Abstract
Proteinuric conditions demonstrate structural and compositional changes of the foot processes and slit diaphragms between podocytes. p130Cas in podocytes serves as an adapter protein anchoring glomerular basement membrane to actin filaments of podocyte cytoskeleton. To investigate the effect of ginseng total saponin (GTS) on the pathologic changes of podocyte p130Cas induced by diabetic conditions, we cultured mouse podocytes under: 1) normal glucose (5 mM, control); 2) high glucose (HG, 30 mM); 3) advanced glycosylation endproducts (AGE)-added; or 4) HG plus AGE-added conditions and treated with GTS. In confocal imaging, p130Cas colocalized with zonula occludens-1 and synaptopodin connecting to F-actin. However, diabetic conditions relocalized p130Cas molecules at perinuclear cytoplasmic area and reduced the intensity of p130Cas. In Western blotting, diabetic conditions, especially HG plus AGE-added condition, decreased cellular p130Cas protein levels at 24 and 48 h. GTS improved such quantitative and qualitative changes. These findings imply that HG and AGE have an influence on the redistribution and amount of p130Cas of podocytes, which can be reversed by GTS.
Keywords: Panax ginseng, Ginseng total saponin, Podocytes, p130Cas, Advanced glycosylation end products
INTRODUCTION
Diabetic nephropathy is one of the serious complications of diabetes mellitus and the leading cause of end-stage renal disease worldwide, however, the predisposing factors and pathogenic mechanisms of diabetic nephropathy have remained unclear [1,2]. Early clinical manifestations of diabetic nephropathy include hyperfiltration and microalbuminuria and progress to overt proteinuria and progressive renal injury. Accompanying morphological changes include enlargement of glomeruli, increase in mesangial matrix, thickening of glomerular basement membrane (GBM), and effacement, denudation, and loss of podocytes [1,2]. Accompanying biochemical alterations with pathological changes lead to increase in glomerular permeability as a result of impaired glomerular filtration structure. These changes would be caused by hyperglycemia, secondary glycated proteins, or irreversible advanced glycosylation end products (AGE) [3,4].
As the glomerular capillary wall functions as an efficient and selective barrier that allows a high flow rate of filtration for plasma water and small solutes, glomerular capillary wall should be a strong but selectively permeable filtration barrier. The glomerular slit diaphragm (SD), a slit between interdigitating foot processes of podocytes, serves as a size-selective barrier and is linked to the actin-based cytoskeleton by adapter proteins, including CD2- associated protein (CD2AP), zonula occludens (ZO)- 1, β-catenin, and podocin and to GBM by p130Cas and integrin [5,6].
p130Cas serves as an ubiquitous docking adapter protein [7] because it contains proline-rich domains, an SH3 domain, and binding motifs for the SH2 domains of v-Crk and v-Src and seems to be involved in integrin-mediated signaling, becoming tyrosine phosphorylated on cell adhesion to extracellular matrix [8] and on flow-induced shear stress [9]. In podocytes, p130Cas localizes diffusely to the cytoplasm with accumulation at ends of F-actin stress fibers at foot processes, where focal adhesion proteins and kinases connect docking proteins to GBM and other adapter proteins including CD2AP connecting p130Cas to SD insertion site [5,6].
The root of Panax ginseng has been widely used for cancer, diabetes, and cardiovascular diseases for thousands of years in oriental countries including Korea [10,11]. Several investigations revealed that ginseng root and its derivatives have been beneficial in both type 2 diabetic patients and healthy individuals [12,13] as well as type 1 and 2 animal diabetic models [11,14,15].
Ginseng has also been reported to be effective in prevention and treatment of diabetic nephropathy of type 1 and type 2 diabetic animal models as followings. In type 1 insulin-dependent diabetic nephropathy animal models induced by streptozotocin, Sun ginseng [16], heat-processed American ginseng [17], and 20(S)-ginsenoside Rg3 [18] ameliorated elevated serum glucose and renal damage. In type 2 insulin-independent diabetic nephropathy animal models, 20(S)-ginsenoside Rg3 also decreased the elevated blood glucose and proteinuria and augmented creatinine clearance in type 2 diabetic Otsuka Long-Evans Tokushima Fatty (OLETF) [19]. Although the renal protective effect of ginseng components in diabetic models has been reported, there were very few reports which have attempted to elucidate the changes of glomerular filtration structures. Recently, we reported that in vitro diabetic conditions induced the distributional change and suppressed the production of ZO-1 protein, thus causing the phenotypical changes and hyperpermeability of podocytes, which can be improved by ginseng total saponin (GTS) [20].
