Abstract
Korean red ginseng, the processed root of Panax ginseng Meyer, has been frequently used for various therapeutic purposes in oriental medicine. The present study investigated the possible effect of Korean red ginseng extract (RGE) for the treatment of liver fibrosis in mice injected with carbon tetrachloride (CCl4) for 4 wk. Liver injuries were assessed by blood biochemistry and histopathology in mice treated with CCl4 alone or CCl4+ RGE (30, 100, and 300 mg/kg). Concomitant treatment with RGE and CCl4 (three times/wk for 4 wk) effectively inhibited liver fibrosis as evidenced by decreases in plasma alanine and aspartate aminotransferases, as well as by the percentages of degenerative regions, numbers of degenerative hepatocytes, and collagen accumulation in hepatic parenchyma. Treatment with CCl4 for 4 wk increased mRNA levels of transforming growth factor β1 and plasminogen activator inhibitor 1 in fibrogenic liver, whereas RGE (30, 100, and 300 mg/kg) significantly blocked the induction of fibrogenic genes by CCl4. Similarly, RGE also prevented transforming growth factor β1-mediated induction of fibrogenic genes in human hepatic stellate cell lines. More importantly, RGE markedly reduced the number of α-smooth muscle actin-positive cells in liver tissue. This study implies that RGE efficaciously protects against the liver fibrosis induced by chronic CCl4 treatment, and may therefore have potential to treat liver disease.
Keywords: Panax ginseng, Korean red ginseng, Liver fibrosis, Transforming growth factor β1, α-smooth muscle actin
INTRODUCTION
Liver fibrosis is the pathophysiological response in the liver to a line of insults such as toxic, infectious, or metabolic agents [1]. Hepatic fibrosis is characterized by excessive accumulation of extracellular matrix (ECM) including collagen, which is found in most types of chronic liver diseases [1,2]. Accumulated ECM proteins form a fibrous scar that contorts the architecture of the liver. In general, chronic liver injury produces fibrosis of the liver, followed by cirrhosis, which is defined by the development of nodules of regenerating hepatocytes [1,2]. Cirrhosis induces hepatocellular dysfunction and may result in hepatocellular carcinoma, which is one of the most common cancers worldwide [3,4]. Despite the importance of liver fibrosis in human health, there is no efficient agent for clinical application to inhibit the progression of liver fibrosis yet.
It has been shown that carbon tetrachloride (CCl4) has been used to induce liver injury in various animals through its direct harmful effects on hepatocytes [5-8]. CCl4 changes the mitochondria and plasma membrane permeability, and forms highly reactive free radicals probably through the CYP2E1 pathway [6]. A single injection of CCl4 causes hepatocyte necrosis in zone 3 of the liver lobule, and repeated treatments over long periods induce chronic liver diseases (e.g., fibrosis, cirrhosis, and cancer) [7,8]. During the development of liver fibrosis, activated hepatic stellate cells (HSCs) have important roles in the progression of the disease as they deposit ECM [1]. The activation of HSCs is mediated by profibrogenic cytokines such as transforming growth factor β1 (TGFβ1) and platelet-derived growth factor (PDGF) [1,2]. TGFβ1 regulates growth and differentiation of HSCs and is a crucial mediator in the fibrogenic process [2,9]. TGFβ1 is also a key stimulator of fibrogenesis after hepatic injuries via ECM deposition through synthesis of ECM proteins and inhibition of collagenase activity [1,2,9].
Korean ginseng (Panax ginseng Meyer) is one of the oldest and most frequently used botanicals in traditional oriental medicine. Korean ginseng extract is recommended for life-enhancing properties and for increasing energy and longevity. Korean red ginseng is P. ginseng that has been heat-processed to enhance its biological and pharmacological activities [10]. Studies have shown that red ginseng has beneficial effects in the treatment of immune diseases, metabolic and neurodegenerative disorders, and neoplasm [11-13]. Especially, it has been reported that ginsenoside Rb1, 25-OCH3-PPD and Rg1 from P. notoginseng inhibit the activation of HSCs and induce their apoptosis [14-16]. However, the effects of Korean red ginseng on liver destruction and on the expression of fibrogenic genes have not been fully established. The present study was designed to verify whether Korean red ginseng extract (RGE) inhibits liver fibrogenesis in the well-established mouse fibrosis model by CCl4 injections for 4 wk. We determined the effects of RGE on hepatocyte protection through blood biochemical and histological analyses. We also assessed collagen accumulation and expression of TGFβ1 in the liver in response to CCl4. In terms of the wide applications of RGE, this finding suggests the potential of Korean red ginseng for the treatment of chronic liver disease as a new drug candidate.
MATERIALS AND METHODS
Materials
RGE was kindly provided by Central Research Institute, Korea Ginseng Corporation (Daejeon, Korea).
