors psychological stability by exciting and staying the mind. Korean red ginseng extract (RGE) contains more ginsenosides than other ginseng, making it the most efficient of all ginsengs [8]. So, Korean RGE is an incomparable natural resource of nutrients for a fine body and mind.
However, sleep disabilities are the greater part generally experienced unfavourable efficiencies of ginseng [9]. To evaluate the role of ginseng in adjusting sleep–wake changes and sleep architecture and to conclude the conceivable mechanisms of NREM-REM sleep regulation, we indagated its impact on the amount of whole sleep and wakefulness, examined power density fluctuations in recorded electroencephalograms (EEG) of concrete sleep–wake stages, and discovered variations in γ-aminobutyric acid (GABA)Aergic receptors of the hypothalamus. Previously, we had been experimented that administering RGE 200 mg/kg for 5 d increased pentobarbital-induced sleep behaviors [10]. Therefore, the current investigation was conducted to assess the change in sleep architecture after long time of administration to determine whether RGE can be used in the remedy of insomnia.
MATERIALS AND METHODS
Ginseng and animals
Red ginseng water extract was kindly provided by Korea Ginseng Corporation (Seoul, Korea). Experiments were performed on 14 adult male SD rats (Samtako, Osan, Korea) weighing between 250 and 350 g. The rats were housed individually with food and water provided ad libitum under an artificial 12 h light/dark cycle (lights on at 7:00 a.m.) and at a constant temperature (22±2℃). The rats were housed in the departmental holding room for one week before testing. All of the rats were maintained in accordance with the National Institute of Toxicological Research and the Korea Food and Drug Administration guidelines for the care and use of laboratory animals.
Surgery
After a minimum 7 d acclimation period, each rat was anesthetized with pentobarbital (50 mg/kg, ip) and implanted with a transmitter (TL10M3-F50-EEE; Data Sciences International, St. Paul, MN, USA) for recording EEG and activity via telemetry as described previously [11]. The body of the transmitter was subcutaneously implanted off the midline and posterior to the scapula. It was attached to the skin with three sutures for stabilization. The transmitters led subcutaneously to the skull, and the bare ends were placed in contact with the dura through holes in the skull (A: 2.0 [Bregma], L: 1.5; P: 7.0 [Bregma], L: 1.5 contra-lateral). The electrodes were anchored to the skull with screws and dental cement. All of the surgical procedures were performed stereotaxically under aseptic conditions.
Red ginseng extract administration
After 7 d of recovery post-surgery, the animals were divided into two groups (control and 200 mg/kg RGE groups) with fourteen rats each. RGE was dissolved in distilled water and orally administrated at 11:00 a.m. once per day for 9 d at a dose of 200 mg/kg. At the end of RGE treatment, the animals were allowed to habituate to a polygraphic recording environment in which they could move freely. Then, polygraphic signs of sleep-wake activities were recorded for 6 h.
Data collection
Telemetric recording of cortical EEG and activity was conducted using procedures similar to previous reports [11]. For the EEG signal, the gain of transmitters was set at -0.5/+0.5 volts per/units ×2, and raw signals generated from the transmitter were in the range of 0.5 to 0.0 Hz. The signals were processed by a Data Sciences analog converter and routed to an analog-to-digital (AD) converter (Eagle PC30, Data Sciences International). The AD converter digitized the EEG and activity signals at 128 Hz. The digitized data were transferred to a computer and graphically displayed by the program. An on-line fast Fourier transformation (FFT) was performed on EEG data. The FFT analysis generated power density values from 0.0 to 20.0 Hz at a resolution of 0.5 Hz. The FFT data were further averaged in the range of 0 to 20 Hz at 10-s intervals. The sleep data and FFT results were saved to the hard disk every 10 s for additional off-line analysis. Movements of the animal in relation to the telemetry receiver generated transistor-transistor logic pulses that were collected and counted as a measure of activity. Data were gathered on the 1st, 5th and 9th days, and oral administration of RGE was performed 24 h before EEG recording. Data were recorded from 11:00 a.m. to 5:00 p.m. EEG and activity were recorded simultaneously.