In this study, we investigated the effect of GTS on the pathologic changes of podocyte p130Cas protein induced by diabetic conditions.
MATERIALS AND METHODS
Cell culture of mouse podocytes
Conditionally immortalized mouse podocytes were kindly provided by Dr. Peter Mundel (University of Harvard, Boston, MA, USA) and were cultured and differentiated as described previously [21]. Briefly, to stimulate podocytes proliferation, cells were cultivated at 33℃ (permissive conditions) in a culture medium supplemented with 10 U/mL mouse recombinant γ-interferon (Roche Mannheim, Germany) to induce expression of temperature-sensitive large T antigen. To induce differentiation, podocytes were maintained at 37℃ without γ-interferon (non-permissive conditions) for at least 2 wk.
Culture additives
Cells were serum-deprived to reduce background 24 h before each experiment, then exposed to glucose and/or AGE. Mouse podocytes were incubated in culture media containing either 5 mM (normal glucose) or 30 mM glucose (high glucose, HG) without insulin. AGE was produced by the technique previously described by Ha et al. [22]. To imitate the long-term diabetic condition, AGE was added (5 μg/mL) and controls were established using unmodified bovine serum albumin (BSA, 5 μg/mL). To exclude the effect of additionally produced glycated proteins in culture conditions, no longer than 48 h of incubation was used. Fetal bovine serum was reduced to 0.5% on the last media change to reduce background caused by serum components before protein extraction. For identification purposes, AGE or BSA was denoted as ‘A’ or ‘B’, and glucose at 5 or 30 mM by ‘5’ or ‘30’, respectively. Briefly describing, each condition means B5, normal; B30, short-term diabetic condition; A5, long-term normoglycemic or aged condition; A30, long-term diabetic condition.
For ginseng treatment podocytes were incubated with GTS at the concentrations of 1 μg/mL for 6, 24, and 48 h. GTS was kindly provided by Korea Ginseng Corporation (Daejeon, Korea).
Confocal image analysis
Podocytes that were grown on type I collagen-coated glass cover slips incubated for 24 h were fixed in 4% paraformaldehyde, permeabilized in phosphate buffer solution, blocked with 10% normal goat serum, and labeled with polyclonal rabbit anti-rat p130Cas antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), monoclonal rabbit anti-rat ZO-1 antibody (Invitrogen, Eugene, OR, USA) or polyclonal rabbit anti-rat synaptopodin (Santa Cruz Biotechnology). Primary antibody-bound specimens were incubated with 1:500 (v/v) Alexa 594 for red (Molecular Probes, Invitrogen)-conjugated respective secondary antibodies at RT for 1 h. F-actin was visualized with TRITC-phalloidin (Sigma Chemical, St. Louis, MO, USA) and nuclei were stained with 2 mM 4’,6-diamidino-2-phenylindole dihydrochloride (Sigma Chemical). Coverslips were mounted in aqueous mountant and viewed with a Fluorescence microscope (BX51; Olympus, Tokyo, Japan).
Western blotting
The confluently grown cell layers incubated with additives for p130Cas and various durations for AGE were extracted and protein concentrations were determined as previously described [22]. For the Western blotting of p130Cas, 30 μg of boiled extracts was applied on 10% SDS-PAGE gels and transferred to polyvinylidene fluoride membranes (Bio-Rad Laboratories, Hercules, CA, USA). Then, the membranes were air-dried and blocked in 3% fat-free milk before incubation with anti-p130Cas antibody. After incubation with horseradish peroxidase–conjugated secondary antibodies (Santa Cruz Biotechnology), bands were detected by using the ECL chemiluminescence system (Amersham Biotech Ltd., Bucks, UK). Data on the densitometric analysis of p130Cas / β-tubulin ratio are expressed as mean±standard deviation.
