Abstract
Korean red ginseng has been shown to possess a variety of biological activities. However, little is known about antiviral activity of ginsenosides of Korean red ginseng. Here, we investigated the protective effect by oral administration of various ginsenosides on the lethal infection of haemagglutinating virus of Japan (HVJ) in mice. In a lethal infection model in which almost all mice infected with HVJ died within 15 days, the mice were administered orally (per os) with 1 mg/mouse of dammarane-type (ginsenoside-Rb1, -Rb2, -Rd, -Re, and -Rg2) or oleanolic acid-type (ginsenoside-Ro) ginsenosides 3, 2, and 1 d before virus infection. Ginsenoside-Rb2 showed the highest protective activity, although other dammarane-type and oleanolic acid-type ginsenosides also induced a significant protection against HVJ. However, neither the consecutive administration with a lower dosage (300 μg/mouse) nor the single administration of ginsenoside-Rb2 (1 mg/mouse) was active. In comparison of the protective activity between ginsenoside-Rb2 and its two hydrolytic products [20(S)- and 20(R)-ginsenoside-Rg3], 20(S)-ginsenoside-Rg3, but not 20(R)-ginsenoside-Rg3, elicited a partial protection against HVJ. The protective effect of ginsenoside-Rb2 and 20(S)-ginsenoside-Rg3 on HVJ infection was confirmed by the reduction of virus titers in the lungs of HVJ-infected mice. These results suggest that ginsenoside-Rb2 is the most effective among ginsenosides from red ginseng to prevent the lethal infection of HVJ, so that this ginsenoside is a promising candidate as a mucosal immunoadjuvant to enhance antiviral activity.
Keywords: Panax ginseng, Ginsenoside-Rb2, Red ginseng, Haemagglutinating virus of Japan, Mucosal adjuvant
INTRODUCTION
Saponins are heterogeneous fractions of natural products that are chemically belonged to glycosides with a triterpenoid, steroidal aglycone or sapogenin. During decades, a great number of biological activities of these saponins have been investigated and their immuno-regulatory effects and adjuvant activities have been reported [1,2]. Furthermore, oral administration of saponins was found to enhance immune response to antigens both parenterally and non-parenterally [3,4], although the precise mechanism of enhancement of mucosal immunity by saponins is not understood.
Ginsenosides derived from Panax ginseng involve two groups of saponins having a distinct structure: 20(S)-protopanaxadiol/triol skeleton (dammarane-type) and oleanolic acid skeleton (oleanolic acid type). The relationship with the structure and biological functions between the two ginsenosides is unclear. Many investigators have demonstrated that ginsenosides have a variety of biological activities including immunomodulatory [5,6] and antitumor [7-9] effect. However, to the best of our knowledge, there is no report on the effect of ginsenosides on the lethal infection by pathogenic viruses in in vivo models. Recently, it was demonstrated that Korean red ginseng and its ginsenosides have beneficial effects on prevention of influenza A (H1N1) virus [10], H1N2 and H3N2 influenza viruses in mice [11], and murine norovirus and feline calicivirus in vitro [12].
Ginsenoside-Rb2, extracted from the root of P. ginseng Meyer, is a dammarane-type saponin which does not induce hemolysis, and possesses diverse biological activities such as regulation of lymphocyte proliferation [13], anti-diabetic, and anti-hyperlipidemic effect [14]. Previously we reported that ginsenoside-Rb2 had antitumor activity to inhibit tumor metastasis and tumor-induced angiogenesis in mice [15].
Haemagglutinating virus of Japan (HVJ) which belongs to the parainfluenza viruses (type 1) and is called Sendai virus, is known to cause a lethal acute respiratory infection in mice, giving rise to complications characteristic of mild rhinitis, moderate tracheitis and severe bronchopneumonia [16]. Immunological studies showed that target tissue of the virus is confined to the epithelial mucosa of the upper respiratory tract, tracheobrochi and bronchioles [17]. In the previous study, we demonstrated that MDP-Lys(L18), a stearoyl derivative of muramyl dipeptide, administered through mucosal routes, i.e. oral and intrarectal administration, augmented non-specific resistance against HVJ, and significantly suppressed the lethal infection of this virus in mice [16].
Considering that ginsenosides have immunomodulatory activity [18] and oral administration of ginsenosides can augment immune responses [19], it is possible that oral administration of ginsenoside-Rb2 enhances the host immunity to protect against the lethal infection of HVJ. In the present study, to examine whether oral administration of ginsenoside-Rb2 is able to enhance host resistance against virus infection, we investigated its activity to protect against the lethal infection of HVJ using an experimental model in mice. And we partially compared the protective activity between ginsenoside-Rb2 and other ginsenosides from Korean red ginseng.
