Abstract
Ginsenoside Rd is a primary constituent of the ginseng rhizome and has been shown to participate in the regulation of diabetes and in tumor formation. Reports also show that ginsenoside Rd exerts anti-oxidative effects by activating anti-oxidant enzymes. Treatment with ginsenoside Rd decreased nitric oxide and prostaglandin E2 (PGE2) in lipopolysaccharides (LPS)-challenged RAW264.7 cells and in ICR mouse livers (5 mg/kg LPS; LPS + ginsenoside Rd [2, 10, and 50 mg/kg]). Furthermore, these decreases were associated with the down-regulations of inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 and of nuclear factor (NF)-κB activity in vitro and in vivo. Our results indicate that ginsenoside Rd treatment decreases; 1) nitric oxide production (40% inhibition); 2) PGE2 synthesis (69% to 93% inhibition); 3) NF-κB activity; and 4) the NF-κB-regulated expressions of iNOS and COX-2. Taken together, our results suggest that the anti-inflammatory effects of ginsenoside Rd are due to the down-regulation of NF-κB and the consequent expressional suppressions of iNOS and COX-2.
Keywords: Panax ginseng, Ginsenoside Rd, Inducible nitric oxide synthase, Cyclooxygenase-2, Prostaglandin E2
INTRODUCTION
The transcription factor nuclear factor (NF)-κB has been studied by many researchers because of its apparent involvement in the inductions of several genes and diseases [1,2]. NF-κB is considered to play a key role in the production of inflammatory mediators, such as, pro-inflammatory cytokines (tumor necrosis factor-α and interleukin-1), cell adhesion molecules (intercellular cell adhesion molecule-1 and vascular cell adhesion molecule-1), immuno-receptors, and acute phase protein production [3]. In resting cells, NF-κB is localized in the cytoplasm as a heterodimer composed of two polypeptides of 50 kDa (p50) and 65 kDa (p65), which are non-covalently associated with cytoplasmic inhibitory proteins, such as IκB. In response to exposure to lipopolysaccharides (LPS), viral infection, the expressions of certain viral products, or other physiological stimuli, IκB undergoes a series of biological events, namely, rapid phosphorylation in its N-terminal domain by a large multi-kinase complex, poly-ubiquitination, and degradation by 26S proteasome, which allows translocation of NF-κB heterodimer to the nucleus [4]. Having reached the nucleus NF-κB activates the transcriptions of several inflammatory enzymes, such as cyclooxygenase (COX)-2 and inducible nitric oxide synthase (iNOS), by interacting with kB sites in their promoter regions. Furthermore, nitric oxide (NO) derived from iNOS and prostaglandin E2 (PGE2), which is synthesized by COX-2, plays a pivotal role in the pathogenesis of acute and chronic inflammation [5]. Studies in a number of cell and animal models have shown that iNOS inhibitors prevent the development of a number of diseases, including experimental allergic encephalomyelitis, atherosclerosis, cancer, inflammatory bowel syndrome, and transplantation rejection [6-8]. Moreover, the inappropriate activation or up-regulation of COX-2 is a characteristic of the majority inflammatory diseases [9], and therefore, the attenuation of the abnormal up-regulations of iNOS and COX-2 is considered a strategy for the treatment and prevention of inflammation conditions and related diseases.
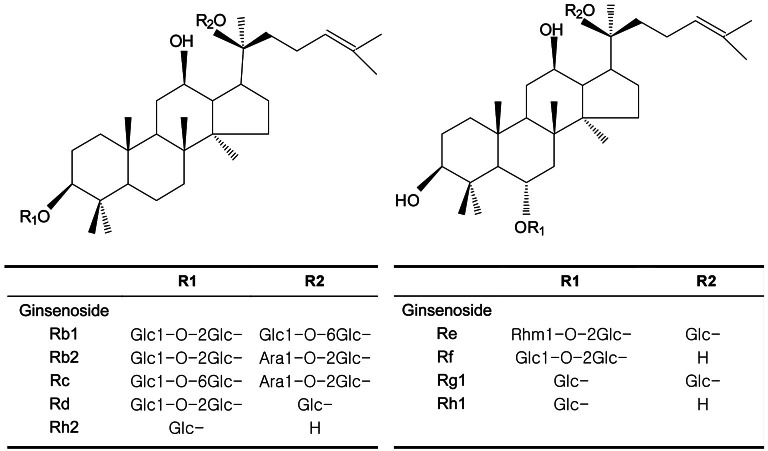
Ginseng (the root of Panax ginseng Meyer, Araliaceae) has been used as a herbal remedy in eastern Asia for thousands of years. In traditional oriental medicine, the roots of ginseng are used to treat dyspepsia, as an aphrodisiac, and to enhance resistance to stressors, such as anxiety and fatigue. The ginsenosides are a component of ginseng preparations, and to date several have been isolated, identified, and characterized, namely, Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rh1, and Rh2.
Furthermore, ginsenosides have been reported to have anti-diabetic [10] and anti-tumor [11] activities and anti-oxidative properties [12,13]. However, the cellular and molecular mechanisms responsible for the anti-inflammatory properties of ginsenosides are not well understood. In the present study, we investigated the mechanism responsible for the anti-inflammatory effect of ginsenoside Rd in RAW264.7 cells and ICR mice.