Statistical analysis
The results are presented as mean values±standard deviation, as required under different conditions. The statistical significance was assessed by nonparametric Kruskal-Wallis ANOVA analysis or Student t-test. A p-value less than 0.05 was considered significant.
RESULTS
Confocal image changes of zonula occludens-1
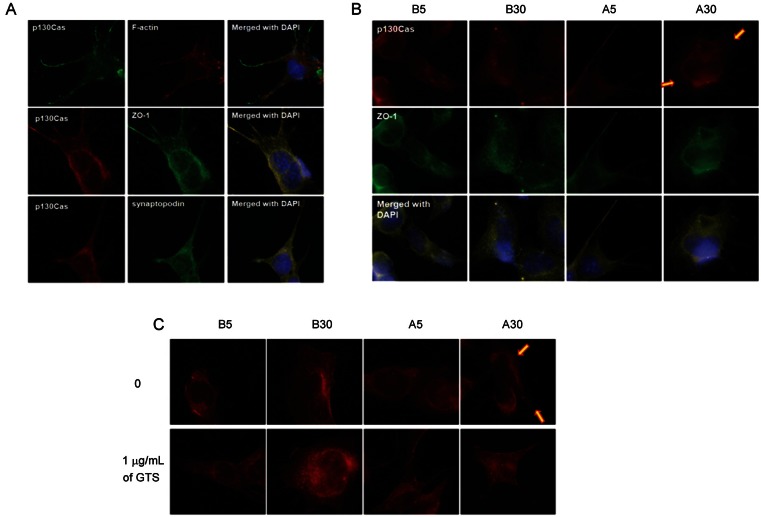
p130Cas stainings are located in peripheral cytoplasm and processes of podocytes, co-localized with ZO-1 and synaptopodin, however, not with actin filaments (Fig. 1A). Therefore, p130Cas connects basal cell membrane to ends of cytoskeleton not directly but indirectly via synaptopodin.
Fig. 1. Localization of p130Cas in podocytes. (A) p130Cas stainings are located in peripheral cytoplasm and processes of podocytes, co-localized with zonula occludens (ZO)-1 and synaptopodin, however, not with actin filaments. (B,C) The intensities of p130Cas stainings are diminished at peripheral cytoplasm in diabetic conditions, especially in more pathologic A30 (arrows). Ginseng total saponin (GTS, 1 μg/mL) improves the distributional changes, therefore, recover p130Cas at peripheral cytoplasm and cellular membrane (×1,000). DAPI, 4’,6-diamidino-2-phenylindole dihydrochloride.
The intensities of p130Cas stainings are diminished at peripheral cytoplasm in diabetic conditions, especially in more pathologic A30 at 24 h (arrows; Fig. 1B, C). GTS (1 μg/mL) improved the distributional change of p130Cas, therefore, recovered p130Cas at peripheral cytoplasm and cellular membrane.