Determination of sleep behaviors and analysis of electroencephalograms power
The amount of time spent in wakefulness, NREM sleep, and REM sleep was determined from digitized data in 10 s intervals using the animal sleep analysis software Sleep-Sign 2.1 (Kissei Comtec, Matsumoto, Japan). Briefly, the software identifies wakefulness as a high-frequency, low-amplitude EEG and NREM by the presence of spindles interspersed with slow waves. EEG power during REM shows significantly reduced lower frequency δ-waves (0.75 to 4.0 Hz) and increased θ-wave activity (5.0 to 9.0 Hz, peak at 7.5 Hz).
Data analysis
The time spent (min) in NREM, REM, and total sleep (NREM + REM) and the number of sleep-wake cycles were processed to obtain 6 h periods for each rat. We further calculated the time spent in each sleep-wake state (wake, NREM, and REM) in each recording. The absolute EEG power was calculated during wakefulness, NREM, and REM. Data were calculated in 0.5 Hz bins from 0.5 to 20 Hz for the entire 6 h recording. Subsequently, EEG power density was evaluated in three selected frequency bands for wakefulness, NREM, and REM (δ-wave, θ-wave, and α-wave [8.0 to 13.0 Hz]).
Western blotting of GABAAreceptors and glutamic acid decarboxylase
Under deep anesthesia (induced by diethyl ether), the animals were decapitated, and the brain was quickly removed and chilled in ice-cold saline. Coronal sections were made using a rodent brain matrix (ASI Instruments, Warren, MI, USA). The hypothalamus was dissected, and samples were immediately frozen on dry ice and stored at -80℃. Frozen tissue samples were homogenized in PRO-PREP protein-extraction solution (Intron Biotechnology Inc., Seongnam, Korea). The homogenate was centrifuged at 13,000 rpm at 4℃ for 20 min, and the supernatant was recovered. The concentration of protein in the supernatant was determined, and the supernatant was then used for Western blot analysis. The concentration of total protein was determined by the modified Lowry method using bovine serum albumin as a standard. The samples were stored at -20℃.
Forty micrograms of protein was added to each lane, and sodium dodecyl sulfate polyacrylamide gel electrophoresis was performed using 10% polyacrylamide gels. Proteins were transferred to polyvinylidene fluoride membranes (Hybond-P; GE Healthcare, Amersham, UK) using a wet transfer system. Immunoblots were incubated with one of the following primary antibodies: rabbit anti-GABAA α1 polyclonal antibody (diluted 1:2,000 in TBS containing 0.5% Tween20; Abcamplc, Cambridge, UK), rabbit anti-GABAA β1 polyclonal antibody (diluted 1:2,500 in TBS contain ing 0.5% Tween20), rabbit anti-GABAA γ3 polyclonal antibody (diluted 1:2,500 in TBS containing 0.5% Tween20) and rabbit anti-glutamic acid decarboxylase (GAD) polyclonal antibody (diluted 1:2,000 in TBS containing 0.5% Tween20). Blots were then washed and incubated with the horseradish peroxidase-conjugated sec ondary antibody: goat anti-rabbit IgG (diluted 1:3,000 in TBS containing 0.5% Tween20). Immunoreactive bands were developed with a BM chemiluminescence detection kit (Roche Diagnostics, Mannheim, Germany). The quantitative analysis of detected bands was performed with densitometric scanning, and all of the values were normalized to the amount of glyceraldehyde 3-phosphate dehydro genase (GADPH) in the sample, which was measured as follows. All of the immunoblots were stripped, incubated with sheep anti-GADPH (diluted 1:2,000 in TBS containing 0.5% Tween20, Abcamplc), subsequently incubated with rabbit anti-sheep IgG (diluted 1:2,000 in TBS containing 0.5% Tween20), and developed to confirm equal protein loading.
Statistical analysis
All statistical analyses were conducted using Sigma- Stat software (SPSS Inc., Chicago, IL, USA). Measures of analysis of variance (ANOVA) were used in the data analysis. After extensive ANOVA testing, post hoc comparisons of means were performed using Tukey’s t-tests. A p-value of less than 0.05 was considered to be significant. The values are expressed as the mean±SEM.