MATERIALS AND METHODS
Materials
The Korean red ginseng extract used was manufactured by the Korea Ginseng Corporation (Seoul, Korea). LPS (from Escherichia coli 0111:B4, CAS registry no. L2630) was obtained from Sigma (St. Louis, MO, USA), 2,7-dichlorodihydrofluorescein diacetate from Molecular Probes (Eugene, OR, USA), and Immobilon-P transfer membranes from Millipore (Bedford, MA, USA). Antibodies for iNOS and COX-2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and enhanced chemiluminescence Western blotting detection reagents were from Amersham Life Science (Arlington Heights, IL, USA). The radio-nucleotide [γ-32P]-ATP was obtained from Amersham (Bucks, UK). All other materials were of the highest grades commercially available. All other compounds were from Sigma unless otherwise stated.
Animals
Male, specific-pathogen free ICR mice (weight 30 to 32 g, 6 wk of age) were purchased from Hyochang Science (Daegu, Korea). Animals were housed in a controlled environment (24℃, 50% to 60% RH) and provided with standard rodent chow and water. This study complied with the Guide for the Care and Use of Laboratory Animals issued by the Institute of Laboratory Animal Resources (ISBN 0-309-05377-3).
Experimental groups
Thirty-six ICR mice were assigned to one of the following six groups: 1) a saline injected group (the control group, n=6); 2) a 5 mg/kg LPS group (n=6); 3) three ginsenoside Rd (at 2, 10, or 50 mg/kg) plus 5 mg/kg LPS groups (ginsenoside+LPS groups, n=6); and 4) a 10 μM dexamethasone plus 5 mg/kg LPS group (the dexamethasone group, n=6). Saline, LPS, ginsenoside, and dexamethasone were administered intraperitoneally and ginsenoside and dexamethasone were administered 2 h prior to LPS treatment. Mice were dissected 4 h after LPS treatment.
Cell culture
RAW264.7 cells (a murine macrophage cell line) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were grown at 37℃ in Dulbecco’s-modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 233.6 mg/mL of glutamine, 72 mg/mL penicillin-streptomycin, 0.25 mg/mL of amphotericin B, and 10% heat-inactivated fetal bovine serum (FBS) in a humidified 5% CO2/95% air atmosphere.
Purification of ginsenosides
Nine fractions of ginsenosides were isolated as described by Kitagawa et al. [14]. Briefly powdered red ginseng (the steamed, dried, powdered roots of P. ginseng produced by the Korean Ginseng and Tobacco Research Institute, Daejeon, Korea) was refluxed in methanol. The extract so obtained was suspended in water and extracted with n-butanol (saturated with water), and the butanol extract was evaporated in vacuo. The powder obtained (total ginsenosides) was dissolved in methanol and extracted with diethylether by stirring. Thus, repeated column chromatography of the ether-soluble portion using a Bondapek C18 column eluted with methanol-water (1:1-7:3) followed by a silica gel column eluted with CHCl3 methanol-water (10:1) provided ginsenosides Rh1 and Rh2. The methanol-soluble portion was subject to silica gel column chromatography eluted with CHCl3-methanol-water (65:35:10, the low phase) and n-butanol-ethylacetate-methanol-water (4:2:1:1, the low phase), followed by Bondapek C18 column chromatography eluted with methanol-water (1:1-7:3) to give ginsenoside Rd. The purities of each of the ginsenosides obtained were determined using melting points, optical rotations, and pos, fast atom bombardment mass spectrometry (FAB-MS). Melting points were determined using a Fisher-John unit, optical rotations using a Jasco DIP-370 Instrument, and pos FAB-MS spectra were obtained using a VG-VSEQ spectrometer (type EBqQ).
Measurement of nitrite (NO) levels
RAW264.7 cells were seeded in 96-well plates at a density of 2.0×105 cells/well. Cells were treated with ginsenoside for 1 h and then incubated for 18 h in DMEM containing 1% FBS with or without 100 ng/mL LPS. Nitrite levels in media were determined using the Griess reaction [15]. Briefly, 100 μL aliquots of cell culture supernatants were reacted with 100 μL of Griess reagent (0.1% [w/v] naphthylethylendiamide dihydrochloride in H2O and 1% [w/v] sulfanilamide in 5% phosphoric acid), and then absorbance was read at 550 nm using an ELISA reader GENios (Tecan Instruments, Salzburg, Austria).
Measurement of prostaglandin E2 production in macrophages
PGE2 production was determined using a modification of the procedure described by Hwang et al. [16]. Briefly, RAW264.7 cells were seeded in 96-well plates at a density of 1.5×104 cells/well, treated with ginsenoside for 1 h, and then incubated for 18 h in DMEM containing 1% FBS with or without 100 ng/mL of LPS. Amount of PGE2 in culture media were determined using a specific Enzyme Immunoassay kit (EIA, Amersham Pharmacia Biotech, UK).
Western blotting
Western blotting was carried out as described previously [17]. Homogenized samples were boiled for 5 min with a gel-loading buffer (0.125 M Tris-HCl, pH 6.8, 4% SDS, 10% 2-mercaptoethanol and 0.2% bromophenol blue) in ratio of 1:1. Total protein-equivalents for each sample were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using 10% acrylamide gels as described by Laemmli [18], and transferred to polyvinylidene fluoride membrane at 15 V for 1 h in a semi-dry transfer system. The membrane was immediately placed into blocking buffer (1% non-fat milk) in 10 mM Tris, pH 7.5, 100 mM NaCl, and 0.1% Tween 20. The blot was allowed to block at room temperature for 1 h. The membrane was incubated with specific primary antibody (COX-2 or iNOS) at 25℃ for 3 h, and followed by a horse radish peroxidase-conjugated anti-rabbit antibody (Santa Cruz, 1:10,000) or anti-goat antibody (Santa Cruz, 1:10,000) at 25℃ for 1 h. Antibody labeling was detected using West-zol Plus and chemiluminescence FluorchemTMSP (Alpha Innotech Corporation, San Leandro, CA, USA). Pre-stained protein markers were used for molecular weight determinations.
