Abstract
Actoprotectors are preparations that increase the mental performance and enhance body stability against physical loads without increasing oxygen consumption. Actoprotectors are regarded as a subclass of adaptogens that hold a significant capacity to increase physical performance. The focus of this article is studying adaptogen herbs of genus Panax (P. ginseng in particular) and their capabilities as actoprotectors. Some animal experiments and human studies about actoprotective properties of genus Panax attest that P. ginseng (administered as an extract) significantly increased the physical and intellectual work capacities, and the data provided suggests that ginseng is a natural source of actoprotectors. Preparations of ginseng can be regarded as potential actoprotectors which give way to further research of its influence on physical and mental work capacity, endurance and restoration after exhaustive physical loads while compared with reference actoprotectors.
Keywords: Panax ginseng, Ginseng, Actoprotector, Memory, Physical work capacity
INTRODUCTION
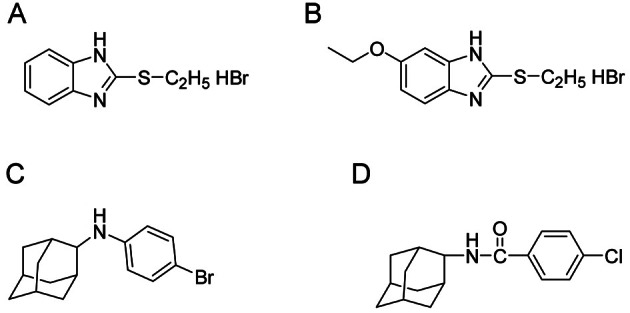
Throughout the 70’s of the 20th century, investigations on a new class of pharmacologically active substances– actoprotectors (aids for improving human’s physical and mental efficiency) were guided by Professor Vladimir Vinogradov. These investigations resulted from the development of the first and the most commonly used actoprotector, bemitil (chemical structure 2-ethylbenzimidazole hydrobromide) (Fig. 1). Later, other actoprotectors were created, such as bromantane [1,2].
Fig. 1. Structures of actoprotectors. (A) Bemitil, (B) ethomersol, (C) bromantane, and (D) chlodantane.
For the last 20 yr, people synthesized and studied other compounds with actoprotecive properties belonging to different chemical classes: thiazoloindole derivatives, 3-hydroxypyridine derivatives, nicotinic acid derivatives, 1-oxa-4-aza-2-silacyclanes etc [3-5]. At the same time, experimental studies and analyses of pharmacological properties of certain herbs proved that some phytochemicals, while having a very low toxicity, are also aids that improve human’s physical and mental efficiency. Among such herbs, there are interesting plant adaptogens: Panax ginseng and other species from genus Panax, Eleutherococcus senticosus, Pfaffia paniculata, Withania somnifera, Schisandra chinensis, Gynostemma pentaphyllum, Rhodiola rosea, etc. [1,6-16].
DEFINITION AND CLASSIFICATION OF ACTOPROTECTORS
Actoprotectors are preparations that enhance the body stability against physical loads without increasing oxygen consumption or heat production, increasing the efficiency factor. Actoprotectors fall under the metabolic drugs of non-consumptive class of action and possibly possessing antihypoxic characteristics at either higher or lower extent. The agents differ from antihypoxants since actoprotectors primarily stimulate protein synthesis and increase working capacity. Moreover, the preparations exert an antihypoxic effect under hypoxic conditions, which may advance as a result of mitochondrion-decreased ability to oxidize substrates under higher physical loads. However, this is not the case in hypoxic conditions of other etiology [1,2].
The principal difference of actoprotectors and psychostimulants (caffeine, sydnocarb, phenamine, methylphenidate, modafinil, adrafinil etc.) is that actoprotectors are agents of non-exhaustive actions. In actions of actoprotectors, there is no increase in oxygen consumption or heat production; differing with nootropic agents–actoprotectors increase not only mental, but physical work capacity as well. The difference between actoprotectors and adaptogens is not so simple. Their characteristics show many similarities. Vinogradov presumed that actoprotectors did not have enough theoretical background to be labeled as a new class of pharmacological compounds. This separation appeared as a result of development of military medicine to improve physical strength [17].
Our opinion about this connection is that the actoprotectors are considered as synthetic (and possibly, natural origin) adaptogens with strong positive influence on physical work capacity. This is the most logical reasoning regarding the classification of actoprotectors. It means, for the convenience of pharmacological classification, some synthetic adaptogens that highly increase the physical performance can be determined as ‘actoprotectors,’ but this term is not applicable for other synthetic adaptogens. For example, benzimidazole derivatives dibazol (bendazol), levamisole and afobazol are all regarded in scientific literature as adaptogens. Dibazol’s adaptogenic action was initially realized in adaptation in difficult environment conditions through immune mechanisms [18-27]. Levamisole’s adaptogenic activity is also connected primarily with adaptive changes in the immune system [28-31]. Afobazol has neuroprotective properties established in vitro on survival of HT-22 neurons in the model of oxidative stress and glutamate toxicity [32], and its adaptogenic action was determined through central nervous system adaptation [33-35]. Since benzimidazole derivatives have adaptogenic properties, these compounds are similar to bemitil, but their influence on physical work capacity is either absent or minimal. This fact does not allow them to be referred as actoprotectors. We think that briefly, actoprotectors can be mentioned as ‘synthetic or natural origin adaptogens with significant capacity to increase physical and mental performance.’
The classic reference actoprotector is bemitil; in its chemical structure, bemitil is 2-ethylthiobenzimidazole hydrobromide (Fig. 1). Currently, only two compounds, among all actoprotectors, are permitted for medical administration: bemitil (commercial name Antihot, certified in Ukraine as dietary supplement) and bromantane (commercial name Ladasten, certified in Russia as a drug) (Fig. 1). Adaptogenic herbs are more available all over the world. In many countries they are certified not only as drugs but also as dietary supplements. It becomes more convenient in the usage for people with active lifestyle or whose professional activity is related with heavy physical and/or mental loads (athletes, military service men, firefighters, crew members, computer operators, night shift doctors and nurses, etc.). Therefore, adaptogenic herbs as potential actoprotectors have not just only theoretical importance for the understanding of mechanisms of their pharmacological action, but also practical applications in sport and occupational medicine.
A primary analysis of actoprotector’s effect under heavy physical loads observed its influence on carbohydrates and energy metabolism: slight decreases of glycogen and creatine phosphate content in the liver and muscles, and of glucose in the blood and lower accumulation of lactates in the tissues and blood, and lower increases in heat production and oxygen consumption. After the period of exertion ended, rehabilitation of the factors under study was accelerated, and indeed, some factors showed super compensation [1,2]. Fig. 2 illustrates the influence of benzimidazole actoprotector on glycogen content in rat liver during recovery.
Fig. 2. Influence of benzimidazole actoprotector (50 mg/kg) on duration of repeated running (% of first running) (A), content of glycogen in liver (B), and total RNA content in liver (C) of rats during recovery after running. 1, initiative parameter (for work capacity) or parameter of intact group (for content of glycogen and RNA); 2, control group; 3, experimental group (actoprotector administration). *p<0.05 in comparison with duration of repeated running of rats from control group [36].
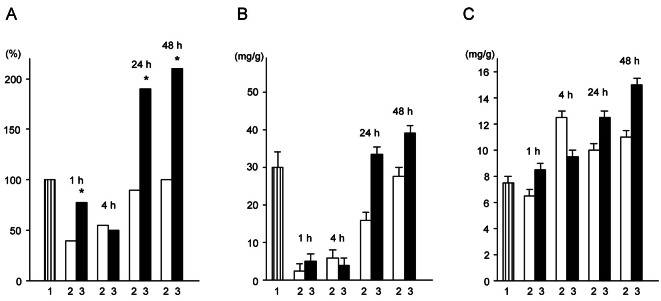
Interestingly, the 4 hour time-point showed a reversed pattern for all of the parameters. The authors assumed that at this point of the recovery period, the energy resources are expended on different biosynthetic processes, leaving the energy supply for muscular activity is severely limited [36]. Thus, in this situation, “switching” of intracellular metabolism to the anabolic reactions, rather than catabolic predominantly, occurs. This is why the work capacity settles at a relatively low level and cannot be increased using pharmacological agents. Bobkov et al. [36] noted the increase of the heart and not the liver glycogen content by the 4th hour of the recovery period, and drew conclusions about the importance of the liver glycogen content to the capacity for work. However, this phenomenon reflects a more complex mechanism that cannot be explained on the aforementioned basis alone and requires further research. The significant decrease of total RNA content in the 4th hour of the recovery period in liver under benzimidazole actoprotector administration is believed to be the evidence behind this reasoning (Fig. 2).
It has been established that the therapeutic effect of actoprotectors (bemitil, as an example) is a function of its complex mechanism entailing cell genome activation, optimization of mitochondrial oxidation, oxidative stress reduction, and stimulation of cellular immune response [1,2].