RESULTS
Effects of red ginseng extract on the number of sleep-wake cycles
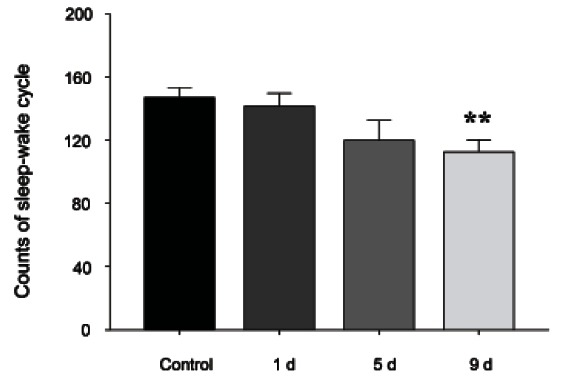
As shown in Fig. 1, on the 1st and 5th day of RGE treatment, no significant changes in sleep-wake cycles were exhibited during a 6 h recording. However, after administering RGE for 9 d, 200 mg/kg doses reduced sleep-wake cycles (p<0.01).
Fig. 1. The effects of 1, 5, and 9 d of red ginseng extract treatment on sleep-wake cycles. Values are expressed as the mean±SEM. **p<0.01 compared with the control.
Effects of red ginseng extract on sleep architecture
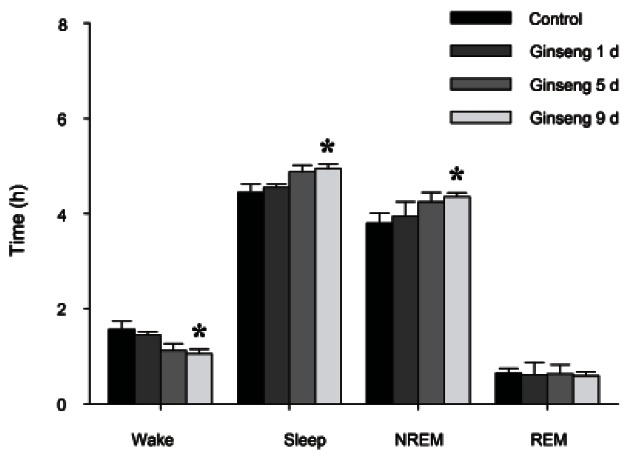
Although no significant changes in sleep architecture were shown on the 1st and 5th day of RGE treatment during a 6 h recording, treatment with RGE for 9 d significantly increased NREM and total sleep but decreased wakefulness (p<0.05) (Fig. 2).
Fig. 2. The effects of 1, 5 and 9 d of red ginseng extract treatment on rat sleep architecture. The data for 200 mg/kg doses are shown. Values are expressed as the mean±SEM. NREM, non-rapid eye movement; REM, rapid eye movement. *p<0.05 compared with the control.
Effects of 1 or 5 d of RGE administration on EEG power density during NREM sleep, REM sleep, and total sleep time
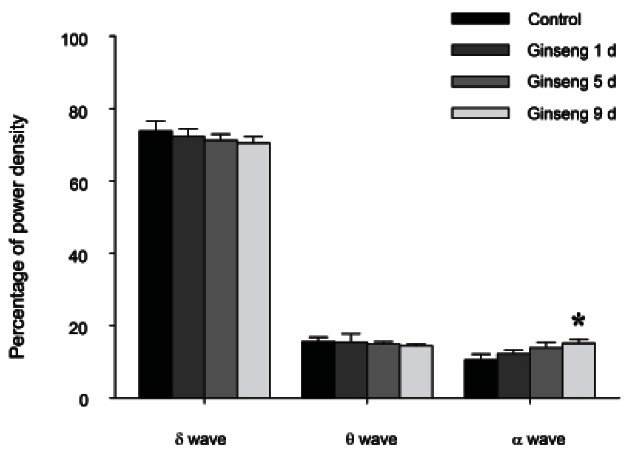
On the 1st and 5th day of treatment with RGE, there were no changes in δ-wave, θ-wave, or α-wave power density during NREM and REM sleep (Fig. 3).
Fig. 3. The effects of 1, 5, and 9 d of red ginseng extract treatment on electroencephalograms (EEG) power density during nonrapid eye movement (NREM) sleep. EEG power densities in δ-wave, θ-wave and α-wave spectral bandwidths were evaluated. The values are expressed as the mean±SEM of EEG power densities in three selected frequency bands for NREM sleep. *p<0.05 compared with the control.