Western blotting of p130Cas in cultured podocyte
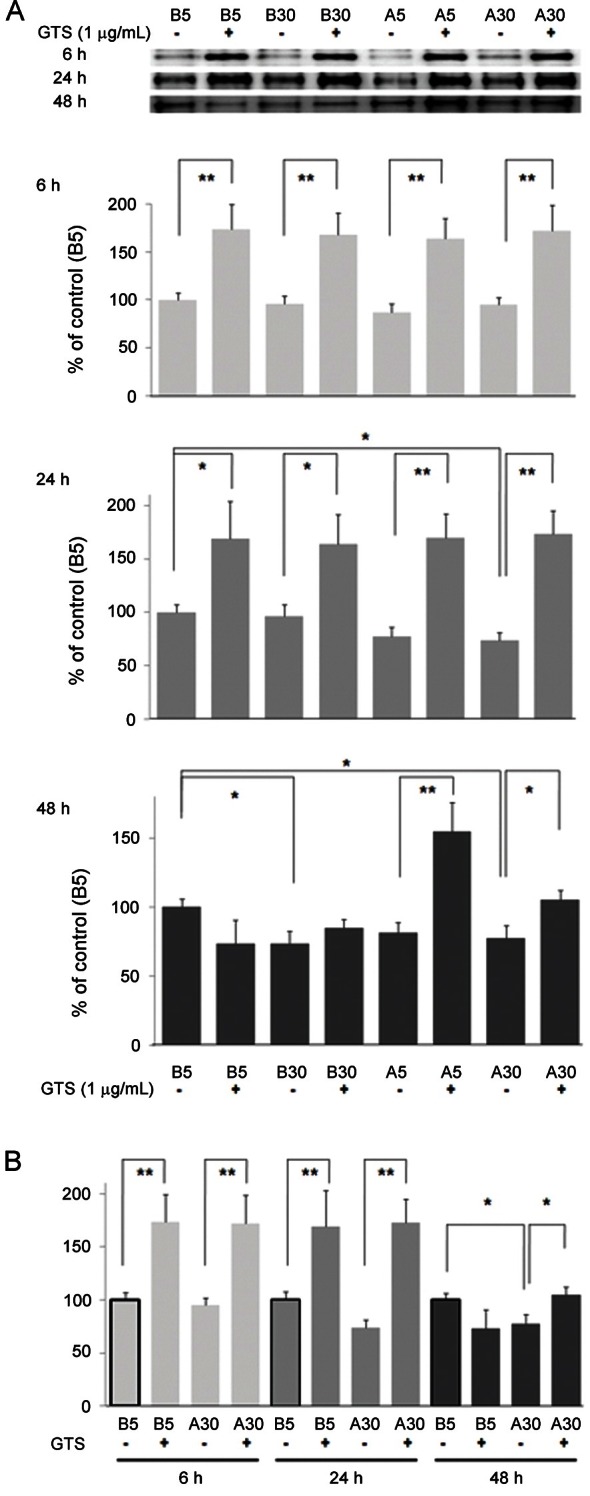
The bands for p130Cas protein at 130 kDa were compared to those of β-tubulin (not shown). Density values for p130Cas protein of representative immunoblots from each group revealed that HG (B30) suppressed p130Cas protein amount by 26.5% at 48 h and A30 condition suppressed p130Cas by 26.8% at 24 h and by 22.7% at 48 h (p<0.05) (Fig. 2A). Decrease in B30 at 48 h incubations but not at shorter incubations (6 and 24 h) might be due to HG itself and subsequently produced glycated proteins. GTS (1 μg/mL) improved change of p130Cas protein amount, significantly (p<0.05 or p<0.01). Notably, GTS upregulates p130Cas of podocytes over control level even in B5, and the remained effect of GTS in A5 condition at 48 h is comparable. Results on B5 and A30 were compared according to exposure times in Fig. 2B.
Fig. 2. Effects of diabetic conditions and ginseng total saponin (GTS) on the p130Cas protein assayed by Western blotting. (A) The major band for p130Cas protein at 130 kDa decreased in A30 conditions at 24 and 48 h and in B30 at 48 h incubations, which improved by GTS (1 μg/mL). Notably, GTS upregulates p130Cas of podocytes over control level even in B5. (B) Results on B5 and A30 were compared according to exposure times. Data on the densitometric analysis of p130Cas/β-tubulin (not shown) ratio are expressed as mean±SD (n=3). Control (100%), the value of B5 without GTS at each incubation time. *p<0.05, **p<0.01 vs. control.
These findings imply that glucose and AGE might have an influence on the redistribution and amount of p130Cas protein of podocytes thereby causing podocyte dysfunction, which could be reversed by GTS.
DISCUSSION
p130Cas protein plays a pivotal role in maintaining the cell adhesion/migration, pro-survival, and cellular signaling by connecting extracellular structure and cytoskeleton [7,8]. In podocytes, p130Cas localizes diffusely to the cytoplasm with accumulation at ends of F-actin stress fibers at foot processes and connected to GBM via focal adhesion kinases (FAKs) and integrin and to SD via adapter proteins including CD2AP [5]. As tyrosine phosphorylation of p130Cas by integrin-mediated adhesion plays a role in controlling actin cytoskeleton organization in response to adhesive and growth factor signaling [23], therefore, p130Cas protein may also play an important role in maintaining the glomerular permeability by connecting podocyte actin cytoskeleton to GBM and SD.
