Abstract
Korean red ginseng water extract (KG-WE) has known beneficial effects on the cardiovascular system via inducting nitric oxide (NO) production in endothelium. Endothelial arginase inhibits the activity of endothelial nitric oxide synthase (eNOS) by substrate depletion, thereby reducing NO bioavailability and contributing to vascular diseases including hypertension, aging, and atherosclerosis. In the present study, we demonstrate that KG-WE inhibits arginase activity and negatively regulates NO production and reactive oxygen species generation in endothelium. This is associated with increased dimerization of eNOS without affecting the protein expression levels of either arginase or eNOS. In a vascular tension assay, when aortas isolated from wild type mice were incubated with KG-WE, NO-dependent enhanced vasorelaxation was observed. Furthermore, KG-WE administered via by drinking water to atherogenic model mice being fed high cholesterol diet improved impaired vascular function. Taken together, these results suggest that KG-WE may exert vasoprotective effects through augmentation of NO signaling by inhibiting arginase. Therefore, KG-WE may be useful in the treatment of vascular diseases derived from endothelial dysfunction, such as atherosclerosis.
Keywords: Panax ginseng, Korean red ginseng water extract, Arginase, Endothelial nitric oxide synthase, Nitric oxide
INTRODUCTION
Ginseng holds an important position in traditional medicine and is cultivated in Korea, China, Japan, the United States, and Canada. Ginseng root has been used as an oriental folk medicine for several thousand years [1]. A large number of reports suggest that the diverse components of ginseng possess numerous pharmacological functions [2-7]. Ginseng has been shown to have several cardioprotective benefits including antihypertensive effects and the attenuation of myocardial hypertrophy and heart failure. Water extract of Korean red ginseng (KG-WE) induces angiogenesis through the activation of the endothelial nitric oxide synthase (eNOS) pathway in human umbilical vein endothelial cells (HUVECs) [8]. Ginsenoside Rb1 [9] and ginsenoside Rg3 [9,10], bioactive components of ginseng, have been implicated in increased nitric oxide (NO) production, which may be dependent on phosphoinositide 3-kinase (PI3K)/Akt activity in endothelial cells. Furthermore, Panax notoginseng saponins inhibit the expression of endothelial adhesion molecules and reduce atherosclerotic lesions in ApoE-/- mice, further evidence supporting the cardioprotective properties of ginseng [11].
The endothelium plays a central role in overall vascular homeostasis by regulating vasoreactivity, oxidation of low-density lipoprotein, platelet activation, leukocyte adhesion, and smooth muscle cell proliferation and migration. Endothelial NO, an important vasoprotective molecule, is a major modulator of these effects, and impaired NO signaling associated with endothelial dysfunction is considered an early marker of vascular diseases. eNOS activity can be increased by post-translational modification such as phosphorylation, protein-protein interactions, and the availability of the cofactor and substrate, L-arginine. Intracellular concentration of L-arginine is regulated by the activity of arginase. This enzyme catalyzes L-arginine into L-ornithine and urea in the last step of the urea cycle. In endothelial cells, arginase can functionally constrain eNOS activity by limiting the availability of L-arginine. In this way, arginase can negatively regulate NO bioavailability. Thus, arginase inhibition actively augments NO production, and this, reportedly, has beneficial effects on normal cardiac function. This system has also been associated with vascular dysfunction typical of atherogenesis, aging, erectile dysfunction, and sickle cell disease [12-20]. Currently, arginase is being embraced as an emerging target for the treatment and the prevention of vascular diseases caused by endothelial dysfunction.
Although KG-WE can induce NO production in endothelial cells, the underlying molecular mechanisms and proteins involved in this pathway have yet to be elucidated. Therefore, we tested whether KG-WE has an inhibitory effect on arginase activity, and whether this effect is associated with endothelium-dependent regulation of vascular function in wild type (WT) and atherosclerotic model (low-density lipoprotein receptor null, LDLR-/-) mice.
MATERIALS AND METHODS
Materials
KG-WE (solid extract 64%, gensenoside Rg1+Rb1 4 mg/g) was obtained from Korea Ginseng Corporation (Chuncheon, Korea) and was directly dissolved in distilled water. Arginase lysates were prepared from livers and kidneys of anesthetized C57BL/6 mice. Mn(III) tetra(4-benzoic acid) porphyrin chloride (MnTBAP) and NG-nitro-L-arginine methyl ester (L-NAME) were obtained from Calbiochem (Rockland, MA, USA). All reagents were purchased from Sigma Aldrich (St. Louis, MO, USA) unless otherwise stated.
Cell culture
HUVECs were purchased from Cascade Biologics (Carlsdad, CA, USA) and were maintained as the supplier’s protocol in Medium230 plus low-serum growth supplement at 37℃ in 5% CO2.
Animal protocol
To determine the effect of KG-WE on vascular reactivity, we studied aortic rings isolated from 20 male C57BL/6J WT mice (10 wk) fed a normal diet (ND), and 25 male LDLR-/- mice fed high-cholesterol diet (HCD; D12108C, Research Diet Inc., New Brunswick, NJ, USA) for 6 wk. Aortic rings from WT mice were incubated with or without KG-WE (15 μmol/L) for 18 h as previously described [21]. LDLR-/- mice were administered KG-WE in the drinking water for 4 wk, during which the mice were started with HCD. Given that each mouse consumed approximately 10 mL water/d, this represented a daily dose of approximately 10 mg/mouse/d of KG-WE.