Electrophoretic mobility shift assay
Electrophoretic mobility shift assay (EMSA) method was used to characterize the binding activities of NF-κB in nuclear extracts [19]. NF-κB oligonuleotide was 5’-GAGAGGCAAGGGGATTCCCTTAGTTAGGA-3' [20]. Protein-DNA binding assays were performed with 10 μg of nuclear protein. To minimize salt on binding, the concentration of salt was adjusted to the same level in all samples. Unspecific binding was blocked by using 1 μg of poly(dI-dC)poly(dI-dC). The binding medium contain 5% glycerol, 1 mM MgCl2, 50 mM NaCl, 0.5 mM ethylenediaminetetraacetic acid (EDTA), 2 mM dithiothreitol, 1% nonyl phenoxypolyethoxylethanol-40, and 10 mM Tris, pH 7.5. In each reaction, 20,000 cpm of radiolabeled probe was included. Samples were incubated at room temperature for 20 min, and the nuclear protein-32P-labeled oligonucleotide complex was separated from free 32P-labeled oligonucleotide by electrophoresis through a 5% native poly-acrylamide gel in a running buffer containing 50 mM Tris, pH 8.0, 45 mM borate, and 0.5 mM EDTA. After separation was achieved, the gel was vacuum dried for autoradiography and exposed to Fuji X-ray film at -80℃ for 1 to 2 d.
Tissue preparation
One gram of liver tissue was homogenized in 10 mL of homogenizing buffer (50 mM potassium phosphate buffer containing 1 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, 1 μM pepstatin, 80 mg/L trypsin inhibitor, pH 7.4) and centrifuged at 900 g at 4℃ for 15 min. The supernatants so obtained were then re-centrifuged at 12,000 g at 4℃ for 15 min to yield a sedimented mitochondrial fraction and cytosol fraction. All fractions were stored at -80℃ until required.
Measurement of NO2- plus NO3- levels in vivo
Total NO2- plus NO3- (NOx) levels were measured using an NO-analyzing system (ENO-20; Eicom Corp., Kyoto, Japan). Samples were deproteinized by adding an equal volume of methanol, and then centrifuged at 12,000 g for 10 min at 4℃ to avoid column occlusion by macromolecules. Nitrite and nitrate were then separated on a polystyrene polymer column, and nitrate was reduced to nitrite by passing its fraction through a cadmium column. The eluate was then mixed with Griess reagent, and absorbance of the purple dye formed was measured at 540 nm using a flow-through spectrophotometer. Concentrations of nitrite and nitrate were determined using a computer system (Power Chrom; Eicom, Kyoto, Japan), which automatically measured the areas of absorbance peaks. The minimal detectable concentrations of nitrite and nitrate using this method were both about 0.01 μM [21].
Statistical analysis
All values in figures are expressed as means±SE (n=6). The analysis was conducted using one-way ANOVA’s post-hoc test, and p-values of <0.05 were considered statistically significant.
RESULTS
Screening of ginsenosides Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rh1, and Rh2 for nitric oxide suppression in RAW264.7 cells
NO is a signaling molecule that plays a critical role in vascular smooth muscle relaxation, reduction of platelet aggregation and adhesion [22]. On the other hand, NO also is synthesized at inflammatory sites by iNOS with nicotinamide adenine dinucleotide phosphate and oxygen as substrates. iNOS is induced in response to LPS, interferon-g, and a variety of pro-inflammatory cytokines [23]. We examined the effects of ginsenoside on NO production in RAW264.7 cells stimulated by LPS, and dexamethasone, a well-known inhibitor of iNOS and COX-2, was used as a positive control.
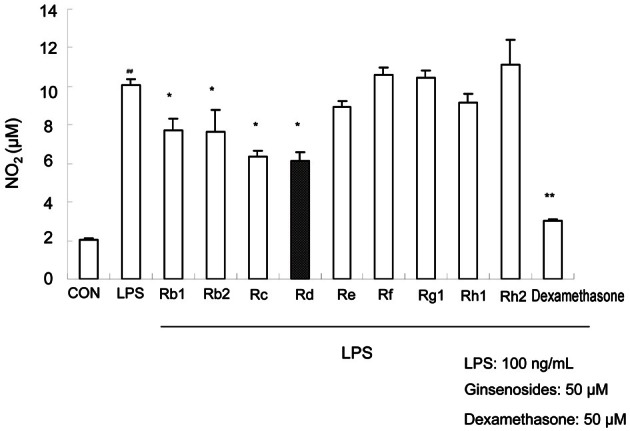
Ginsenosides Rd, Rc, and Rb2 (Fig. 1) were found to be the effective components of ginseng; about 40% inhibition of NO production was achieved versus LPS treatment alone (Fig. 2). As shown in Fig. 2, the nitrite level in culture medium was markedly increased from 2.05±0.05 μM to 10.08±0.29 μM 18 h after treatment with LPS. Nine ginsenosides were screened to test their anti-inflammatory effects. In subsequent experiments, we decided to use ginsenoside Rd, because little is known of its effects.
Fig. 1. Structures of ginsenosides.
Fig. 2. Effect of ginsenosides on lipopolysaccharides (LPS)-induced nitrite production in RAW264.7 cells. Cells were treated with ginsenosides (50 μM) for 1 h and then treated with LPS (100 ng/mL) for 18 h. Nitrite levels were determined by the Griess reaction, as described in Materials and Methods section. The values shown are means±SE of three tests. Statistical significance: *p<0.05 and **p<0.01 vs. LPS-stimulated cells and ##p<0.01 vs. control (non-stimulated cells). CON, control.
