Abstract
Polyphenol oxidase (PPO) was purified from fresh ginseng roots using acetone precipitation, carboxymethyl (CM)-Sepharose chromatography, and phenyl-Sepharose chromatography. Two isoenzymes (PPO 1 and PPO 2) were separated using an ion-exchange column with CM-Sepharose. PPO 1 was purified up to 13.2-fold with a 22.6% yield. PPO 2 bound to CM-Sepharose, eluted with NaCl, and was purified up to 22.5-fold with a 17.4% yield. PPO 2 was further chromatographed on phenyl-Sepharose. The molecular weight of the purified PPO 2 from fresh ginseng was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and was about 40 kDa. The optimum temperature and pH were 20℃ and 7.0, respectively, using catechol as a substrate. Pyrogallol showed the highest substrate specificity. The effect of a PPO inhibitor showed that its activity increased slightly in the presence of a low concentration of citric acid. High concentrations of acidic compounds and sulfite agents significantly inhibited purified ginseng PPO 2.
Keywords: Panax ginseng, Polyphenol oxidase, Purification, Catechol, Carboxymethyl-Sepharose
INTRODUCTION
Polyphenol oxidase (PPO) or tyrosinase is a metalloenzyme that contains a dinuclear copper center. These enzymes seem to be universally distributed in animals, plants, and some fungi and bacteria, and are responsible for melanization in animals and the browning reaction in plants [1]. These are also called phenolase and catecholase depending on substrate specificity. They utilize molecular oxygen to catalyze the oxidation of phenolic compounds in which either one or two reactions occur. PPO carries out monophenol hydroxylation, which involves hydroxylase and cresolase activities (EC 1. 14. 18. 1), and oxidation of o-dihydroxyphenols into o-quinones, which involves laccase and catecholase activities (EC 1. 10. 3. 1) [2-4]. The physiological functions of plant and fungal PPO are not well understood. Melanins produced by fungal PPO contribute to spore formation and fungal virulence [2,4]. In higher plants, PPO plays a role in defense against pathogens and insects. This plant defense mechanism is provided by reactive oxygen species via oxidation of o-quinones [5]. PPO is important to industry, because it affects food quality by oxidizing polyphenols in edible vegetables and fruits. PPO may find applications in the biosensor and drug industries, as well as in phenolic compound degradation. PPO has been used to synthesize value-added products such as 3,4-dihydroxy-ʟ- phenylalanine (ʟ-DOPA) for treatment of Parkinson’s disease [6]. PPO from potato or fungi has been used to degrade organic contaminants [7].
Korean ginseng (Panax ginseng Meyer) has been used as a medical and pharmaceutical herb for many kinds of diseases and as a functional health food in Asian countries [8,9]. Ginseng root is usually dried for storage or used like many vegetables. But, the browning reactions presumed to be due to oxidation of phenolic compounds by PPO and peroxidase (PO) in this plant occur during the drying process. Brown or black pigment can be produced enzymatically and chemically in plant tissues. The browning reaction is also involved in considerable economic loss due to changes in color, flavor, and nutritional values, resulting in chemical changes in fresh ginseng [10].
Many studies have characterized PPO from difference sources such as apple [11], grape [12], potato [13], and fungi such as Boletus erythropus [14] and Trichoderma reesei [15]. Although PPO from various plants and fungi has been studied for many years, ginseng PPO has not been studied in detail.
In this study, PPO was purified from ginseng root using ion-exchange chromatography and hydrophobic interaction chromatography to identify optimal pH, optimal temperature, substrate specificity, and the molecular weight of the purified ginseng PPO. The purification and characterization of ginseng PPO may help in korean ginseng studies for the food industries and processed food using ginseng.
