Abstract
This study examined the effect of fermented ginseng (FG) on memory impairment and β-amyloid (Aβ) reduction in models of Alzheimer’s disease (AD) in vitro and in vivo. FG extract was prepared by steaming and fermenting ginseng. In vitro assessment measured soluble Aβ42 levels in HeLa cells, which stably express the Swedish mutant form of amyloid precursor protein. After 8 h incubation with the FG extract, the level of soluble Aβ42 was reduced. For behavioral assessments, the passive avoidance test was used for the scopolamine-injected ICR mouse model, and the Morris water maze was used for a transgenic (TG) mouse model, which exhibits impaired memory function and increased Aβ42 level in the brain. FG extract was treated for 2 wk or 4 mo on ICR and TG mice, respectively. FG extract treatment resulted in a significant recovery of memory function in both animal models. Brain soluble Aβ42 levels measured from the cerebral cortex of TG mice were significantly reduced by the FG extract treatment. These findings extract was prepared by steaming and fermenting ginseng. of Aβ42 protein, which results in enhanced behavioral memory function, thus, suggesting that FG extract may be an effective preventive or treatment for AD.
Keywords: Panax ginseng, Fermented ginseng, Alzheimer’s disease, Beta-amyloid, Animal model
INTRODUCTION
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder diagnosed clinically by loss of cognition and memory, and pathologically by the increase of β-amyloid (Aβ) and deposition of Aβ plaques in the brain [1,2]. Increased proteolytic degradation of β-amyloid precursor protein (APP), the precursor for Aβ, is considered the primary cause of the disease process [3]. Overpopulated Aβ peptides tend to oligomerize, which leads to polymerization, conformational change, fibrillation, and deposition of fibrillar Aβ plaques within the brain parenchyma [4]. Currently, many attempts have been made to identify or develop agents capable of controlling Aβ-related mechanisms of AD [5]. However, there still remains no cure for AD, and the majority of currently approved treatments offer only symptomatic relief at best. This has triggered a thorough investigation of herbal therapeutics that has long been utilized in traditional medicines to treat aging and memory loss.
For thousands of years, ginseng, commonly known as Panax ginseng Meyer, has been used in traditional medicine as a general body tonic [6]. Ginseng has also been used regularly for a multitude of applications with associative functions. An example is the use of ginseng as a central nervous system stimulant to improve cognitive function, including marked effects on memory and learning as well as ameliorating confusion and forgetfulness often encountered with aging [7]. Based on previous research, ginsenosides are the main molecular components that are effective in the treatment of cognitive impairments [8], including the amelioration of Aβ plaques [9-10]. However, various other components of P. ginseng, including polysaccharides, peptides, polyacetylenic alcohols, and fatty acids, have also been identified as biologically active [11].
In an effort to determine a safer and more effective agent with increased amounts of various ginsenosides, fermented ginseng (FG) was developed. FG contains high levels of Rg3, Rg5, and Rk1 that have been bio-converted from Re, Rg1, and Rb1. Recently, FG was found to have hypolipidemic and hypoglycemic effects [12,13] as well as anti-cancer [14], anti-inflammatory [15,16], and therapeutic effects on allergic rhinitis in clinical patients [17]. However, there are no reports on the effect of FG on cognition impairment or AD. Therefore, in the present study, the effect of FG on chemically-induced amnesia mouse model and enhancement of Aβ-related symptoms in AD transgenic (TG) mice were investigated, as well as in vitro investigation of mutated HeLa cells in an effort to reduce Aβ levels.
MATERIALS AND METHODS
Preparation and analysis of fermented ginseng extract
Six-year-old root of P. ginseng Meyer was purchased from a traditional medicine market in Hongcheon, Korea. Well-being LS Co. Ltd. (Gangneung, Korea) manufactured the FG extract through steaming, drying, and fermentation processes. Briefly, the ginseng root was steamed for 15 min at 121℃ and dried until the water content was below 12%. Next, 10 g of the processed root were extracted with 200 mL of distilled water for 2 h and inoculated with 3% Lactobacillus fermentum (1×108 cfu/g) for 16 h of fermentation at 42℃, 80 rpm. The FG extract was then sterilized with an autoclave at 121℃ for 15 min. For comparison, dried ginseng (DG) extract was also prepared by a 22 h water extraction with 10 g of dried ginseng root (water content less than 12%) and 200 mL of distilled water. FG and DG extracts were freeze-dried and analyzed for their active components by HPLC using YL9100 HPLC system (Younglin, Anyang, Korea) that was equipped with a YL9160 PDA detector and an ELSD ZAM 3000 detector (Schambeck SFD GmbH, Bad Honnef, Germany).