Animal and treatment
C57BL6 mice were obtained from Oriental Bio (Seongnam, Korea) and acclimatized for 1 wk. Mice (N=5/group) were concomitantly treated with CCl4 with or without RGE for 4 wk. To induce liver fibrosis, CCl4 dissolved in olive oil (10%) was intraperitoneally injected (2 mL/kg) into the mice three times per week for 4 wk. RGE (30, 100, or 300 mg/kg RGE) dissolved in water was administered orally 30 min before CCl4 injection. After the multiple CCl4 or CCl4+RGE administrations, the mice were sacrificed on day 28, and the livers were excised. Blood was collected and assayed using an automated blood biochemistry analyzer (Abbott Laboratories, Abbott Park, IL, USA).
Cell culture and treatment
The immortalized human HSCs cell line, LX-2 cells, was kindly provided by Dr. SL Friedman (Mount Sinai School of Medicine, New York, NY, USA). The cells were maintained in Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum, 100 U/mL penicillin and 100 μg/mL streptomycin at 37℃ in a humidified atmosphere containing 5% CO2. To examine the effect of RGE on the induction of fibrogenic genes, cells were pretreated with 0.3 or 1 mg/mL RGE for 30 min and then further incubated with 5 ng/mL TGFβ1 (R&D Systems, Minneapolis, MN, USA) for 12 h.
Histological process
Liver samples from the left lateral and median lobes were separated and fixed in 10% neutral buffered formalin, then embedded in paraffin, sectioned (3 to 4 μm) and stained with hematoxylin and eosin for general observation or with Masson’s trichrome for collagen fibers; the histopathological profiles of each sample were then observed under a light microscope (Nikon, Tokyo, Japan). The percentage of degenerative regions in the liver showing centrolobular necrosis, congestion, fibrosis, and inflammatory cell infiltration in hepatic lobules (%/mm2 of hepatic parenchyma) were calculated by automated image analysis (DMI-300 Image Processing; DMI, Daegu, Korea). In addition, the number of hepatocytes showing degenerative changes, such as necrosis, acute cellular swelling (ballooning), and severe fatty changes (cells/1,000 hepatocytes) was also calculated with collagen deposited percentages (%/mm2 of hepatic parenchyma) in uniform areas, using an automated image analyzer. The certified histopathologist was blinded to the group distribution when this analysis was made.
Realtime polymerase chain reaction analyses
Total RNA was isolated from liver tissue or LX-2 cells by using Trizol reagent (Invitrogen, Carlsbad, CA, USA). The RNA (2 μg each) was reverse-transcribed using oligo-d(T)16 primers to obtain cDNA. TGFβ1 and plasminogen activator inhibitor 1 (PAI-1) genes were amplified by using specific primers. Realtime polymerase chain reaction (PCR) was carried out according to the manufacturer’s instructions (7500 system, ABI). The relative levels of mouse TGFβ1 (sense: 5’- GCCCTGGATACCAACTATTGC-3’, antisense: 5’-GCAGGAGCGCACAATCATGTT-3’, 327 bp), mouse PAI-1 (sense: 5’-GACACCCTCAGCATGTTCATC-3’, antisense: 5’-AGGGTTGCACTAAACATGTCAG-3’, 208 bp), human TGFβ1 (sense: 5’-GGCAGTGGTTGAGCCGTGGA-3’, antisense: TGTTGGACAGCTGCTCCACCT, 531 bp) and human PAI-1 (sense: 5’-TCGTCCAGCGGGATCTGA-3’, antisense: 5’-CCTGGTCATGTTGCCTTTC-3’, 512 bp) were normalized to the level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). After PCR amplification, the melting curve of each amplicon was determined to verify its accuracy [17].
Immunohistochemistry
Liver sections were deparaffinized and then pretreated with 10 mM citrate buffer (pH 6.0). After inactivation of endogenous peroxidase and blocking with normal horse serum (Vector Lab Inc., Burlingame, CA, USA), tissue sections were incubated with primary antibody against α-smooth muscle actin (α-SMA) (Sigma-Aldrich, St. Louis, MO, USA) overnight. Sections were then stained using avidin-biotin complex methods (Vector Lab Inc.). Cells occupied by over 10% of α-SMA immunoreactivities, in terms of density, were regarded as positive, and the numbers of positive cells were calculated in the both lateral and median lobes of the liver in the central vein regions by automated image analyzer (DMI-300, DMI) [17,18].
Data analysis
One-way analysis of variance procedures was used to assess significant differences among treatment groups. For each significant treatment effect, the Newman-Keuls test was utilized to compare multiple group means.