MATERIALS AND METHODS
Reagents
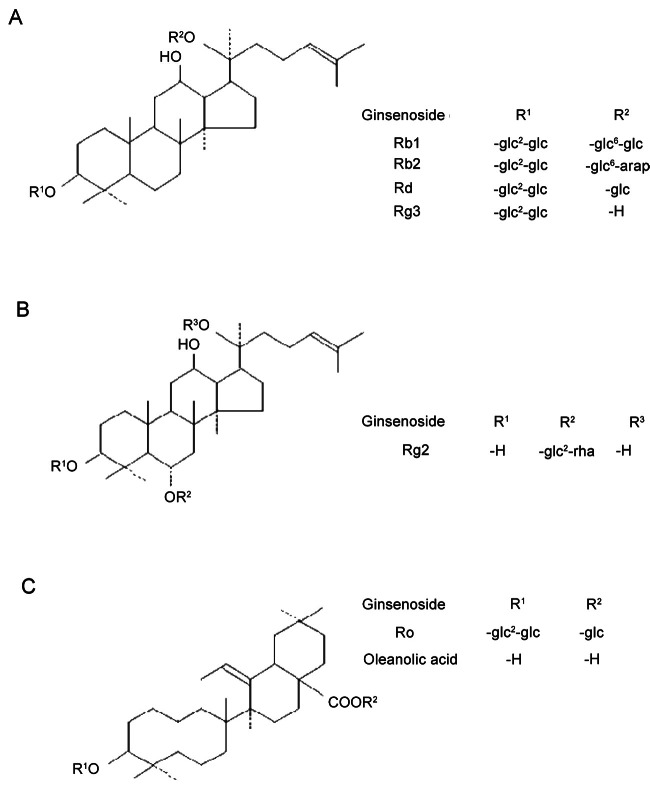
Authentic ginsenosides used in this study were kindly provided by the Korea Ginseng Corporation (Daejeon, Korea). All ginsenosides were obtained from the roots of 6-year old P. ginseng Meyer in Korea. The purity of these ginsenosides was above 99.9% as estimated by high performance liquid chromatography [20]. The chemical structures of ginsenosides used in this study are shown in Fig. 1. An appropriate amount of each ginsenoside was suspended in phosphate-buffered saline (PBS) before use.
Fig. 1. The chemical structures of dammarane-type and oleanolic acid-type ginsenosides. (A) Dammarane type ginsenosides: 20(S)-protopanaxadiol. (B) Dammarane type ginsenosides: 20(S)-protopanaxatriol. (C) Oleanolic acid-type ginsenosides.
Animals
Specific pathogen-free BALB/c mice were purchased from SLC (Hamamatsu, Japan) and used at the age of 7 to 8 wk. The mice were housed in plastic cages in vinylfilm isolators. Water and pelleted diets were supplied ad libitum. The mice were treated according to the Laboratory Animal Control Guidelines of Institutional Animal Care and Use Committee of Konyang University.
Virus and cells
HVJ was purchased from Flow Laboratories (Rockville, MD, USA). After 13 passages of this virus in C3H/He mice, the lungs were homogenized in PBS to make a 20% suspension as described previously [16]. The supernatant was dispersed in ampoules of 0.5 mL amounts, and stored as seed virus suspension at -80℃. The titer of this seed virus was determined by the hemadsorption (HAD) test as described (1.7×106 HAD/mL). The 50% lethal dose (LD50) of the seed virus in BALB/c mice was calculated as 1.3×104 HAD/LD50/0.03 mL by intranasal (i.n.) inoculating the virus into the mice. The LLCMK-2 cell line, derived from a rhesus monkey kidney cell, was cultivated in Eagle’s minimum essential medium (EMEM) supplemented with 3 mM glutamine, 0.07% sodium bicarbonate and 5% fetal calf serum.
Protection against haemagglutinating virus of Japan infection
For each experiment, ampoules of seed virus were thawed and 2 LD50 (2.6×104 HAD/0.03 mL/mouse) of the virus suspension was inoculated i.n. under anaesthesia. In this model, almost all mice infected with HVJ died within 15 d after infection. Ginsenosides was administered per os (p.o.) various days before HVJ infection. The survival rates were monitored for up to 20 d after infection.