In vitro soluble β-amyloid 42 detection
A HeLa cell line stably expressing the Swedish mutant form of APP (APPswe) was maintained in Dulbecco’s modified Eagle medium supplemented with 10% heat-inactivated fetal bovine serum, as previously described [18]. Briefly, the cells were plated at 70% to 80% confluence in 60 mm dishes. The cells were pre-incubated with FG or DG extract 200 and 400 μg/mL concentrations for 8 h. Then, media was collected, and cell debris was removed by centrifugation at 12,000 rpm for 5 min. The levels of Aβ42 in conditioned media were determined using sandwich ELISA (human Aβ42, #KHB3482; BioSource International Inc., Camarillo, CA, USA) according to the manufacturer’s protocol.
Animals
For the scopolamine-induced amnesia model, 7- week-old male ICR mice were obtained from Orient Bio Inc. (Seongnam, Korea). Male APP/PS1 double-TG mice (strain name, B6C3-Tg(APPswe/PSEN1dE9)85Dbo/J; stock number 004462) used in this study were obtained from Jackson Laboratory (Bar Harbor, ME, USA) at the age of 4 months old. TG mice expressed a chimeric mouse/human APP containing the K595N/M596L Swedish mutations and a mutant human presenilin-1 carrying the exon 9-deleted variant under the control of mouse prion promoter elements, which directs the transgene expression predominantly to central nervous system neurons. All animals were housed in solid bottom cages with pellet food and water available ad libitum. The mice were also maintained on a 12/12 h light-dark cycle in a temperature and humidity-controlled room (22℃, 50%). The animal protocols used in this study were in accordance with the Korea Institute of Science and Technology Animal Care Committee guidelines.
Step-through passive avoidance test
A two-compartment step-through passive avoidance apparatus (Jeung Do Bio & Plant Co., Seoul, Korea) was used for the test. The apparatus was divided into bright and dark compartments (10×12×15 cm3 each) by a wall with a guillotine door. The bright compartment was illuminated by a fluorescent light (8 W). Eight weeks old ICR mice were randomly grouped and administered 400 or 800 mg/kg/10 mL oral doses of FG extract. The other groups were administered saline. After 2 wk of FG extract pre-treatment, the mice were injected with 1 mg/kg of scopolamine or vehicle by intra peritoneal injection 45 min prior to the training session. During the training session, the mice were placed in the bright compartment and allowed to explore for 30 s, at which point the guillotine door was raised to allow the mice to enter the dark compartment. When the mice entered the dark compartment, the guillotine door was closed, and an electrical foot shock (0.3 mA) was delivered for 3 s, and the mice were then placed back into their cages. After 24 h, a test session was performed. During the test session, the mice were placed in the bright compartment and allowed to explore for 30 s, and then, the guillotine door was raised. The latency to enter the dark compartment was recorded for up to 300 s.
Morris water maze test
Starting at the age of 7 months old, TG mice were randomly grouped and administered the FG extract in doses of 400 or 800 mg/kg/10 mL for 4 mo. The other groups were administered saline during the administration period. The Morris water maze (MWM) consists of a circular pool (120 cm in diameter, and 60 cm deep) filled with water at 24℃ to 26℃ to a depth of 40 cm and made opaque (white in color) with water soluble non-toxic paint. A non-visible escape platform, 8 cm in diameter, was submerged approximately 1 cm below the water’s surface in the center of the designated target quadrant. The two phases of the MWM tests, acquisition and retention, were conducted for 7 consecutive days. During the 6 consecutive days of the acquisition phase, each TG mouse was run for total of 3 trials per day, with a 2 h inter-trial interval. The mice were given a maximum of 90 s to find the platform and remain seated for at least 5 s. If the mouse was unable to find the platform within the 90 s time frame, it was placed directly on the platform for 5 s and then returned to its cage. For each trial, the mouse was placed under one of the 4 visual cues in a random order. During the acquisition phase, escape latency, the amount of time (in seconds) it took the mouse to find the hidden platform, was measured. For those mice that did not find the hidden platform in the allotted time, a score of 90 s was given. The retention phase of the MWM occurred on the day immediately following the last day of the acquisition phase. During the retention phase, the platform was removed, and the mice were given 120 s to explore the pool, and the duration of time spent in the target quadrant, which had contained the escape platform during the acquisition phase, was measured. After the test, the mice were removed from the pool and returned to their cages.
In vivo soluble β-amyloid 42 detection
For in vivo detection of brain soluble Aβ42, the TG mice that had undergone behavioral tests were anesthetized and decapitated, and the cerebral cortex was dissected from the exposed brains. Brain samples were stored at -80℃ until use. The brain samples (100 mg) were homogenized in Tris-buffered saline solution (20 mM Tris, 137 mM NaCl, pH 7.4) containing a complete protease inhibitors tablet (catalog no. P8340; Sigma, St. Louis, MO, USA). The extraction ratio (brain tissue:Tris-buffered saline) was 1:5 or 1:10 (w/v). The tissue homogenates were centrifuged at 100,000 g for 1 h at 4℃. Soluble Aβ42 levels in the resulting supernatants were determined using the same sandwich ELISA kit used for the in vitro detection.