RESULTS
Effects of Korean red ginseng extract on hepatocyte injury
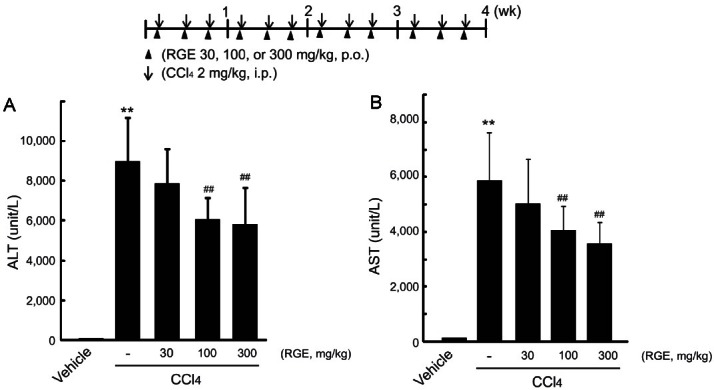
CCl4 exerts liver toxicity through the formation of highly reactive toxic metabolites produced by oxidative metabolism via CYP2E1, which induces severe centrilobular hepatocyte necrosis and subsequent fibrosis in the liver [6-8]. First, we determined the effects of RGE against the liver injury induced by CCl4. Alanine aminotransferases (ALT) and aspartate aminotransferases (AST) are cytosolic enzymes present mainly in the liver, and an increase in plasma ALT or AST indicates damage to the liver [19]. Intraperitoneal injection of CCl4 at a dose of 2 mL/kg (three times per week) for 4 wk successfully induced hepatocyte damage and liver injury in mice, as evidenced by distinct increases in the plasma levels of ALT and AST (Fig. 1A, B). Concomitant treatment with RGE at doses of 30, 100, and 300 mg/kg (three times per week, per oral) significantly decreased the levels of the plasma aminotransferases increased by CCl4 treatments.
Fig. 1. Effects of Korean red ginseng extract (RGE) on liver function in carbon tetrachloride (CCl4)-treated fibrotic mice. Mice (N=5/group) were injected with 10% CCl4 in olive oil (2 mL/kg) intraperitoneally three times per week and administered RGE (30 to 300 mg/kg) orally three times per week for 4 wk (inset). After 4-week treatment, the activities of alanine aminotransferase (ALT) (A) and aspartate aminotransferase (AST) (B) were assayed by using an automated blood chemistry analyzer. Data represent the mean±SD of 5 mice (significant compared to vehicle-treated group, **p<0.01; significant compared to CCl4-treated group, ##p<0.01). p.o., per oral; i.p., intraperitoneal.
Histopathological analysis
To determine the hepatoprotective effects of RGE against CCl4, we conducted histological examination of the extent of liver damage. Vehicle-treated control mice had no pathological changes both in the lateral and median lobes (Fig. 2A, B).
Fig. 2. The representative histological profiles of the liver sections. The liver sections from lateral (A) and median (B) lobes were stained with hematoxylin and eosin (H&E) in mice treated with vehicle, carbon tetrachloride (CCl4), CCl4+ Korean red ginseng extract (RGE) 30 mg/kg, CCl4+RGE 100 mg/kg, or CCl4+RGE 300 mg/kg. Accumulated collagen in liver sections was stained by Masson’s trichrome staining. The scale bars indicate 160 μm.
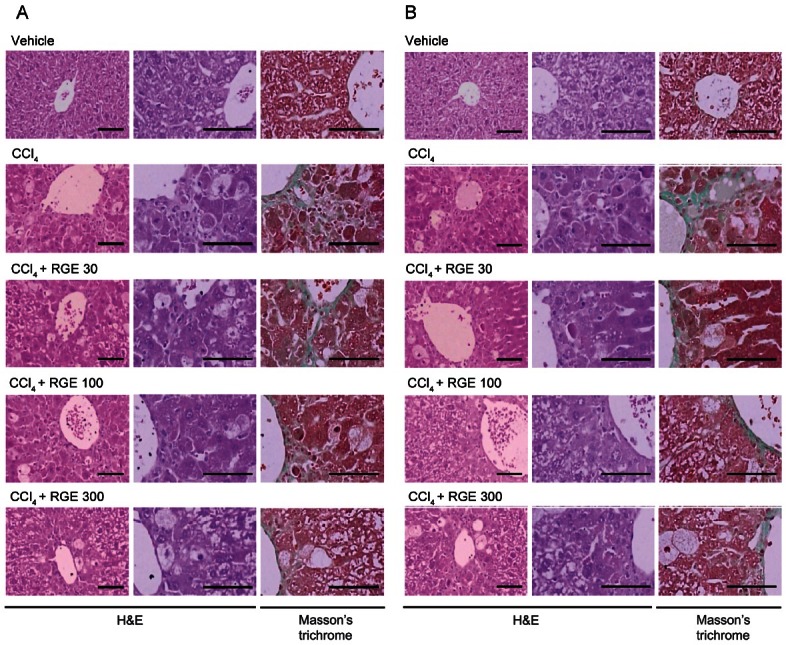
As progress of CCl4 intoxication, degenerative changes in the liver-centrolobular necrosis including ballooning of hepatocytes, deposition of lipid droplets in hepatocytes (fatty changed cells), and infiltration of inflammatory cells were detected with collagen depositions. These CCl4-related hepatic damages were reconfirmed by histomorphometry as the percentages of degenerative regions, the numbers of degenerative hepatocytes, and the percentages of collagen deposits in the hepatic parenchyma, which were significantly (p<0.01) increased in CCl4-treated mice compared with vehicle-treated mice. These histological approaches verified that treatment of mice with RGE (30, 100, and 300 mg/kg, per oral) had a beneficial effect against CCl4-induced liver injury in both the lateral and median lobes of the liver (Fig. 2A, B; Table 1). Increases in histomorphometrical scores for regions and cell numbers of degenerative hepatocytes by CCl4 were all inhibited by concomitant treatment with RGE, which reduced the scores by 50% (Table 1). Maximal inhibition of hepatocyte degeneration was obtained at both 100 and 300 mg/kg of RGE, which had similar effects in the aspect of hepatocyte protection (Table 1).