MATERIALS AND METHODS
Materials
Six-year-old ginseng (P. ginseng Meyer) root was obtained from the Anseong Ginseng Nonghyup (Anseong, Korea) and stored at -80℃ before use. Miracloth was purchased from Calbiochem (San Diego, CA, USA). Phenyl-Sepharose 4-Fast Flow and carboxymethyl (CM)- Sepharose-Fast Flow were obtained from Pharmacia (Uppsala, Sweden). All other reagents including PPO inhibitors were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Polyphenol oxidase assay and protein determination
PPO was assayed in 50 mM HEPES-KOH, pH 7.0, containing catechol as a substrate at 20℃ by measuring the initial rate of quinone formation. The PPO assay mixture contained 50 mM HEPES-KOH, pH 7.0, 30 mM catechol, and 0.1 mL of enzyme in 1.5-mL. Reaction mixtures were incubated at 20℃ for 30 min. Absorbances at 420 nm were measured spectrophotometrically. One unit of PPO activity was defined as the change in absorbance of 0.001 per min. Activity measurements were carried out in duplicate. Proteins were measured by the Bradford microprotein assay [16]. Bovine serum albumin was used as the standard.
Electrophoresis
Enzyme purity was determined during the purification steps using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Electrophoresis was carried out according to the method of Laemmli [17]. The separating gel concentration was 15% with a 4% stacking gel. Gels were stained with Coomassie blue.
Substrate specificity
Substrate specificity was determined using five different substrates. Activity was measured by monitoring wavelength. Hydroquinone was used as the monophenolic substrate, whereas ʟ -DOPA, chlorogenic acid, and catechol were used as diphenolic substrates. Pyrogallol was used as the triphenolic substrate. In the case of hydroquinone, chlorogenic acid, catechol and pyrogllol as substrates, 420 nm was used as the detecting wavelength and in the case of ʟ-DOPA, 475 nm was used. A 10 mM substrate solution was prepared in 50 mM HEPES-KOH, pH 7.0.
Optimal temperature and pH on ginseng polyphenol oxidase activity
Optimal temperature was determined by adding 0.1 ml of the purified enzyme to 1.4 mL of 30 mM catechol in 50 mM HEPES-KOH, pH 7.0. The mixture was incubated at 10℃ to 70℃ for 30 min. Optimal pH was determined by adding 0.1 mL of the purified enzyme to 1.4 mL of 30 mM catechol in 50 mM HEPES-KOH (pH 5.0 to 9.0).
Inhibitor studies of ginseng polyphenol oxidase activity
A ginseng PPO standard assay mixture was tested with four different inhibitors (kojic acid, ascorbic acid, sodium metabisulfite, and citric acid) at two different concentrations of 1 and 10 mM. These inhibitors were prepared in 50 mM HEPES-KOH, pH 7.0 and 30 mM catechol was used for the substrate.
Purification of ginseng polyphenol oxidase
All purification steps were performed at 4℃. The crude enzyme extract for PPO purification was prepared using the procedure of Ozel et al. [14], with some modifications. Fifty grams of ginseng root were frozen in liquid nitrogen and ground to a fine powder with a Waring blender. The ginseng powder was suspended in 200 mL of buffer (50 mM sodium acetate, pH 5.0 containing 20 mM ascorbic acid, 1 mM benzamidine-HCl, and 0.5 mM phenylmethylsulfonyl fluoride). The slurry was homogenized at 12,000 rpm for 5 min with a Polytron PT 3000 homogenizer (Kinematica AG, Lucerne, Switzerland) and filtered through two layers of Miracloth (Calbiochem). The filtrate was centrifuged at 8,000 rpm for 30 min at 4℃. Acetone was added to 30% of the supernatant and centrifuged at 8,000 rpm for 20 min at 4℃. After centrifugation, the supernatant was collected, acetone was added to 50%, and centrifuged as described above. The precipitate was dissolved in 10 mL of 50 mM sodium acetate buffer, pH 5.0. The crude enzyme solution was loaded onto a CM-Sepharose column (2.5×25 cm) previously equilibrated with 50 mM sodium acetate buffer (the equilibration buffer). The column was washed with equilibration buffer to remove unbound protein. After column washing, the buffer was changed stepwise to 0.5 M NaCl with equilibration buffer containing NaCl, and bound proteins were eluted. The eluted fractions (4 mL/tube) showing higher PPO activities were collected, and the fractions were used for further purification. The collected fractions were adjusted to 1 M ammonium sulfate before loading. The sample containing 1.0 M ammonium sulfate was loaded onto the phenyl-Sepharose 4-Fast Flow column (1.5×20 cm), previously equilibrated with the above buffer containing 1.0 M ammonium sulfate. The column was eluted with the same buffer without ammonium sulfate. The higher PPO activity fractions were collected.