Statistical analysis
All results are presented as the mean±SEM. The overall significance of experimental results was examined by Levene’s test for variance homogeneity then one-way analysis of variance and the two-tail Dunnet’s t-test. Differences between the groups were considered significant at p<0.05 with the appropriate Bonferroni correction for multiple comparisons.
RESULTS
Analysis of fermented ginseng and dried ginseng extracts
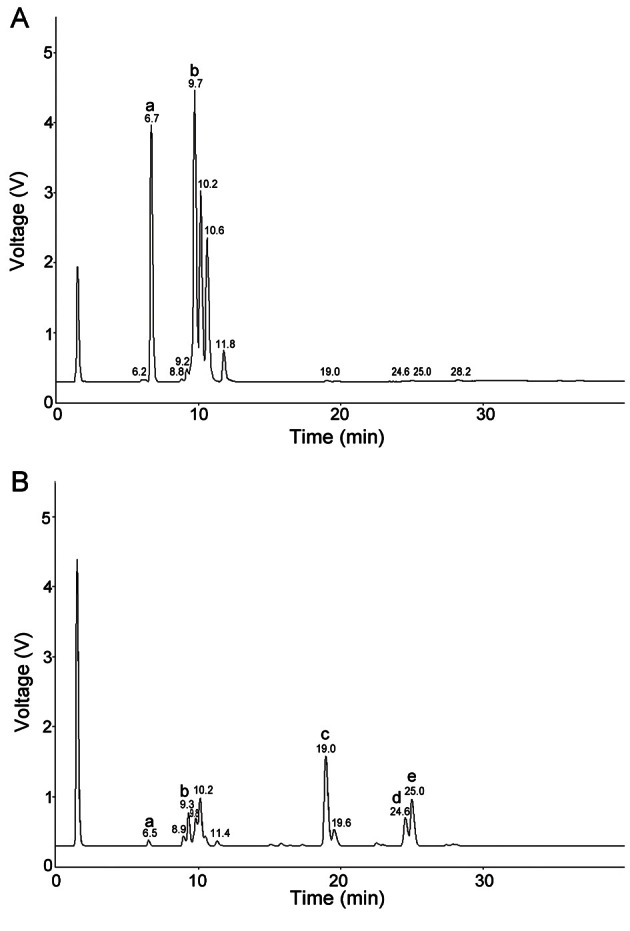
According to Fig. 1, 1 g of DG extract contained undetectable amount of Rg3, Rg5, and Rk1 (Fig. 1A). However, these ginsenosides, Rg3, Rg5, and Rk1, were significantly increased in 1 g of FG extract to 11.9 mg, 7.9 mg, and 6.1 mg, respectively (Fig. 1B). In contrast, DG extract contained higher amounts of Re and Rg1 (31.4 mg) and Rb1 (40.6 mg) compared with the FG extract containing 4.0 mg and 5.7 mg, respectively.
Fig. 1. HPLC analysis of ginsenoside contents in dried ginseng (A) and fermented ginseng (B) extracts. HPLC analysis for ginsenosides was conducted using YL9100 HPLC system, equipped with a YL9160 PDA detector and a ELSD ZAM 3000 detector. Designations of a, b, c, d, and e indicate Re+Rg1, Rb1, Rg3, Rk1, and Rg5, respectively.
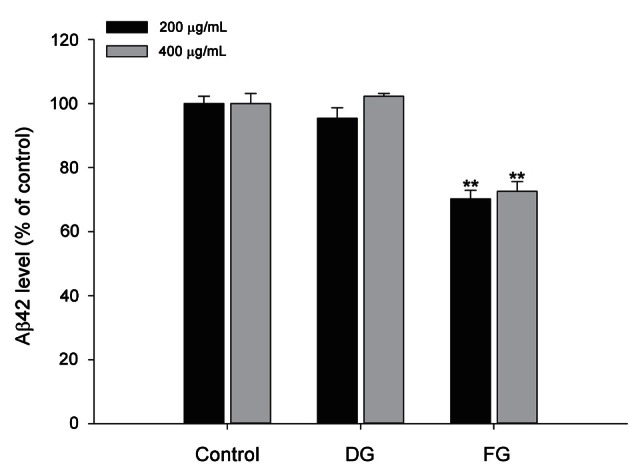
Level of β-amyloid 42 in fermented ginseng and dried ginseng extract-treated HeLa cell line
APPswe-mutant HeLa cells exhibited a significantly increased level of Aβ42. DG extract treatment showed no significant effect on the level of Aβ42 expression. However, FG extract 200 or 400 μg/mL treatment resulted in a significant reduction of Aβ42 level to 70.2±2.7% and 72.6±3.0% compared with the control group, respectively (Fig. 2).