Table 1.
Changes on the histomorphometrical analysis of liver
| Groups | Percentages of degenerative regions (%/mm2 of hepatic parenchyma) | Numbers of degenerative hepatocytes (cells/1000 hepatocytes) | Collagen deposited percentages (%/mm2 of hepatic parenchyma) |
|---|---|---|---|
|
| |||
| Lateral lobes | |||
| Vehicle | 0.20±0.12 | 5.80±2.05 | 0.34±0.11 |
| CCl4 | 66.90±15.10** | 159.20±58.41** | 11.86±3.48** |
| CCl4 + RGE 30 mg/kg | 42.94±14.70**,## | 103.20±29.45** | 4.72±1.20**,## |
| CCl4 + RGE 100 mg/kg | 30.64±16.05**,## | 89.60±20.90**,## | 3.82±0.94**,## |
| CCl4 + RGE 300 mg/kg | 28.40±10.83**,## | 91.20±11.80**,## | 3.34±1.32**,## |
| Median lobes | |||
| Vehicle | 0.84±1.00 | 13.60±10.33 | 0.32±0.19 |
| CCl4 | 57.48±12.05** | 168.80±30.78** | 10.04±0.97** |
| CCl4 + RGE 30 mg/kg | 41.04±8.52**,# | 112.80±33.75**,## | 6.92±1.27**,## |
| CCl4 + RGE 100 mg/kg | 34.70±12.11**,# | 94.40±30.54**,## | 5.16±1.27**,## |
| CCl4 + RGE 300 mg/kg | 35.42±9.86**,# | 102.40±23.43**,## | 5.12±2.55**,## |
All values were expressed as mean±SD of five mice (significant as compared to vehicle-treated group, **p<0.01; significant as compared to CCl4-treated group ##p<0.01, #p<0.05).
CCl4, carbon tetrachloride; RGE, red ginseng extract.
Effects of Korean red ginseng extract on collagen deposition
It has been shown that CCl4 induces liver fibrosis [8]. The extent of liver fibrosis was histologically examined after 4 wk of CCl4 or CCl4+RGE treatments. Mice treated with CCl4 exhibited marked fibrosis in the liver (Fig. 2 and Table 1). Masson’s trichrome staining, which was used to assess collagen deposition, revealed that mice treated with CCl4 showed typical morphological changes of fibrosis in the liver (Fig. 2) [18]. Treatment of mice with RGE significantly decreased the extent of liver fibrosis. Statistical analysis confirmed that the liver fibrosis induced by CCl4 was inhibited by RGE, as evidenced by decreases in collagen deposition. Treatment with 300 mg/kg of RGE distinctly inhibited the scores of collagen deposition in the lateral lobes (i.e., 11.86 vs. 3.34%/mm2 of hepatic parenchyma) (Table 1).