Arginase activity assay
Tissue lysates were prepared using lysis buffer (50 mM Tris-HCl, pH7.5, 0.1 mM EDTA and protease inhibitors) by homogenization at 4℃ followed by centrifugation for 20 min at 14,000 ×g at 4℃. The supernatants were used to assay for arginase activity as previously described [22].
Nitrate/nitrite measurement
NO was estimated by Griess reaction based upon the concentration of nitrate/nitrite (NOx) after conversion of nitrate to nitrite by nitrate reductase using a NO assay kit (Calbiochem). The concentration of NOx in HUVECs was expressed as μmol/mg protein.
Western blotting analysis
Aortic vessels from C57BL/6 mice (10 wk) were homogenized in homogenization buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Nonidet P-40, 1 mM EDTA, 1 μg/mL of leupeptin, 1 μg/mL of pepstatin, 1 μg/mL of aprotinin, 1 mM phenylmethylsulfonylflouride, 1 mM sodium orthovanadate, and 1 mM NaF) and centrifuged for 30 min at 14,000 ×g. The protein concentration of the supernatant was analyzed by the Bradford method. Proteins (100 μg) were separated in a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred to a nitrocellulose membrane. The blots were incubated with a polyclonal anti-arginase II (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-eNOS (BD Bioscience, San Jose, CA, USA), or anti-β-tubulin (BD Bioscience) antibodies followed by the secondary antibody (Bio-Rad, Hercules, CA, USA). The signals were detected using an enhanced chemiluminescence detection reagent with X-ray films.
Determination of endothelial nitric oxide synthase dimerization
Dimers and monomers of eNOS were separated using low-temperature sodium dodecyl sulfate-polyacrylamide gel electrophoresis as previously described [23]. Band intensities were analyzed using National Institutes of Health ImageJ software.
Estimation of nitric oxide or reactive oxygen species generation in isolated mice aorta using 4-amino-5-methylamino-2’,7’-difluorofluorescein or dihydroethidium
NO and reactive oxygen species (ROS) production were estimated at different time intervals as described previously [21].
Aortic vascular tension assay
The study was approved in accordance with Guide for the Care and Use of Laboratory Animals (Institutional Review Board, Kangwon National University). Male C57BL/6J mice were anesthetized using isoflurane and the thoracic aorta was rapidly removed. The aortas were placed in ice-cold oxygenated Krebs-Ringer bicarbonate solution (NaCl 118.3, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, CaCl2 1.6, NaHCO3 25, glucose 11.1 [in mM]) and cleared of adherent connective tissues. The mouse aortas were cut into 1.5-mm rings and suspended between two wire stirrups (150 μm) in a myograph (Multi Myograph System DMT-620) in 10 mL Krebs-ringer (95% O2-5% CO2, pH7.4, 37℃). One stirrup was connected to a three-dimensional micromanipulator, and the other was attached to a force transducer. The rings were passively stretched at 10-minute intervals in increments of 100 mg to reach optimal tone (600 mg). After the arterial rings were stretched to their optimal resting tone, the contractile response to 100 mM KCl was determined. U46619 (10-8 M) was applied and pre-constricted the vessels for 15 min, and KG-WE was then added to determine relaxation activities in the presence or absence of eNOS inhibitor, L-NAME (10-5 M), for 10 min. To further confirm the vasorelaxation activity in a NO-dependent manner, the inhibitor of guanylate cyclase (1H-[1,2,4]oxadizolo[4,3-a]quinoxalin-1-one, ODQ; 1 μM) was added at the end of experiments.
Statistics
All data are represented as mean±standard deviation of at least four independent experiments. An unpaired Student’s t-test or one-way analysis of variance (ANOVA) was used to assess significant differences. A value of p<0.05 was accepted as significant.
RESULTS
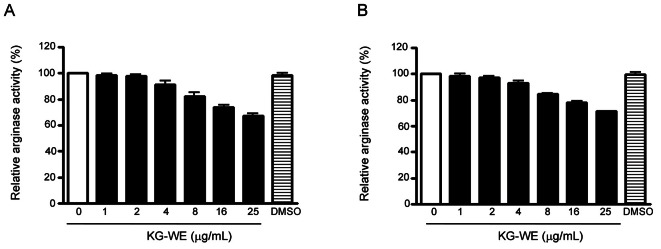
Korean red ginseng water extract inhibited arginase I and II activities in a dose-dependent manner
During a screening procedure on medicinal plants with the aim of finding novel arginase inhibitors, KG-WE was shown to significantly inhibit arginase activity. Enzyme solutions of arginase I and II were prepared from liver and kidney lysates of C57BL/6 mice, respectively. The expression of arginase isoforms was previously confirmed by western blot analysis (data not shown). KG-WE significantly inhibits arginase I and II in a dose-dependent manner. Statistically significant inhibition of both arginase I and II was achieved at KG-WE concentrations greater than 4 μg/mL The activities of arginase I after incubation with 4, 16, and 25 μg/mL KG-WE were 91.0±3.5%, 73.6±1.7%, and 67.3±1.6% of baseline, respectively (p<0.01, one-way ANOVA test) (Fig. 1A). The activities of arginase II after incubation with 4, 16, and 25 μg/mL KG-WE were 93.3±1.7%, 78.3±0.7%, and 71.3±0.3% of baseline, respectively (p<0.01, one-way ANOVA test) (Fig. 1B).
Fig. 1. Effect of Korean red ginseng water extract (KG-WE) on the activities of arginase I and II. KG-WE incubation inhibits the activity of arginase I (A, liver) and II (B, kidney) in a dose-dependent manner (n=9 from 3 independent experiments; p<0.01 by one-way ANOVA). Dimethyl sulfoxide (DMSO, 10 μL) was used as a control.