COMPOSITION OF GINSENG PREPARATIONS AND THEIR STANDARDIZATION
Recently P. ginseng (known also as ginseng and Korean ginseng) is one of the most well-known and studied adaptogens. It is the most studied among plants belonging to genus Panax. It is grown in China, Korea, Japan, and Russia while having a long-time (some thousands years) history of its administration in oriental medicine. Nowadays, P. ginseng as a dietary and medicinal custom is not only in Asia (especially Korea and China), but is also used world-wide. Ginseng is available in many forms: whole root, root powder (white ginseng), steamed root powder (red ginseng), heat processed root powder (sun ginseng), steamed and dried roots for 5 d and 9 times respectively (black ginseng), teas, tinctures, and standardized root extracts containing known and reproducible amounts of ginseng saponins in every batch [37-40]. In some countries, ginseng preparations are produced from P. ginseng cells cultivated in cell cultures. Other species from genus Panax (P. bipinnatifidus, P. japonicus, P. notoginseng, P. pseudoginseng, P. quinquefolius, P. stipuleanatus, P. trifolius, P. vietnamensis, P. wangianus, P. zingiberensis) are not as well-known as P. ginseng, but they are also used in oriental medicine.
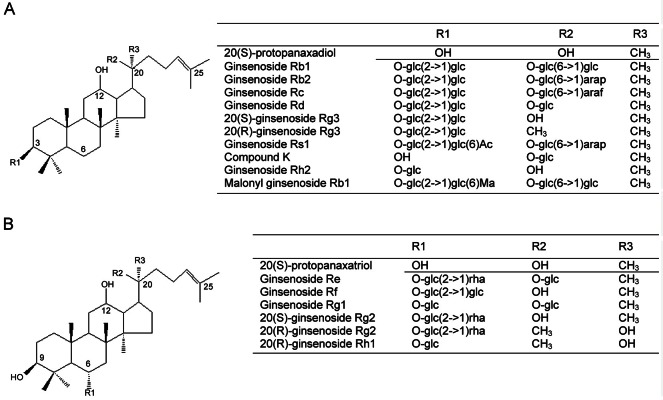
Among the diverse constituents of ginseng, steroid-like phytochemicals with adaptogenic properties named ginsenosides have been found to be major components responsible for their biological and pharmacological actions. Ginsenosides are a special group of triterpenoid saponins that can be classified into two main groups by the skeleton of their aglycones: panaxadiol group (Rb1, Rb2, Rb3, Rc, Rd, Rg3, Rh2, and Rs1) and panaxatriol group (Re, Rf, Rg1, Rg2, and Rh1) [41] (Fig. 3).
Fig. 3. Chemical structure of ginsenosides. (A) Protopanaxadiol-type ginsenoside, (B) protopanaxatriol-type ginsenoside. glc, b-D-glucopyranosyl; arap, a-L-arabinoyranosyl; araf, a-L-arabinofuranosyl; Ac, acetyl; Ma, malonyl; rha, a-L-rhamnopyranosyl.
Ginsenosides are found nearly exclusively in Panax species. More than 150 naturally occurring ginsenosides have been isolated from roots, leaves/stems, fruits, and/or flower buds of ginseng. Ginsenosides have been the target to many researches as they are believed to be the main active components behind the claims of ginseng’s efficacy. These steroid-like phytochemicals, which are known to counter the negative influence of stress, are beneficial for health property. The glycosides act on the adrenal glands, helping to prevent adrenal hypertrophy and excess corticosteroid production in response to stress. Ginsenosides increase protein synthesis and the activity of neurotransmitters in the brain. Ginseng stimulates the formation of blood vessels and improves blood circulation in the brain, thereby improving memory and cognitive abilities. Ginseng is also used as treatments of diabetes, migraines, infections, cancer, radiation and chemotherapy protection, sleep aid, and appetite stimulation [42-44].
The ginsenosides content in ginseng preparations can vary depending on the species, the age, portion of the plant, the preservation method, the season of harvest, and the extraction method [45-47]. For example, comparative study on the ginsenosides of 47 samples of ginseng products derived from different Panax species was conducted using a reverse-phase HPLC method. The results showed that the ginsenoside compositions in ginseng products of different origins were considerably variable [48]. Total saponin contents varied by 10-fold from the highest product to the lowest one. Chikusetsu-ninjin derived from P. japonicus (Japan) was found to have the highest content (192.80-296.18 mg/g) and a product from P. ginseng to be the lowest (5.78-15.63 mg/g). Two main groups suggested by phytochemical data were clearly observed: group I mainly containing dammarane saponins consisted of P. ginseng, P. quinquefolius, P. notoginseng, P. vietnamensis and P. vietnamensis var. fuscidiscus and group II containing a large amount of oleanolic acid saponins was composed of P. japonicus (Japan), P. zingiberensis, P. japonicus (China), P. japonicus var. angustifolius, P. japonicus var. major, and P. stipuleanatus. The ratios of the subtotal of dammarane saponins to that of oleanolic acid saponins were found to be >1.9 and <0.25 for groups I and II, respectively [48]. The product samples derived from the same botanical origin revealed similar constituent patterns, in other words, each Panax taxon showed its own characteristic chromatographic profile, which appeared in the specific shape of an 11-direction radar graph constructed on the basis of the result of quantitative analysis [48]. Similarities of chemical constitution were seen among the closely phylogenetically-related taxa, including P. ginseng and P. quinquefolius, P. vietnamensis and P. vietnamensis var. fuscidiscus, P. japonicus (China) and its varieties, except for P. japonicus (Japan) and P. zingiberensis [48].
As mentioned above, except for ginsenosides, plants from genus Panax contain other active compounds (carbohydrates including polysaccharides, vitamins, alkaloids, fat soluble components, organic acids, microelements and macroelements, etc.) which make important contributions in their pharmacological activity, but the quantity and composition of these compounds differ among the different species [49-53].
All together these facts explain why pharmacological properties of plants from genus Panax are similar, but not the same; moreover, pharmacological activity of different preparations from the same part (roots, leaves etc.) of the same species can be different depending on the season of harvest and the extraction method. Finally, differences between different ginseng preparations can have an influence not just only in their potency, but also in kinds of their pharmacological activity. Recently, the most widely standardized ginseng extracts, both commercially and for research purposes, are G115 [54] and products of Korea Ginseng Corporation (Seoul, Korea), concentrated aqueous extracts from P. ginseng root, which are standardized to contain a certain amount of ginsenosides.
Poor standardization can cause difficulties in evaluation of data received from the animal experiments and human studies related with pharmacological activity of adaptogens including ginseng. It means that sometimes data from different laboratories connected with pharmacological properties (including neurocognitive and actoprotective activity) of preparations from the same species cannot be compared (or, at least, their comparison is very complicated). In conclusion, data on pharmacological activity of preparations received from different species from genus Panax should be evaluated separately.
EFFECT ON EXERCISE PERFORMANCE
Analyses of scientific literature connected with influence of preparations from plants of genus Panax on physical work capacity is more complicated when compared with that of their influence on cognitive functions. Similar to the situation with memory and attention, most studies are connected with preparations from P. ginseng. A few group use P. quinquefolius, P. notoginseng and P. japonicas as well.
Results of many animal experiments attest that P. ginseng preparations can significantly increase physical work capacity. Administration preparations with different qualities from this plant in different dosages increase exhaustion time for swimming in mice [55-58] and rats [59] and exhaustion time for treadmill running in rats [60-62]. Short-term (4 d), although not acute, treatment with complex of ginseng saponins (10 and 20 mg/kg/d) significantly prolongs the aerobic endurance of non-trained rats exercising at approximately 70% VO2max [63]. Wang et al. [64] established that PEC (the oral liquid which consists of P. quinquefolius, Epimedium brevicornum, S. chinensis Bail and Cervus eplaphus) administration could prolong swimming duration of mice in water tank and increase the tolerant ability against oxygen-deficiency.
However at the same time, results of some other experimental studies show no significant influence of ginseng on physical work capacity. Martinez and Staba [65] established that saponin extracts from different kinds of P. ginseng (Korean red, Shiu-Chi red, Kirin red and Sanchi ginseng) and P. quinquefolius (Canadian, American white and American red ginseng) have no influence on exhaustion time for swimming in rats.
The term ‘ergogenic’ stems from the Greek roots ‘ergon’ and ‘genes,’ meaning ‘work’ and ‘born,’ respectively. Any means of enhancing energy production or utilization may be described as an ergogenic aid [66]. It defines ergogenic aids as substances, foods, or training methods that enhance energy production, in use or recovery, while providing athletes with a competitive advantage. Regarding to herbs currently being used to enhance physical performance, Bucci [67] subscribed that they can have different reasons for use including their adaptogenic properties, testosterone-like (anabolic) effect, stimulating effect on central nervous system, effect on capacity to increase endogenous testosterone production (testosterone booster), and alpha-adrenergic agonist properties etc. A wide understanding of ergogenic aid makes this term a practical definition in the field of sport science and sport medicine but not pharmacology.
According to modern pharmacological classification, P. ginseng and most other herbs from genus Panax should be definite adaptogens due to their ability to increase physical work capacity in a healthy person, this being one of the important components of adaptogens’ action [1]. However on the other hand, different adaptogens have different capacities to increase physical performance. That is why adaptogens with the strongest potency to increase physical work capacity are referred to actoprotecors. According to conventional wisdom [2], it is reasonable to refer agents from actoprotectors’ class to synthetic adaptogens and to regard their strong actoprotective effect as one of their components of adaptogenic action. It should be stressed that the focus of this study is on the ability to enhance physical work capacity and not on the adaptogens’ origins, synthetic or natural. According to this point of view, adaptogens of natural origin also can be referred to actoprotectors if they have potent influence on physical work capacity.
Some mechanisms of antifatigue action can be included into adaptogen action; but other mechanisms can be different. As we see regarding to ginseng, its actoprotective properties are very discussible, but antifatigue properties have even more evidence supporting this along with proofs.