The proteinuric condition, regardless of the underlying diagnosis, demonstrates ultrastructural changes in the visceral GEpC (podocytes) with retraction and effacement of the highly specialized interdigitating foot processes, which were accompanied by the alterations of SD and linking molecules even before the onset of proteinuria [5,6]. There were very limited reports on the change of p130Cas in pathologic conditions till now. As an adapter protein, the immunofluorescent density of p130Cas increased around the capillary loop of human membranous nephropathy, however, not of minimal change disease [24]. They speculated that the increase of p130Cas might be as a result of tyrosine phosphorylation of its constituent proteins. In patients and animal models with immune-mediated glomerular diseases, increased tyrosine phosphorylation within focal adhesion proteins, increased Pyk2, and FAK activation have been reported [25-27]. Recently, we found a reduced expression of p130Cas by puromycin aminonucleoside which could induce the similar pathologic findings of minimal change disease [28] and in diabetic model of this study. Therefore, we suggest that the changes in the expression of podocyte p130Cas would be a cause or a consequence in podocyte injury, although the expression of podocyte p130Cas could be different according to the pathophysiologic mechanisms leading to podocytopathy.
HG strongly inhibited adhesion of podocytes to basement membrane and reduced α3β1 integrin mRNA and protein expression [29]. Recently, integrin α1/Akita double-knockout mice on a BALB/c background developed albuminuria, GBM thickening, and exacerbated glomerulosclerosis after diabetic injury [30]. As tyrosine phosphorylation of p130Cas would be induced by integrin-mediated adhesion [23], we suggest that podocyte p130Cas might be up-regulated by tyrosine phosphorylation in immune-mediated glomerular diseases, however, reduced with a suppression of integrin in diabetic condition.
There were several reports on the effects of P. ginseng root and its derivatives on human and experimental diabetic nephropathy, however, there were a few reports on podocyte, a major glomerular filtration component using cultured podocytes. Focusing on glomerular filtration structures, Zhang et al. [31] found that ginsenoside Rgl improved the pathologic diabetic changes of glomerular filtration, such as, GBM thickness and podocytopenia with the reduction of urine protein and serum creatine. As ginsenoside Rgl also improved the overexpressed levels of serum monocyte chemotactic protein-1 and tumor necrosis factor-α, which correlated with the improved clinical and pathologic indices. Recently, we reported that both HG- and AGE-added condition induce the distributional change and suppress the production of ZO-1 protein, thus causing the phenotypical changes and hyperpermeability of podocytes, which can be improved by GTS [20].
In conclusion, the results of our in vitro study document that both HG- and AGE-added condition induce the distributional change and suppress the production of podocyte p130Cas, which can be improved by GTS, therefore, GTS will be helpful to prevent the podocyte change in diabetic nephropathy.
Acknowledgments
This study was supported by the grants from the Korean Society of Ginseng (2010) and Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2012-0004731). The author thanks EM Ahn for their technical assistances and Dr. Peter Mundel for mouse podocytes.