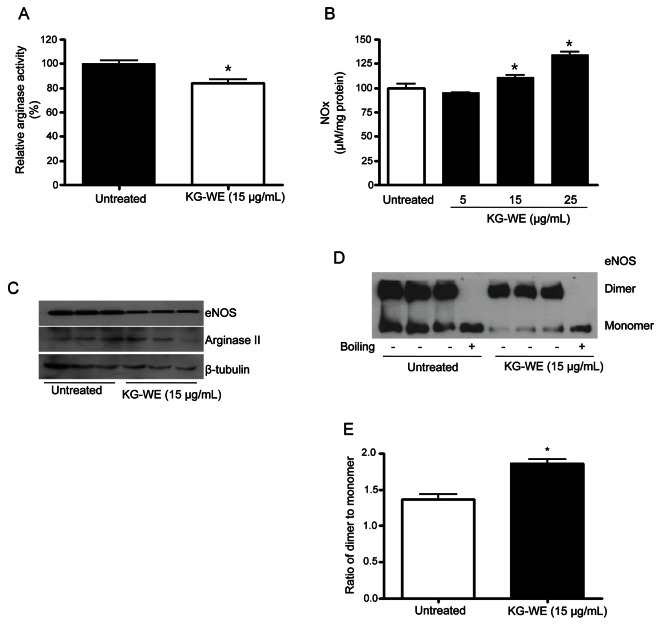
Arginase inhibition increases NOx generation and enhances the formation of eNOS dimer in HUVECs
Given that arginase negatively regulates NOS activity by limiting L-arginine bioavailability, we tested whether KG-WE-induced inhibition of arginase was associated with an increase in NOx generation. Furthermore, we assayed proteins levels of eNOS and arginase II, the dominant arginase isoform in human endothelial cells, and eNOS dimerization as a representative mechanism of NOS activation. In HUVECs, KG-WE incubation (15 μg/mL) for 18 h significantly inhibited arginase activity (74±6%, p<0.01) (Fig. 2A).
Fig. 2. Korean red ginseng water extract (KG-WE)-dependent arginase inhibition increases nitrate/nitrite (NOx) production by enhancing the formation of endothelial nitric oxide synthase (eNOS) dimer in human umbilical vein endothelial cells (HUVECs). HUVECs were treated with KG-WE (15 μg/mL) for 18 h and KG-WE significantly inhibits arginase activity (A, * vs. untreated, p<0.01, n=4). Inhibition of arginase activity negatively increases NOx production (B, * vs. untreated, p<0.05, n=4). Protein levels of arginase II and eNOS were not changed by KG-WE incubation for 18 h (C, n=3). Low-temperature electrophoresis and western blot analysis for eNOS shows that incubation with KG-WE induces a decrease in the amount of eNOS monomer (D) and an increase in the ratio of dimer to monomer (E, * vs. untreated, p<0.01, n=6). Boiled samples were used as a control.
The decreased arginase activity was associated with increased NOx generation. Treatment with KG-WE at 15 and 25 μg/mL increases NOx generation to 111.3±3.1% and 134.2±5.3%, respectively (p<0.05) (Fig. 2B). Next, we tested for changes in protein expression levels. As shown in Fig. 2C, KG-WE incubation for 18 h did not induce a significant change in the expression levels of either eNOS or arginase II. However, KG-WE increased the ratio of dimer to monomer of eNOS from 1.3±0.1 to 1.9±0.1 (p<0.01) (Fig. 2E) by enhancing dimer formation and decreasing the amount of monomer (Fig. 2D).
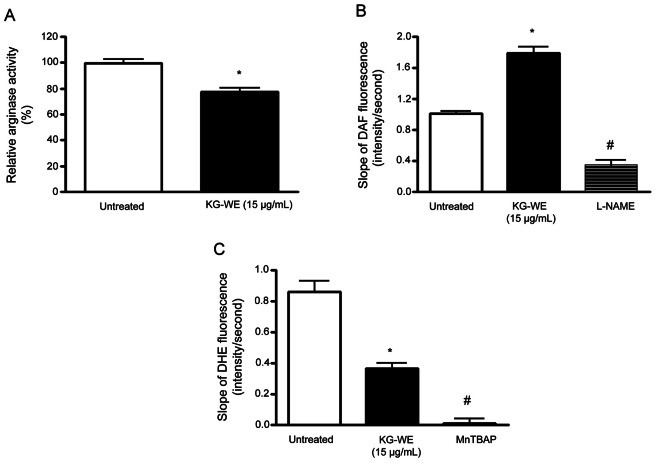
KG-WE-dependent arginase inhibition increases NO generation and decreases ROS production in isolated mice aorta
We next tested whether increased NOx generation by arginase inhibition in HUVECs translated into redox regulation in the endothelium of mice aorta. Isolated aortic vessels were incubated with KG-WE as described in the Methods. KG-WE markedly inhibited arginase activity compared to control (77.5±2.8% vs. 100±1.7%, p<0.05).