From the results of experimental and human studies on the influence of ginseng preparations on physical performance and restoration after loads, there are many other controversies besides those mentioned about critical points of protocols and experimental techniques, including administration of different quality and composition ginseng supplements.
Administration of ginseng preparations on physical work capacity showed controversial results in animal experiments. Detail analyses of experimental results demonstrated no influence by ginseng based on the swimming exhaustion time of rats and mice indicating critical points. For example, in one study some experimental groups included very few animals (3-4) [65]. In this study, results of the swimming test generally showed variable swimming time (205-592 min). It cannot be excluded that such various individual results can possibly be connected with methodological defects because of the difficulties in managing swimming test (water of room temperature was preferred to avoid gas sorption on hair but could not use fresh tap water) along with insufficient quantity of animals in some experimental groups. In Jung et al.’s study [57], very high doses of ginseng extract were used (500 mg/kg/d) during a long time period (4 wk), although it is well-known that too high of dose of adaptogens can lead to inverse effects [1].
Results from numerous human studies, which are summarized in Table 1, are also controversial. Similar to animal experiments, interpretation of these studies is complicated since variable methodology was applied. For example, a study by Knapik et al. (cited in [67]) demonstrated that ginseng supplementation had no effect but had a very small sample size (5 athletes in experimental group and 6 in placebo group). Ziemba et al. [68] established that ginseng administration does improve psychomotor performance during exercise without affecting exercise capacity in their study with soccer players, but used non-specific method for this kind of sport test (incremental bicycle ergometer exercise test). Pieralisi et al. [69] demonstrated substantial ergogenic effects but for ginseng combined with dimethylaminoethanol bitartrate, vitamins, minerals, and trace element. Kulaputana et al. [70] established that ginseng supplementation does not exert an ergogenic property on aerobic fitness enhancement in well-fit individuals with 60 young men (30 in experimental and 30 in control group), but used non-standardized 100% ginseng instead of any standardized ginseng preparation such as G115, products of Korea Ginseng Corporation. or any other standardized extract. Two separate studies Forgo et al, 1981 and 1982 respectively (both cited in [67]), showed significant change in aerobic capacity, lactate level, and heart rate under ginseng administration but failed to show either placebo or control conditions.
Table 1.
Results of human studies with plants from genus Panax on physical performance (adapted with modifications from [67])
| Study (reference) | Subject (n) | Study design | Subject age range | Daily dose | Preparation type | Study duration | Effects (statistically significant unless otherwise stated) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| P. ginseng | |||||||
| Dorling et al., 1980 (cited in [67]) | 60 | DB, PC | 22-80 yr | # | G115 | 12 wk | Improved visual and auditory reaction times, postexercise recovery (stair climbing), 2-handcoordination, alertness, and subjective assessments |
| Forgo et al., 1981 (cited in [67]) | 20 | NC | 18-31 yr | 200 mg | G115 | 9 wk | Increased aerobic capacity; reduced lactate production, and heart rate |
| Forgo et al., 1981 [71] | 120 | DB | 30-60 yr | 200 mg | G115 | 12 wk | Improved vital capacity, forced expiration volume, maximum expiratory flow, maximal breathing capacity, work output; NS for serum LH, FSH, testosterone, estradiol, blood chemistries |
| Forgo et al., 1982 (cited in [67]) | 30 | NC | Elite young athletes | 200 mg | Standardized extract, 4% or 7% ginsenoside content | 9 wk | Improved aerobic capacity; reduced lactate production, and heart rate; NS for difference between 4% and 7% ginsenoside content |
| Forgo, 1983 [72] | 30 Elite athletes | DB, PC | 19-31 yr | 200 mg | G115 | 9 wk | Improved oxygen uptake, maximal breathing capacity, vital capacity, and forced expiration volume; reduced lactate production ,and heart rate; NS for serum LH, testosterone, and cortisol |
| Knapik et al., 1983 (cited in [67]) | 11 Marathon runners | DB, PC | # | 2,000 mg | 1.5% glycosides | 4 wk | NS for R values, glucose, lactate, free fatty acids, glycerol, insulin, cortisol, and growth hormone |
| Teves et al., 1983 (cited in [67]) | 12 Marathon runners | DB, PC | 22±1 yr | 2,000 mg | 1.5% glycosides | 4 wk | NS for run time to exhaustion, aerobic capacity, heart rate, VE, and RPE |
| Murano et al., 1984 (cited in [67]) | 65 | NC | 18-21, 38-70 yr | 2 capsules for 30 d,1 capsule for 30 d | ARM229 standardized extract | 60 d | Older group: improved performance in Cooper test (12-min run time); younger group: NS trend in Cooper and Harvard step tests |
| Forgo et al., 1985 (cited in [73]) | 28 Elite athletes | DB, PC | 20-30 yr | 200 mg | G115 | 9 wk | Improved oxygen uptake, forced expiration volume, vital capacity, visual reaction times, and heart rates |
| Ng et al., 1986 (cited in [67]) | 214 | # | # | # | # | # | Improved endurance, maximal oxygen uptake, postexercise recovery, simple reaction time |
| Macareg et al., 1986 (cited in [67]) | 12 | R, DB,PC, CO | # | # | # | # | NS for time to exhaustion, glucose, and lactate |
| Von Ardenne et al., 1987 [74] | 10 | NC | 50 yr | 200 mg | G115 | 4 wk | Improved resting PO2 uptake (arteriovenous difference) by 29% |
| Tesch et al., 1987 (cited in [67]) | 38 | PC | 50-54 yr | 80 mg | Standardized extract, vitamins, minerals | 8 wk | Improved heart rate and lactate production (> 180 W), RPE (60, 80, 120 W workloads); NS for lactate production up to 180 W |
| McNaughton et al., 1989 (cited in [67]) | 15 F, 15 M | R, DB, PC, CO | # | 1,000 mg | Ginseng root powder | 6 wk | Improved aerobic capacity, pectoral strength (27%), quadriceps strength (18%), postexercise recovery; NS for grip strength |
| Gribaudo et al., 1990 (cited in [75]) | 12 M | R, DB, PC, CO | Young | 1,000 mg | Ginseng + fenu greek | 15 d | Improved total work output, NS for lactate |
| Gribaudo et al., 1991 (cited in [75]) | 14 Well trained amateur cyclists | R, DB, CO | # | 1,000 mg | Ginseng + fenu greek | 30 d | Improved maximal work, VO2 max, anaerobic threshold, NS for lactate |
| Pieralisi et al., 1991 [69] | 50 | R, DB, PC, CO | 21-47 yr | 200 mg | Standardized extract plus DMAE, vitamins, minerals | 6 wk | Improved total work load, time to exhaustion, aerobic capacity, ventilation, oxygen consumption, carbon dioxide production, lactate production, and heart rate; NS for RER |
| Van Schepdael 1993 (cited in [67]) | 43 F triathletes | R, DB, PC, CO | 24-36 yr | 400 mg | G115 | 20 wk | Prevented loss of physical fitness after 10 wk |
| Engels et al., 1995 (cited in [67]) | 19 F | DB | 26±1 yr | 200 mg | G115 | 8 wk | NS for exercise recovery (heart rate, lactate production, oxygen consumption, and ventilation) |
| Caso Marasco et al., 1996 [76] | 625 | R, DB, PC | 18-65 yr | 200 mg | Standardized extract plus minerals, vitamins | 12 wk | Improved quality of life, prevention of increased body weight and high blood pressure |
| Engels et al., 1997 [78] | 36 M | R, DB, PC | # | 200 or 400 mg | G115 | 8 wk | NS for oxygen consumption, RER, RPE, lactate, and heart rate during exercise |
| Lifton et al., 1997 [77] | 7 M, 4 F well trained | DB, CO | # | 3 g | # | 13 d | NS for heart rate max, VO2 max, total workload |
| Allen et al., 1998 [79] | 8 F, 20 M | R, DB, PC, CO | 23±3 yr | 200 mg | 7% ginsenoside standardized extract | 3 wk | NS for oxygen uptake, exercise time, workload, lactate production, hematocrit, heart rate, ratings of perceived exertion at150 W, 200 W, or peak |
| Kolokouri et al., 1999 [80] | 24 F | DB, PC | Adult | 400 mg | # | 8 wk | NS for peak anaerobic power output, fatigue reate |
| Ziemba et al., 1999 [68] | 15 Soccer players | DB | 19.07±0.62 yr | 350 mg | Ginseng preparation | 6 wk | Improved psychomotor performance during bicycle ergometer exercise without affecting exercise capacity |
| Engels et al., 2001 [81] | 24 F | DB | # | 400 mg | G115 | 8 wk | No ergogenic benefits during and in the recovery from short, supramaximal exercise |
| Cardinal et al., 2001 [82] | 83 Adults (40 F, 43 M) | R, DB, PC, CO | Mean age, 25.7 | 200 or 400 mg | G115 | 8 wk | No evidence that chronic ginseng supplementation enhancing affect or mood in healthy young adults |
| Kang et al., 2002 [83] | 8 College students | R, PC | Young | 20 g | Ginseng root extract | Acute administration, after exercise | NS after and during 2 h recovery period for cortisol, testosterone, hGH, insulin-like growth factor |
| Kim et al., 2005 [84] | 7 M | # | # | 6 g | Standardized extract | 8 wk | Ginseng supplementation has ergogenic properties in facilitating recovery from exhaustive exercise |
| Engels et al., 2003 [85] | 27 | R, DB, PC | # | 400 mg | G115 | 8 wk | No changes in secretory IgA at rest and after an exercise induced state of homeostatic disturbance. No evidence for improvement of physical performance and heart rate recovery of individuals undergoing repeated bouts of exhausting exercise |
| Kulaputana et al., 2007 [70] | 60 M | # | 17-22 yr | 3 g | 100% ginseng | 8 wk | No changes of lactate threshold and physical performances in physically active men |
| Ping et al., 2011 [86] | 9 Heat adapted recreational runners | R, DB, PC | 25.4±6.9 yr | 200 mg | # | Acute administration, 1 h before running | No influence on the endurance running performance of the heat-adapted male recreational runners in the heat |
| Jung et al., 2011 [87] | 18 M | # | Young | 20 g | Korean red ginseng extract | 7 d | Reduced exercise-induced muscle damage and inflammatory responses, resulting in improvements in insulin sensitivity |
| P. quinquefolius | |||||||
| Morris et al., 1996 [88] | 1 F, 7 M | R, DB, PC | 27±5 yr | 8 or 16 mg/kg | Water-ethanol extract | 7 d | NS for cycle time to exhaustion and physiologic responses |
| Biondo et al., 2008 [89] | # | # | # | 1,125 mg | P. quinquefolius standardized extract | 35 d | No influence on exercise-induced changes in plasma concentrations of lactate, insulin, cortisol, or growth hormone |
| P. notoginseng | |||||||
| Liang et al., 2005 [90] | 29 Untrained adults | # | 20-35 yr | 1,350 mg | # | 30 d | Improves endurance time to exhaustion, and lowered mean blood pressure and VO2 during endurance exercise |
#, data not listed or unavailable; CO, crossover; DB, double-blind; DMAE, dimethylaminoethanol; F, female; FSH, follicle stimulating hormone; LH, luteinizing hormone; M, male; NC, not controlled; PANAS, positive and negative affect schedule; PC, placebo-controlled; POMS, profile of mood survey; R, randomized; RER, respiratory exchange ratio; RPE, ratings of perceived exertion; SB, single-blind; VE, expiratory ventilation.