References
- 1.Parving HH, Mauer M, Fioretto P, Rossing P, Ritz E. Diabetic nephropathy. In: Brenner BM, ed. The kidney. WB Saunders; Philadelphia: 2011. pp. 1411–1454. [Google Scholar]
- 2.Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, Chugh S, Danesh FR. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med (Maywood) 2008;233:4–11. doi: 10.3181/0705-MR-134. [DOI] [PubMed] [Google Scholar]
- 3.Sharma K, Ziyadeh FN. Biochemical events and cytokine interactions linking glucose metabolism to the development of diabetic nephropathy. Semin Nephrol. 1997;17:80–92. [PubMed] [Google Scholar]
- 4.Vlassara H. Protein glycation in the kidney: role in diabetes and aging. Kidney Int. 1996;49:1795–1804. doi: 10.1038/ki.1996.270. [DOI] [PubMed] [Google Scholar]
- 5.Mundel P, Kriz W. Structure and function of podocytes: an update. Anat Embryol (Berl) 1995;192:385–397. doi: 10.1007/BF00240371. [DOI] [PubMed] [Google Scholar]
- 6.Asanuma K, Mundel P. The role of podocytes in glomerular pathobiology. Clin Exp Nephrol. 2003;7:255–259. doi: 10.1007/s10157-003-0259-6. [DOI] [PubMed] [Google Scholar]
- 7.O’Neill GM, Fashena SJ, Golemis EA. Integrin signalling: a new Cas(t) of characters enters the stage. Trends Cell Biol. 2000;10:111–119. doi: 10.1016/s0962-8924(99)01714-6. [DOI] [PubMed] [Google Scholar]
- 8.Nojima Y, Morino N, Mimura T, Hamasaki K, Furuya H, Sakai R, Sato T, Tachibana K, Morimoto C, Yazaki Y, et al. Integrin-mediated cell adhesion promotes tyrosine phosphorylation of p130Cas, a Src homology 3-containing molecule having multiple Src homology 2-binding motifs. J Biol Chem. 1995;270:15398–15402. doi: 10.1074/jbc.270.25.15398. [DOI] [PubMed] [Google Scholar]
- 9.Okuda M, Takahashi M, Suero J, Murry CE, Traub O, Kawakatsu H, Berk BC. Shear stress stimulation of p130(cas) tyrosine phosphorylation requires calcium-dependent c-Src activation. J Biol Chem. 1999;274:26803–26809. doi: 10.1074/jbc.274.38.26803. [DOI] [PubMed] [Google Scholar]
- 10.Park HJ, Kim DH, Park SJ, Kim JM, Ryu JH. Ginseng in traditional herbal prescriptions. J Ginseng Res. 2012;36:225–241. doi: 10.5142/jgr.2012.36.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SK, Park JH. Trends in ginseng research in 2010. J Ginseng Res. 2011;35:389–398. doi: 10.5142/jgr.2011.35.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sotaniemi EA, Haapakoski E, Rautio A. Ginseng therapy in non-insulin-dependent diabetic patients. Diabetes Care. 1995;18:1373–1375. doi: 10.2337/diacare.18.10.1373. [DOI] [PubMed] [Google Scholar]
- 13.Vogler BK, Pittler MH, Ernst E. The efficacy of ginseng. A systematic review of randomized clinical trials. Eur J Clin Pharmacol. 1999;55:567–575. doi: 10.1007/s002280050674. [DOI] [PubMed] [Google Scholar]
- 14.Chung SH, Choi CG, Park SH. Comparisons between white ginseng radix and rootlet for antidiabetic activity and mechanism in KKAy mice. Arch Pharm Res. 2001;24:214–218. doi: 10.1007/BF02978260. [DOI] [PubMed] [Google Scholar]
- 15.Yuan HD, Shin EJ, Chung SH. Anti-diabetic effect and mechanism of Korean red ginseng in C57BL/KsJ db/db mice. J Ginseng Res. 2008;32:187–193. [Google Scholar]
- 16.Kang KS, Kim HY, Yamabe N, Nagai R, Yokozawa T. Protective effect of sun ginseng against diabetic renal damage. Biol Pharm Bull. 2006;29:1678–1684. doi: 10.1248/bpb.29.1678. [DOI] [PubMed] [Google Scholar]
- 17.Kim HY, Kang KS, Yamabe N, Nagai R, Yokozawa T. Protective effect of heat-processed American ginseng against diabetic renal damage in rats. J Agric Food Chem. 2007;55:8491–8497. doi: 10.1021/jf071770y. [DOI] [PubMed] [Google Scholar]