RESULTS AND DISCUSSION
Purification of polyphenol oxidase from ginseng
Ginseng PPO was purified using acetone precipitation, ion-exchange chromatography, and hydrophobic interaction chromatography. Two types of ginseng PPO were obtained from the CM-Sepharose column. Table 1 shows the ginseng PPO purification steps. During the PPO extraction procedure, proteinase inhibitors such as phenylmethylsulfonyl fluoride and benzamidine as well as a reducing agent such as ascorbic acid for inhibiting the browning reaction were added. Acetone precipitation as the first step of the ginseng PPO purification was effective for removing low molecular weight proteins and lipid-soluble compounds. A total of 95% of the proteins were removed during this process, and the PPO recovery was 101% (Table 1).
Table 1.
Purification of ginseng polyphenol oxidase
| Fraction | Volume (mL) | Total protein (mg) | Total activity (unit) | Specific activity (unit/mg) | Yield (%) | Purification fold |
|---|---|---|---|---|---|---|
|
| ||||||
| Crude extract | 200 | 64.6 | 1,200 | 18.6 | 100 | 1 |
| Dissolved 50% acetone-precipitated pellet | 9.5 | 3.2 | 1,216 | 380 | 101.3 | 20.4 |
| Carboxymethyl-Sepharose | ||||||
| Peak I | 12 | 1.1 | 271.5 | 246.8 | 22.6 | 13.2 |
| Peak II1) | 8 | 0.5 | 209.3 | 418.6 | 17.4 | 22.5 |
| Phenyl-Sepharose | 0.7 | 0.003 | 12.0 | 4,000 | 1 | 215 |
Purification was based on 50 g of wet ginseng powder.
1)Only peak II was chromatographed on phenyl-Sepharose.
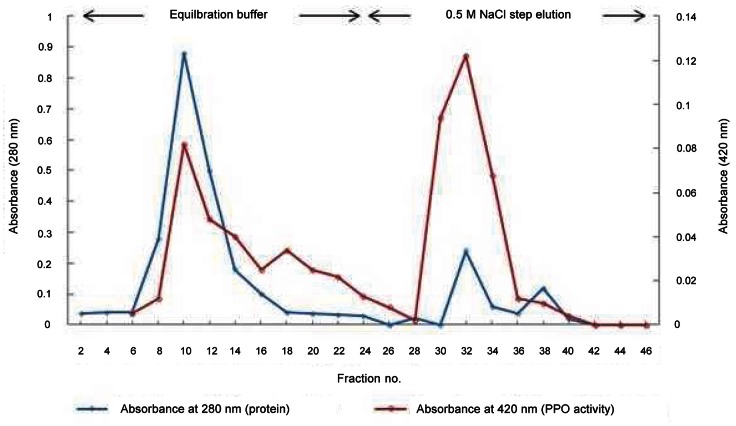
Higher than a 100% yield might be due to removal of the inhibitors in the crude extract. The enzyme solution was applied to the CM-Sepharose column, from which two types of ginseng PPO (PPO 1 and PPO 2) were obtained. PPO 1, which is the unbound form, was eluted with equilibration buffer without NaCl, and PPO 2 was eluted with a buffer containing 0.5 M NaCl (Fig. 1).