As shown in Fig. 3B, KG-WE-dependent arginase inhibition resulted in an increase of NO generation (1.8±0.8 vs. 1.0±0.7, average slope of 4-amino-5-methylamino-2’,7’-difluorofluorescein [DAF] fluorescence, p<0.01). This increase was acutely blocked by incubation with NOS inhibitor, L-NAME (1.8±0.8 vs. 3.4±1.1, average slope of DAF fluorescence, p<0.01). These results are consistent with data from KG-WE-treated HUVECs. We wished to determine whether increased NO generation upon arginase inhibition contributes to reduced ROS production. Therefore, we measured O2·- production by using O2·--sensitive fluorescence dye, dihydroergotamine (DHE), in the endothelium of KG-WE-treated aorta. Preincubation with KG-WE (15 μg/mL) significantly decreased the time-dependent DHE fluorescence (0.9±0.1 vs. 0.3±0.1, p<0.01 in average slope of DHE fluorescence) (Fig. 3C) and the signal of DHE fluorescence was completely prevented with MnTBAP (slope<0.01, # vs. KG-WE, p<0.01).
Fig. 3. Arginase inhibition by Korean red ginseng water extract (KG-WE) increases nitric oxide (NO) production and decreases reactive oxygen species (ROS) generation in isolated mice aorta. (A) Preincubation of isolated aortic rings with KG-WE (15 μg/mL, 18 h) resulted in a decrease of arginase activity (* vs. untreated, p<0.05, n=6). (B) Time-lapse NO-dependent changes in fluorescence of NO-dependent dye, 4-amino-5-methylamino-2’,7’-difluorofluorescein (DAF) (5 μmol/L), were measured in pretreated aortic rings (endothelial side up; 5 μmol/L). The cumulative data are shown as the slope of DAF fluorescence over 3 min. The effect of KG-WE on the slope of the DAF fluorescence was determined (* vs. untreated, p<0.01, n=6) and then NG-nitro-L-arginine methyl ester (L-NAME, 10-5 mol/L) was added (# vs. KG-WE, p<0.01, n=6). (C) ROS production was determined using O2--sensitive fluorescence dye, dihydroergotamine (DHE). KG-WE treatment resulted in a decrease of the slope of DHE fluorescence (* vs. untreated, p<0.01, n=6) and treatment with Mn(III) tetra(4-benzoic acid) porphyrin chloride (MnTBAP, 10-6 mol/L) as a scavenger of ROS quenched ROS-dependent DHE fluorescence (# vs. KG-WE, p<0.01, n=6).
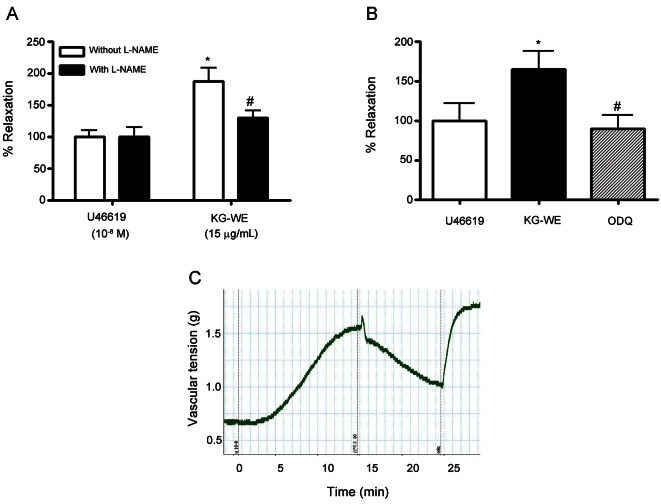
Korean red ginseng water extract induces nitric oxide-dependent vessel relaxation in mice aorta
Next, we determined whether the KG-WE-dependent increase in NO production contributes to vessel relaxation by measuring vascular tension. As shown in Fig. 4A, KG-WE treatment acutely induced the relaxation of vessels that were preconstricted with U46619 in the absence of NOS inhibitor, L-NAME, from 100.0±12.6% to 187.3±27.9% (p<0.01). However, inhibition of NO synthesis by L-NAME (10-5 mol/L) blocked the vasodilating effect of KG-WE (# vs. KG-WE treatment without L-NAME, 129.2±17.9% vs. 187.3±27.9%, p<0.05). We further tested the NO-dependent relaxation effect of KG-WE by blocking the NO-dependent cGMP pathway. The soluble guanylate cyclase inhibitor, ODQ, significantly constricted vessels which were relaxed by KG-WE treatment (* vs. U46619, 165.0±22.1% vs. 100.0±21.4%, p<0.01; # vs. KG-WE, 89.7±15.3% vs. 165.0±22.1%, p<0.01) (Fig. 4B). Fig 4C shows representative traces of the KG-WE and ODQ response.
Fig. 4. Korean red ginseng water extract (KG-WE) induces nitric oxide-dependent vessel relaxation. (A) KG-WE treatment induces the relaxation of vessels pre-constricted by U46619 (10-8 mol/L). This was prevented by incubation with the nitric oxide synthase inhibitor, NG-nitro-L-arginine methyl ester (L-NAME, 10-5 mol/L) (A, * vs. U46619 without L-NAME, p<0.01, n=8; # vs. KG-WE without L-NAME, p<0.05, n=8). (B) Inhibition of soluble guanylate cyclase with 1H-[1,2,4]oxadizolo[4,3-a]quinoxalin-1-one (ODQ, 10-6 mol/L) resulted in the constriction of KG-WE-relaxed vessels (* vs. U46619, p<0.01, n=8; # vs. KG-WE, p<0.01, n=8). The representative response was shown in (C).