Forgo [72] did, however, extend these studies with a double blind placebo-controlled investigation into the effects of 9 weeks administration of G115, G115 plus tocopherol, or placebo, on physiological and hormonal measures (luteinizing hormone, testosterone, and cortisol) in athletes. He reported the significant increase in oxygen uptake and significant decreases in both exercise blood lactates and heart rate, but no change in hormone levels for both of the active treatments in comparison to placebo [72]. This was followed by a further double blind study investigating the duration of the effects of 9 weeks administration of G115 (100 mg twice daily) during exercise. Results reported a significant increase in oxygen uptake and forced expiratory volume and significant decrease in heart rate and visual reaction times. Some of these differences persisted at 3 weeks at the end of administration of G115 (Forgo and Schimert, cited in [73]). Liang et al. [90] reported that 30 d administration of P. notoginseng improves endurance time of exhaustion, and lowers mean blood pressure and VO2 during endurance exercise in healthy untrained adults.
Other studies do not support the view on ginseng as supplementation increases physical work capacity. Ping et al. [86] reported that acute P. ginseng supplementation does not affect the endurance of running performance of the heat-adapted male recreational runners in the heat. Morris et al. [88] found that 1week administration of two different doses of ginseng does not show better effect on any of the physiological indices under investigation (oxygen, free fatty acids, lactate, and glucose) than placebo in a placebo-controlled, cross-over study. Allen et al. [79] reported, in a randomized double-blind, placebo-controlled study involving 28 healthy young adults, that the administration of 200 mg ginseng extract for 21 d did not significantly affect heart rate or perceived exertion at 150 and 200 W ergometric exercise and claimed that it did not affect VO2, exercise time, workload, plasma lactate, or hematocrit at peak levels of exercise. Similarly, Engels et al. did not establish any increasing of physical work capacity after its course administration in a many-years series of ergogenic properties of G115 [81]. Thus, Engels and Wirth [78] failed to demonstrate any effect of 8-weeks administration of ginseng on O2 consumption, respiratory exchange ratio, minute ventilation, blood lactic acid levels, heart rate, or perceived effort in a randomized double blind placebo-controlled G115 trial involving 36 healthy men. Engels et al. [81] found no effect of 400 mg/d G115 for 8 wk on supramaximal exercise performance and postexercise heart rate in 19 healthy women. Engels et al. [85] failed to demonstrate improvement of physical performance and heart rate recovery of individuals undergoing repeated bouts of exhausting exercise of 8-weeks administration of ginseng (G115, 400 mg/d) in a double-blind, placebo-controlled, randomized study involving 38 active healthy women. Morris et al. [88] found that no influence of short (7 d) administration of P. quinquefolius water-ethanol extract (8 or 16 mg/kg/d) on cycle time to exhaustion and physiologic responses for loads in healthy people.
Direct increasing of physical work capacity after acute or course administration (actoprotective properties) should be separated from increasing of recovery speed after previous physical or other heavy loads for the convenience of administration of ergogenic aids in practice of sport preparedness as well as for pharmacological study of their mechanism of action. The property of increasing of recovery speed after previous physical or other heavy loads can be defined as ‘antifatigue activity’ in healthy people. Drugs and dietary supplements with antifatigue properties are very important in some types of sports with demand for repeated performance after short intervals (academic rowing, kayak and canoe paddling, different styles of wrestling, etc.). Positive influence of P. ginseng on parameters of recovery after exhaustive physical loads has been proved by numerous animal experiments since the 1970’s.
The first article published in 1974 [91] studied the effects of extracts obtained from P. ginseng root (50-200 mg/kg intraperitoneal, immediately following the exercise) on recovery from exhaustion (four hour oscillation movements) using six methods: exploratory movement (EM), hole cross (HC), rotating rod (RR), sliding angle (SA), spring balance (SB) and rectal temperature (RT) tests. Water extract significantly accelerated the recovery after EM, increased motor activity index in EM test, and elevated rectal temperature. However, water extract decreased the index in HC test and grip tone in SB test. Antifatigue effects of ginsenoside Rg1 were shown in every test. Lipophilic fraction significantly sped up the recovery from fatigue in EM, RR, RT, and SB tests while delaying recovery in HC and SA tests. Neutral saponin fraction had no effect on recovery in the 6 tests [91]. Later, Banerjee and Izquierdo [92] studied antistress and antifatigue properties of P. ginseng preparation on Swiss albino mice which were exposed to various experimental models of stress in comparison with piracetam. Both ginseng and piracetam were administered chronically in drinking water for 16 to 18 d as well as acutely 30 to 60 min prior to the experiments by injection. Reactivity of the mice, loss of body weight, amount of feces, length of endurance, and incidence of mortality were graded and measured. Both piracetam and ginseng treatment provided good protection against electroshock stress when compared to untreated mice. Fighting scores, incidence of tonic convulsion, and mortality were significantly less in the treated groups. In the heat stress experiments, both piracetam and ginseng provided significant protection to the heat-exposed mice. In the fatigue stress of forced swim test, ginseng treatment was provided to be effective on adaptation to fatigue and ginseng also increased the endurance in both male and female mice. Piracetam, on the other hand, only showed some antifatigue effects in the male mice. In the locomotor activity tests, ginseng did not depress motility, while piracetam did as described in the later part of the tests.
From human studies on healthy people and clinical studies on patients who suffered from fatigue, the antifatigue property of P. ginseng preparations can be confirmed. Dorling et al. (cited in [67]) reported that administration of ginseng extract during 12-weeks improves postexercise recovery. Later, postexercise recovery under ginseng administration was confirmed by Ng and Ng (cited in [67]), McNaughton et al. (cited in [67]), and by other researches. Dai et al. [93] evaluated the effect of the traditional Chinese medicine Tongxinluo and ginseng which had a good effect on the excess fatigue rats using metabolomics approach. A metabolomics study was performed on the excess fatigue rats treated with traditional Tongxinluo or ginseng based on ultra-fast liquid chromatography coupled with ion trap-time of flight mass spectrometry. The plasma metabolic profiling data of the control rats, excess fatigue rats, and excess fatigue rats treated with Tongxinluo or ginseng were acquired. The orthogonal partial least squares analysis was applied for the multivariate statistics, leading to the discovery of important differential metabolites distinguishing the excess fatigue rats treated with Tongxinluo or ginseng from the control rats and excess fatigue rats. The results showed that tryptophan, bile acid, and lysophosphatidylcholine metabolism were disturbed in the excess fatigue rats. The metabolic pattern including the related metabolic pathways of the rats, after being treated with Tongxinluo or ginseng, was adjusted towards the normal state.
Clinical studies of P. ginseng administration in treatment of unexplained chronic fatigue of unknown etiology demonstrated as effective in 56% cases [94]; P. ginseng and P. quinquefolius preparations can be regarded also as perspective compounds to improve cancer-related fatigue [95,96].
In the last 20 yr of scientific literature, the question “Is P. ginseng an ergogenic aid?” has been widely discussed [67,97-101]. Based on modern understanding of pharmacology terms and definitions, it seems logical to concretize this debate by dividing it into two questions: “Is P. ginseng an actoprotector” and “Is P. ginseng an antifatigue supplementation?” Such division has a principal character and needs separate explanation.
Analyses of data from properly controlled studies on P. ginseng preparations (Table 1) allow us to make the preliminary conclusion that the controversies related to this study are connected with different doses, duration of courses being used in different studies, and physical condition of subjects who participated in these studies.