Fig. 1. Elution profile of the ginseng polyphenol oxidase (PPO) by carboxymethyl (CM)-Sepharose column chromatography. A crude enzyme solution was applied to the CM-Sepharose column, and PPO was eluted with a step gradient from 0 to 0.5 M NaCl. Each fraction was 4 mL, PPO activity was determined, and highly active fractions were collected.
A Coomassie-stained gel of the two PPO activity peaks was shown in Fig. 2. The unbound fractions (PPO 1) showed the contaminant protein, which was located around 60-kDa. The bound fractions (PPO 2) do not seem to contain the 60-kDa contaminant protein. PPO 1 and PPO 2 resulted in 22.6% and 17.4% recovery, and a 13.2-fold and 22.5-fold purification, respectively. Fractions eluted with NaCl were used for the further purification step, which was hydrophobic interaction chromatography on phenyl-Sepharose. Hydrophobic interaction chromatography has been used to purify PPO from other sources including mamey [18], longan fruit [19], and apple [20]. The peak from the phenyl-Sepharose column without salt showed the highest activity, whereas the 1.0 M ammonium sulfate fractions did not show any activity. The purified PPO from the phenyl-Sepharose column could be visualized only after concentrating the fractions by acetone precipitation. Ginseng PPO 2 was purified 215-fold from the crude extract, resulting in about 3 μg of the enzyme from 50 g of ginseng powder. The overall yield for ginseng PPO was 1% from the crude extract. The reason for the low purification yield was partly due to the two types of ginseng PPO from the CM-Sepharose column chromatography, and only PPO 2 was used for the final purification step. The molecular weight of the purified PPO was determined by SDS-PAGE and was about 40 kDa (Fig. 3).
Fig. 2. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the ginseng polyphenol oxidase (PPO) resolved by carboxymethyl-Sepharose chromatography. The gel was stained for proteins with Coomassie blue. Lane 1, molecular weight markers; lane 2, dissolved acetone pellet; lanes 3 to 5, flow-though fractions (PPO 1); lanes 6 to 8, eluted fractions with 0.5 M NaCl (PPO 2).
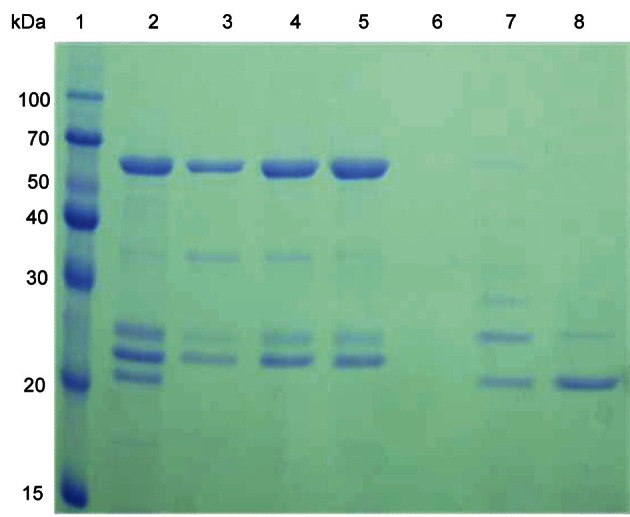
Fig. 3. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the ginseng polyphenol oxidase (PPO) at various stages of purification. Lane 1, molecular weight markers; lane 2, crude extract of ginseng powder; lane 3, carboxymethyl-Sepharose peak 2 (PPO 2); lane 4, phenyl-Sepharose peak concentrated using acetone precipitation. Arrow in the right margin indicates the purified PPO from ginseng root.