Effects of 9 d of RGE treatment on EEG power density during NREM sleep, REM sleep, and total sleep time
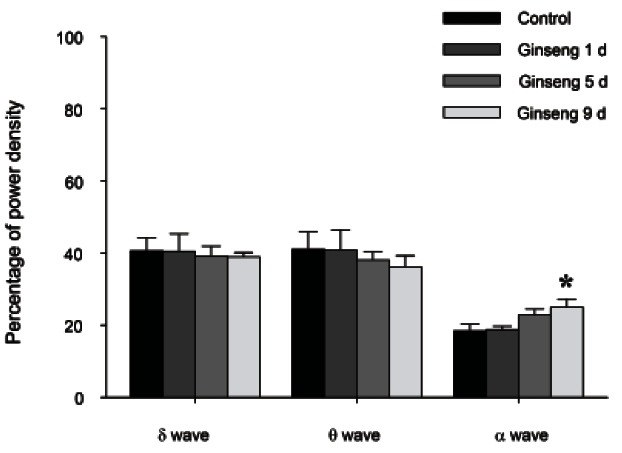
RGE treatment (200 mg/kg) for 9 d showed no significant changes in δ-wave or θ-wave power density during NREM and REM sleep, whereas α-wave power density was increased during NREM sleep and REM sleep (p<0.05) (Fig. 4).
Fig. 4. The effects of 1, 5, and 9 d of red ginseng extract treatment on electroencephalograms (EEG) power density during rapid eye movement (REM) sleep. EEG power densities in δ-wave, θ-wave and α-wave spectral bandwidths were evaluated. The values are expressed as the mean±SEM of EEG power densities in three selected frequency bands for the REM sleep. *p<0.05 compared with the control.
Effects of RGE administration on GABAAreceptors and GAD protein expression in the hypothalamus
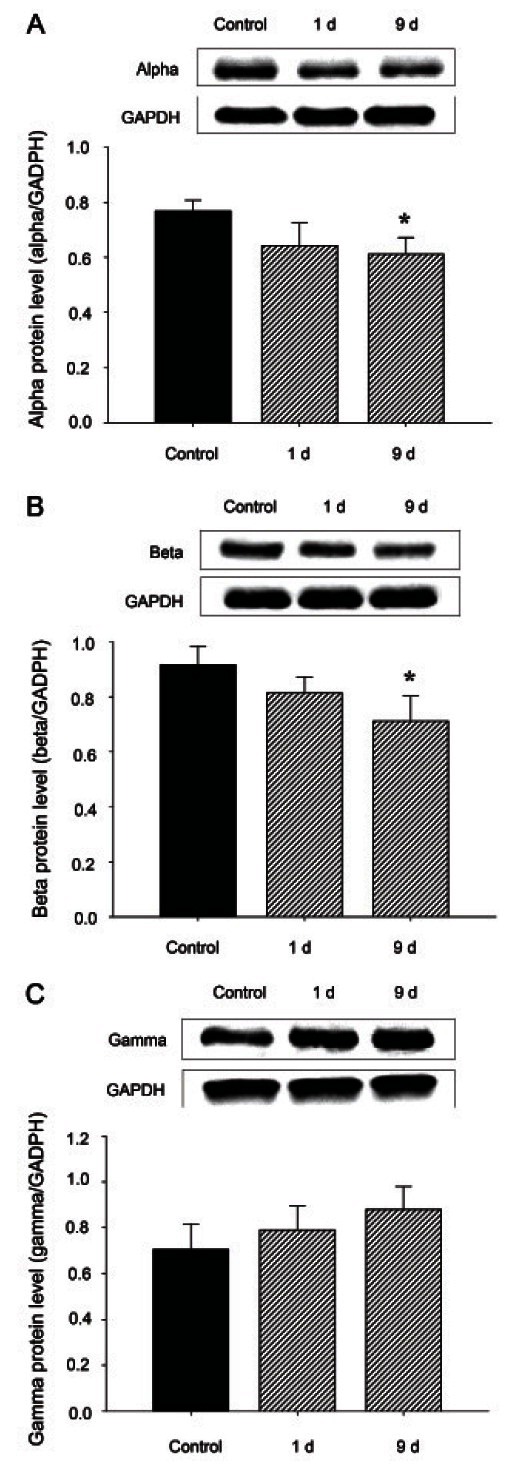
We examined the protein levels of GAD and the GABAAreceptor α-, β- and γ-subunits in rats. As indicated in Figs. 5 and 6, 9 d of treat ment with 200 mg/kg RGE reduced the expres sion of GABAA α- and β-subunits (Fig. 6A, B) but not γ-subunits (Fig. 6C) in the rat hypothalamus compared with controls. RGE treatment with 200 mg/kg for 9 d significantly augmented the protein levels of GAD 65/67 (Fig. 5).