Enhancement of physical work capacity was only observed in studies when: 1) higher doses of ginseng supplementation (over 200 mg/kg/d of standardized extract) were used, 2) there were longer durations of study (not less than 8 wk), 3) had larger subjects’ number, indicating greater statistical power, and 4) subjects were in relatively poor physical condition.
One of the explanations that ginseng supplementation has no positive influence on physical performance was made by Ferrando et al. [102]. In their experiments, the effects of the ginseng extract on various biochemical and hematological parameters in male Wistar rats subjected to a treadmill exercise protocol were studied for 12 wk. The results showed increase in the hematological parameters. The increase was the largest for the animals treated with the extract during the third month of the study. The exercise alone also led to the increase in these parameters, while the combination of both exercise and ginseng extract produced smaller increase. This study shows a clear physiological response due to the ginseng extract administration that reproduces many of the effects obtained after long-term exercise. Authors made conclusion that the combination of exercise and treatments seems to support the theory that there is no clear synergic effect when compared with the performance of exercise. In other work, the same authors established that treatment with P. ginseng increases the capillary density and the mitochondrial content of the red gastrocnemius muscle of rats. The results suggest that prolonged treatment with P. ginseng increases the capillary density and the oxidative capacity of the muscles with greater aerobic potential in a manner similar to the performance of physical exercise. Although when exercise and treatment are combined, the effects are not potentiated [103]. This explanation can be accepted as one possibility, but it generally cannot be a foundation for the conclusion that any effects of physical training and ginseng supplementation may be obtained separately but not when combined.
The facts mentioned above allow us to conclude that P. ginseng (and other plants from genus Panax, possibly) can be regarded as a potential actoprotector and antifatigue preparation with further research of its influence on physical work capacity, endurance and restoration after exhaustive physical loads in comparing with reference actoprotector bemitil.
Possible biochemical mechanisms for increasing the endurance after ginseng administration are connected with its influence on 1) carbohydrate and fat metabolism [57,63,104-106], 2) immune and endocrine mechanisms [60,83,87], and 3) regulation of oxidative balance [106-111].
It seems possible, that in realization of the actoprotective and antifatigue properties of ginseng preparations, saponins are not the only components that have big importance. Polysaccharides also play a large role. Wang et al. [52] established that ginseng polysaccharides have anti-fatigue activity, reflecting in the effects on the physiological markers for fatigue. The acidic polysaccharide is more potent than the neutral polysaccharide.
It seems logical that such controversies are connected with different doses, duration of courses used in different studies, as well as with administration of different quality and composition of ginseng supplements. Such a conclusion also allows ginseng to be regarded as potential actoprotector and allows for further research on its influence on physical work capacity, endurance and restoration after exhaustive physical loads in being compared to reference actoprotector, bemitil.
EFFECTS OF GINSENG ON COGNITIVE FUNCTIONS
Numerous animal experiments and clinical studies about the influence of ginseng preparations (mainly preparations from P. ginseng) on cognitive functions show less controversial results compared to studies connected with exercise performance. Several of them show memory improvement after ginseng administration.
Various memory-impairment models (aged animals, scopolamine-induced memory deficit, ethanol-induced memory deficit, electroconvulsive shock-induced memory disturbances, muscarinic-induced memory deficit, dopamine-induced memory deficit, brain ischemia, cerebral infarct, sham or medial prefrontal cortex lesions, reserpine-induced orofacial dyskinesia, beta-amyloid-induced amnesia model, etc.) have been used to evaluate the effects of ginseng and its active ingredients on a person’s learning and memory. Results of experimental studies on normal animals and on mentioned above animal models are summarized in Table 2.
Table 2.
Results of animal experiments with separated ginsenosides and preparations of plants from genus Panax on cognitive functions
| Study (reference) | Preparation type | Animals and experimental model | Daily dose and study duration | Briefly results |
|---|---|---|---|---|
|
| ||||
| Separated ginsenosides | ||||
| Zhang et al., 1990 [112] | Rg1 and Rb1 | Mice, rats; one trial avoidance learning method | # | Rg1 and Rb1 improved acquisition, consolidation and retrieval of memory improved by amnestic agents |
| Benishin et al., 1991 [113] | Rb1 | Rats, scopolamine-induced memory deficit | # | Rb1 partially prevented memory deficits |
| Ma et al., 1993 [114] | 20(S)-ginsenoside-Rg2 | Male Wistar rats, two-way active avoidance method | 20 mg/kg i.p., repeatedly | Positive influence on memory function |
| Li et al., 1999 [115] | Pseudoginsenoside-F11 from P. quinquefolium | Mice, rats; scopolamine-induced memory deficit | 1-4 mg/kg/d (i.p.), once time or 5 d course | Compound antagonized the memory dysfunction induced by scopolamine |
| Yamazaki et al., 2001 [116] | Panaxynol | Mice, scopolamine-induced memory deficit | 20 mg/kg/d, i.p., for 3 d | Improvement of scopolamine-induced memory deficit |
| Bao et al., 2005 [117] | Rg3(R), Rg3(S) and Rg5/Rk1 (a mixture of Rg5 and Rk1, 1:1, w/w) | Mice, ethanol and scopolamine-induced memory deficit | p.o., course 4 d | Rg3(S) and Rg5/Rk1 significantly reversed the memory dysfunction induced by ethanol or scopolamine |
| Yang et al., 2009 [118] | Rh2 | Mice, scopolamine-induced memory deficit | 40 mg/kg, p.o. 1 h before the first trial session at every consecutive day | Rh2 ameliorated scopolamine-induced learning deficit in mice |
| Wang et al., 2010 [119] | Rg1 and Rb1 | Mice, scopolamine-induced memory deficit | 6 and 12 mg/kg, i.p., 7 d | Multiple administrations of Rg1 and Rb1 are effective in improving memory deficiency induced by scopolamine. Rg1 and Rb1 ameliorated cognition-deficiency in mice with dementia. Rg1 showed stronger effects than Rb1. Both Rg1 and Rb1 increased acetylcholine levels in the hippocampus, but Rg1 inhibited acetylcholinesterase activity while Rb1 had no effect on its activity. Both Rg1 and Rb1 inhibited the decrease of 5-HT induced by scopolamine, but Rb1 was more active than the same dose of Rg1. Rg1 appears to be more potent than Rb1 in improving acquisition impairment. |
| P. ginseng | ||||
| Lasarova et al., 1987 [120] | G115 | Rats, electroconvulsive shock-induced memory disturbances | 30 mg/kg/d (p.o.), 10 d | Tendency for elimination of the memory-impairing effect of electroconvulsive shock. |
| Petkov et al., 1987 [121] | G115 | Rats, “shuttle-box” method for active avoidance | 3, 10, 30, 100 and 300 mg/kg/d (p.o.), 10 d | The results show that ginseng at appropriate doses improves learning, memory and physical capabilities. Bell-shaped dose-effect curves, reported with other nootropic drugs, were obtained. |
| Zhang et al., 1987 [122] | # | Mice | # | Induction of memory facilitation |
| Jaenicke et al., 1991 [123] | # | Female rats of two groups (6 and 27 mo), passive avoidance test | 30 mg/kg/d (p.o.), 13 d | Increasing of learning ability in older rats |
| Petkov et al., 1992 [124] | Standardized extracts: from stem and leaves (GL), and from roots (G115) | Rats with undisturbed memory and in rats with experimentally-impaired memory (electroconvulsive shock); methods for active avoidance (shuttle-box) and passive avoidance (step-down, step-through), the water-maze method and the method for studying exploratory behavior | Multiple administration | G115 exerted favorable effects on learning and memory and on the higher nervous activity as a whole; GL had, in the majority of cases, an effect weaker than that of G115 or was without effect at all |
| Petkov et al., 1993 [125] | G115 | Young (aged 3 months) and old (aged 26 months) rats; conditioned-reflex methods with punishment or positive reinforcement for active and passive avoidance (shuttle-box, step-down, step-through, and water maze) | 17, 50, and 150 mg/kg/d (p.o.), 7 d before training | Positive influence on memory effects, similar to those of nootropic drugs |
| Nitta et al., 1995 [126] | Standardized extract | Aged Fischer 344 rats | 8 g/kg/d, p.o. for 12-33 d | Subchronic treatment with ginseng extract improves spatial cognitive impairment in aged rats |