Fig. 2. Effect of dried ginseng (DG) and fermented ginseng (FG) extracts on the level of soluble β-amyloid (Aβ)42 protein in the amyloid precursor protein (APP)-transfected HeLa cell line. APP-transfected HeLa cells were incubated with 200 μg/mL and 400 μg/mL of DG or FG extracts for 8 h. Soluble Aβ42 level was measured from the supernatant of conditioned media using the sandwich ELISA method as described in the Materials and Methods. The data represent the mean±SEM (n=3). ** Significantly different (p<0.01) from the control group.
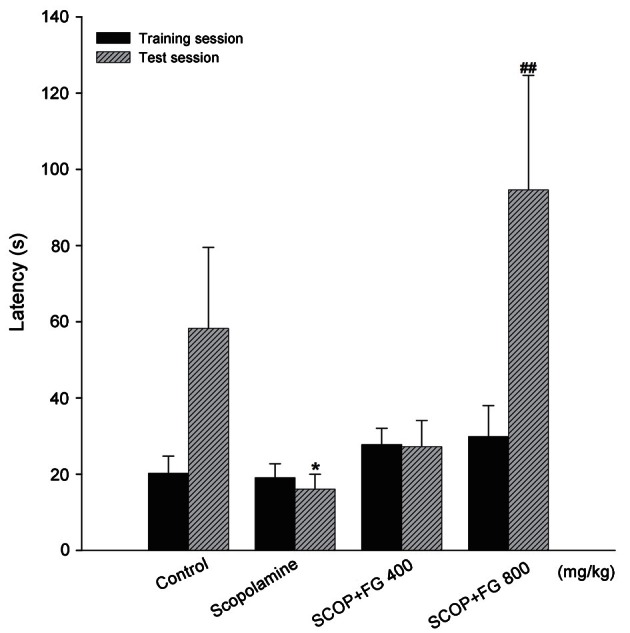
Effect of fermented ginseng extract on performance of the step-through passive avoidance test
ICR mice with scopolamine-induced amnesia were subjected to training with an electrical foot shock session followed by a test session to measure their passive avoidance behavior. As shown in Fig. 3, latency to enter the dark compartment during the training session was not significantly different between the experimental groups. In contrast, during the test session, which was 24 h after the electric foot shock, the retencey to re-enter the dark compartment significantly decreased in the scopolamine-induced amnesia mouse group (16.11±3.87 s) compared with the control group (58.25±21.28 s). The FG extract treatment (800 mg/kg) significantly ameliorated the effect of scopolamine and increased the re-entry time to 94.62±30.01 s.
Fig. 3. Effect of fermented ginseng (FG) extract on step through passive avoidance test. FG extract (400 or 800 mg/kg, per os, daily) was administered to 8-weeks-old ICR mice for 2 wk and 30 min prior to intra peritoneal scopolamine injection (1 mg/kg). Then, the mice underwent the training session for the passive avoidance test 45 min after the scopolamine injection. After 24 h, the test session was performed, and the latency to enter the dark compartment was recorded for up to 300 s. The data represent the mean±SEM (n=10). SCOP, scopolamine treated. *Significantly different (p<0.05) from the control group. ##Significantly different (p<0.01) from the scopolamine control group.
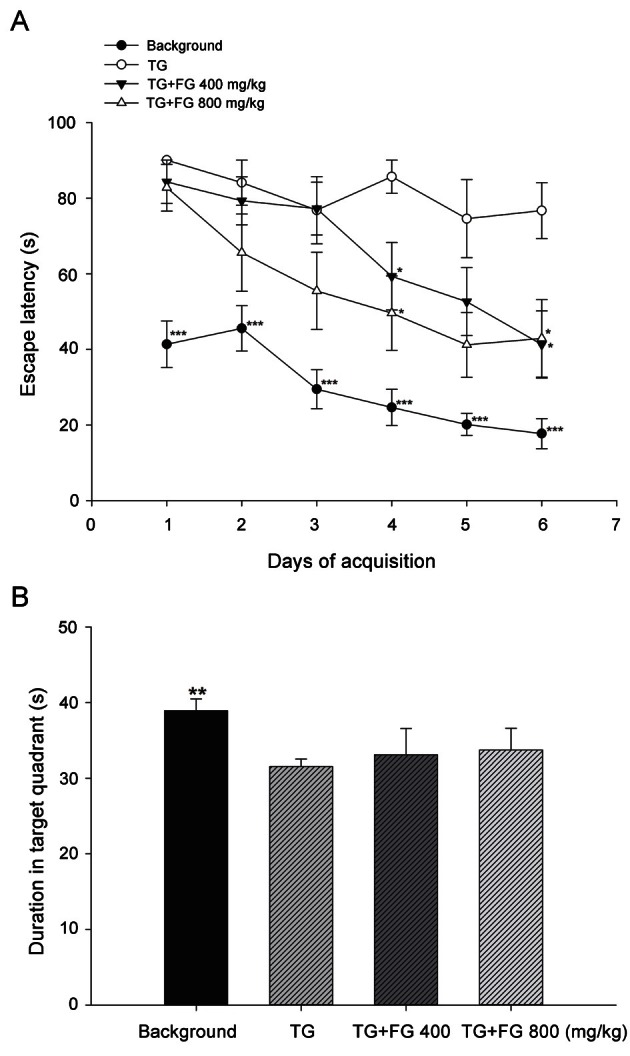
Effect of fermented ginseng extract on performance of the Morris water maze test
To assess spatial memory function, 6 d of acquisition phase was conducted with TG mice. As shown in Fig. 4A, non-TG background mouse group showed a significant increase of memory function from the 1st day (41.36±6.19 s) to the 6th day (17.75±3.98 s) compared with the TG mouse group. In contrast, TG mice showed no progress in finding the escape platform from the 1st day (90.06±0.00 s) to the 6th day (76.69±7.39 s). FG extract treatments, 400 and 800 mg/kg, exhibited significant dose-dependent enhancement of escape latency starting from 84.30±5.75 and 82.76±6.19 s on the 1st day to 41.30±8.90 and 42.90±10.25 s on the 6th day, respectively. Subsequently, spatial memory was tested on the 7th day during the retention phase.