Isolation of haemagglutinating virus of Japan from the lungs
Groups of three BALB/c mice were inoculated i.n. with HVJ (2.6×104 HAD/mouse). The lungs and tracheae removed from the mice 5 d after infection, were added to 0.5 mL of RPMI-1640 medium with 5% fetal bovine serum and homogenized as described [16]. After centrifugation at 650 g (2000 revs min-1) for 10 min, the supernatants were massed up to 1 mL with RPMI-1640 and stored at -80℃. The virus titer of each homogenate was determined by plaque forming units (PFU) on the monolayer of LLCMK-2 cells. The monolayer of LLCMK-2 cells on 24-well spot slides was infected with 1,000-fold diluted each homogenate (20 μL/well) for 1 h. After wash, the cells were incubated for 1 d under an overlay medium containing 1.5% carboxymethyl cellulose sodium salt and EMEM. Each well was reacted with 200-fold mouse immune serum to HVJ which was prepared in our laboratory, and then 500-fold fluorescein isothiocynate-conjugated goat anti-mouse IgG+A+M (H+L) (Zymed, CA, USA) for 1 h. The number of foci of each well was counted under a fluorescent microscope.
Statistical analysis
Statistical significance was determined by Mann-Whiteny U-probability test or Student’s two-tailed t-test.
RESULTS
Effect of various ginsenosides on haemagglutinating virus of Japan infection
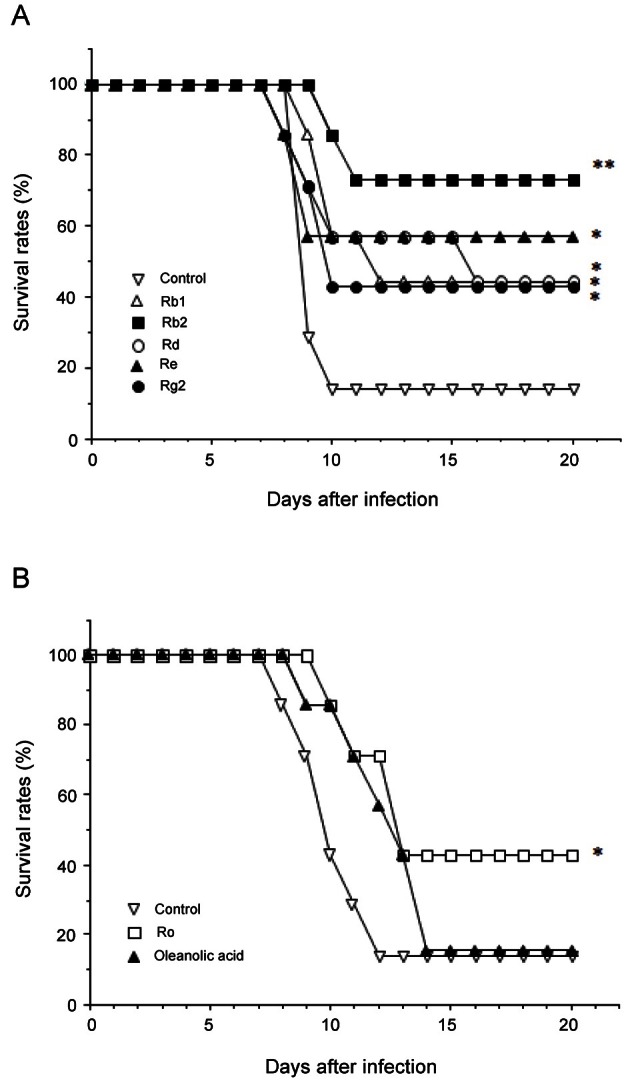
To examine the protective effect of oral administration of various types of ginsenosides on HVJ infection, we investigated their activity to prevent the lethal infection of this virus that causes a severe pneumonitis in mice. Five dammarane-type (ginsenoside-Rb1, -Rb2, -Rd, -Re, and -Rg2) and one oleanolic acid-type (ginsenoside-Ro) saponin fractions (1 mg/mouse) were administered orally into the mice 3, 2, and 1 d before the lethal infection of HVJ, and the survival rates of ginsenoside-treated mice were compared with that of HVJ-infected control mice.
As shown in Fig. 2A, the mice treated with all dammarane-type ginsenosides protected against HVJ, showing a higher survival rates ranging from 41.8% to 71.4% compared with the control (14.3%). Similarly, oleanolic acid-type saponin ginsenoside-Ro, but not oleanolic acid which is its aglycone, was also active, even though its survival rate was relatively low (42.8%) (Fig. 2B). Of particular significance was the finding that ginsenoside-Rb2 showed the highest survival rate (71.4%) among dammarane-type as well as oleanolic acid-type ginsenosides.