Effects of Korean red ginseng extract on transforming growth factor β1 and plasminogen activator inhibitor 1 expressions
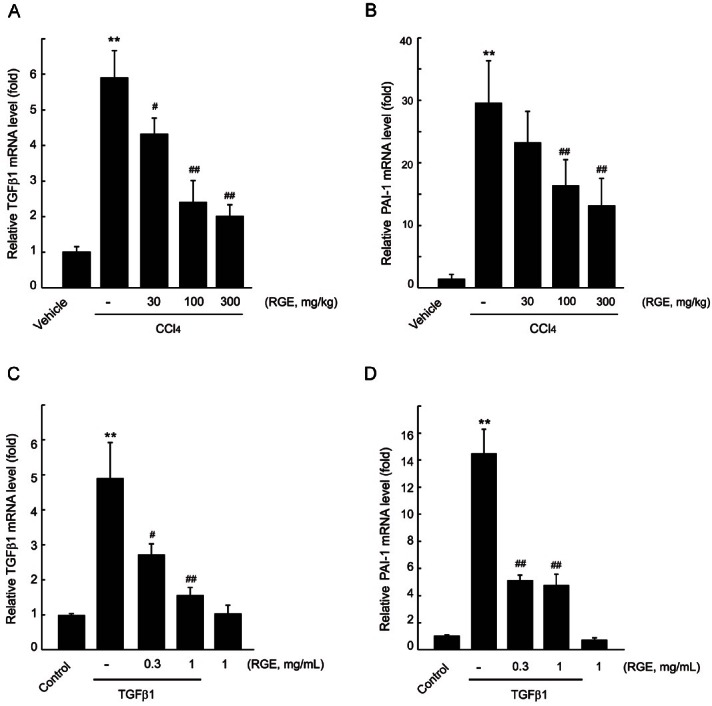
TGFβ1 is an important regulator of fibrogenesis in various organs, and expression of TGFβ1 protein is regulated by TGFβ1 translational process and post-translational modification [2,9]. It has been shown that quantification of TGFβ1 mRNA level is generally accepted as a marker of the TGFβ1 activity [20]. TGFβ1 is involved in the induction of PAI-1 as a major regulator, and PAI-1 is related to organ fibrosis and metabolic disorders [21,22]. Hence, we determined the expressions of TGFβ1 and PAI-1 mRNA in the liver by realtime PCR analysis. Treatment with CCl4 for 4 wk increased the mRNA levels of TGFβ1 and PAI-1 by 6- and 30-fold, respectively. RGE treatment at doses of 30, 100, and 300 mg/kg prevented the elevation of TGFβ1 and PAI-1 mRNA levels in the liver (Fig. 3A, B). To validate the effect of RGE on induction of fibrogenic genes, we used immortalized human HSCs, LX-2 cells. TGFβ1 treatment (5 ng/mL, 12 h) in LX-2 cells increased the expression of TGFβ1 and PAI-1, and those were also significantly inhibited by 0.3 and 1 mg/mL RGE pretreatment (Fig. 3C, D).
Fig. 3. Effects of Korean red ginseng extract (RGE) on fibrogenic gene expressions. Realtime polymerase chain reaction (PCR) was assessed to investigate mRNA level of transforming growth factor β1 (TGFβ1) (A) and plasminogen activator inhibitor 1 (PAI-1) (B) in the liver. Data represent the mean±SD of 5 mice (significant compared to vehicle-treated group, **p<0.01; significant compared to carbon tetrachloride [CCl4]-treated group, #p<0.05 or ##p<0.01). The effect of RGE on TGFβ1-induced TGFβ1 (C) and PAI-1 (D) expressions in LX-2 cells was also monitored by realtime PCR analysis. Data represent the mean±SD of three separated experiments (significant compared to vehicle-treated cells, **p<0.01; significant compared to TGFβ1-treated cells, #p<0.05 or ##p<0.01).
Immunohistochemistry of α-smooth muscle actin
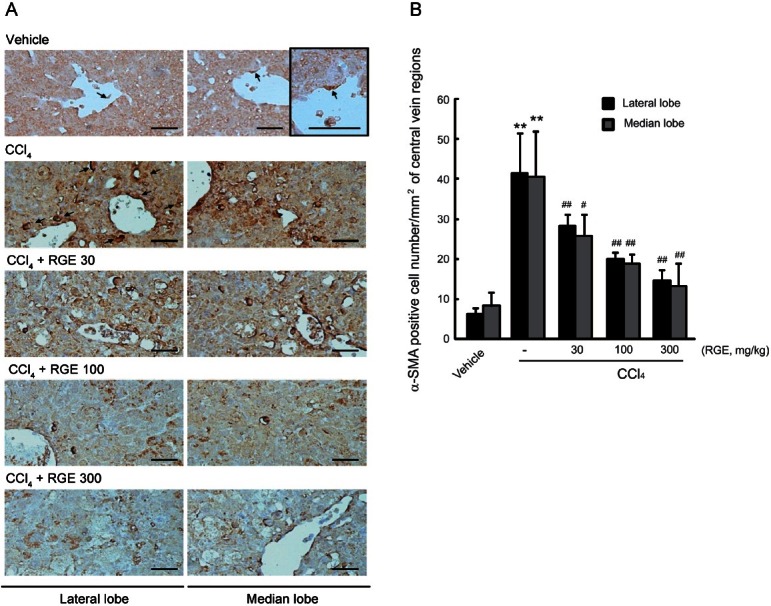
α-SMA is a key marker of liver fibrosis [17]. In previous studies, activated HSCs were stained with α-SMA, and their localization in the liver was around the accumulated fibers [17,23]. In this study, we assessed the immunohistochemistry of α-SMA in the fibrotic liver induced by CCl4 injection for 4 wk. Administration of CCl4 markedly induced the expression of α-SMA in both the lateral and median lobes of the livers (Fig. 4A). However, oral treatment with RGE (30, 100, and 300 mg/kg) significantly decreased the number of α-SMA-positive cells in the dose dependent manner (Fig. 4B).