Fig. 4. Effect of fermented ginseng (FG) extract during the Morris water maze test. The FG extract (400 or 800 mg/kg, daily) or saline was administered orally to 7-months-old transgenic (TG) mice for 4 mo and 1 h before the first trial of each acquisition day. The acquisition phase (A) consisted of 6 consecutive days in which each TG mouse accomplished total of 3 trials with a 2 h inter-trial interval. During the acquisition phase, the escape latency, the amount of time in seconds that it took the mouse to find the hidden platform was measured. The retention phase (B) occured the day immediately following the last day of the acquisition phase. During retention phase, the platform was removed, and the mice were allotted 120 s to explore the pool, and the duration of the time spent in the target quadrant, which had contained the escape platform during the acquisition phase, was measured. The data represent the mean±SEM (n=7). *p<0.05, **p<0.01,***p<0.001 from the TG control group.
As shown in Fig. 4B, the duration in the target quadrant, which had contained the escape platform, significantly decreased in the TG mouse group (31.53±0.99 s) compared with the non-TG background mouse group (38.92±1.56 s). However, FG extract 400 and 800 mg/kg treated groups showed a tendency to increase the duration in target quadrant up to 33.10±3.47 and 33.72±2.88 s, respectively, compared with the untreated TG mouse group.
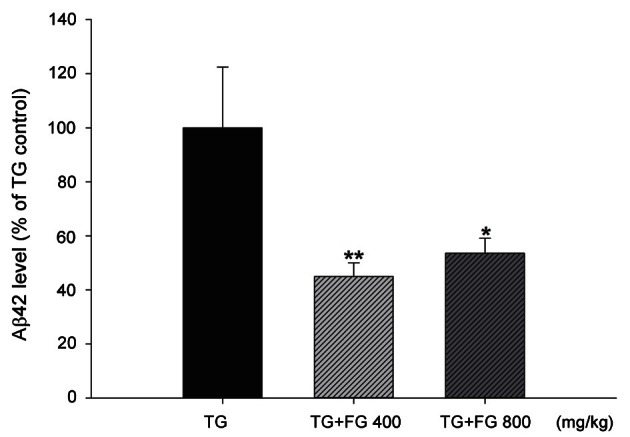
Level of β-amyloid 42 in fermented ginseng-treated transgenic mice
Aβ42, a toxic form of Aβ that increases in AD, increased dramatically in the cerebral cortex of TG mouse group compared with the non-TG background mice. However, FG extract 400 and 800 mg/kg treatment significantly reduced the level of Aβ42 down to 44.95±5.08% and 53.59±5.50%, respectively (Fig. 5).
Fig. 5. Effect of fermented ginseng (FG) extract on the level of soluble β-amyloid (Aβ) 42 protein in the mouse cerebral cortex. Transgenic (TG) mouse brain tissue was collected after behavioral test at 11 mo of age. The brain samples (100 mg) were homogenized in Tris-buffered saline solution (20 mM Tris, 137 mM NaCl, pH 7.4) containing complete protease inhibitors tablets. The extraction ratio (brain tissue:Tris-buffered saline) was 1:5 or 1:10 (w/v). The tissue homogenates were centrifuged at 100,000 g for 1 h at 4℃. Soluble Aβ42 level in the resulting supernatants were measured using the sandwich ELISA kit according to the manufacturer’s protocol. The data represent the mean±SEM (n=7). *p<0.05, **p<0.01 from the TG control group.