Fig. 2. Protective effect of oral administration of various types of ginsenosides on haemagglutinating virus of Japan (HVJ) infection. Seven BALB/c mice per group were administered per os with dammarane-type (A) or oleanolic acid-type (B) ginsenosides (1 mg) 3, 2 and 1 d before intranasal inoculation of HVJ (2.6×104 hemadsorption/mouse). The survival rates were monitored for up to 20 d after infection. *p<0.05, **p<0.01, compared with untreated control (infection only) by Mann-Whitney U-test.
Protective effect ginsenoside-Rb2 on haemagglutinating virus of Japan infection
Since the consecutive administration of ginsenoside-Rb2 was the most effective to prevent HVJ infection, we next examined the relationship between its protective effect and the number of times being administered. Single administration of ginsenoside-Rb2 (1 mg) 3, 2, or 1 d before HVJ infection resulted in a lower survival rate (0% to 28.5%) than that of the multiple administration for 3 d before infection (71.4%) (Fig. 3A).
Fig. 3. Administration frequency and dosage of ginsenoside-Rb2 to induce the effective protection against haemagglutinating virus of Japan (HVJ). In analysis for the number of times being administered, the mice were administered per os with 1 mg of ginsenoside-Rb2 on the indicated days before HVJ infection (A). Optimal dosage of ginsenoside-Rb2 to induce its protective effect on HVJ infection was examined in an experiment in which the mice were administered p.o. with 300 μg or 1 mg of ginsenoside-Rb2 3, 2, and 1 d before virus infection (B). The survival rates were monitored for up to 20 d after infection. *p<0.05, **p<0.01, compared with untreated control (infection only) by Mann-Whitney U-test.
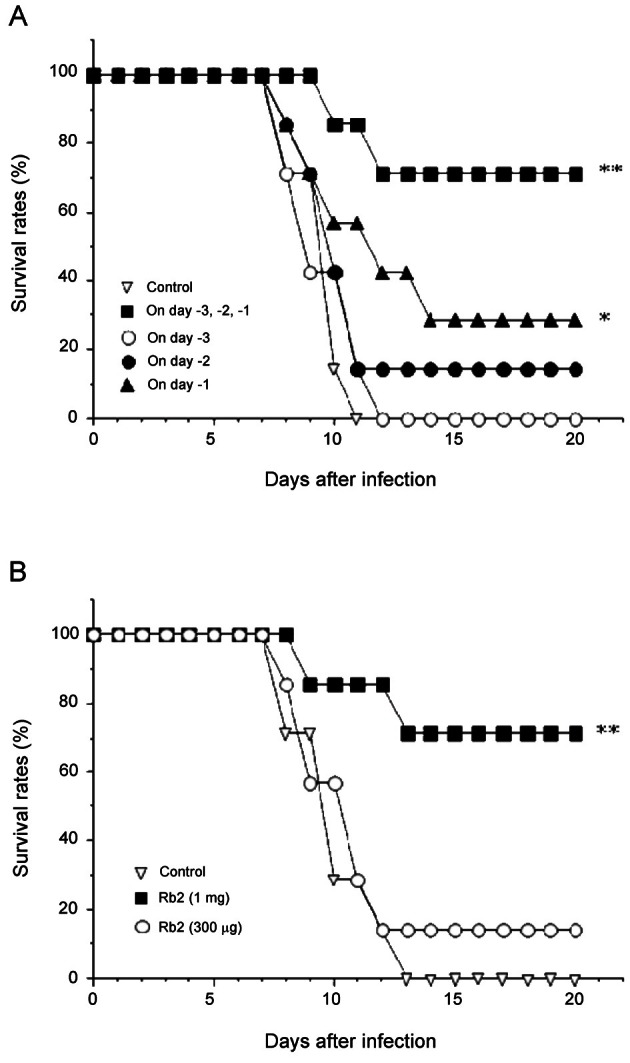
According to the results from Fig. 2A and Fig. 3A that treatment with ginsenoside-Rb2 (1 mg) 3, 2, and 1 d before infection manifested the most prophylactic effect on HVJ infection, we examined whether a relatively lower dosage (300 μg) of ginsenoside-Rb2 was also active at the same administration schedule. Unfortunately, multiple administration of ginsenoside-Rb2 at the dosage of 300 μg had no effect (Fig. 3B). These results suggest that oral administration of ginsenoside-Rb2 is effective for the prevention of HVJ infection, and that its prophylactic effect on HVJ infection is dependent upon the dosage and the number of times being administered.