Fig. 4. Effect of Korean red ginseng extract (RGE) on the level of α-smooth muscle actin (α-SMA)-positive cells. (A) Representative immunohistochemical staining of α-SMA-positive cells (arrows) in lateral and median lobes. The scale bars indicate 160 μm. (B) Changes in the number of α-SMA-immunoreactive cells in the hepatic central vein regions. Data represent the mean±SD of 5 mice (significant compared to vehicle-treated animal **p<0.01; significant compared to carbon tetrachloride [CCl4]-treated animal #p<0.05 or ##p<0.01).
DISCUSSION
A line of animal models of liver fibrosis and cirrhosis has been generated using chemicals (e.g., CCl4 or dimethylnitrosamine). CCl4-induced liver fibrosis is a well-established model in terms of its detrimental effects and mechanistic basis in animals [5,8,24]. Using this model, we demonstrated that administration of RGE prevented the development of liver fibrosis induced by CCl4 in mice. We incorporated two approaches: 1) histological analysis of liver fibrosis and 2) quantitative measurement of fibrogenic gene expression by PCR and immunohistochemistry.
Liver fibrosis is a pathological process in response to chronic liver injury such as alcohol, cholestasis, chronic hepatitis, and drugs, and is the final result of the wound-healing process in the tissue [1,2]. After an acute liver injury (e.g., CCl4), hepatocytes regenerate and/or replace the degenerative cells, which involves the inflammatory process. If the hepatic injuries are repeated, the parenchymal cells are replaced with abundant ECM, of which alterations in quantity and composition are major characteristics of liver fibrosis [1,2,9]. In the present study, CCl4 successfully induced liver fibrosis, as indicated by parameters of hepatocyte damage (i.e., plasma ALT and AST; hepatocyte degeneration) and collagen deposition (i.e., Masson’s trichrome staining). Concomitant treatment with RGE significantly inhibited regions and cell numbers of degenerative hepatocytes as well as collagen deposition induced by CCl4, showing that RGE has anti-fibrotic effects in mice.
Several lines of study indicate that TGFβ1 plays a key role in increasing the synthesis of ECM proteins and cellular receptors for various matrix proteins [9]. In the process of liver fibrogenesis, increased TGFβ1 levels stimulate expression of PAI-1 [25]. Induction of PAI-1 contributes to accumulation of ECM as a result of matrix metalloproteinase inhibition, which results in the progression of tissue remodeling [21,22]. Therefore, elevations in the levels of TGFβ1 and PAI-1 in the liver are a feature of hepatic fibrosis. In this fibrosis model, CCl4 markedly induced TGFβ1 and PAI-1 mRNA levels in the liver, and treatment with RGE significantly inhibited the ability of CCl4 to increase the levels of TGFβ1 and PAI-1 expression in the fibrotic liver. Moreover, it has been shown that TGFβ1 stimulates collagen synthesis during fibrogenesis from fibroblast and myofibroblast cells (e.g., activated stellate cells) [1,2]. Therefore, the inhibitory effects of RGE on ECM accumulation and the fibrotic genes might result in regulation of HSC transactivation.
HSCs are the major source of the ECM proteins characterizing liver fibrosis [1,2,9]. Although quiescent HSCs show no fibrogenic phenotype in the normal state, repeated damage and fibrogenic cytokines (e.g., TGFβ1) stimulate transactivation of HSCs to myofibroblasts, which play an important role in wound healing and organ structure remodeling [2,26]. Activation of HSCs generally shows major phenotype changes, including increased expression of α-SMA, a marker of transdifferentiation [17,23,26]. In this study, RGE actively decreased the number of α-SMA-positive cells in fibrotic liver induced by CCl4, indicating that RGE inhibited transactivation of HSCs in fibrotic liver. In addition, RGE antagonized significant increases in TGFβ1 and PAI-1 mRNA expressions, which play a key role in activating HSCs. These results, in conjunction with the marked inhibition of CCl4-induced collagen disposition by RGE, imply that the anti-fibrotic effects of RGE might result from both inhibition of fibrotic gene expression and transdifferentiation of HSCs.