Fig. 5. The expression of glutamic acid decarboxylase (GAD) protein in the rat hypothalamus after 1, 5, and 9 d of red ginseng extract (RGE) treatment. Hypothalamus membrane extracts from rats treated as controls or with RGE were analyzed by Western blotting. A representative image is shown in each case. The intensity of the immunoreactive bands of 3 to 4 independent experiments was measured by densitometry scanning and normalized using glyceraldehyde 3-phosphate dehydrogenase (GADPH) as a standard (bar graph). The results are presented as the percentage immunoreactivity detected in the hypothalamus with respect to the GADPH proteinloading control. Values are expressed as the mean±SEM. **p<0.01 compared with the control.
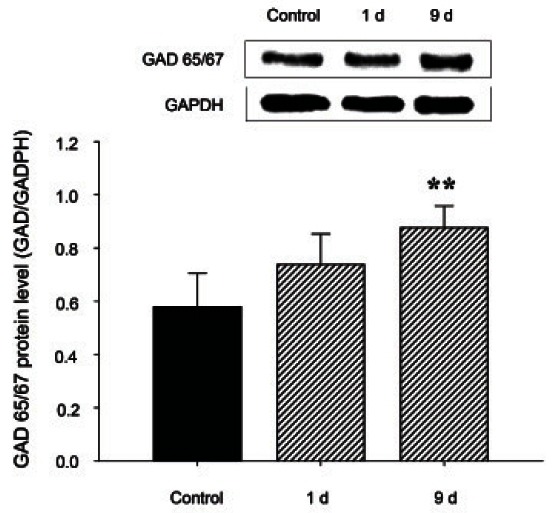
Fig. 6. The expression of γ-aminobutyric acid (GABA)A receptor alpha (A), beta (B), and gamma (C) subunits in the rat hypothalamus after 1, 5, and 9 d of red ginseng extract (RGE) treatment. GABAA receptor subunits in the hypothalamus of RGE-treated rats after 1, 5, and 9 d of treatment were analyzed by Westernblotting. The intensity of the immunoreactive bands of 3 to 4 independent experiments was measured by densitometry scanning and normalized using glyceraldehyde 3-phosphate dehydrogenase (GADPH) as a standard (bar graph). The results are presented as the percentage immunoreactivity detected in the hypothalamus with respect to the GADPH proteinloading control. Values are expressed as the mean±SEM. *p<0.05 compared with the control.
DISCUSSION
Our inquiry implied that RGE increased total and NREM sleep in rodent and declined wakefulness. In the current study, we indicated that after 5 or 9 d of continuous RGE treatment, only 9 d of treatment preeminently augmented NREM and total sleep and diminished wakefulness. These data indicate that RGE modulates sleep architecture, notably NREM sleep. Analogously, RGE regulated sleep-wake cycles during long periods of administration. Furthermore, on the 1st and 5th days, the oral administration of RGE failed to alter the number of sleep-wake cycles for a 12 h recording period. On the other hand, RGE treatment for 9 d reduced the sleepwake cycle. The above findings are somewhat coincide with a past report [12], where the amount of wakefulness and slow-wave sleep (SWS) for a 12 h light period was reduced and enhanced, respectively, by RGE administration, while REM sleep was scarcely influenced.