Effect of 20(R)- and 20(S)-ginsenoside-Rg3 on haemagglutinating virus of Japan infection
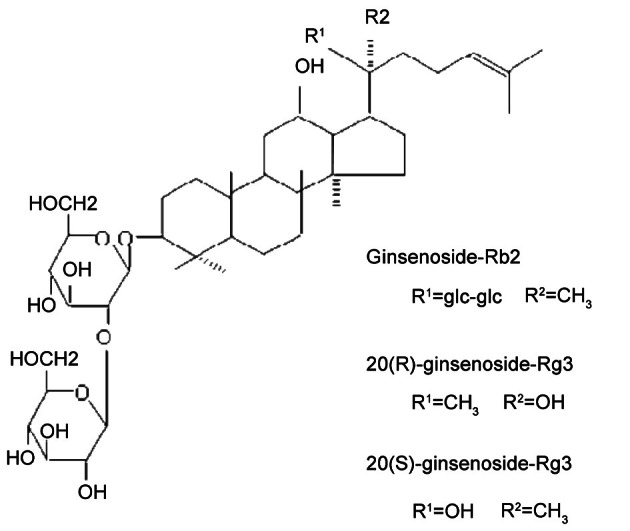
In the previously study, we demonstrated that two epimeric saponin preparations such as 20(R)- and 20(S)-ginsenoside-Rg3 which are hydrolytic products of ginsenoside-Rb2 (Fig. 4) inhibited tumor metastasis in mice, and that their antitumor activity was almost same with that of ginsenoside-Rb2 [8].
Fig. 4. The chemical structures of ginsenoside-Rb2 and its hydrolytic products.
Here, to address whether oral administration of 20(R)- and 20(S)-ginsenoside-Rg3 induce the protective effect on virus infection as ginsenoside-Rb2 did, we assessed their activity to protect against the lethal infection of HVJ in mice. In oral administration 3, 2, and 1 d prior to HVJ infection, 20(S)-ginsenoside-Rg3 showed a significant protective activity, even though its survival rate was much lower than that of ginsenoside-Rb2 (42.8% and 71.4%, respectively) (Fig. 5). However, 20(R)-ginsenoside-Rg3 in a epimeric structure of 20(S)-ginsenoside-Rg3 was not active.
Fig. 5. Comparison of the protective effect on haemagglutinating virus of Japan (HVJ) between ginsenoside-Rb2 and its hydrolytic products. Seven BALB/c mice per group were administered per os with 1 mg of ginsenoside-Rb2, 20(R)- or 20(S)-ginsenoside-Rg3 3, 2, and 1 d before HVJ infection. The survival rates were monitored for up to 20 d after infection. *p<0.05, **p<0.01, compared with untreated control (infection only) by Mann-Whitney U-test.
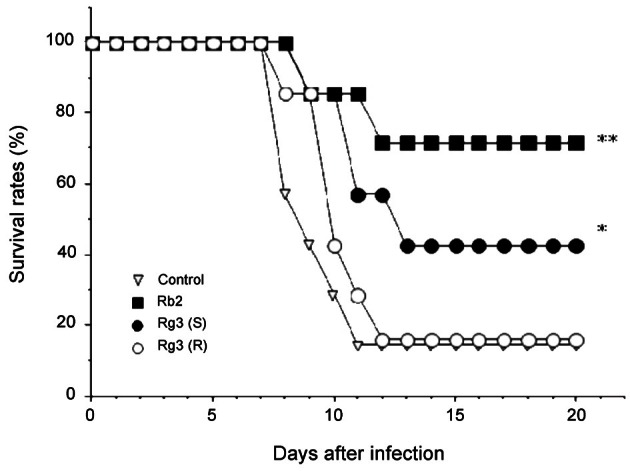
Growth inhibition of haemagglutinating virus of Japan by ginsenoside-Rb2 and 20(S)-ginsenoside-Rg3 in vivo
Next, we tried to compare the growth of HVJ in the lungs between non- and ginsenoside-treated mice. In the previous study, we found that the mice infected i.n. with 2 LD50 (2.6×104 HAD/mouse) of HVJ showed the highest virus titers in the lungs 5 d after infection. Therefore, we collected the lungs of HVJ-infected mice 5 d after virus infection, and determined virus titers in the lungs of the mice by counting the number of PFU on LLCMK-2 cells. The mice administered with ginsenoside-Rb2 as well as 20(S)-ginsenoside-Rg3 significantly suppressed the growth of HVJ in the lungs (Fig. 6).