| Nitta et al., 1995 [127] | P. ginseng ethanol extract and its WSF and LSF fractions | Rats; scopolamine-induced disruption of radial maze performance | P. ginseng ethanol extract, WSF and LSF - 2-8 g dried root/kg, 90 min before testing | P. ginseng ethanol extract and WSF improved the maze performance disrupted by scopolamine in a dose-dependent manner, but LSF failed to attenuate the disruption. Ginseng extract possesses a beneficial effect regarding spatial cognitive impairment and that the water-soluble fraction of ginseng extract mainly contributes to the effect of the ethanol extract |
| Wang et al., 1995 [128] | Root saponins | Normal male Wistar rats | 50 mg/kg/d (ig.), 7 d | Ginseng root saponins facilitate the learning and memory of normal male Wistar rats and significantly raise the levels of biogenic monoamines in their brain |
| Zhao et al., 1998 [129] | Crude ginseng extract | Normal and brain-damaged (sham or medial prefrontal cortex lesions) rats | 40 or 80 mg/kg daily during 30 d after operation | administration of the higher dose resulted in better performance in the learning paradigm |
| Jin et al., 1999 [130] | Root saponins with a low PD/PT (1.24) and high PD/PT (1.46) ratio | Mice, scopolamine-induced memory deficit | 50 and 100 mg/kg (intraperitoneally) before training | The two saponins improved the scopolamine-induced learning impairment at both dosages. The two saponins did not show a favorable effect on learning and memory in normal mice. Korean red ginseng saponin with a low PD/PT ratio had an improving effect on spatial working memory, but the saponin with a high PD/PT ratio did not |
| Hsieh et al., 2000 [131] | # | Rats, scopolamine-induced memory deficit | 1-week course (p.o.) | Improvement of the scopolamine-induced learning and memory deficit |
| Petkov et al., 2003 [132] | G115 | Rats, experimentally-impaired memory (by alcohol or by muscarinic- and dopamine-receptor antagonists) model | # | Favorable effects on learning and memory. These effects varied with the dose and administration schedules, with the rat strain and with the behavioral method |
| Kurimoto et al., 2004 [133] | Nonsaponin fraction of red ginseng | Aged rats | # | Nonsaponin fraction of red ginseng contains important substances to improve learning and memory in aged rats and that this amelioration by nonsaponin might be attributed partly to augmentation of long-term potentiation in the hyppocampal CA3 subfield |
| Lee et al., 2010 [134] | Methanol extracts of wild and cultivated ginseng | Rats; scopolamine-induced memory deficit | 7 days at 30 min before scopolamine injection (2 mg/kg, i.p.) | Wild ginseng demonstrates a significant neuroprotective effect against scopolamine-induced neuronal and cognitive impairment |
| Sanghavi et al., 2011 [135] | Standardized extract | Rats; reserpine-induced orofacial dyskinesia | 100 and 200 mg/kg, p.o., 3 wk | Korean ginseng extract could be useful in the treatment of drug-induced dyskinesia and amnesia |
| P. quinquefolium | ||||
| Sloley et al., 1999 [136] | Standardized extract (HT-1001) | Sprague-Dawley rats; scopolamine-induced memory deficit | 200 mg/kg/d (p.o.), 8 d | HT-1001 demonstrates a capacity to protect against scopolamine-induced memory deficits (protected against scopolamine-induced amnesia and increased choline uptake in synaptosomal preparations; did not alter brain concentrations of norepinephrine, dopamine, serotonin, 3,4-dihydroxyphenylacetic acid or 5-hydroxyindoleactic acid) |
| Zhong et al., 2000 [137] | Red ginseng powder (Ginseng Radix rubura, Seikansho, Kobe, Japan) | Young (10-12 wk) and aged (28-32 mo) Fisher-344 rats; brain ischemia model | # | Red ginseng ameliorates learning and memory deficits through effects on the central nervous system, partly through effects on the hippocampal formation |
| Wang et al., 2006 [138] | Ginsenisides (containing both protopanaxadiol- and protopanaxtriol-type saponins), isolated from the dry roots of P. quinquefolium by 80% alcohol extraction and column chromatography; the yield in ginsenosides (versus dry root weight) was found to be 4.2%–5.1% | Male Sprague-Dawley rats (3-4 months old); beta-amyloid-induced amnesia model | 80 mg/kg/d p.o., 5 d before icv beta-amyloid injection and 7 d afterward; or the same dose 12 d but only after β-amyloid injection | Ginsenosides pre-treatment can functionally prevent the beta-amyloid-induced memory loss possibly by minimizing the inhibitory effect of beta-amyloid on hippocampal cholinergic transmission |
| Chatterjee et al., 2012 [139] | Standardized extract | Male Swiss albino mice | 12.5-200 mg/kg, p.o. | P. quinquefolium extract administration (100 mg/kg) blocked ketamine induced memory impairment in the passive avoidance paradigm |
| P. notoginseng | ||||
| Hsieh et al., 2000 [131] | # | Rats, scopolamine-induced memory deficit | 1 week course (p.o.) | Improvement of the scopolamine-induced learning and memory deficit |
| Chuang et al., 2008 [140] | PNB | Rats, cerebral infarct model | 0.5 g/kg/d, p.o., 3 d per week for 4 wk | PNB attenuates impairment of learning and memory functions and increases ED1, BDNF and beta-secretase immunoreactive cells in chronic stage ischemia-reperfusion injured rats |
| Zhong et al., 2011 [141] | PNS | SAMP8 | High and low doses of PNS | PNS can improve the abilities of learning and memory of SAMP8, the mechanism may be relevant to down-regulating the expression of APP gene at transcriptional level |
| P. pseudoginseng | ||||
| Zhang et al., 1987 [122] | # | Mice | # | Induction of memory facilitation |
#, data not listed or unavailable; i.p., intraperitoneal; p.o., per os; WSF, water-soluble fractions; LSF, lipid-soluble fractions; PD, protopanaxadiol; PT, protopanaxatriol; PNB, P. notoginseng Burk; BDNF, brain derivative neurotrophic factor; PNS, P. notoginseng saponins; SAMP8, senescence accelerated mouse prone 8.
In most studies, independent of experimental model used, course administration of P. ginseng preparations had as a final result with significant antiamnaestic effect. In addition, positive results for cognitive functions enhancement were received from preparations of P. quinquefolium [136,137,139], P. notoginseng [131,140,141] and P. pseudoginseng [122]. However, different fractions or doses of ginseng extract have been shown to impair learning. For example, Saito et al. [142] found that extracts of ginseng inhibits conditioned avoidance response and discrimination behavior on pole climbing and shuttle box tests. Similarly, Petkov and Mosharrof [121] found that high doses of G115 impaired, rather than improved, conditioned reflex activity, and Takagi et al. [143] and Takagi et al. [144] demonstrated decreasing exploratory activity and a specific blocking action of conditioned responses following the administration of a crude ginsenoside fraction.
Some experiments were provided with isolated components of ginseng preparations, first with ginsenosides Rg1 and Rb1. In passive avoidance test, Rg1 improved learning and memory acquisition, consolidation, and retrieval, indicating that Rg1 can improve all stages of memory [112]. Later, to study the effect of Rg1 on one’s learning and memory loss induced by β-amyloid, passive avoidance and performance in the Morris water maze were assayed after the final treatment. Ginsenoside Rg1 significantly decreased the latency and swimming distance, improved corresponding changes in search strategies in the Morris water maze, and increased the step-through latency [145]. In other studies, Rg1 significantly improved memory deficits in aged rats, ovariectomized rats, and cerebral ischemia-reperfusion rats (Qiu et al., cited in [146]; Chen et al., cited in [146]). Rb1 also improved spatial cognitive performance of rats in Morris water maze [147]. These results showed that ginseng extract and ginsenosides Rg1 and Rb1 facilitated acquisition and retrieval of memory. Moreover, ginsenosides Rb1 and Rg1 also antagonized memory loss and cognitive deficit under various pathological conditions, such as cerebral ischemia and dementia (Qiu et al., cited in [146]). At the same time, other ginsenosides like Rg3(S), Rg5/Rk1 [117], and Rh2 [118] and even nonsapongin fraction of red ginseng [133] also have antiamnaestic properties and may have some importance on prevention of memory impairment or treatment of memory disorders.
Results of most human studies, connected with evaluation of influence of ginseng supplementation on cognitive functions, are summarized in Table 3. Some of these studies show positive effects of ginseng preparations even at acute [148-150] or short course [151,152] administration. Poor standardization of different preparations unfortunately does not allow for the comparing of results from different studies. In this connection, it is necessary to put attention on row of studies provided with G115 [152-160].
Table 3.