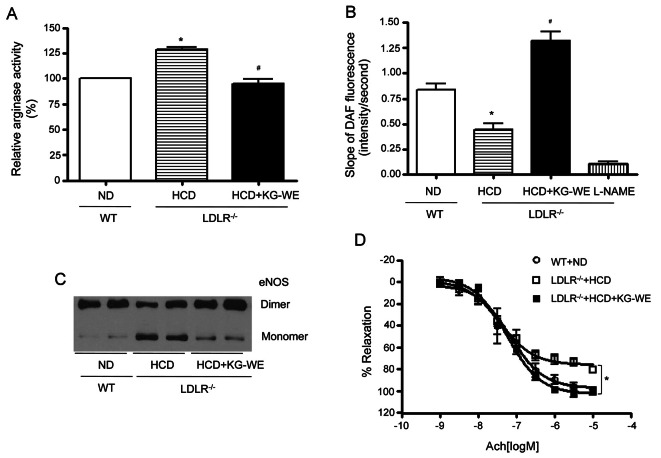
Arginase inhibition by Korean red ginseng water extract improves endothelial function in LDLR-/- mice fed high-cholesterol diet
Given the above data shows that KG-WE improves endothelial function by inhibiting arginase in WT mice, we hypothesized that KG-WE could restore the impaired endothelial function observed in HCD-induced atherogenic model mice (LDLR-/- mice). HCD induced an increase in arginase activity in LDLR-/- mice (128.4±5.3 vs. 100±0%, p<0.01) (Fig. 5A) that was reversed by administration of KG-WE (# vs. LDLR-/-+HCD, 94.8±10.1 vs. 128.4±5.3, p<0.01). Inhibiting arginase activity by KG-WE resulted in an increase in NO production (* vs. WT+ND, 0.45±0.12 vs. 0.84±0.12, p<0.01; # vs. LDLR-/- +HCD, 1.32±0.17 vs. 0.45±0.12, slope of DAF fluorescence, p<0.01) (Fig. 5B). This increase in NO production was associated with enhanced eNOS coupling (Fig. 5C). Next, we performed a vascular tension assay using the endothelium-dependent vasorelaxant, acetylcholine, after preconstriction of vessels by U46619. Interestingly, KG-WE administration restored the impaired endothelial function by increasing the Emax value from 75.81±2.30 vs. 102.5±2.54% (*, LDLR-/-+HCD vs. LDLR-/-+HCD+ KG-WE, p<0.01) (Fig. 5D).
Fig. 5. Korean red ginseng water extract (KG-WE) ameliorates vascular function in atherosclerotic model animal. (A) Arginase activity was significantly increased in aortic vessels from low-density lipoprotein receptor null (LDLR-/-) mice fed high-cholesterol diet (HCD) for 6 wk. This activity was blocked by the administration of KG-WE (10 mg/mouse/d). *p<0.01, #p<0.01. n=4 mice. (B) Endothelial nitric oxide (NO) was measured in mouse aorta (en face, endothelial side up) by monitoring the change of the slope of 4-amino-5-methylamino-2’,7’-difluorofluorescein fluorescence, *p<0.01, #p<0.01. NG-nitro-L-arginine methyl ester (L-NAME, 10 μmol/L) was used as a control. n=6. (C) Endothelial nitric oxide synthase (eNOS) uncoupling induced by HCD was markedly restored with the administration of KG-WE. (D) Vessels were preconstricted to 50% to 75% of contractile Emax with U46619 (10-8 mol/L), and cumulative dose responses to Ach were obtained. The vasodilation Emax Ach was markedly attenuated in aortic rings from LDLR-/- fed HCD compared with wild type (WT) mice fed normal diet (ND); whereas KG-WE administration enhanced the vasodilation response. LDLR-/-+HCD vs. LDLR-/-+HCD+ KG-WE, *p<0.01, n=8.
DISCUSSION
Endothelial arginase can constrain the activity of eNOS by depleting the critical substrate, L-arginine. In turn, this reduces NO bioavailability and contributes to vascular diseases such as hypertension, aging, and atherosclerosis [12,23-26]. Here, we show that KG-WE enhances NO generation, reduces ROS production by inhibiting arginase activity, and induces the vasorelaxation in isolated aortic vessels. Furthermore, administering KG-WE via drinking water to atherosclerotic prone (LDLR-/-) mice restored HCD-dependent endothelial dysfunction by enhancing eNOS coupling.
KG-WE has been extensively studied and its consumption is progressively increasing. Ginseng has been shown to have beneficial effects in the treatment of various diseases, including thrombosis, hyperlipidemia, cancer, and atherosclerosis [11,27-30]. In the vasculature, it is well-documented that KG-WE has vasoprotective effects by eliminating superoxide that is derived from nicotinamide adenine dinucleotide phosphate oxidase [31]. This promotes endothelial cell proliferation and protection from H2O2-dependent cell death [32,33] and induces heme oxygenase-1 expression [34]. Ginseng extract exerts a direct vasodilatory effect by releasing NO in an endothelium-dependent manner [35]. Furthermore, the beneficial effects of ginseng on vascular system may be dependent on the activation of Akt/PI3K signal transduction [8], inhibition of angiotensin converting enzyme [36], and inhibition of calcium ion influx resulting from the blockade of the calcium ion channel [37]. Here, we demonstrate that arginase is a novel target protein for KG-WE. We show that KG-WE inhibits arginase activity and is associated with increased NO production and decreased ROS generation in both physiological and pathophysiological conditions. The increased NO bioavailability induced by KG-WE resulted in vasodilation in response to endothelium-dependent vasodilator, acetylcholine.