Fig. 6. Inhibitory effect of ginsenoside-Rb2 and 20(S)-ginsenoside-Rg3 on the growth of haemagglutinating virus of Japan (HVJ) in the lungs in mice. Three BALB/c mice per group were administered per os with each of the indicated ginsenosides (1 mg) 3, 2, and 1 d before HVJ infection. Mice were killed 5 d after virus infection. Virus titers in the lungs of mice were determined by counting the number of plaque forming units (PFU) on LLCMK-2 cells. *p<0.05, **p<0.01, compared with untreated control (infection only) by Student’s two-tailed t-test.
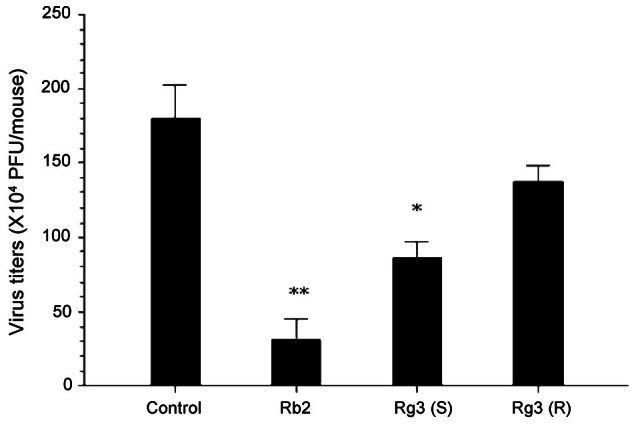
Especially the inhibitory activity of gionsenoside-Rb2 was higher than that of 20(S)-ginsenoside-Rg3. However, 20(R)-ginsenoside-Rg3 that had no effect on the lethal infection of HVJ did not inhibit the growth of this virus in the lungs. These results suggest that oral administration of ginsenoside-Rb2 and 20(S)-ginsenoside-Rg3 significantly protects against HVJ infection through inhibition of viral growth in the lungs, and also indicate that, among ginsenoside-Rb and its hydrolytic products such as 20(R)-ginsenoside-Rg3 and 20(S)-ginsenoside-Rg3, ginsenoside-Rb2 has the highest protective activity to inhibit the lethal infection of HVJ.
DISCUSSION
A variety of approaches to enhance specific immunity against mucosal pathogens have been tried by administration of various types of antigens [16]. However, there are few reports on enhancement of nonspecific protection against mucosal infections by mucosal immunoadjuvant. Considering the distinctive feature of mucosal immune system against foreign antigens in the host, it may be possible that the stimulation of mucosal tissues by a potent immunoadjuvant effectively augments nonspecific resistance against mucosal pathogens. Since many studies have shown that the antigen stimulation of a mucosa-associated lymphoid tissue (MALT) induced a potential activation of mucosal immunity of different mucosal tissues, the concept of common mucosal immune system (CMIS) has been introduced [21,22]. Especially, oral administration to stimulate mucosal immunity against infectious diseases has been shown to be convenient, relatively safe, and taking advantage of the mass of MALT.
It has been well known that saponin preparations from red ginseng possess immunomodulatory activities such as the enhancement of cytokine induction, cell-mediated immunity, and natural killer cell activity, although not all studies on the immunomodulatory activities of ginseng-derived saponins have been positive [18,23,24]. In our previous study indicating a possibility that a dammarane-type saponin ginsenoside-Rb2 acts as a mucosal immunoadjuvant, we showed that p.o. administration of this saponin into tumor-bearing mice markedly inhibited the lung metastasis of B16-BL6 melanoma cells, by enhancing nonspecific resistance to tumor cells in mice [8]. In those regards, we tried to investigate the stimulatory effect of an immunoadjuvant on mucosal immunity, using the combination of ginsenoside-Rb2 as an immunoadjuvant and infection model by mucosal infectious HVJ.
Here, we demonstrated that oral administration of ginsenoside-Rb2 prior to virus challenge enhanced host resistance against HVJ that causes severe acute infection in the lungs. The most effective protection of HVJ infection by ginsenoside-Rb2 was obtained from an administration condition in which mice were consecutively administered 3, 2, and 1 d before virus infection in a dose of 1 mg/mouse (Fig. 2). In addition, ginsinoside-Rb2 was shown to have the highest activity to protect against HVJ infection among dammarane-type (ginsenoside-Rb1, -Rb2, Rd, Re, and Rg2) and oleanolic acid-type ginsenoside (ginsenoside-Ro), even though other types of ginsenosides showed a partial activity (Fig. 1). Interestingly, in comparison between ginsenoside-Rb2 and its hydrolytic products, 20(S)-ginsenoside-Rg3 partially inhibited the lethal infection of HVJ, but 20(R)-ginsenoside-Rg3, an epimer of 20(S)-ginsenoside-Rg3, was not active (Fig. 5). These results suggest that the protective effect on HVJ infection by oral administration of ginsenosides is associated with the characteristic of chemical structures of these saponins.