Results of human studies with plants from genus Panax on neurocognitive function (adapted with modifications from [67])
| Study (reference) | Subject (n) | Study design | Subject age range | Daily dose | Preparation type | Study duration | Effects (statistically significant unless otherwise stated) |
|---|---|---|---|---|---|---|---|
|
| |||||||
| P. ginseng | |||||||
| Kochmareva, 1958 [148] | 122 | CO, PC, SB | Students | 2 mL | Tincture | Acute | Increased quality and quantity of mental work performed |
| Medvedev, 1963 [149] | 13 | CO, PC, SB | 21-23 yr | 2 mL | Tincture | Acute | Decreased errors in data sent by radio operators 1 h after drug uptake |
| Popov et al., 1973 [150] | 32 M | DB, PC | 21-23 yr | 2-mL extract | 40% ethanol tincture | Acute | Decreased errors in radio transmission of coded messages (17% compared with 31%); NS for number of characters transmitted |
| Sandberg, 1974 (cited in [67]) | 30 | DB, PC | Students | # | # | # | NS for spiral maze tracing test, letter cancellation test |
| Revers et al., 1976 (cited in [67]) | # | DB | Elderly | # | G115 | 90 d | Improved vitality, alertness, rigidity, concentration, visual-motor coordination, positive outlook, visual and auditory reaction times |
| Simon et al., 1977 [161] | 36 | # | Elderly | # | G115 | 90 d | Improved concentration, and mental accuracy; NS for attention |
| Bae, 1978 (cited in [162]) | 32 | DB, DC | 21-23 yr | # | # | # | Reduced telegraphy mistakes (17% compared with 31%); NS for mental concentration, coordination |
| Schmidt, 1978 (cited in [162]) | 540 | PC | # | # | # | # | Improved subjective and objective indexes; normalized blood glucose and blood pressure |
| Dorling et al., 1980 (cited in [67]) | 60 | DB, PC | 22-80 yr | # | G115 | 12 wk | Improved visual and auditory reaction times, hand coordination, alertness, and subjective assessments |
| Sandberg, 1980 (cited in [67]) | 60 | DB, PC | # | # | 2 Types of standardized extract | 12 wk | Improved spiral maze tracing test, letter cancellation test, and oxygen metabolism (15-min step test) |
| Johnson, 1980 (cited in [162]) | 38 | # | Dental students | # | # | # | NS for mathematics performance, blood cortisol and epinephrines, proofreading error detection, mood, and fatigue indexes |
| Forgo et al., 1981 [71] | 120 | DB | 30-60 yr | 200 mg | G115 | 12 wk | Improved vital capacity, forced expiration volume, maximum expiratory flow, maximal breathing capacity, reaction times, subjective assessments of mood, work output, sleep, concentration, vitality; NS for serum LH, FSH, testosterone, estradiol, blood chemistries |
| Hallstrom et al. 1978 [151] | 12 Nightshift nurses | DB, PC, CO | 21-27 yr | 1200 mg | Korean white ginseng powder | 3 d | Improved tapping rate test; NS for mood, somatic symptoms, blood glucose (all trends); negative effects on sleep quality |
| D’Angelo et al., 1986 [163] | 32 | DB, PC | 20-24 yr | 200 mg | G115 | 12 wk | Improved mental arithmetic calculations; NS trend for attention, choice reaction time, auditory reaction time; NS for tapping test, recognition, and visual reaction time |
| Zhao, 1990 [164] | 481 | # | 50-85 yr | 150 mg | Sugar-coated tablets of GRS | 2 months | GRS possessed antisenility effect and marked effect on relieving the symptoms of aging, adjusting organic metabolism and improving physiological function, etc., such as promoting memory, raising the amount of white cells and improving organic immunity function. |
| Wiklund et al., 1994 [165] | 390 | PC | Middle-age | 200 mg | G115 + vitamins, minerals | 12 wk | Improved alertness, relaxation, appetite, overall score, and general well-being (3 scales) |
| Smith et al., 1995 (cited in [67]) | 19 F | DB | 26 ±1 yr | 200 mg | G115 | 8 wk | NS for POMS and PANAS (psychological tests) and RPE |
| Sorensen et al., 1996 [166] | 112 Healthy volunteers | R, DB, PC | >40 yr | 400 mg | G115 | 8-9 wk | Faster reaction times, better abstract thinking; NS for memory, concentration, well-being |
| Ziemba et al., 1999 [68] | 15 Soccer players | DB | 19.07±0.62 yr | 350 mg | Ginseng preparation | 6 wk | Improved psychomotor performance during bicycle ergometer exercise without affecting exercise capacity |
| Kennedy et al., 2001 [152] | 40 | R, DB, PC, CO | Young | 200, 400, and 600 mg | G115 | 7 d | Significant improvement in “quality of memory” and the associated “secondary memory” factor at all-time points following 400 mg of ginseng. Both the 200 and 600 mg doses were associated with a significant decrement of the “speed of attention” factor at later testing times only |
| Lee et al., 2008 [167] | 97 | # | Alzheimer’s disease patients | 4.5 g/d | P. ginseng powder | 12 wk | P. ginseng is clinically effective in the cognitive performance of Alzheimer disease patients |
| Reay et al., 2010 [154] | 30 | R, DB, PC, CO | Healthy young adults (22.87±4.01) | 200 and 400 mg | G115 | 8 d | No evidence of additional benefits, or attenuation of acute effects following repeated ingestion of G115 |
| Heo et al., 2011 [168] | 61 | # | Alzheimer ‘s disease patients 50-80 yr | 4.5, 9 g | Korean red ginseng | 96 wk | Improvement of cognitive deficit in Alzheimer ‘s disease patients |
| Yeo et al., 2012 [169] | 15 M | R, DB, PC | Healthy young adults | 4.5 g | Korean red ginseng | 2 wk | Decreased latency in event-related potential test associated with improved cognitive function |
| P. quinquefolius | |||||||
| Scholey et al., 2010 [170] | 32 | R, DB, PC, CO | Healthy young adults | 100, 200, 400 mg | Cereboost (P. quinquefolius standardized to 10.65% ginsenosides) | Acute administration | This study has identified robust working memory enhancement following administration of Cereboost |
#, data not listed or unavailable; CO, crossover; DB, double-blind; DMAE, dimethylaminoethanol; F, female; FSH, follicle stimulating hormone; GRS, ginseng-rhizome saponin; LH, luteinizing hormone; M, male; NC, not controlled; PANAS, positive and negative affect schedule; PC, placebo-controlled; POMS, profile of mood survey; R, randomized; RER, respiratory exchange ratio; RPE, ratings of perceived exertion; SB, single-blind; VE, expiratory ventilation.
Effects of acute administration of G115 on cognition in young healthy individuals were evaluated in randomized double blind placebo-controlled studies [152,156-158] where ginseng differentially improved scores on a ‘secondary memory’ factor (a composite of four memory tasks). In the first study, doses of 200, 400, and 600 mg of G115 were administered. Enhancement of ‘secondary memory’ was found following 400 mg at four post-dose testing sessions while the lower and higher dosage reduced performance on the ‘speed of attention’ factor [152]. Kennedy et al. [158] replicated the finding that 400 mg dosage improved ‘secondary memory.’ A later study assessed the effects of 200 and 400 mg ginseng during sustained cognitive demand-repeated cycles of Serial Threes, Serial Sevens, and the Bakan Rapid Visual Information Processing (RVIP) task. Serial Sevens performance was improved by the 200 mg dose [159]. In a follow-up study, the same dose improved Serial Threes and RVIP performance [160]. It appears that P. ginseng or its constituents are capable of producing tangible cognitive enhancing effects and that 200 to 400 mg appears to be the optimal dose range for young healthy adults when administered acutely prior to a cognitive test.
Course administration of ginseng preparations in most studies also showed positive effect on cognitive functions (Table 3).
Detail analyses of mechanisms to determine underlying the positive impact on cognition under ginseng administration is out of the focus of this article. They are already described in numerous review articles published in recent literature [146,156,171-180]. It is necessary to subscribe that among these mechanisms is the capacity of ginsenosides to potentiate the cholinergic system in central nervous system [112,113,119,181,182]. Other important neurotransmitters for learning, memory, and cognitive functions involved in mechanism action of ginsenosides are glutamate [183,184] and 5-HT [175].
Similar to studies connected with physical performance, it seems logical that such inconsistencies in the results of different studies are connected with different doses, duration of courses used in different studies, as well as with administration of different quality and composition ginseng supplements. Despite that, most studies show positive influences of ginseng supplementation on intellectual work capacity in normal subjects and those of decreased cognitive functions. Such conclusion also allows ginseng to be regarded as potential actoprotector and opens the way for further research of its influence on mental work capacity and cognitive functions in comparing with reference actoprotector, bemitil, and reference nootropic drug, piracetam.
CONCLUSION
Related to the capacity of many plant adaptogens to increase physical and mental performance, the question that arose about actoprotectors class including not just only synthesized but also natural origin compounds has importance for fundamental theory of pharmacological science and practical administration of many phytochemicals.
The ginsenosides content in ginseng preparations can vary depending on the species, the age and part of the plant, the preservation method, the season of harvest, and the extraction method. In this connection, poor standardization can cause some difficulties in the evaluation of data received from animal experiments and human studies about the pharmacological activity of adaptogens including ginseng.
Despite these difficulties, large quantities of data received from animal experiments and human studies allow for making some preliminary conclusions about potential actoprotective properties of ginseng preparations: 1) results of some animal experiments and human studies attest that P. ginseng (administered as extract) can significantly increase physical and intellectual work capacity and the data allow ginseng to be referred as an actoprotector of natural origin; 2) results related to the influence of ginseng on physical performance are more controversial than those connected with its influence on intellectual work capacity; and 3) ginseng preparations can be regarded as potential actoprotectors and allow for further research of its influence on physical and mental work capacity, endurance, and restoration after exhaustive physical loads in comparing with reference actoprotectors (bemitil) and nootropic drugs (piracetam).
Pharmacological activity of preparations received from different species from genus Panax should be evaluated separately, but extract preparation should be standardized. Comparison of ginseng preparations with preparations of other adaptogenic herbs will allow the decision of the most effective herbal actoprotectors. Composition based on additive effects of herbal actoprotectors and synthesized ones can become the more useful in clinical and preventive medicine.
Acknowledgments
This work was supported by the Korea Research Foundation Grant (MRC, 2010-0029355) funded by the Korean government (MEST).