Inhibitory effects of ginsenoside Rd on NO2- plus NO3- levels and inducible nitric oxide synthase expression activity
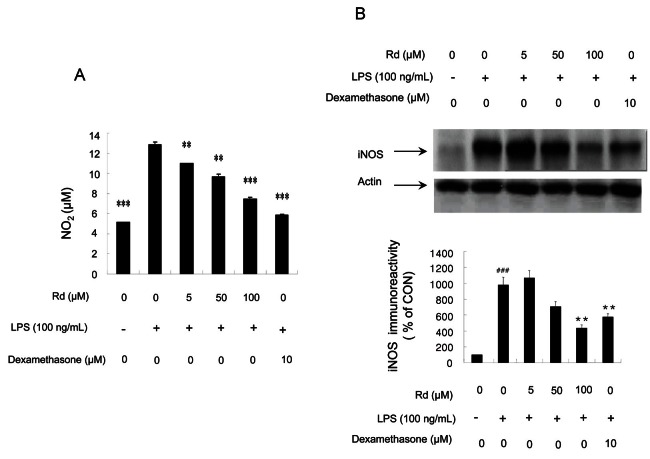
In order to determine whether ginsenoside Rd inhibits iNOS expression in vitro, we investigated iNOS activities after adding ginsenoside Rd to LPS-stimulated RAW264.7 cells. NOx levels in culture medium were significantly elevated from 5.170±0.002 μM to 12.81±0.85 μM 18 h after treatment with LPS, but after treatment with ginsenoside Rd (at 5, 50, and 100 μM), dose-dependent decreases in nitrite levels were observed in cultured cells, peaking at 100 mM of the ginsenoside Rd treatment (Fig. 3A).
Fig. 3. Effects of ginsenoside Rd on lipopolysaccharides (LPS)-induced nitrite production and inducible nitric oxide synthase (iNOS) expression in RAW264.7 cells. (A) RAW264.7 cells were treated with various concentrations of ginsenoside Rd (5, 50, and 100 μM) and 100 ng/mL of LPS. Nitrite levels were determined using the Griess reaction. (B) Cells were treated with various concentrations of ginsenoside Rd (5, 50, and 100 μM) for 1 h and then 100 ng/mL of LPS was added for 18 h. Total cellular proteins (50 μg) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride membranes, and detected using specific antibodies, as described in the Methods. iNOS expression was determined by densitometry and normalized versus β-actin. For both (A) and (B), values are expressed as means±SE of three tests. Statistical significance: *p<0.05, **p<0.01, and ***p<0.001 vs. LPS-stimulated cells and ##p<0.01, ###p<0.001 vs. control (non-stimulated cells). CON, control.
iNOS protein expressions in LPS-stimulated macrophages were examined by Western blotting. Densitometer scans of respective blots showed that RAW264.7 cells did not express detectable levels of iNOS protein when incubated in medium alone for 18 h (Fig. 3B), whereas iNOS expression increased dramatically in cells treated with LPS treatment (100 ng/mL) for 18 h. Furthermore, LPS-induced iNOS expression was significantly abolished when ginsenoside Rd was added at a concentration of 100 μM.
Inhibitory effects of ginsenoside Rd on prostaglandin E2 and cyclooxygenase-2 protein levels
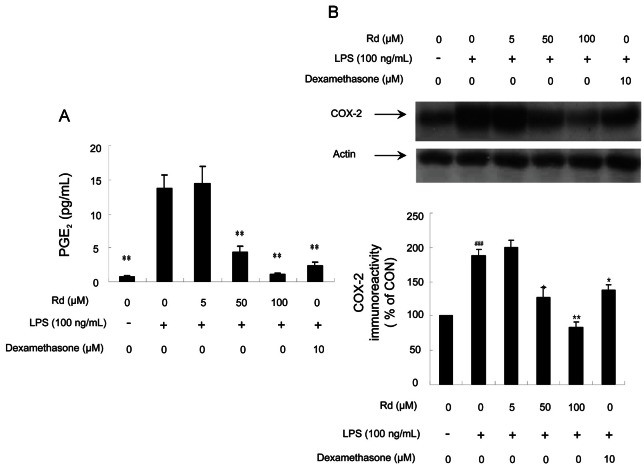
COX is a key enzyme in the generation of prostanoids (PGs) and other eicosanoids from arachidonic acid [24], and two COX isozymes, COX-1 and COX-2, have been identified. In most tissues, COX-1 is considered the constitutive isoform that catalyzes the synthesis of PGs. On the other hand, COX-2 is an inducible isoform and is expressed after an inflammatory stimulus, such as LPS, inflammatory cytokines, growth factors, or tumor promoters. Furthermore, COX-2 is responsible for the production of large amounts of pro-inflammatory PGE2 [25]. In this study, the effects of ginsenoside Rd on PGE2 production and COX-2 expression were investigated.
Fig. 4B shows that LPS-mediated COX-2 expression in RAW264.7 cells was effectively inhibited by ginsenoside Rd at 50 μM, as determined by densitometry. Furthermore, 100 μM of ginsenoside Rd was more effective than 10 μM of dexamethasone. We next examined PGE2 levels. Fig. 4A shows that ginsenoside Rd markedly suppressed PGE2 production in LPS-stimulated RAW264.7 cells. In fact, PGE2 levels were inhibited by 69% to 93% by 50 to 100 μM of ginsenoside Rd, whereas dexamethasone at 10 μM inhibited PGE2 production by 83%.