In the analysis of the inhibition of virus growth in vivo, the protective activity acquired from oral administration of ginsenoside-Rb2 was involved in the suppression of virus growth in the lungs. The involvement was clearly supported by the results of Fig. 6 that the mice administered p.o. with ginsenoside-Rb2 prior to virus infection exhibited prominently lower titers of HVJ in the lungs 5 d after virus infection. Considering that the severity of infection caused by HVJ is dependent upon the extent of virus replication in its infection site, it is of note that oral administration of ginsenoside-Rb2 inhibited virus growth in the lungs, the different mucosal route from that of ginsenoside-Rb2 administration. In this regard, it is possible that the protective effect on HVJ infection by oral administration of ginsenoside-Rb2 may result from enhancement of mucosal immunity based on CMIS.
In the present study, we demonstrated that oral administration of ginsenoside-Rb2 is a potent mucosal immunoadjuvant to enhance nonspecific resistance against the lethal infection of HVJ by potentiating mucosal immunity and that its protective effect was associated with the inhibition of virus growth in the lungs. Further study to elucidate the mechanisms involved in activation of mucosal immune cells by ginsenoside-Rb2 and the relationship between the chemical structures and biological activities of ginsenosides derived from red ginseng is now on the way.
Acknowledgments
This work was carried out with the support of Cooperative Research Program for Agriculture Science & Technology Development (project no. PJ007777) from Rural Development Administration, Republic of Korea. We are grateful to Dr. I. Azuma, Dr. K. Matsuzawa, and Dr. K. Samukawa for their skillful technical advices.
References
- 1.Song X, Hu S. Adjuvant activities of saponins from traditional Chinese medicinal herbs. Vaccine. 2009;27:4883–4890. doi: 10.1016/j.vaccine.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 2.Sun HX, Xie Y, Ye YP. Advances in saponin-based adjuvants. Vaccine. 2009;27:1787–1796. doi: 10.1016/j.vaccine.2009.01.091. [DOI] [PubMed] [Google Scholar]
- 3.Kirk DD, Rempel R, Pinkhasov J, Walmsley AM. Application of Quillaja saponaria extracts as oral adjuvants for plant-made vaccines. Expert Opin Biol Ther. 2004;4:947–958. doi: 10.1517/14712598.4.6.947. [DOI] [PubMed] [Google Scholar]
- 4.Pickering RJ, Smith SD, Strugnell RA, Wesselingh SL, Webster DE. Crude saponins improve the immune response to an oral plant-made measles vaccine. Vaccine. 2006;24:144–150. doi: 10.1016/j.vaccine.2005.07.097. [DOI] [PubMed] [Google Scholar]
- 5.Park D, Bae DK, Jeon JH, Lee J, Oh N, Yang G, Yang YH, Kim TK, Song J, Lee SH, et al. Immunopotentiation and antitumor effects of a ginsenoside Rg3-fortified red ginseng preparation in mice bearing H460 lung cancer cells. Environ Toxicol Pharmacol. 2011;31:397–405. doi: 10.1016/j.etap.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Yu JL, Dou DQ, Chen XH, Yang HZ, Guo N, Cheng GF. Protopanaxatriol-type ginsenosides differentially modulate type 1 and type 2 cytokines production from murine splenocytes. Planta Med. 2005;71:202–207. doi: 10.1055/s-2005-837817. [DOI] [PubMed] [Google Scholar]
- 7.Dong H, Bai LP, Wong VK, Zhou H, Wang JR, Liu Y, Jiang ZH, Liu L. The in vitro structure-related anti-cancer activity of ginsenosides and their derivatives. Molecules. 2011;16:10619–10630. doi: 10.3390/molecules161210619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mochizuki M, Yoo YC, Matsuzawa K, Sato K, Saiki I, Tono-oka S, Samukawa K, Azuma I. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3, of red ginseng. Biol Pharm Bull. 1995;18:1197–1202. doi: 10.1248/bpb.18.1197. [DOI] [PubMed] [Google Scholar]