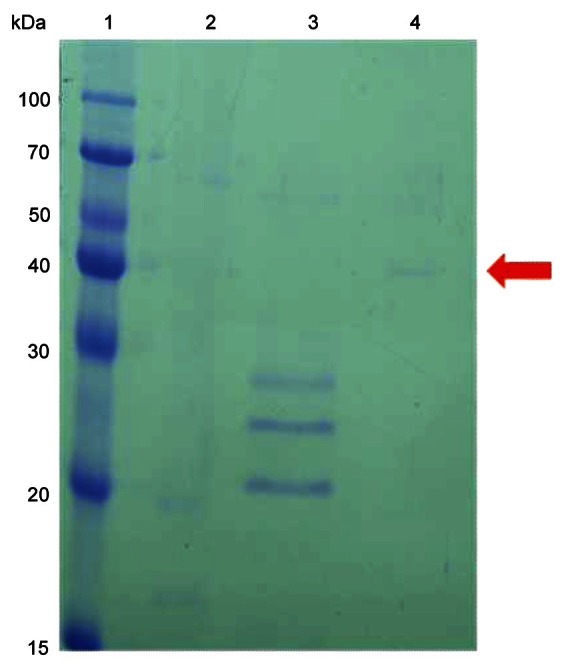
According to studies on PPO purification from various fruits and vegetables, some PPO isoforms might exist. In fact, various plant and fungal species have several isoforms with different molecular weight PPO. These isoforms can be distinguished based on biochemical properties such as optimal pH, optimal temperature, pI, and substrate specificity. According to the report by Flurkey and Inlow [2], PPO is proteolytically cleaved in some plant tissues, in which certain C-terminal or N-terminal peptides are cleaved. As a result, the reported PPO molecular weight was less than those calculated based on the nucleotide sequence. Because ginseng PPO cDNA has not been reported yet, the purified molecular weight of the PPO from this study may not provide the physiological process of ginseng.
Effect of temperature and pH on the purified ginseng polyphenol oxidase activity
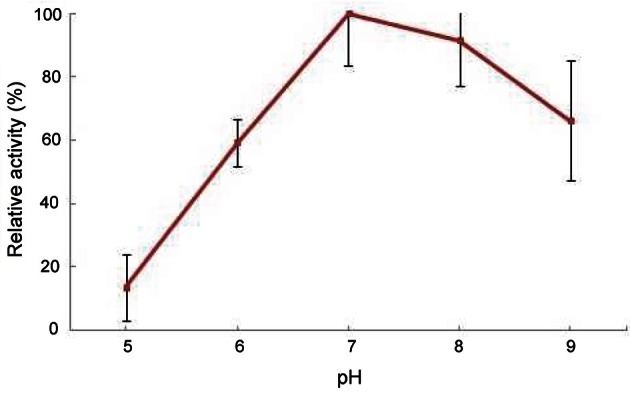
PPO activity increased to a maximum at 20℃ (Fig. 4). Ginseng PPO had thermal stability at 10℃ to 70℃ (data not shown). Optimal temperatures of some PPOs using catechol are 25℃ to 45℃ [4,21]. The optimal pH was 7.0 (Fig. 5). According to previous reports, the optimum pH of PPOs from various plants and vegetables is commonly 4.0 to 8.0. The optimum pH for PPO from plant sources differs depending on the substrate [4]. Ginseng PPO showed higher activity around neutral pH using catechol as a substrate, as in various fruits such as longan, pineapple, and kiwi [22,23].
Fig. 4. Optimal temperature of the purified ginseng polyphenol oxidase.
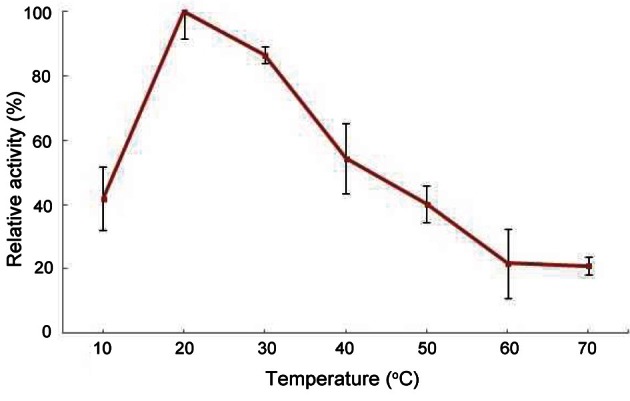
Fig. 5. Optimal pH of the purified ginseng polyphenol oxidase.