Ginsenosides, the major active ingredients of ginseng, are mostly triterpene glycosides. The effects of ginsenosides on endothelial cells have been reported. Ginsenoside Rg3 inhibits the expression of adhesion molecules and of inflammatory cytokines that play important roles in atherogenesis [10] and prevents mitochondrial caspase pathway activation [38]. Another ginsenoside, Rb1, protects endothelial cells from oxidized-low density lipoprotein-induced-necrosis [39] and regulates the proliferation and migration of endothelial cells [40]. Furthermore, protopanaxatriol, another ginsenoside, modulates intracellular redox status [32]. These effects of ginsenosides may be explained by increased NO bioavailability because NO in endothelium is well-documented as an important vasoprotective molecule. In this study, we demonstrate that arginase inhibition by KG-WE leads to increased NO bioavailability and decreased ROS production, both of which are important players in the maintenance of the nitroso-redox balance in endothelial cells.
KG-WE-dependent arginase inhibition contributed to an increase of NO generation and decease of ROS production in isolated mice aortas (Fig. 3). These effects resulted in the induction of NO-dependent vasorelaxation (Fig. 4). These results are consistent with previous observations that arginase inhibition accentuates NO release in rat aortic endothelium [12], bovine pulmonary endothelial cells [41], and porcine coronary artery model [42]. The main mechanism of KG-WE-dependent NO release is likely due to increased eNOS coupling resulting from increased L-arginine availability (Fig. 2), in line with previous data [23]. During eNOS uncoupling, electrons flow from the reductase domain in the heme to molecular oxygen rather than L-arginine, resulting in O2·- production rather than NO.
Arginase is present as two isoforms: arginase I, or the hepatic isoform, is located in the cytosol; and arginase II, or the extrahepatic isoform, is localized to the mitochondrial. During physiological homeostasis, arginase I catalyzes the final step of urea cycle that allows for the excretion of excess nitrogen in the form of urea. Arginase I is also expressed in extrahepatic tissues, such as lung, to regulate L-arginine homeostasis [43]. Together with arginase I, arginase II also serves to regulate L-arginine homeostasis and the biosynthetic pathways of L-ornithine from L-arginine. These pathways include NO biosynthesis, L-proline biosynthesis to support collagen production, and polyamine biosynthesis to facilitate cellular proliferation. Since increased arginase activity is associated with aberrant L-arginine homeostasis and/or NO biosynthesis, arginase is an increasingly important pharmaceutical target for the management of various diseases, including cardiovascular disease such as atherosclerosis, cystic fibrosis, and cancer tumor growth.
While an active compound was not identified, we present a novel mechanism in which KG-WE inhibits arginase activity, increases NO generation by enhancing eNOS dimer formation, and induces vasorelaxation in an NO-dependent manner. Importantly, the beneficial effects of KG-WE in the pathophysiological atherosclerosis were also demonstrated. These finding suggest that KG-WE possesses therapeutic potential for cardiovascular diseases associated with endothelial dysfunction.
Acknowledgments
This work was supported by the Basic Science Research Program of the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2011-0026173).
References
- 1.Chevallier A. Encyclopedia of herbal medicine. Dorling Kindersley; New York: 2000. [Google Scholar]
- 2.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 3.Cho WC, Chung WS, Lee SK, Leung AW, Cheng CH, Yue KK. Ginsenoside Re of Panax ginseng possesses significant antioxidant and antihyperlipidemic efficacies in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2006;550:173–179. doi: 10.1016/j.ejphar.2006.08.056. [DOI] [PubMed] [Google Scholar]
- 4.Helms S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 2004;9:259–274. [PubMed] [Google Scholar]
- 5.Hu SY. A contribution to our knowledge of ginseng. Am J Chin Med (Gard City N Y) 1977;5:1–23. doi: 10.1142/s0192415x77000026. [DOI] [PubMed] [Google Scholar]
- 6.Lee TK, Johnke RM, Allison RR, O’Brien KF, Dobbs LJ Jr. Radioprotective potential of ginseng. Mutagenesis. 2005;20:237–243. doi: 10.1093/mutage/gei041. [DOI] [PubMed] [Google Scholar]
- 7.Yuan HD, Kim JT, Kim SH, Chung SH. Ginseng and diabetes: the evidences from in vitro, animal and human studies. J Ginseng Res. 2012;36:27–39. doi: 10.5142/jgr.2012.36.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YM, Namkoong S, Yun YG, Hong HD, Lee YC, Ha KS, Lee H, Kwon HJ, Kwon YG, Kim YM. Water extract of Korean red ginseng stimulates angiogenesis by activating the PI3K/Akt-dependent ERK1/2 and eNOS pathways in human umbilical vein endothelial cells. Biol Pharm Bull. 2007;30:1674–1679. doi: 10.1248/bpb.30.1674. [DOI] [PubMed] [Google Scholar]