Several molecular targets to prevent HSC activation have been actively studied, and those might be associated with the therapeutic target of Korean red ginseng. Among them, it has been reported that ginsenoside Rg1 reduces the expression of PDGF receptor beta by attenuating nuclear factor kB activity, which might inhibit HSC proliferation [16]. In addition, several lines of evidence indicate that adenosine monophosphate-activated protein kinase, which is also activated both by ginseng and its active components [27-29], inhibits profibrogenic gene induction by interrupting the interaction between Smad 3 and coactivator p300 [30], and blocks PDGF-mediated proliferation in HSCs [31]. Therefore, the effects of RGE on upstream signaling molecules and the pharmacological target of RGE in HSCs remain to be established. The exact mechanistic basis of RGE needs to be clarified in the future.
In summary, our research results verify that RGE exerts anti-fibrotic effects in mice. Treatment with RGE for 4 wk inhibited hepatocyte degeneration and collagen accumulation. Moreover, administration of RGE markedly blocked TGFβ1 and PAI-1 expression as well as immunohistochemistry of α-SMA, one of the representative markers of HSC transactivation. The findings presented here imply that the effects of RGE on liver fibrosis might result, at least in part, from inhibition of HSC activation. This study offers the possibility of RGE treatment for fibrotic liver disease, although its effects on liver disease might be preventive in nature.
Acknowledgments
This work was supported by a 2010 grant from the Korean Society of Ginseng and by the National Research Foundation of Korea funded by the Korean government (MEST) (grant no. 2012-0009405).
References
- 1.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 4.Simonetti RG, Camma C, Fiorello F, Politi F, D’Amico G, Pagliaro L. Hepatocellular carcinoma. A worldwide problem and the major risk factors. Dig Dis Sci. 1991;36:962–972. doi: 10.1007/BF01297149. [DOI] [PubMed] [Google Scholar]
- 5.Tamayo RP. Is cirrhosis of the liver experimentally produced by CCl4 and adequate model of human cirrhosis? Hepatology. 1983;3:112–120. doi: 10.1002/hep.1840030118. [DOI] [PubMed] [Google Scholar]
- 6.Manibusan MK, Odin M, Eastmond DA. Postulated carbon tetrachloride mode of action: a review. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2007;25:185–209. doi: 10.1080/10590500701569398. [DOI] [PubMed] [Google Scholar]
- 7.Shi J, Aisaki K, Ikawa Y, Wake K. Evidence of hepatocyte apoptosis in rat liver after the administration of carbon tetrachloride. Am J Pathol. 1998;153:515–525. doi: 10.1016/S0002-9440(10)65594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pierce RA, Glaug MR, Greco RS, Mackenzie JW, Boyd CD, Deak SB. Increased procollagen mRNA levels in carbon tetrachloride-induced liver fibrosis in rats. J Biol Chem. 1987;262:1652–1658. [PubMed] [Google Scholar]
- 9.Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76–99. doi: 10.1111/j.1582-4934.2006.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HY, Kang KS, Yamabe N, Yokozawa T. Comparison of the effects of Korean ginseng and heat-processed Korean ginseng on diabetic oxidative stress. Am J Chin Med. 2008;36:989–1004. doi: 10.1142/S0192415X08006417. [DOI] [PubMed] [Google Scholar]
- 11.Kaneko H, Nakanishi K. Proof of the mysterious efficacy of ginseng: basic and clinical trials. Clinical effects of medical ginseng, Korean red ginseng: specifically, its anti-stress action for prevention of disease. J Pharmacol Sci. 2004;95:158–162. doi: 10.1254/jphs.fmj04001x5. [DOI] [PubMed] [Google Scholar]
- 12.Heo JH, Lee ST, Oh MJ, Park HJ, Shim JY, Chu K, Kim MH. Improvement of cognitive deficit in Alzheimer’s disease patients by long term treatment with Korean red ginseng. J Ginseng Res. 2011;35:457–461. doi: 10.5142/jgr.2011.35.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seo EY, Kim WK. Red ginseng extract reduced metastasis of colon cancer cells in vitro and in vivo. J Ginseng Res. 2011;35:315–324. doi: 10.5142/jgr.2011.35.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu YL, Wan Y, Jin XJ, OuYang BQ, Bai T, Zhao YQ, Nan JX. 25-OCH3-PPD induces the apoptosis of activated t-HSC/Cl-6 cells via c-FLIP-mediated NF-κB activation. Chem Biol Interact. 2011;194:106–112. doi: 10.1016/j.cbi.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Lo YT, Tsai YH, Wu SJ, Chen JR, Chao JC. Ginsenoside Rb1 inhibits cell activation and liver fibrosis in rat hepatic stellate cells. J Med Food. 2011;14:1135–1143. doi: 10.1089/jmf.2010.1485. [DOI] [PubMed] [Google Scholar]