Substrate specificity of the purified ginseng polyphenol oxidase activity
Substrate specificities of the purified ginseng PPO are shown in Table 2. Although hydroquinone was used as a substrate (Table 2), ginseng PPO did not use the monohydroxy phenolic compounds such as tyrosine and vanillic acid (data not shown). However, ginseng PPO did use dihydroxy and trihydroxyphenol compounds such as catechol, ʟ-DOPA, hydroquinone, and pyrogallol under the present assay conditions. Interestingly, activity increased approximately 27% compared to maximum activity using pyrogallol as a substrate, than that using catechol as the substrate.
Table 2.
Substrate specificity of purified ginseng polyphenol oxidase
| Substrate (10 mM) | Relative activity (%) |
|---|---|
|
| |
| Hydroquinone | 70 |
| 3,4-Dihydroxy-ʟ-phenylalanine | 13.61) |
| Chlorogenic acid | 68 |
| Catechol | 100 |
| Pyrogallol | 127 |
1)Absorbance was monitored at 475 nm.
Different plants and fruits have various natural phenolic compounds [4]. Previous reports have shown that white and red ginseng have seven phenolic compounds, including vanillic acid, trans-ᴘ-coumaric, ᴘ-hydroxybenzoic acid, salicylic acid, genistic acid, trans-ferulic acid, and caffeic acid [24-26]. Among them, vanillic acid and caffeic acid are useful as substrates for PPO from grape, sunflower, and peach [27-29]. In this experiment, catechol was used as the substrate for the assay.
Inhibitor studies
The effect of inhibitors on the purified ginseng PPO is shown in Table 3. As previously reported, PPO was inhibited by two types of inhibition such as interactions with copper in the enzyme and with the binding site for the phenolic substrates. Ginseng PPO activity was inhibited by ascorbic acid, sodium metabisulfite, and kojic acid, using catechol as the substrate. In contrast, 1 mM citric acid showed slight activation rather than inhibition for the ginseng PPO. In general, acids and reducing agents also inhibit PPO [1,4]. Ascorbic acid, used to inhibit the browning reaction, is commonly utilized as a reducing agent in the food industry. Some organic acids play a role by reducing the intermediate quinones back to diphenolic compounds and inhibiting PPO activity under acidic environmental conditions. Other organic acids such as kojic acid and citric acid inhibit PPO by chelating the copper ion in the PPO active site [30,31]. Additionally, sulfiting agents are commonly used to inhibit the browning reaction in vegetables and plants. Sulfiting agents interact with the intermediate quinone, resulting in the formation of sulfoquinone and preventing synthesis of the brown pigment by PPO [32]. In the inhibitor studies, sodium metabisulfite and kojic acid at 1 and 10 mM showed 13.5%, 8.2% and 80.2%, and 59.6% activity, respectively (Table 3).
Table 3.
Effect of inhibitors on the enzyme activity of purified ginseng polyphenol oxidase
| Inhibitor | Concentration (mM) | Relative activity (%) |
|---|---|---|
|
| ||
| Kojic acid | 1 | 80.2 |
| 10 | 59.6 | |
| Sodium metabisulfite | 1 | 13.5 |
| 10 | 8.2 | |
| Ascorbic acid | 1 | 0 |
| 10 | 0 | |
| Citric acid | 1 | 103.1 |
| 10 | 5.5 | |
Ascorbic acid showed 100% inhibition at both concentrations. However, citric acid stimulated PPO activity slightly rather than inhibited it at the low concentration. It has been reported that citric acid lower than 0.02 M stimulated PPO activity of fresh-cut Chinese water chestnut in an in vitro assay and also browning in apple slices was not inhibited in an in vivo assay with a low concentration of citric acid [33,34]. In the present experiment, 1 mM citric acid did not show any inhibition for ginseng PPO.