Fig. 4. Effects of ginsenoside Rd on lipopolysaccharides (LPS)-induced prostaglandin E2 (PGE2) production and cyclooxygenase (COX)-2 expression in RAW264.7 cells. (A) Cells were treated with the indicated concentrations of ginsenoside Rd (5, 50, and 100 μM) for 1 h and then LPS (100 ng/mL) was added for 18 h. PGE2 concentrations in culture media was measured by enzyme immunoassay. (B) Cells were treated with various concentrations of ginsenoside Rd (5, 50, and 100 μM) for 1 h and then LPS (100 ng/mL) was added for 18 h. Total cellular proteins (50 μg/lane) were separated on 8% sodium dodecyl sulfate-polyacrylamide gels and blotted with antibody specific for iNOS. For both (A) and (B), values are expressed as means±SE of three tests. Statistical significance: *p<0.05 and **p<0.01 vs. LPS-stimulated cells and ###p<0.0001 vs. control (non-stimulated cells). CON, control.
Effects of ginsenoside Rd on lipopolysaccharides-induced nuclear factor-κB activation in macrophage cell line
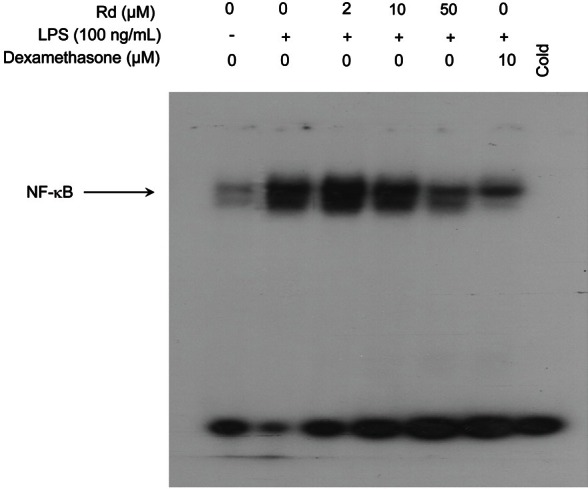
A report showed that NF-κB can be activated by LPS in macrophage cell lines [26]. Since NF-κB plays an important role in the expressions of, iNOS and COX-2, we examine whether NF-κB activity is modulated by ginsenoside Rd treatment in LPS-induced cells. A DNA binding assay was used to assess the ability of ginsenoside Rd to reduce the ability of active NF-κB to bind to a radio-labeled oligonucleotide containing kB DNA elements using EMSA. Nuclear extracts from LPS-stimulated cells were incubated with increasing concentrations of ginsenoside Rd for 30 min at 37℃ (Fig. 5). The binding of NF-κB to DNA in the presence of LPS only was markedly increased in a small proportion of macrophages. However, ginsenoside Rd inhibited NF-κB binding to DNA dose-dependently and significantly inhibited NF-κB binding to DNA at a concentration of 50 μM. This result demonstrates that NF-κB DNA binding can be inhibited by ginsenoside Rd in a dose-dependent manner.
Fig. 5. Effect of ginsenoside Rd on lipopolysaccharides (LPS)-induced nuclear factor-κB (NF-κB) binding in RAW264.7 cells. Nuclear extracts from LPS-stimulated RAW264.7 cells were treated with different concentrations of ginsenoside Rd for 30 min at 37℃ in vitro and analyzed for NF-κB binding by electrophoretic mobility shift assays as described in Methods. The arrow indicates the gel location of NF-κB bound to DNA.
Inhibitory effects of ginsenoside Rd on NOx levels and on the expressions of inducible nitric oxide synthase, and cyclooxygenase-2 in vivo
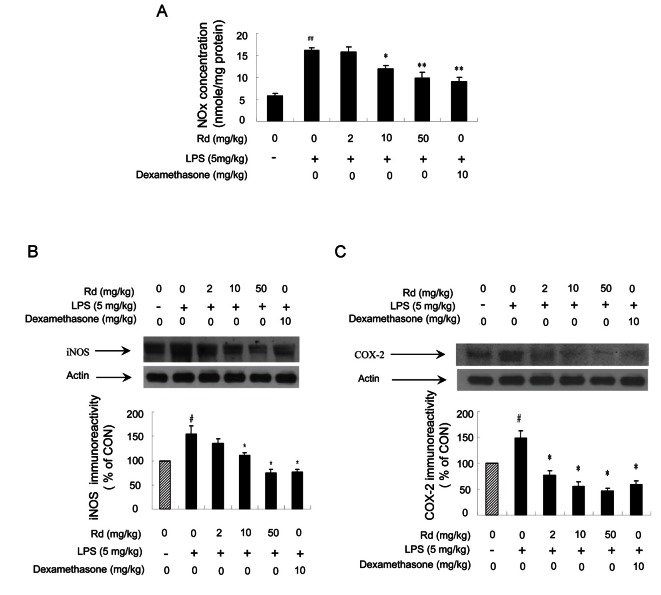
We performed in vivo experiments to confirm the results obtained in vitro. Total NOx levels in mouse liver were determined using an NO-analyzer, as described in the Materials and Methods section. Mice were injected with three different concentrations of ginsenoside Rd (2, 10, and 50 mg/kg) and 2 h later were injected with LPS (5 mg/kg). Mice were sacrificed 4 h after LPS treatment. Total NOx levels in liver were much increased in LPS-injected mice, but pretreatment with ginsenoside Rd dose-dependently inhibited NOx level increases (Fig. 6A).