In addition, even though RGE administration had alike effects on total sleep time and sleep efficacy in our investigations, it had dissimilar effects on sleep architecture. The dissimilarity among these inquiries may be due to differences in administration plans. In addition, a previous report by Kitaoka et al. [13] indicated that dissimilar species of ginseng normally have different ginsenoside constituent. Supplementarily the ginsenoside constituent of ginseng can vary according to the species, the age and section of the plant, the conservation method, the period of harvest, and the extraction process [14,15]. The total amount and the frequency of sleep rely on the activity figures of cortical EEG waves [12]. Standardized ginseng extract G115 has been indicated to create a desynchronizing effect on an EEG [16]. Using a double-blind, placebo-controlled, balanced crossover test in 15 lusty volunteers, Kennedy et al. [17] described that ginseng resulted in considerable declines in frontal ‘eyes closed’ theta and beta activity, with an extra decline in the alpha wave bandwidth, which illustrated that Panax ginseng can immediately regulate cerebroelectrical activity. Several innocent saponins segregated from P. ginseng Meyer likewise had regular delirifacients on EEG and the action and EEG stimulus reaction induced by electrical irritation in the midbrain in cats [18]. Few papers have examined the role of RGE in EEG power density in mammals. Hence, we analyzed the influence of RGE on brain waves in rats. Even though 1 and 5 d of RGE treatment did not affect any wave power density, 9 d of RGE treatment enhanced alpha activity during NREM sleep and REM sleep. The amount of EEG analysis allowed the extraction of significant functional parameters, like as slow-wave activity (or delta activity) for NREM sleep, which includes constituents of the EEG signal in the frequency range of 0.5 to 4.5 Hz [19]. REM sleep and waking are dominated in animals (i.e., rodents) by a noticeable activity in the theta (6 to 9 Hz) frequency range, although the functional importance of slow-wave and REM sleep modulation is not well-known. Some studies in humans indicated that the EEG activity of the α-wave activity (frequency range of 8 to 13 Hz) might be a indicator of REM sleep homeostasis [20]. Nevertheless, it is indicated that α-wave activity may not be related to the REM sleep homeostasis of rats [21].
GABAA receptors function as sleep modulation. Some compounds interfering with the sleep-wake cycle, containing barbiturates and benzodiazepines, are agonists acting at various combining sites on the GABAA receptors [22]. The mechanism proposed suggests that GABAergic neurons from the basal forebrain and preoptic area project to the posterior lateral hypothalamus [23]. Moreover, evidence gained using microdialysis detection ways indicates that extracellular levels of GABA are increased for sleep [24]. Pharmacological tests have proved that enhancing GABAA receptor activity improves SWS [25].
Previously, Sugiyama et al. [26] proved that ginseng radix activated intracelluar chloride channel mediated by the GABAA receptor. More inquiry has verified the opinion that some of the behavioral efficiencies of ginseng at least take place through the variation of the GABAA receptor. For instance, social isolation commonly lead to a stress-evoked reduction in the duration of sleep induced by pen tobarbital. The capability of majonoside-R2 (a principal ocotillol-type saponin content of Vietnamese ginseng) to reverse this effect is mediated by the neurosteroid sites on the GABAA receptors in mice [27]. Besides, Kimura et al. [28] reported that ginsenosides interact through ligand combining to the GABAA and GABAB receptors. However, Lee et al. [29] indicated that not all behavioral actions of ginseng saponins are caused by the modulation of GABAA receptor activation. To indagate whether the efficacy of RGE administration on sleep behavior related the GABAAergic system, we indagated the protein levels of GAD 65/67 and GABAA receptor α-, β-, and γ-subunits in the rat hypothalamus. Our observations indicated that 9 days of repeated RGE treatment decreased the expression level of GABAA α- and β- but not γ-subunits in the rat hypothalamus; Although, in the 1 d administration groups, the expression of GABAA α-, β- and γ-subunits and GAD 65/67 was not affected in rats treated with 200 mg/kg RGE. These results presumed that the modulation of sleep behavior by a long period of RGE treatment involves a decrease in the expression level of GABAA receptor α- and β- but not γ-subunits. Increased GABA production may be significant for a long time RGE treatment. Commonly, our data demonstrate that RGE modulates sleep via the GABAAergic systems [13,26].
In 1979, ginseng abuse syndrome (GAS) was coined as the result of an inquiry of people who had been using a variety of ginseng preparations for at least 1 mo. It was reported that administration of ginseng for more than 1 mo induced GAS. Some patients underwent GAS, defined as hypertension, erethism, somnipathy, pimple and diarrhea. However, one subject underwent hypotension, feebleness and quiver when ginseng use was suddenly stopped. Ginseng was used to treat “hot syndromes”, such as stress, insomnia, palpitations, and headache. Recently, it was reported that ginseng induces sleep in mammals [4]. Ginseng would also be a good candidate for the treatment of insomnia.
In summary, RGE enhances total sleep and NREM sleep and reduces the number of sleep-wake cycles. The α-wave activities in cortical EEG were increased in sleep architecture by RGE. The current inquiry illustrated that RGE regulated sleep-wake cycles and sleep architecture in a time-dependent manner. In ad dition, the regulation of sleep behavior by RGE administration for 9 d may relate a decline in the expression level of GABAA receptors composed mainly of α- and β- but not γ-subunits. As a result, longer RGE administration may modulate sleep behavior by increasing GABA production.