Acknowledgments
This study was supported by the Chung-Ang University Research Scholarship Grant to support Jae-Joon Kim (2011-2012). This study was also partly supported by the ‘Industry and Academy Collaboration for Gyeonggi-Ginseng’ Program, We greatly acknowledge Professor Jong-Ki Kim (Department of Integrative Plant Science, Chung-Ang University) for helpful Discussions and Anseong Ginseng Nonghyup for the kind gift of the 6 year-old Gyeonggi ginseng root used in this study.
References
- 1.Mayer AM. Polyphenol oxidases in plants and fungi: going places? A review. Phytochemistry. 2006;67:2318–2331. doi: 10.1016/j.phytochem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Flurkey WH, Inlow JK. Proteolytic processing of polyphenol oxidase from plants and fungi. J Inorg Biochem. 2008;102:2160–2170. doi: 10.1016/j.jinorgbio.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Van Gelder CW, Flurkey WH, Wichers HJ. Sequence and structural features of plant and fungal tyrosinases. Phytochemistry. 1997;45:1309–1323. doi: 10.1016/s0031-9422(97)00186-6. [DOI] [PubMed] [Google Scholar]
- 4.Yoruk R, Marshall MR. Physicochemical properties and function of plant polyphenol oxidase: a review. J Food Biochem. 2003;27:361–422. [Google Scholar]
- 5.Thipyapong P, Stout MJ, Attajarusit J. Functional analysis of polyphenol oxidases by antisense/sense technology. Molecules. 2007;12:1569–1595. doi: 10.3390/12081569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pialis P, Saville BA. Production of ʟ-DOPA from tyrosinase immobilized on nylon 6,6: enzyme stability and scaleup. Enzyme Microb Technol. 1998;22:261–268. [Google Scholar]
- 7.Hou MF, Tang XY, Zhang WD, Liao L, Wan HF. Degradation of pentachlorophenol by potato polyphenol oxidase. J Agric Food Chem. 2011;59:11456–11460. doi: 10.1021/jf202236c. [DOI] [PubMed] [Google Scholar]
- 8.Yeo HB, Yoon HK, Lee HJ, Kang SG, Jung KY, Kim L. Effects of Korean red ginseng on cognitive and motor function: a double-blind, randomized, placebo-controlled trial. J Ginseng Res. 2012;36:190–197. doi: 10.5142/jgr.2012.36.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park SU, Ahn DJ, Jeon HJ, Kwon TR, Lim HS, Choi BS, Baek KH, Bae H. Increase in the contents of ginsenosides in raw ginseng roots in response to exposure to 450 and 470 nm light from light-emitting diodes. J Ginseng Res. 2012;36:198–204. doi: 10.5142/jgr.2012.36.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker JR, Ferrar PH. Diphenol oxidases, enzyme-catalysed browning and plant disease resistance. Biotechnol Genet Eng Rev. 1998;15:457–498. doi: 10.1080/02648725.1998.10647966. [DOI] [PubMed] [Google Scholar]
- 11.Janovitz-Klapp A, Richard F, Nicolas J. Polyphenoloxidase from apple, partial purification and some properties. Phytochemistry. 1989;28:2903–2907. [Google Scholar]
- 12.Sanchez-Ferrer A, Bru R, Cabanes J, Garcia-Carmona F. Characterization of catecholase and cresolase activities of monastrell grape polyphenol oxidase. Phytochemistry. 1989;27:319–321. [Google Scholar]
- 13.Kwon DY, Kim WY. Purification of the glycosylated polyphenol oxidase from potato tuber. J Biochem Mol Biol. 1996;29:163–168. [Google Scholar]
- 14.Ozel A, Colak A, Arslan O, Yildirim M. Purification and characterization of a polyphenol oxidase from Boletus erythropus and investigation of its catalytic efficiency in selected organic solvents. Food Chem. 2010;119:1044–1049. [Google Scholar]