Fig. 6. Effects of ginsenoside Rd on the expressions of lipopolysaccharides (LPS)-induced nitric oxide (NO) metabolites, inducible nitric oxide synthase (iNOS), and cyclooxygenase (COX)-2 in vivo. (A) Different concentrations of ginsenoside Rd (2, 10, and 50 mg/kg) were injected intraperitoneally into ICR mice and 2 h later mice were treated with LPS (5 mg/kg, intraperitoneally). Mice were sacrificed 4 h after the LPS injection. The inhibitory effects of ginsenoside Rd on NO2- plus NO3- (NOx) generation were determined as described in Materials and Methods section. The values shown are means±SE for 6 mice. Statistical significance: *p<0.05 and **p<0.01 vs. LPS-stimulated mice and ###p<0.001 vs. control (non-stimulated mice). (B) Total liver proteins (50 mg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride membranes, and detected with specific antibodies as described in Materials and Methods section. Values are means±SEs. Statistical significance: *p<0.05 vs. LPS-stimulated cells and #p<0.05 vs. control (non-stimulated cells). (C) Total cellular proteins (50 mg/lane) were separated on 8% sodium dodecyl sulfate-polyacrylamide gel and blotted with antibody specific for COX-2. Values are means±SE. Statistical significance: *p<0.05 vs. LPS-stimulated cells and #p<0.05 vs. control (non-stimulated cells).
Furthermore, whereas naïve mice showed no detectable iNOS protein in liver, iNOS protein was highly expressed in LPS (5 mg/kg) treated mice. Pretreatment with ginsenoside Rd at 2, 10, or 50 mg/kg reduced LPS-induced expression (Fig. 6B). In addition, we examined whether ginsenoside Rd also affects COX-2 expression. The levels of COX-2 protein were examined by immunoblot analysis using a specific anti-COX-2 antibody. In LPS (5 mg/kg) treated mouse livers, COX-2 was highly expressed, whereas pretreatment with ginsenoside Rd markedly reduced LPS-induced COX-2 expression (Fig. 6C). Taken together, these in vivo results confirmed the observed in vitro effects of ginsenoside Rd on iNOS and COX-2.
DISCUSSION
Studies show that nitric oxide is a principle mediator of a wide range of toxic oxidative reactions, such as the initiation of lipid peroxidation, the inhibition of mitochondrial respiratory chain enzymes, the inhibition of membrane sodium/potassium ATP-ase activity, and the inactivation and oxidative modifications of proteins [27]. Furthermore, all of these toxic effects are related to acute and chronic inflammation [28,29]. In our previously studies, we also found that nitric oxide contributes to vascular inflammation [30,31], and thus, the excessive expression of iNOS, a precursor of NO, is likely to be implicated in the pathogeneses of many inflammatory diseases. Recently, natural occurring phytochemicals have been shown to reduce the undesirable expression of iNOS [32,33], which suggests that suitable phytochemicals could be used to treat inflammation. Similarly, the present study shows that ginsenoside Rd reduces NO production by inhibiting iNOS expression in LPS-activated murine macrophages and in vivo.
COX-2 expression has been reported to weaken antioxidant capacity in mouse macrophages [34,35], and oxidative stress is known to regulate and exacerbate inflammation. Therefore, we used RAW264.7 cells in this study to evaluate the effect of ginsenoside Rd on the transcriptional activities of COX-2. Our results show that COX-2 activity and levels of its by-product PGE2 are decreased by ginsenoside Rd.
NF-κB activation is known to be associated with the up-regulations of the expressions of iNOS and COX-2 mediated by LPS. To elucidate the molecular actions of ginsenoside Rd, we examined its ability to inhibit the production of iNOS and COX-2. EMSA revealed that the activity of NF-κB binding to the consensus sequence of kB was inhibited by ginsenoside Rd in LPS-activated RAW264.7 cells.
Several other studies have attempted to elucidate the inhibitory effects of ginsenoside on iNOS expression and NO production in vitro and in vivo. For example, Park et al. [36] suggested that ginsenoside Rb1 modulates NO and PGE2 biosynthesis in RAW264.7 cells induced by LPS, and in a later study found that in mice, ginsenoside Rh1 possesses anti-inflammatory activity due to its ability to inhibit the expressions of iNOS and COX-2 [37]. Ginsenoside Rd has been reported to have an inhibitory effect on NO production in C6 rat glioma cells [38], and recently, was shown to attenuate oxidative stress [39]. These results support the view that ginsenoside Rd inhibits the pro-inflammatory mediators, iNOS and COX-2.
The above findings raise the question as to the nature of any potential pathway of initiated by ginsenoside Rd. In our previous study, we found that ginsenosides Rh1 and Rh2 mediate the nuclear translocation of glucocorticoid receptor (GR) and to the differentiation of teratocarcinoma stem cells [40], which suggests that the GR signal cascade provides a means whereby ginsenoside Rd could act therapeutically.
Based on our findings, we conclude that the anti-inflammatory property of ginsenoside Rd is due to the expressional inhibitions of iNOS and COX-2. These effects of ginsenoside Rd were further supported by its effectiveness to modulate pro-inflammatory NF-κB activity. iNOS and COX-2 are known to play pivotal roles in the pathogenesis of acute and chronic inflammation, and thus, the inhibition of the abnormal up-regulations of iNOS and COX-2 provides a molecular basis for the therapeutic effect of ginsenoside Rd on inflammation and inflammatory diseases. Furthermore, ginsenoside Rd inhibited PGE2- and NO-triggered inflammatory responses in vitro and in vivo, and since the aberrant over-expressions of COX-2 and iNOS are implicated in the pathogenesis of various inflammation-related diseases, the results of this study indicate that ginsenoside Rd may have applications for the treatment of inflammatory disorders.