Therefore, RGE administration widely modulated the power spectral densities of EEGs, even though no dose-dependent tendencies were determined. Furthermore, our data allude that the regulation of these sleep behaviors by RGE relates the GABAAergic system. These results are assumed that the mechanism through which RGE modulates EEG and sleep behavior may be far more muddled than previous perspectives have implied. However, whether RGE influences sleep behavior by modulating EEG power densities remains to be further analyzed.
Acknowledgments
This work was supported by the grant from the Korean Society of Ginseng funded by Korean Ginseng Corporation (2010).
References
- 1.Datta S, Maclean RR. Neurobiological mechanisms for the regulation of mammalian sleep-wake behavior: reinterpretation of historical evidence and inclusion of contemporary cellular and molecular evidence. Neurosci Biobehav Rev. 2007;31:775–824. doi: 10.1016/j.neubiorev.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Datta S, Hobson JA. The rat as an experimental model for sleep neurophysiology. Behav Neurosci. 2000;114:1239–1244. doi: 10.1037//0735-7044.114.6.1239. [DOI] [PubMed] [Google Scholar]
- 3.Blanco-Centurion C, Xu M, Murillo-Rodriguez E, Gerashchenko D, Shiromani AM, Salin-Pascual RJ, Hof PR, Shiromani PJ. Adenosine and sleep homeostasis in the basal forebrain. J Neurosci. 2006;26:8092–8100. doi: 10.1523/JNEUROSCI.2181-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim CS, Han JY, Kim SH, Hong JT, Oh KW. Herbs for the treatment of insomnia. Biomol Ther. 2011;19:274–281. [Google Scholar]
- 5.Kim CS, Jo YJ, Park SH, Kim HJ, Han JY, Hong JT, Cheong JH, Oh KW. Anti-stress effects of ginsenoside Rg3-standardized ginseng extract in restraint stressed animals. Biomol Ther. 2010;18:219–225. [Google Scholar]
- 6.Lee SP, Honda K, Rhee YH, Inoue S. Chronic intake of Panax ginseng extract stabilizes sleep and wakefulness in food-deprived rats. Neurosci Lett. 1990;111:217–221. doi: 10.1016/0304-3940(90)90371-f. [DOI] [PubMed] [Google Scholar]
- 7.Xiang YZ, Shang HC, Gao XM, Zhang BL. A comparison of the ancient use of ginseng in traditional Chinese medicine with modern pharmacological experiments and clinical trials. Phytother Res. 2008;22:851–858. doi: 10.1002/ptr.2384. [DOI] [PubMed] [Google Scholar]
- 8.Kim SK, Park JH. Trends in ginseng research in 2010. J Ginseng Res. 2011;35:389–398. doi: 10.5142/jgr.2011.35.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi HK, Seong DH, Rha KH. Clinical efficacy of Korean red ginseng for erectile dysfunction. Int J Impot Res. 1995;7:181–186. [PubMed] [Google Scholar]
- 10.Yang SL, Nam SY, Han JY, Kim JC, Lee K, Jin TH, Oh KW, Eun JS. Alterations of spontaneous sleep architecture and cortical electroencephalogram power spectra by red ginseng extract via GABAAergic systems. J Ginseng Res. 2010;34:304–313. [Google Scholar]
- 11.Sanford LD, Yang L, Liu X, Tang X. Effects of tetrodotoxin (TTX) inactivation of the central nucleus of the amygdala (CNA) on dark period sleep and activity. Brain Res. 2006;1084:80–88. doi: 10.1016/j.brainres.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Rhee YH, Lee SP, Honda K, Inoue S. Panax ginseng extract modulates sleep in unrestrained rats. Psychopharmacology (Berl) 1990;101:486–488. doi: 10.1007/BF02244226. [DOI] [PubMed] [Google Scholar]
- 13.Kitaoka K, Uchida K, Okamoto N, Chikahisa S, Miyazaki T, Takeda E, Sei H. Fermented ginseng improves the first-night effect in humans. Sleep. 2009;32:413–421. doi: 10.1093/sleep/32.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee DY, Cho JG, Lee MK, Lee JW, Lee YH, Yang DC, Baek NI. Discrimination of Panax ginseng roots cultivated in different areas in Korea using HPLC-ELSD and principal component analysis. J Ginseng Res. 2011;35:31–38. doi: 10.5142/jgr.2011.35.1.031. [DOI] [Google Scholar]