- 18.Kang KS, Yamabe N, Kim HY, Park JH, Yokozawa T. Therapeutic potential of 20(S)-ginsenoside Rg(3) against streptozotocin-induced diabetic renal damage in rats. Eur J Pharmacol. 2008;591:266–272. doi: 10.1016/j.ejphar.2008.06.077. [DOI] [PubMed] [Google Scholar]
- 19.Kang KS, Yamabe N, Kim HY, Park JH, Yokozawa T. Effects of heat-processed ginseng and its active component ginsenoside 20(S)-Rg3 on the progression of renal damage and dysfunction in type 2 diabetic Otsuka Long-Evans Tokushima Fatty rats. Biol Pharm Bull. 2010;33:1077–1081. doi: 10.1248/bpb.33.1077. [DOI] [PubMed] [Google Scholar]
- 20.Ha TS, Choi JY, Park HY, Lee JS. Ginseng total saponin improves podocyte hyperpermeability induced by high glucose and advanced glycosylation endproducts. J Korean Med Sci. 2011;26:1316–1321. doi: 10.3346/jkms.2011.26.10.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mundel P, Reiser J, Zuniga Mejia Borja A, Pavenstadt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- 22.Ha TS, Song CJ, Lee JH. Effects of advanced glycosylation endproducts on perlecan core protein of glomerular epithelium. Pediatr Nephrol. 2004;19:1219–1224. doi: 10.1007/s00467-004-1590-1. [DOI] [PubMed] [Google Scholar]
- 23.Di Stefano P, Cabodi S, Boeri Erba E, Margaria V, Bergatto E, Giuffrida MG, Silengo L, Tarone G, Turco E, Defilippi P. P130Cas-associated protein (p140Cap) as a new tyrosine-phosphorylated protein involved in cell spreading. Mol Biol Cell. 2004;15:787–800. doi: 10.1091/mbc.E03-09-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bains R, Furness PN, Critchley DR. A quantitative immunofluorescence study of glomerular cell adhesion proteins in proteinuric states. J Pathol. 1997;183:272–280. doi: 10.1002/(SICI)1096-9896(199711)183:3<272::AID-PATH914>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 25.Takagi C, Ueki K, Ikeuchi H, Kuroiwa T, Kaneko Y, Tsukada Y, Maezawa A, Mitaka T, Sasaki T, Nojima Y. Increased expression of cell adhesion kinase beta in human and rat crescentic glomerulonephritis. Am J Kidney Dis. 2002;39:174–182. doi: 10.1053/ajkd.2002.29912. [DOI] [PubMed] [Google Scholar]
- 26.Morino N, Matsumoto T, Ueki K, Mimura T, Hamasaki K, Kanda H, Naruse T, Yazaki Y, Nojima Y. Glomerular overexpression and increased tyrosine phosphorylation of focal adhesion kinase p125FAK in lupus-prone MRL/MP-lpr/lpr mice. Immunology. 1999;97:634–640. doi: 10.1046/j.1365-2567.1999.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma H, Togawa A, Soda K, Zhang J, Lee S, Ma M, Yu Z, Ardito T, Czyzyk J, Diggs L, et al. Inhibition of podocyte FAK protects against proteinuria and foot process effacement. J Am Soc Nephrol. 2010;21:1145–1156. doi: 10.1681/ASN.2009090991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ha TS, Choi JY, Park HY. Puromycin aminonucleoside modulates p130Cas of podocytes. Korean J Pediatr. 2012;55:371–376. doi: 10.3345/kjp.2012.55.10.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Gui D, Chen Y, Mou L, Liu Y, Huang J. Astragaloside IV improves high glucose-induced podocyte adhesion dysfunction via alpha3beta1 integrin upregulation and integrin-linked kinase inhibition. Biochem Pharmacol. 2008;76:796–804. doi: 10.1016/j.bcp.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 30.Yu L, Su Y, Paueksakon P, Cheng H, Chen X, Wang H, Harris RC, Zent R, Pozzi A. Integrin α1/Akita double-knockout mice on a Balb/c background develop advanced features of human diabetic nephropathy. Kidney Int. 2012;81:1086–1097. doi: 10.1038/ki.2011.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang LN, Xie XS, Zuo C, Fan JM. Effect of ginsenoside Rgl on the expression of TNF-alpha and MCP-1 in rats with diabetic nephropathy. Sichuan Da Xue Xue Bao Yi Xue Ban. 2009;40:466–471. [PubMed] [Google Scholar]