Acknowledgments
This work was supported by a grant in 2011 from the Korean Society of Ginseng and by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A090582). This study was financially supported by the 2012 Post-Doc Development Program of Pusan National University
References
- 1.Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 2.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 3.Adams J, Stein R. Novel inhibitors of the proteasome and their therapeutic use in inflammation. Annu Rep Med Chem. 1996;31:279–288. [Google Scholar]
- 4.Chen YQ, Ghosh S, Ghosh G. A novel DNA recognition mode by the NF-kappa B p65 homodimer. Nat Struct Biol. 1998;5:67–73. doi: 10.1038/nsb0198-67. [DOI] [PubMed] [Google Scholar]
- 5.Baldwin AS Jr. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 6.Mann JR, Backlund MG, DuBois RN. Mechanisms of disease: inflammatory mediators and cancer prevention. Nat Clin Pract Oncol. 2005;2:202–210. doi: 10.1038/ncponc0140. [DOI] [PubMed] [Google Scholar]
- 7.Song SB, Tung NH, Quang TH, Ngan NT, Kim KE, Kim YH. Inhibition of TNF-α-mediated NF-κB transcriptional activity in HepG2 cells by dammarane-type saponins from Panax ginseng leaves. J Ginseng Res. 2012;36:146–152. doi: 10.5142/jgr.2012.36.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 9.Hilmy M, Campbell R, Bartlett JM, McNicol AM, Underwood MA, McMillan DC. The relationship between the systemic inflammatory response, tumour proliferative activity, T-lymphocytic infiltration and COX-2 expression and survival in patients with transitional cell carcinoma of the urinary bladder. Br J Cancer. 2006;95:1234–1238. doi: 10.1038/sj.bjc.6603415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shang W, Yang Y, Jiang B, Jin H, Zhou L, Liu S, Chen M. Ginsenoside Rb1 promotes adipogenesis in 3T3-L1 cells by enhancing PPARgamma2 and C/EBPalpha gene expression. Life Sci. 2007;80:618–625. doi: 10.1016/j.lfs.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 11.Wakabayashi C, Hasegawa H, Murata J, Saiki I. In vivo antimetastatic action of ginseng protopanaxadiol saponins is based on their intestinal bacterial metabolites after oral administration. Oncol Res. 1997;9:411–417. [PubMed] [Google Scholar]
- 12.Xie JT, Shao ZH, Vanden Hoek TL, Chang WT, Li J, Mehendale S, Wang CZ, Hsu CW, Becker LB, Yin JJ, et al. Antioxidant effects of ginsenoside Re in cardiomyocytes. Eur J Pharmacol. 2006;532:201–207. doi: 10.1016/j.ejphar.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Shen T, Lee J, Park MH, Lee YG, Rho HS, Kwak YS, Rhee MH, Park YC, Cho JY. Ginsenoside Rp1, a ginsenoside derivative, blocks promoter activation of iNOS and COX-2 genes by suppression of an IKKb-mediated NF-kB pathway in HEK293 cells. J Ginseng Res. 2011;35:200–208. doi: 10.5142/jgr.2011.35.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitagawa I, Yoshikawa M, Yoshihara M, Hayashi T, Taniyama T. Chemical studies of crude drugs (1). Constituents of ginseng radix rubra. Yakugaku Zasshi. 1983;103:612–622. [PubMed] [Google Scholar]
- 15.Kim H, Lee HS, Chang KT, Ko TH, Baek KJ, Kwon NS. Chloromethyl ketones block induction of nitric oxide synthase in murine macrophages by preventing activation of nuclear factor-kappa B. J Immunol. 1995;154:4741–4748. [PubMed] [Google Scholar]
- 16.Hwang BY, Lee JH, Koo TH, Kim HS, Hong YS, Ro JS, Lee KS, Lee JJ. Furanoligularenone, an eremophilane from Ligularia fischeri, inhibits the LPS-induced production of nitric oxide and prostaglandin E2 in macrophage RAW264.7 cells. Planta Med. 2002;68:101–105. doi: 10.1055/s-2002-20250. [DOI] [PubMed] [Google Scholar]
- 17.Go EK, Jung KJ, Kim JM, Lim H, Lim HK, Yu BP, Chung HY. Betaine modulates age-related NF-kappaB by thiol-enhancing action. Biol Pharm Bull. 2007;30:2244–2249. doi: 10.1248/bpb.30.2244. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Kerr LD. Electrophoretic mobility shift assay. Methods Enzymol. 1995;254:619–632. doi: 10.1016/0076-6879(95)54044-x. [DOI] [PubMed] [Google Scholar]
- 20.Kim HJ, Kim KW, Yu BP, Chung HY. The effect of age on cyclooxygenase-2 gene expression: NF-kappaB activation and IkappaBalpha degradation. Free Radic Biol Med. 2000;28:683–692. doi: 10.1016/s0891-5849(99)00274-9. [DOI] [PubMed] [Google Scholar]
- 21.Kimura S, Watanabe K, Yajiri Y, Motegi T, Masuya Y, Shibuki K, Uchiyama S, Homma T, Takahashi HE. Cerebrospinal fluid nitric oxide metabolites in painful diseases. Neuroreport. 1999;10:275–279. doi: 10.1097/00001756-199902050-00013. [DOI] [PubMed] [Google Scholar]