DISCUSSION
AD is an age-related neurodegenerative disorder with progressive cognitive dysfunction and characterized by presence of senile plaques in the brain. Aβ is the major component of senile plaques and is considered to have a causal role in the development and progress of AD [19]. Over the years, various research studies have reported, P. ginseng, especially ginsenosides such as Rg1 and Rb1, to exhibit neuroprotection against AD with respect to the Aβ-related symptoms [20-24]. However, other ginsenosides such as Rg3, Rg5, and Rk1 have not been widely studied in the area of neurodegenerative disease. The FG extract used in this study was compared with DG extract due to their different compositions of ginsenosides. We found that FG extract consists of less Re, Rg1, and Rb1 and more Rg3, Rg5, and Rk1 compared with the DG extract. Based on our pilot study, the comparison of various kinds of ginsenosides on the in vitro Aβ reduction effect resulted in Rg3 exhibiting a stronger effect compared with the same concentration of Rg1 (data not shown). The soluble Aβ42 level that was measured in vitro also revealed that the FG extract was more effective at reducing Aβ42 protein than the DG extract. These results suggest that ginsenoside Rg3, Rg5, and Rk1, or the combination of these ginsenosides needs to be further investigated in AD-related models.
To confirm our hypothesis that the FG extract would be effective in animal models of AD, we used two animal models that exhibit amnesia and Aβ accumulation. Through many previous studies, scopolamine treatment is a well-supported model of the learning and memory symptoms found with increasing age and dementia [25-27]. Cholinergic deficits are neuropathological occurrences that are consistently associated with memory loss and are correlated with the severity of AD [28].
In the present study, we first attempted to examine the effect of FG extract on memory impairment induced by scopolamine injection using the step-through passive avoidance test; this test is a useful tool for the estimation of standard learning and memory function [29,30]. The result of this behavioral study indicates that FG extract has a significant ameliorating effect on the scopolamine-induced memory dysfunction, which indicates FG as a possible candidate as a symptomatic treatment for AD.
Although a symptomatic treatment can be effective in delaying the cognitive impairment in AD patients, it is not a fundamental treatment for the disease. Thus, further investigations on the causal mechanism for AD, such as Aβ formation, are needed.
AD-related TG mice with APP and presenilin-1 mutation exhibit increased Aβ formation and Aβ plaques in the brain, which leads to severe cognitive malfunction [31,32]. We attempted to examine the effect of FG extract on TG mice to reveal the effect of prolonged treatment of FG on Aβ formation.
The MWM test, a well-known behavioral task used in various studies [33] to measure spatial memory, was also used in this study. Compared with TG control group, FG extract-treated groups showed consistent learning of the escape platform in the water maze. On the last day of the acquisition phase, the FG extract-treated groups showed a significantly decreased escape latency, which indicates an increased ability to remember the position of escape platform. On the 7th day of the experiment, the retention phase test was conducted. The non-TG background mouse group showed significantly increased swimming time in the target quadrant where the escape platform existed during the previous 6 experimental days. Compared with the non-TG background mouse group, TG control mice showed significantly decreased time spent in the target quadrant. The FG extract treatment, however, showed a tendency to increase the average time spent to find the escape platform compared with the TG control mouse group. In addition, during the probe phase, numbers of zone transition of the swimming mouse from other quadrant areas to target quadrant decreased to 74% compared to non-TG background mouse group. The FG extract treated groups recovered the zone transition rate up to 89% (data not shown).
Because of the spatial memory enhancing effect of FG in the TG mice, we assumed that the effect could be caused by an Aβ reduction in the brain. Therefore the toxic Aβ42 protein, which can induce formation of Aβ plaques in the AD brain [34,35], was measured in the brains of TG mice.
TG control mouse group exhibited significantly increased amount of Aβ42 protein level in the cerebral cortex compared with the non-TG background mouse group. However, FG extract treatment significantly ameliorated the Aβ42 protein level in the brains of the mice.
These results suggest that FG extract can ameliorate memory impairment, which is a symptom that can be found in AD. Furthermore, restoration of cognitive function is partially due to the reduction of Aβ42 protein accumulation in the brain, which was due to the prolonged treatment of FG extract containing Rg3, Rg5, and Rk1, as well as Rg1 and Rb1.
Further research on the mechanisms of FG effects and clinical investigations in AD may lead to FG being a candidate for treatment of AD.
Acknowledgments
This work was funded and supported by the Ministry of Knowledge Economy, Korea (project no. 2M25740), the Korea Institute of Science and Technology, Korea (project no. 2Z03550) and Well-being LS Co., Ltd., Korea.