- 9.Yu J, Eto M, Akishita M, Kaneko A, Ouchi Y, Okabe T. Signaling pathway of nitric oxide production induced by ginsenoside Rb1 in human aortic endothelial cells: a possible involvement of androgen receptor. Biochem Biophys Res Commun. 2007;353:764–769. doi: 10.1016/j.bbrc.2006.12.119. [DOI] [PubMed] [Google Scholar]
- 10.Hien TT, Kim ND, Pokharel YR, Oh SJ, Lee MY, Kang KW. Ginsenoside Rg3 increases nitric oxide production via increases in phosphorylation and expression of endothelial nitric oxide synthase: essential roles of estrogen receptor-dependent PI3-kinase and AMP-activated protein kinase. Toxicol Appl Pharmacol. 2010 doi: 10.1016/j.taap.2010.05.008. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Wan JB, Lee SM, Wang JD, Wang N, He CW, Wang YT, Kang JX. Panax notoginseng reduces atherosclerotic lesions in ApoE-deficient mice and inhibits TNF-alpha-induced endothelial adhesion molecule expression and monocyte adhesion. J Agric Food Chem. 2009;57:6692–6697. doi: 10.1021/jf900529w. [DOI] [PubMed] [Google Scholar]
- 12.Berkowitz DE, White R, Li D, Minhas KM, Cernetich A, Kim S, Burke S, Shoukas AA, Nyhan D, Champion HC, et al. Arginase reciprocally regulates nitric oxide synthase activity and contributes to endothelial dysfunction in aging blood vessels. Circulation. 2003;108:2000–2006. doi: 10.1161/01.CIR.0000092948.04444.C7. [DOI] [PubMed] [Google Scholar]
- 13.Bivalacqua TJ, Hellstrom WJ, Kadowitz PJ, Champion HC. Increased expression of arginase II in human diabetic corpus cavernosum: in diabetic-associated erectile dysfunction. Biochem Biophys Res Commun. 2001;283:923–927. doi: 10.1006/bbrc.2001.4874. [DOI] [PubMed] [Google Scholar]
- 14.Bivalacqua TJ, Liu T, Musicki B, Champion HC, Burnett AL. Endothelial nitric oxide synthase keeps erection regulatory function balance in the penis. Eur Urol. 2007;51:1732–1740. doi: 10.1016/j.eururo.2006.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, Schimel DM, Cochard AE, Wang X, Schechter AN, et al. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood. 2007;109:3088–3098. doi: 10.1182/blood-2006-08-039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris CR, Poljakovic M, Lavrisha L, Machado L, Kuypers FA, Morris SM Jr. Decreased arginine bioavailability and increased serum arginase activity in asthma. Am J Respir Crit Care Med. 2004;170:148–153. doi: 10.1164/rccm.200309-1304OC. [DOI] [PubMed] [Google Scholar]
- 17.Nelin LD, Wang X, Zhao Q, Chicoine LG, Young TL, Hatch DM, English BK, Liu Y. MKP-1 switches arginine metabolism from nitric oxide synthase to arginase following endotoxin challenge. Am J Physiol Cell Physiol. 2007;293:C632–C640. doi: 10.1152/ajpcell.00137.2006. [DOI] [PubMed] [Google Scholar]
- 18.Steppan J, Ryoo S, Schuleri KH, Gregg C, Hasan RK, White AR, Bugaj LJ, Khan M, Santhanam L, Nyhan D, et al. Arginase modulates myocardial contractility by a nitric oxide synthase 1-dependent mechanism. Proc Natl Acad Sci U S A. 2006;103:4759–4764. doi: 10.1073/pnas.0506589103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White AR, Ryoo S, Li D, Champion HC, Steppan J, Wang D, Nyhan D, Shoukas AA, Hare JM, Berkowitz DE. Knockdown of arginase I restores NO signaling in the vasculature of old rats. Hypertension. 2006;47:245–251. doi: 10.1161/01.HYP.0000198543.34502.d7. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, Gao X, Potter BJ, Cao JM, Zhang C. Anti-LOX-1 rescues endothelial function in coronary arterioles in atherosclerotic ApoE knockout mice. Arterioscler Thromb Vasc Biol. 2007;27:871–877. doi: 10.1161/01.ATV.0000259358.31234.37. [DOI] [PubMed] [Google Scholar]
- 21.Woo A, Min B, Ryoo S. Piceatannol-3’-O-beta-D-glucopyranoside as an active component of rhubarb activates endothelial nitric oxide synthase through inhibition of arginase activity. Exp Mol Med. 2010;42:524–532. doi: 10.3858/emm.2010.42.7.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryoo S, Lemmon CA, Soucy KG, Gupta G, White AR, Nyhan D, Shoukas A, Romer LH, Berkowitz DE. Oxidized low-density lipoprotein-dependent endothelial arginase II activation contributes to impaired nitric oxide signaling. Circ Res. 2006;99:951–960. doi: 10.1161/01.RES.0000247034.24662.b4. [DOI] [PubMed] [Google Scholar]
- 23.Ryoo S, Gupta G, Benjo A, Lim HK, Camara A, Sikka G, Lim HK, Sohi J, Santhanam L, Soucy K, et al. Endothelial arginase II: a novel target for the treatment of atherosclerosis. Circ Res. 2008;102:923–932. doi: 10.1161/CIRCRESAHA.107.169573. [DOI] [PubMed] [Google Scholar]
- 24.Andrawis N, Jones DS, Abernethy DR. Aging is associated with endothelial dysfunction in the human forearm vasculature. J Am Geriatr Soc. 2000;48:193–198. [PubMed] [Google Scholar]
- 25.Demougeot C, Prigent-Tessier A, Marie C, Berthelot A. Arginase inhibition reduces endothelial dysfunction and blood pressure rising in spontaneously hypertensive rats. J Hypertens. 2005;23:971–978. doi: 10.1097/01.hjh.0000166837.78559.93. [DOI] [PubMed] [Google Scholar]