- 9.Musende AG, Eberding A, Wood CA, Adomat H, Fazli L, Hurtado-Coll A, Jia W, Bally MB, Tomlinson Guns ES. A novel oral dosage formulation of the ginsenoside aglycone protopanaxadiol exhibits therapeutic activity against a hormone-insensitive model of prostate cancer. Anticancer Drugs. 2012;23:543–552. doi: 10.1097/CAD.0b013e32835006f5. [DOI] [PubMed] [Google Scholar]
- 10.Kim JY, Kim HJ, Kim HJ. Effect of oral administration of Korean red ginseng on influenza A (H1N1) virus infection. J Ginseng Res. 2011;35:104–110. [Google Scholar]
- 11.Yoo DG, Kim MC, Park MK, Song JM, Quan FS, Park KM, Cho YK, Kang SM. Protective effect of Korean red ginseng extract on the infections by H1N1 and H3N2 influenza viruses in mice. J Med Food. 2012;15:855–862. doi: 10.1089/jmf.2012.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee MH, Lee BH, Jung JY, Cheon DS, Kim KT, Choi CS. Antiviral effect of Korean red ginseng extract and ginsenosides on murine norovirus and feline calicivirus as surrogates for human norovirus. J Ginseng Res. 2011;35:429–435. doi: 10.5142/jgr.2011.35.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho JY, Kim AR, Yoo ES, Baik KU, Park MH. Ginsenosides from Panax ginseng differentially regulate lymphocyte proliferation. Planta Med. 2002;68:497–500. doi: 10.1055/s-2002-32556. [DOI] [PubMed] [Google Scholar]
- 14.Lee KT, Jung TW, Lee HJ, Kim SG, Shin YS, Whang WK. The antidiabetic effect of ginsenoside Rb2 via activation of AMPK. Arch Pharm Res. 2011;34:1201–1208. doi: 10.1007/s12272-011-0719-6. [DOI] [PubMed] [Google Scholar]
- 15.Sato K, Mochizuki M, Saiki I, Yoo YC, Samukawa K, Azuma I. Inhibition of tumor angiogenesis and metastasis by a saponin of Panax ginseng, ginsenoside-Rb2. Biol Pharm Bull. 1994;17:635–639. doi: 10.1248/bpb.17.635. [DOI] [PubMed] [Google Scholar]
- 16.Fukushima A, Yoo YC, Yoshimatsu K, Matsuzawa K, Tamura M, Tono-oka S, Taniguchi K, Urasawa S, Arikawa J, Azuma I. Effect of MDP-Lys(L18) as a mucosal immunoadjuvant on protection of mucosal infections by Sendai virus and rotavirus. Vaccine. 1996;14:485–491. doi: 10.1016/0264-410x(95)00236-t. [DOI] [PubMed] [Google Scholar]
- 17.Faisca P, Desmecht D. Sendai virus, the mouse parainfluenza type 1: a longstanding pathogen that remains up-to-date. Res Vet Sci. 2007;82:115–125. doi: 10.1016/j.rvsc.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Bomford R, Stapleton M, Winsor S, Beesley JE, Jessup EA, Price KR, Fenwick GR. Adjuvanticity and ISCOM formation by structurally diverse saponins. Vaccine. 1992;10:572–577. doi: 10.1016/0264-410x(92)90435-m. [DOI] [PubMed] [Google Scholar]
- 19.Stewart-Tull DE. The theory and practical application of adjuvants. Wiley and Sons; Chichester: 1994. [Google Scholar]
- 20.Samukawa KI, Yamashita H, Matsuda H, Kubo M. Simultaneous analysis of saponins in ginseng radix by high performance liquid chromatography. Chem Pharm Bull (Tokyo) 1995;43:137–141. [Google Scholar]
- 21.McGhee JR, Mestecky J, Dertzbaugh MT, Eldridge JH, Hirasawa M, Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10:75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- 22.Yuki Y, Kiyono H. New generation of mucosal adjuvants for the induction of protective immunity. Rev Med Virol. 2003;13:293–310. doi: 10.1002/rmv.398. [DOI] [PubMed] [Google Scholar]
- 23.Du XF, Jiang CZ, Wu CF, Won EK, Choung SY. Synergistic immunostimulating activity of pidotimod and red ginseng acidic polysaccharide against cyclophosphamide-induced immunosuppression. Arch Pharm Res. 2008;31:1153–1159. doi: 10.1007/s12272-001-1282-6. [DOI] [PubMed] [Google Scholar]
- 24.Yun YS, Moon HS, Oh YR, Jo SK, Kim YJ, Yun TK. Effect of red ginseng on natural killer cell activity in mice with lung adenoma induced by urethan and benzo(a)pyrene. Cancer Detect Prev Suppl. 1987;1:301–309. [PubMed] [Google Scholar]