- 15.Kim MH, Lee YC, Choi SY, Cho CW, Rho J, Lee KW. The changes of ginsenoside patterns in red ginseng processed by organic acid impregnation pretreatment. J Ginseng Res. 2011;35:497–503. doi: 10.5142/jgr.2011.35.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samira MM, Attia MA, Allam M, Elwan O. Effect of the standardized ginseng extract G115 on the metabolism and electrical activity of the rabbit’s brain. J Int Med Res. 1985;13:342–348. doi: 10.1177/030006058501300608. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy DO, Scholey AB, Drewery L, Marsh VR, Moore B, Ashton H. Electroencephalograph effects of single doses of Ginkgo biloba and Panax ginseng in healthy young volunteers. Pharmacol Biochem Behav. 2003;75:701–709. doi: 10.1016/s0091-3057(03)00120-5. [DOI] [PubMed] [Google Scholar]
- 18.Kaku T, Miyata T, Uruno T, Sako I, Kinoshita A. Chemico-pharmacological studies on saponins of Panax ginseng C. A. Meyer. II. Pharmacological part. Arzneimittelforschung. 1975;25:539–547. [PubMed] [Google Scholar]
- 19.Winsky-Sommerer R. Role of GABAA receptors in the physiology and pharmacology of sleep. Eur J Neurosci. 2009;29:1779–1794. doi: 10.1111/j.1460-9568.2009.06716.x. [DOI] [PubMed] [Google Scholar]
- 20.Roth C, Achermann P, Borbely AA. Alpha activity in the human REM sleep EEG: topography and effect of REM sleep deprivation. Clin Neurophysiol. 1999;110:632–635. doi: 10.1016/s1388-2457(98)00060-1. [DOI] [PubMed] [Google Scholar]
- 21.Bjorvatn B, Fagerland S, Ursin R. EEG power densities (0.5-20 Hz) in different sleep-wake stages in rats. Physiol Behav. 1998;63:413–417. doi: 10.1016/s0031-9384(97)00460-5. [DOI] [PubMed] [Google Scholar]
- 22.Sieghart W. Structure and pharmacology of gammaaminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 23.Henny P, Jones BE. Vesicular glutamate (VGlut), GABA (VGAT), and acetylcholine (VACht) transporters in basal forebrain axon terminals innervating the lateral hypothalamus. J Comp Neurol. 2006;496:453–467. doi: 10.1002/cne.20928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nitz D, Siegel JM. GABA release in posterior hypothalamus across sleep-wake cycle. Am J Physiol. 1996;271(6 Pt 2):R1707–R1712. doi: 10.1152/ajpregu.1996.271.6.R1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottesmann C. GABA mechanisms and sleep. Neuroscience. 2002;111:231–239. doi: 10.1016/s0306-4522(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 26.Sugiyama K, Muteki T, Kano T. The Japanese herbal medicine ‘saiko-keishi-to’ activates GABAA receptors of rat sensory neurons in culture. Neurosci Lett. 1996;216:147–150. doi: 10.1016/0304-3940(96)13000-7. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen TT, Matsumoto K, Yamasaki K, Watanabe H. Majonoside-R2 reverses social isolation stress-induced decrease in pentobarbital sleep in mice: possible involvement of neuroactive steroids. Life Sci. 1997;61:395–402. doi: 10.1016/s0024-3205(97)00396-2. [DOI] [PubMed] [Google Scholar]
- 28.Kimura T, Saunders PA, Kim HS, Rheu HM, Oh KW, Ho IK. Interactions of ginsenosides with ligand-bindings of GABAA and GABAB receptors. Gen Pharmacol. 1994;25:193–199. doi: 10.1016/0306-3623(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 29.Lee SH, Yang SC, Park JK, Jung MW, Lee CJ. Reduction of electrically evoked neural activity by ginseng saponin in rat hippocampal slices. Biol Pharm Bull. 2000;23:411–414. doi: 10.1248/bpb.23.411. [DOI] [PubMed] [Google Scholar]