- 15.Selinheimo E, Saloheimo M, Ahola E, Westerholm-Parvinen A, Kalkkinen N, Buchert J, Kruus K. Production and characterization of a secreted, C-terminally processed tyrosinase from the filamentous fungus Trichoderma reesei. FEBS J. 2006;273:4322–4335. doi: 10.1111/j.1742-4658.2006.05429.x. [DOI] [PubMed] [Google Scholar]
- 16.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Palma-Orozco G, Ortiz-Moreno A, Dorantes-Alvarez L, Sampedro JG, Najera H. Purification and partial biochemical characterization of polyphenol oxidase from mamey (Pouteria sapota). Phytochemistry. 2011;72:82–88. doi: 10.1016/j.phytochem.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Jiang YM. Purification and some properties of polyphenol oxidase of longan fruit. Food Chem. 1999;66:75–79. [Google Scholar]
- 20.Ni Eidhin D, Murphy E, O’Beirne D. Polyphenol oxidase from apple (Malus domestica Borkh. cv Bramley’s Seedling): purification strategies and characterization. J Food Sci. 2006;71:C51–C58. [Google Scholar]
- 21.Wititsuwannakul D, Chareonthiphakorn N, Pace M, Wititsuwannakul R. Polyphenol oxidases from latex of Hevea brasiliensis: purification and characterization. Phytochemistry. 2002;61:115–1121. doi: 10.1016/s0031-9422(02)00234-0. [DOI] [PubMed] [Google Scholar]
- 22.Das JR, Bhat SG, Gowda LR. Purification and characterization of a polyphenol oxidase from the Kew cultivar of India pineapple fruit. J Agric Food Chem. 1997;45:2031–2035. [Google Scholar]
- 23.Park EY, Luh BS. Polyphenol oxidase of kiwifruit. J Food Sci. 1985;50:678–684. [Google Scholar]
- 24.Han BH, Park MH, Han YN. Studies on the antioxidant components of Korean ginseng (III): identification of phenolic acids. Arch Pharmacal Res. 1981;4:53–58. [Google Scholar]
- 25.Park MK, Park JH, Kim KH, Han SB, Han BH. Analysis of aromatic acids in Panax ginseng by gas chromatography. Yakhak Hoeji. 1994;38:389–393. [Google Scholar]
- 26.Wee JJ, Heo JN, Kim MJ. Analysis of phenolic components in Korean red ginseng by GC/MS. Korean J Ginseng Sci. 1996;20:284–290. [Google Scholar]
- 27.Flurkey WH, Jen JJ. Purification of peach polyphenol oxidase in the presence of added protease inhibitors. J Food Biochem. 1980;4:29–41. [Google Scholar]
- 28.Lee CY, Smith NL, Pennesi AP. Polyphenoloxidase from DeChaunac grapes. J Sci Food Agric. 1983;34:987–991. [Google Scholar]
- 29.Raymond J, Rakariyatham N, Azanza JL. Purification and some properties of polyphenoloxidase from sunflower seeds. Phytochemistry. 1993;34:927–931. [Google Scholar]
- 30.Eskin NA, Henderson HM, Townsend RJ. Browning reactions in foods. In: Eskin NA, Henderson HM, Townsend RJ, eds. Biochemistry of foods. Academic Press; New York: 1971. pp. 69–83. [Google Scholar]
- 31.Chen JS, Wei CI, Marshall MR. Inhibition mechanism of kojic acid on polyphenol oxidase. J Agric Food Chem. 1991;39:1897–1901. [Google Scholar]
- 32.Ashie IN, Simpson BK, Smith JP. Mechanisms for controlling enzymatic reactions in foods. Crit Rev Food Sci Nutr. 1996;36:1–30. doi: 10.1080/10408399609527716. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Y, Pen L, Li J. Use of citric acid for shelf life and quality maintenance of fresh-cut Chinese water chestnut. J Food Eng. 2004;63:325–328. [Google Scholar]
- 34.Lu S, Luo Y, Turner E, Feng H. Efficacy of sodium chlorite as inhibitor of enzymatic browning in apple slices. Food Chem. 2007;104:824–829. [Google Scholar]