- 26.Ming XF, Barandier C, Viswambharan H, Kwak BR, Mach F, Mazzolai L, Hayoz D, Ruffieux J, Rusconi S, Montani JP, et al. Thrombin stimulates human endothelial arginase enzymatic activity via RhoA/ROCK pathway: implications for atherosclerotic endothelial dysfunction. Circulation. 2004;110:3708–3714. doi: 10.1161/01.CIR.0000142867.26182.32. [DOI] [PubMed] [Google Scholar]
- 27.Jin YR, Yu JY, Lee JJ, You SH, Chung JH, Noh JY, Im JH, Han XH, Kim TJ, Shin KS, et al. Antithrombotic and antiplatelet activities of Korean red ginseng extract. Basic Clin Pharmacol Toxicol. 2007;100:170–175. doi: 10.1111/j.1742-7843.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 28.Kwak YS., Kyung JS., Kim JS., Cho JY., Rhee MH. Anti-hyperlipidemic effects of red ginseng acidic polysaccharide from Korean red ginseng. Biol Pharm Bull. 2010;33:468–472. doi: 10.1248/bpb.33.468. [DOI] [PubMed] [Google Scholar]
- 29.Park BJ, Lim YS, Lee HJ, Eum WS, Park J, Han KH, Choi SY, Lee KS. Anti-oxidative effects of Phellinus linteus and red ginseng extracts on oxidative stress-induced DNA damage. BMB Rep. 2009;42:500–505. doi: 10.5483/bmbrep.2009.42.8.500. [DOI] [PubMed] [Google Scholar]
- 30.Wong VK, Cheung SS, Li T, Jiang ZH, Wang JR, Dong H, Yi XQ, Zhou H, Liu L. Asian ginseng extract inhibits in vitro and in vivo growth of mouse lewis lung carcinoma via modulation of ERK-p53 and NF-κB signaling. J Cell Biochem. 2010;111:899–910. doi: 10.1002/jcb.22778. [DOI] [PubMed] [Google Scholar]
- 31.Kim CS, Park JB, Kim KJ, Chang SJ, Ryoo SW, Jeon BH. Effect of Korea red ginseng on cerebral blood flow and superoxide production. Acta Pharmacol Sin. 2002;23:1152–1156. [PubMed] [Google Scholar]
- 32.Kwok HH, Ng WY, Yang MS, Mak NK, Wong RN, Yue PY. The ginsenoside protopanaxatriol protects endothelial cells from hydrogen peroxide-induced cell injury and cell death by modulating intracellular redox status. Free Radic Biol Med. 2010;48:437–445. doi: 10.1016/j.freeradbiomed.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 33.Nakajima S, Uchiyama Y, Yoshida K, Mizukawa H, Haruki E. The effects of ginseng radix rubra on human vascular endothelial cells. Am J Chin Med. 1998;26:365–373. doi: 10.1142/S0192415X98000403. [DOI] [PubMed] [Google Scholar]
- 34.Yang HN, Lee SE, Jeong SI, Park CS, Jin YH, Park YS. Up-regulation of heme oxygenase-1 by Korean red ginseng water extract as a cytoprotective effect in human endothelial cells. J Ginseng Res. 2011;35:352–359. doi: 10.5142/jgr.2011.35.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeon BH., Kim CS., Kim HS., Park JB., Nam KY., Chang SJ. Effect of Korean red ginseng on blood pressure and nitric oxide production. Acta Pharmacol Sin. 2000;21:1095–1100. [PubMed] [Google Scholar]
- 36.Persson IA, Dong L, Persson K. Effect of Panax ginseng extract (G115) on angiotensin-converting enzyme (ACE) activity and nitric oxide (NO) production. J Ethnopharmacol. 2006;105:321–325. doi: 10.1016/j.jep.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 37.Guan YY, Zhou JG, Zhang Z, Wang GL, Cai BX, Hong L, Qiu QY, He H. Ginsenoside-Rd from Panax notoginseng blocks Ca2+ influx through receptor- and store-operated Ca2+ channels in vascular smooth muscle cells. Eur J Pharmacol. 2006;548:129–136. doi: 10.1016/j.ejphar.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Min JK, Kim JH, Cho YL, Maeng YS, Lee SJ, Pyun BJ, Kim YM, Park JH, Kwon YG. 20(S)-ginsenoside Rg3 prevents endothelial cell apoptosis via inhibition of a mitochondrial caspase pathway. Biochem Biophys Res Commun. 2006;349:987–994. doi: 10.1016/j.bbrc.2006.08.129. [DOI] [PubMed] [Google Scholar]
- 39.He F, Guo R, Wu SL, Sun M, Li M. Protective effects of ginsenoside Rb1 on human umbilical vein endothelial cells in vitro. J Cardiovasc Pharmacol. 2007;50:314–320. doi: 10.1097/FJC.0b013e3180cab12e. [DOI] [PubMed] [Google Scholar]
- 40.Leung KW, Pon YL, Wong RN, Wong AS. Ginsenoside-Rg1 induces vascular endothelial growth factor expression through the glucocorticoid receptor-related phosphatidylinositol 3-kinase/Akt and beta-catenin/T-cell factor-dependent pathway in human endothelial cells. J Biol Chem. 2006;281:36280–36288. doi: 10.1074/jbc.M606698200. [DOI] [PubMed] [Google Scholar]
- 41.Chicoine LG, Paffett ML, Young TL, Nelin LD. Arginase inhibition increases nitric oxide production in bovine pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L60–L68. doi: 10.1152/ajplung.00194.2003. [DOI] [PubMed] [Google Scholar]
- 42.Zhang C, Hein TW, Wang W, Chang CI, Kuo L. Constitutive expression of arginase in microvascular endothelial cells counteracts nitric oxide-mediated vasodilatory function. FASEB J. 2001;15:1264–1266. doi: 10.1096/fj.00-0681fje. [DOI] [PubMed] [Google Scholar]
- 43.North ML, Khanna N, Marsden PA, Grasemann H, Scott JA. Functionally important role for arginase 1 in the airway hyperresponsiveness of asthma. Am J Physiol Lung Cell Mol Physiol. 2009;296:L911–L920. doi: 10.1152/ajplung.00025.2009. [DOI] [PubMed] [Google Scholar]