References
- 1.Chauhan NB, Sandoval J. Amelioration of early cognitive deficits by aged garlic extract in Alzheimer’s transgenic mice. Phytother Res. 2007;21:629–640. doi: 10.1002/ptr.2122. [DOI] [PubMed] [Google Scholar]
- 2.Heo JH, Lee ST, Oh MJ, Park HJ, Shim JY, Chu K, Kim MH. Improvement of cognitive deficit in Alzheimer’sdisease patients by long term treatment with Korean red ginseng. J Ginseng Res. 2011;35:457–461. doi: 10.5142/jgr.2011.35.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selkoe DJ. Toward a comprehensive theory for Alzheimer’s disease. Hypothesis: Alzheimer’s disease is caused by the cerebral accumulation and cytotoxicity of amyloid beta-protein. Ann N Y Acad Sci. 2000;924:17–25. doi: 10.1111/j.1749-6632.2000.tb05554.x. [DOI] [PubMed] [Google Scholar]
- 4.Selkoe DJ. Cell biology of protein misfolding: the examples of Alzheimer’s and Parkinson’s diseases. Nat Cell Biol. 2004;6:1054–1061. doi: 10.1038/ncb1104-1054. [DOI] [PubMed] [Google Scholar]
- 5.Yoon SS, Jo SA. Mechanisms of amyloid-β peptide clearance: potential therapeutic targets for Alzheimer’s disease. Biomol Ther (Seoul) 2012;20:245–255. doi: 10.4062/biomolther.2012.20.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeo HB, Yoon HK, Lee HJ, Kang SG, Jung KY, Kim L. Effects of Korean red ginseng on cognitive and motor function: a double-blind, randomized, placebo-controlled trial. J Ginseng Res. 2012;36:190–197. doi: 10.5142/jgr.2012.36.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jesky R, Hailong C. Are herbal compounds the next frontier for alleviating learning and memory impairments? An integrative look at memory, dementia and the promising therapeutics of traditional chinese medicines. Phytother Res. 2011;25:1105–1118. doi: 10.1002/ptr.3388. [DOI] [PubMed] [Google Scholar]
- 8.Zhao H, Li Q, Zhang Z, Pei X, Wang J, Li Y. Long-term ginsenoside consumption prevents memory loss in aged SAMP8 mice by decreasing oxidative stress and up-regulating the plasticity-related proteins in hippocampus. Brain Res. 2009;1256:111–122. doi: 10.1016/j.brainres.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 9.Tohda C, Matsumoto N, Zou K, Meselhy MR, Komatsu K. Abeta(25-35)-induced memory impairment, axonal atrophy, and synaptic loss are ameliorated by M1, A metabolite of protopanaxadiol-type saponins. Neuropsychopharmacology. 2004;29:860–868. doi: 10.1038/sj.npp.1300388. [DOI] [PubMed] [Google Scholar]
- 10.Sano M, Grossman H, Van Dyk K. Preventing Alzheimer’s disease: separating fact from fiction. CNS Drugs. 2008;22:887–902. doi: 10.2165/00023210-200822110-00001. [DOI] [PubMed] [Google Scholar]
- 11.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 12.Trinh HT, Han SJ, Kim SW, Lee YC, Kim DH. Bifidus fermentation increases hypolipidemic and hypoglycemic effects of red ginseng. J Microbiol Biotechnol. 2007;17:1127–1133. [PubMed] [Google Scholar]
- 13.Jeon WJ, Oh JS, Park MS, Ji GE. Anti-hyperglycemic effect of fermented ginseng in type 2 diabetes mellitus mouse model. Phytother Res. 2012 doi: 10.1002/ptr.4706. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Park JW, Lee JC, Ann S, Seo DW, Choi WS, Yoo YH, Park SK, Choi JY, Um SH, Ahn SH, et al. A fermented ginseng extract, BST204, inhibits proliferation and motility of human colon cancer cells. Biomol Ther (Seoul) 2011;19:211–217. [Google Scholar]
- 15.Yuan HD, Chung SH. Fermented ginseng protects streptozotocin-induced damage in rat pancreas by inhibiting nuclear factor-kappaB. Phytother Res. 2010;24(Suppl 2):S190–S195. doi: 10.1002/ptr.3076. [DOI] [PubMed] [Google Scholar]
- 16.Seo JY, Lee JH, Kim NW, Her E, Chang SH, Ko NY, Yoo YH, Kim JW, Seo DW, Han JW, et al. Effect of a fermented ginseng extract, BST204, on the expression of cyclooxygenase-2 in murine macrophages. Int Immunopharmacol. 2005;5:929–936. doi: 10.1016/j.intimp.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Jung JW, Kang HR, Ji GE, Park MS, Song WJ, Kim MH, Kwon JW, Kim TW, Park HW, Cho SH, et al. Therapeutic effects of fermented red ginseng in allergic rhinitis: a randomized, double-blind, placebo-controlled study. Allergy Asthma Immunol Res. 2011;3:103–110. doi: 10.4168/aair.2011.3.