- 16.Geng J, Peng W, Huang Y, Fan H, Li S. Ginsenoside-Rg1 from Panax notoginseng prevents hepatic fibrosis induced by thioacetamide in rats. Eur J Pharmacol. 2010;634:162–169. doi: 10.1016/j.ejphar.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Cho IJ, Kim YW, Han CY, Kim EH, Anderson RA, Lee YS, Lee CH, Hwang SJ, Kim SG. E-cadherin antagonizes transforming growth factor β1 gene induction in hepatic stellate cells by inhibiting RhoA-dependent Smad3 phosphorylation. Hepatology. 2010;52:2053–2064. doi: 10.1002/hep.23931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho IJ, Kim SH, Kim SG. Inhibition of TGFbeta1-mediated PAI-1 induction by oltipraz through selective interruption of Smad 3 activation. Cytokine. 2006;35:284–294. doi: 10.1016/j.cyto.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Rej R. Aspartate aminotransferase activity and isoenzyme proportions in human liver tissues. Clin Chem. 1978;24:1971–1979. [PubMed] [Google Scholar]
- 20.Kruse JJ, Bart CI, Leer JW, Wondergem J. Detection and quantitative measurement of transforming growth factor-beta1 (TGF-beta1) gene expression using a semi-nested competitive PCR assay. Cytokine. 1999;11:179–185. doi: 10.1006/cyto.1998.0413. [DOI] [PubMed] [Google Scholar]
- 21.Seki T, Imai H, Uno S, Ariga T, Gelehrter TD. Production of tissue-type plasminogen activator (t-PA) and type-1 plasminogen activator inhibitor (PAI-1) in mildly cirrhotic rat liver. Thromb Haemost. 1996;75:801–807. [PubMed] [Google Scholar]
- 22.Hagiwara H, Kaizu K, Uriu K, Noguchi T, Takagi I, Qie YL, Seki T, Ariga T. Expression of type-1 plasminogen activator inhibitor in the kidney of diabetic rat models. Thromb Res. 2003;111:301–309. doi: 10.1016/j.thromres.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Kang KW, Kim YG, Cho MK, Bae SK, Kim CW, Lee MG, Kim SG. Oltipraz regenerates cirrhotic liver through CCAAT/enhancer binding protein-mediated stellate cell inactivation. FASEB J. 2002;16:1988–1990. doi: 10.1096/fj.02-0406fje. [DOI] [PubMed] [Google Scholar]
- 24.Fujii T, Fuchs BC, Yamada S, Lauwers GY, Kulu Y, Goodwin JM, Lanuti M, Tanabe KK. Mouse model of carbon tetrachloride induced liver fibrosis: histopathological changes and expression of CD133 and epidermal growth factor. BMC Gastroenterol. 2010;10:79. doi: 10.1186/1471-230X-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM. Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–3100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee HJ, Lee YH, Park SK, Kang ES, Kim HJ, Lee YC, Choi CS, Park SE, Ahn CW, Cha BS, et al. Korean red ginseng (Panax ginseng) improves insulin sensitivity and attenuates the development of diabetes in Otsuka Long-Evans Tokushima fatty rats. Metabolism. 2009;58:1170–1177. doi: 10.1016/j.metabol.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Lee S, Lee MS, Kim CT, Kim IH, Kim Y. Ginsenoside Rg3 reduces lipid accumulation with AMP-activated protein kinase (AMPK) activation in HepG2 cells. Int J Mol Sci. 2012;13:5729–5739. doi: 10.3390/ijms13055729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim AD, Kang KA, Zhang R, Lim CM, Kim HS, Kim DH, Jeon YJ, Lee CH, Park J, Chang WY, et al. Ginseng saponin metabolite induces apoptosis in MCF-7 breast cancer cells through the modulation of AMP-activated protein kinase. Environ Toxicol Pharmacol. 2010;30:134–140. doi: 10.1016/j.etap.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Lim JY, Oh MA, Kim WH, Sohn HY, Park SI. AMP-activated protein kinase inhibits TGF-β-induced fibrogenic responses of hepatic stellate cells by targeting transcriptional coactivator p300. J Cell Physiol. 2012;227:1081–1089. doi: 10.1002/jcp.22824. [DOI] [PubMed] [Google Scholar]
- 31.Caligiuri A, Bertolani C, Guerra CT, Aleffi S, Galastri S, Trappoliere M, Vizzutti F, Gelmini S, Laffi G, Pinzani M, et al. Adenosine monophosphate-activated protein kinase modulates the activated phenotype of hepatic stellate cells. Hepatology. 2008;47:668–676. doi: 10.1002/hep.21995. [DOI] [PubMed] [Google Scholar]