- 22.Soloviev A, Lehen’kyi V, Zelensky S, Hellstrand P. Nitric oxide relaxes rat tail artery smooth muscle by cyclic GMP-independent decrease in calcium sensitivity of myofilaments. Cell Calcium. 2004;36:165–173. doi: 10.1016/j.ceca.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Napoli C, de Nigris F, Williams-Ignarro S, Pignalosa O, Sica V, Ignarro LJ. Nitric oxide and atherosclerosis: an update. Nitric Oxide. 2006;15:265–279. doi: 10.1016/j.niox.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Giuliano F, Warner TD. Origins of prostaglandin E2: involvements of cyclooxygenase (COX)-1 and COX-2 in human and rat systems. J Pharmacol Exp Ther. 2002;303:1001–1006. doi: 10.1124/jpet.102.041244. [DOI] [PubMed] [Google Scholar]
- 25.Yamada M, Niki H, Yamashita M, Mue S, Ohuchi K. Prostaglandin E2 production dependent upon cyclooxygenase-1 and cyclooxygenase-2 and its contradictory modulation by auranofin in rat peritoneal macrophages. J Pharmacol Exp Ther. 1997;281:1005–1012. [PubMed] [Google Scholar]
- 26.Bayon Y, Ortiz MA, Lopez-Hernandez FJ, Gao F, Karin M, Pfahl M, Piedrafita FJ. Inhibition of IkappaB kinase by a new class of retinoid-related anticancer agents that induce apoptosis. Mol Cell Biol. 2003;23:1061–1074. doi: 10.1128/MCB.23.3.1061-1074.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu BP, Chung HY. Oxidative stress and vascular aging. Diabetes Res Clin Pract. 2001;54 Suppl 2:S73–S80. doi: 10.1016/s0168-8227(01)00338-2. [DOI] [PubMed] [Google Scholar]
- 28.Cuzzocrea S. Role of nitric oxide and reactive oxygen species in arthritis. Curr Pharm Des. 2006;12:3551–3570. doi: 10.2174/138161206778343082. [DOI] [PubMed] [Google Scholar]
- 29.Marriott HM, Ali F, Read RC, Mitchell TJ, Whyte MK, Dockrell DH. Nitric oxide levels regulate macrophage commitment to apoptosis or necrosis during pneumococcal infection. FASEB J. 2004;18:1126–1128. doi: 10.1096/fj.03-1450fje. [DOI] [PubMed] [Google Scholar]
- 30.Chung HY, Sung B, Jung KJ, Zou Y, Yu BP. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006;8:572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- 31.Lee C, Miura K, Liu X, Zweier JL. Biphasic regulation of leukocyte superoxide generation by nitric oxide and peroxynitrite. J Biol Chem. 2000;275:38965–38972. doi: 10.1074/jbc.M006341200. [DOI] [PubMed] [Google Scholar]
- 32.Yadav PN, Liu Z, Rafi MM. A diarylheptanoid from lesser galangal (Alpinia officinarum) inhibits proinflammatory mediators via inhibition of mitogen-activated protein kinase, p44/42, and transcription factor nuclear factor-kappa B. J Pharmacol Exp Ther. 2003;305:925–931. doi: 10.1124/jpet.103.049171. [DOI] [PubMed] [Google Scholar]
- 33.Rose P, Won YK, Ong CN, Whiteman M. Beta-phenylethyl and 8-methylsulphinyloctyl isothiocyanates, constituents of watercress, suppress LPS induced production of nitric oxide and prostaglandin E2 in RAW 264.7 macrophages. Nitric Oxide. 2005;12:237–243. doi: 10.1016/j.niox.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Kim JW, Zou Y, Yoon S, Lee JH, Kim YK, Yu BP, Chung HY. Vascular aging: molecular modulation of the prostanoid cascade by calorie restriction. J Gerontol A Biol Sci Med Sci. 2004;59:B876–B885. doi: 10.1093/gerona/59.9.b876. [DOI] [PubMed] [Google Scholar]
- 35.Baek BS, Kim JW, Lee JH, Kwon HJ, Kim ND, Kang HS, Yoo MA, Yu BP, Chung HY. Age-related increase of brain cyclooxygenase activity and dietary modulation of oxidative status. J Gerontol A Biol Sci Med Sci. 2001;56:B426–B431. doi: 10.1093/gerona/56.10.b426. [DOI] [PubMed] [Google Scholar]
- 36.Park EK, Shin YW, Lee HU, Kim SS, Lee YC, Lee BY, Kim DH. Inhibitory effect of ginsenoside Rb1 and compound K on NO and prostaglandin E2 biosyntheses of RAW264.7 cells induced by lipopolysaccharide. Biol Pharm Bull. 2005;28:652–656. doi: 10.1248/bpb.28.652. [DOI] [PubMed] [Google Scholar]
- 37.Park EK, Choo MK, Han MJ, Kim DH. Ginsenoside Rh1 possesses antiallergic and anti-inflammatory activities. Int Arch Allergy Immunol. 2004;133:113–120. doi: 10.1159/000076383. [DOI] [PubMed] [Google Scholar]
- 38.Choi SS, Lee JK, Han EJ, Han KJ, Lee HK, Lee J, Suh HW. Effect of ginsenoside Rd on nitric oxide system induced by lipopolysaccharide plus TNF-alpha in C6 rat glioma cells. Arch Pharm Res. 2003;26:375–382. doi: 10.1007/BF02976694. [DOI] [PubMed] [Google Scholar]
- 39.Yokozawa T, Satoh A, Cho EJ. Ginsenoside-Rd attenuates oxidative damage related to aging in senescence-accelerated mice. J Pharm Pharmacol. 2004;56:107–113. doi: 10.1211/0022357022449. [DOI] [PubMed] [Google Scholar]
- 40.Lee YN, Lee HY, Lee YM, Chung HY, Kim SI, Lee SK, Park BC, Kim KW. Involvement of glucocorticoid receptor in the induction of differentiation by ginsenosides in F9 teratocarcinoma cells. J Steroid Biochem Mol Biol. 1998;67:105–111. doi: 10.1016/s0960-0760(98)00080-6. [DOI] [PubMed] [Google Scholar]