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landman N, Jeong SY, Shin SY, Voronov SV, Serban G, Kang MS, Park MK, Di Paolo G, Chung S, Kim TW. Presenilin mutations linked to familial Alzheimer’sdisease cause an imbalance in phosphatidylinositol 4,5-bisphosphate metabolism. Proc Natl Acad Sci U S A. 2006;103:19524–19529. doi: 10.1073/pnas.0604954103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alkam T, Nitta A, Mizoguchi H, Itoh A, Nabeshima T. A natural scavenger of peroxynitrites, rosmarinic acid, protects against impairment of memory induced by Abeta(25-35). Behav Brain Res. 2007;180:139–145. doi: 10.1016/j.bbr.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Fang F, Chen X, Huang T, Lue LF, Luddy JS, Yan SS. Multi-faced neuroprotective effects of Ginsenoside Rg1 in an Alzheimer mouse model. Biochim Biophys Acta. 2012;1822:286–292. doi: 10.1016/j.bbadis.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi YQ, Huang TW, Chen LM, Pan XD, Zhang J, Zhu YG, Chen XC. Ginsenoside Rg1 attenuates amyloid-beta content, regulates PKA/CREB activity, and improves cognitive performance in SAMP8 mice. J Alzheimers Dis. 2010;19:977–989. doi: 10.3233/JAD-2010-1296. [DOI] [PubMed] [Google Scholar]
- 22.Wang YH, Du GH. Ginsenoside Rg1 inhibits beta-secretase activity in vitro and protects against Abeta-induced cytotoxicity in PC12 cells. J Asian Nat Prod Res. 2009;11:604–612. doi: 10.1080/10286020902843152. [DOI] [PubMed] [Google Scholar]
- 23.Zhao R, Zhang Z, Song Y, Wang D, Qi J, Wen S. Implication of phosphatidylinositol-3 kinase/Akt/glycogen synthase kinase-3β pathway in ginsenoside Rb1’s attenuation of beta-amyloid-induced neurotoxicity and tau phosphorylation. J Ethnopharmacol. 2011;133:1109–1116. doi: 10.1016/j.jep.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Liu J, Zhang Z, Bi P, Qi Z, Zhang C. Anti-neuroinflammation effect of ginsenoside Rbl in a rat model of Alzheimer disease. Neurosci Lett. 2011;487:70–72. doi: 10.1016/j.neulet.2010.09.076. [DOI] [PubMed] [Google Scholar]
- 25.Araujo JA, Studzinski CM, Milgram NW. Further evidence for the cholinergic hypothesis of aging and dementia from the canine model of aging. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:411–422. doi: 10.1016/j.pnpbp.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Fischer W, Chen KS, Gage FH, Bjorklund A. Progressive decline in spatial learning and integrity of forebrain cholinergic neurons in rats during aging. Neurobiol Aging. 1992;13:9–23. doi: 10.1016/0197-4580(92)90003-g. [DOI] [PubMed] [Google Scholar]
- 27.Tariot PN, Patel SV, Cox C, Henderson RE. Age-related decline in central cholinergic function demonstrated with scopolamine. Psychopharmacology (Berl) 1996;125:50–56. doi: 10.1007/BF02247392. [DOI] [PubMed] [Google Scholar]
- 28.Giacobini E. The cholinergic system in Alzheimer disease. Prog Brain Res. 1990;84:321–332. doi: 10.1016/s0079-6123(08)60916-4. [DOI] [PubMed] [Google Scholar]
- 29.Bejar C, Wang RH, Weinstock M. Effect of rivastigmine on scopolamine-induced memory impairment in rats. Eur J Pharmacol. 1999;383:231–240. doi: 10.1016/s0014-2999(99)00643-3. [DOI] [PubMed] [Google Scholar]
- 30.Fibiger HC. Cholinergic mechanisms in learning, memory and dementia: a review of recent evidence. Trends Neurosci. 1991;14:220–223. doi: 10.1016/0166-2236(91)90117-d. [DOI] [PubMed] [Google Scholar]
- 31.Epis R, Gardoni F, Marcello E, Genazzani A, Canonico PL, Di Luca M. Searching for new animal models of Alzheimer’s disease. Eur J Pharmacol. 2010;626:57–63. doi: 10.1016/j.ejphar.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 32.Woodruff-Pak DS. Animal models of Alzheimer’s disease: therapeutic implications. J Alzheimers Dis. 2008;15:507–521. doi: 10.3233/jad-2008-15401. [DOI] [PubMed] [Google Scholar]
- 33.Sharma S, Rakoczy S, Brown-Borg H. Assessment of spatial memory in mice. Life Sci. 2010;87:521–536. doi: 10.1016/j.lfs.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galimberti D, Scarpini E. Alzheimer’s disease: from pathogenesis to disease-modifying approaches. CNS Neurol Disord Drug Targets. 2011;10:163–174. doi: 10.2174/187152711794480438. [DOI] [PubMed] [Google Scholar]
- 35.Evin G, Barakat A, Masters CL. BACE: therapeutic target and potential biomarker for Alzheimer’s disease. Int J Biochem Cell Biol. 2010;42:1923–1926. doi: 10.1016/j.biocel.2010.08.017. [DOI] [PubMed] [Google Scholar]