References
- 1.Oliynyk SA, Gunina LM, Seifulla RD. Pharmacology of sports. Olimpiyskaya Literatura; Kyiv: 2010. [Google Scholar]
- 2.Oliynyk S, Oh S. The pharmacology of actoprotectors: practical application for improvement of mental and physical performance. Biomol Ther. 2012;20:444–445. doi: 10.4062/biomolther.2012.20.5.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gavreev AI, Marysheva VV, Shabanov PD. The actoprotective action of thiazoloindole antihypoxic agents. Eksp Klin Farmakol. 2010;73:25–30. [PubMed] [Google Scholar]
- 4.Iasnetsov VV, Tsublova EG, Iasnetsov VV, Karsanova SK, Skachilova SIa. Actiprotective and antihypoxic action of new heteroaromatic antioxidants. Aviakosm Ekolog Med. 2011;45:51–54. [PubMed] [Google Scholar]
- 5.Kurochka AV, Agafonova OV, Losev AS, Mamaeva EA, Bylikin SY, Negrebetsky VV, Kramarova EP, Shipov AG, Baukov YI. Six- and seven-membered 1-oxa-4-aza-2-silacyclanes as possible correctors of adaptational mechanisms. Met Based Drugs. 1998;5:25–33. doi: 10.1155/MBD.1998.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panossian A, Wikman G. Evidence-based efficacy of adaptogens in fatigue, and molecular mechanisms related to their stress-protective activity. Curr Clin Pharmacol. 2009;4:198–219. doi: 10.2174/157488409789375311. [DOI] [PubMed] [Google Scholar]
- 7.Panossian AG, Oganessian AS, Ambartsumian M, Gabrielian ES, Wagner H, Wikman G. Effects of heavy physical exercise and adaptogens on nitric oxide content in human saliva. Phytomedicine. 1999;6:17–26. doi: 10.1016/S0944-7113(99)80030-0. [DOI] [PubMed] [Google Scholar]
- 8.Panossian A, Wagner H. Stimulating effect of adaptogens: an overview with particular reference to their efficacy following single dose administration. Phytother Res. 2005;19:819–838. doi: 10.1002/ptr.1751. [DOI] [PubMed] [Google Scholar]
- 9.Kuo J, Chen KW, Cheng IS, Tsai PH, Lu YJ, Lee NY. The effect of eight weeks of supplementation with Eleutherococcus senticosus on endurance capacity and metabolism in human. Chin J Physiol. 2010;53:105–111. doi: 10.4077/cjp.2010.amk018. [DOI] [PubMed] [Google Scholar]
- 10.Aslanyan G, Amroyan E, Gabrielyan E, Nylander M, Wikman G, Panossian A. Double-blind, placebo-controlled, randomised study of single dose effects of ADAPT-232 on cognitive functions. Phytomedicine. 2010;17:494–499. doi: 10.1016/j.phymed.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Mendes FR, Carlini EA. Brazilian plants as possible adaptogens: an ethnopharmacological survey of books edited in Brazil. J Ethnopharmacol. 2007;109:493–500. doi: 10.1016/j.jep.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Azizov AP, Seifulla RD. The effect of elton, leveton, fitoton and adapton on the work capacity of experimental animals. Eksp Klin Farmakol. 1998;61:61–63. [PubMed] [Google Scholar]
- 13.Zhang GL, Deng JP, Wang BH, Zhao ZW, Li J, Gao L, Liu BL, Xong JR, Guo XD, Yan ZQ, et al. Gypenosides improve cognitive impairment induced by chronic cerebral hypoperfusion in rats by suppressing oxidative stress and astrocytic activation. Behav Pharmacol. 2011;22:633–644. doi: 10.1097/FBP.0b013e32834afef9. [DOI] [PubMed] [Google Scholar]
- 14.Long BB. The effects of gynostemma on sports ability of mice. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2010;26:339–340. [PubMed] [Google Scholar]
- 15.Grodzinskij AM. Phytoergonomics. Naukova Dumka; Kyiv: 1989. [Google Scholar]
- 16.Grinevich MA. Information search for perspective medicinal plants: an experience of studies on the traditional medicine of Eastern Asia with the aid of computer. Nauka; Leningrad: 1990. [Google Scholar]
- 17.Vinogradov VM, Krivoruchko BI. Pharmacological defence of the brain from hypoxia. pharmacol Biol Narcol. 2001;1:27–37. [Google Scholar]
- 18.Sidorova NV, Kiikova OI. Dibazol: a remedy for prophylaxis of acute respiratory infections among students of military colleges. Zh Mikrobiol Epidemiol Immunobiol. 2000;(6):122–124. [PubMed] [Google Scholar]
- 19.Udintsev SN, Shakhov VP, Borovskoi IG, Ibragimova SG. The effect of low concentrations of adaptogen solutions on the functional activity of murine bone marrow cells in vitro. Biofizika. 1991;36:105–108. [PubMed] [Google Scholar]
- 20.Novikov VS, Bortnovskii VN. Effect of dibazol on indices of nonspecific resistance in human subjects in a hermetically sealed enclosure. Kosm Biol Aviakosm Med. 1985;19:68–71. [PubMed] [Google Scholar]
- 21.Zarudii FS. Effect of obzidan, tropaphen, adrenaline and euphylline on histamine bronchospasm in guinea pigs. Farmakol Toksikol. 1984;47:81–84. [PubMed] [Google Scholar]
- 22.Rusin VI. Influence of muscle training, adaptation to cold and dibazol administration on the resistance of certain tissues. Fiziol Zh SSSR Im I M Sechenova. 1967;53:431–437. [PubMed] [Google Scholar]
- 23.Rusin VI. Resistance to cold and heat in animals receiving dibazol or subjected to muscular training and acclimatization. Fiziol Zh SSSR Im I M Sechenova. 1963;49:359–365. [PubMed] [Google Scholar]
- 24.Rusin VI. The effect of prolonged dibazol administration on the growth and resistance of white mice and their offspring. Fiziol Zh SSSR Im I M Sechenova. 1963;49:632–638. [PubMed] [Google Scholar]
- 25.Rusin VI. The effect of dibazol and adaptation to muscular work and cold on animals with the Ehrlich tumor. Vopr Onkol. 1963;18:60–66. [PubMed] [Google Scholar]
- 26.Rusin VI. On adaptation to cold and heat in muscular training and in dibazol administration. Patol Fiziol Eksp Ter. 1962;6:63–65. [PubMed] [Google Scholar]
- 27.Rusin VI. Role of the adaptation to low temperatures and dibazol in increased resistance of mice to adverse factors. Fiziol Zh SSSR Im I M Sechenova. 1962;48:195–200. [PubMed] [Google Scholar]
- 28.Voskanian NA, Dzhikidze EK, Pochkhura MA. Immunological status of monkeys during acclimatization and its correction with levamisole. Zh Mikrobiol Epidemiol Immunobiol. 1986;(3):62–65. [PubMed] [Google Scholar]
- 29.Alvarez-Pellitero P, Sitja-Bobadilla A, Bermudez R, Quiroga MI. Levamisole activates several innate immune factors in Scophthalmus maximus (L.) Teleostei. Int J Immunopathol Pharmacol. 2006;19:727–738. doi: 10.1177/039463200601900403. [DOI] [PubMed] [Google Scholar]
- 30.Chen LY, Lin YL, Chiang BL. Levamisole enhances immune response by affecting the activation and maturation of human monocyte-derived dendritic cells. Clin Exp Immunol. 2008;151:174–181. doi: 10.1111/j.1365-2249.2007.03541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fabrizi F, Dixit V, Messa P, Martin P. Meta-analysis: levamisole improves the immune response to hepatitis B vaccine in dialysis patients. Aliment Pharmacol Ther. 2010;32:756–762. doi: 10.1111/j.1365-2036.2010.04410.x. [DOI] [PubMed] [Google Scholar]
- 32.Zenina TA, Gavrish IV, Melkumyan DS, Seredenina TS, Seredenin SB. Neuroprotective properties of afobazol in vitro. Bull Exp Biol Med. 2005;140:194–196. doi: 10.1007/s10517-005-0443-7. [DOI] [PubMed] [Google Scholar]
- 33.Uyanaev AA, Fisenko VP. Studies of long-term noopept and afobazol treatment in rats with learned helplessness neurosis. Bull Exp Biol Med. 2006;142:202–204. doi: 10.1007/s10517-006-0327-5. [DOI] [PubMed] [Google Scholar]
- 34.Litvintsev SV, Davydov AT, Uspenskii IP, Zagrebel’nyi IA, Balukova EV. Using of aphobazol in the treatment of adaptation disorder in the contract service men, dismissed from the armed forces. Voen Med Zh. 2007;328:28–29. [PubMed] [Google Scholar]
- 35.Bogdan NG, Kolotilinskaia NV, Nadorov SA, Iarkova MA, Badyshtov BA. Effect of afobazole on the psychophysiological state of healthy volunteers. Eksp Klin Farmakol. 2011;74:8–12. [PubMed] [Google Scholar]
- 36.Bobkov IG, Vinogradov VM, Katkov VP, Losev SS, Smirnov AV. Pharmacological correction of tirednass. Meditsina; Moscow: 1984. [Google Scholar]
- 37.Chung HS, Lee YC, Rhee YK, Lee SY. Consumer acceptance of ginseng food products. J Food Sci. 2011;76:S516–S522. doi: 10.1111/j.1750-3841.2011.02399.x. [DOI] [PubMed] [Google Scholar]
- 38.Yap KY, Chan SY, Weng Chan Y, Sing Lim C. Overview on the analytical tools for quality control of natural product-based supplements: a case study of ginseng. Assay Drug Dev Technol. 2005;3:683–699. doi: 10.1089/adt.2005.3.683. [DOI] [PubMed] [Google Scholar]
- 39.Yun TK. Brief introduction of Panax ginseng C.A. Meyer. J Korean Med Sci. 2001;16 Suppl:S3–S5. doi: 10.3346/jkms.2001.16.S.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Court WE. Ginseng: the history of an insignificant plant. Pharm Hist (Lond) 2000;30:38–44. [PubMed] [Google Scholar]
- 41.Tachikawa E, Kudo K, Harada K, Kashimoto T, Miyate Y, Kakizaki A, Takahashi E. Effects of ginseng saponins on responses induced by various receptor stimuli. Eur J Pharmacol. 1999;369:23–32. doi: 10.1016/s0014-2999(99)00043-6. [DOI] [PubMed] [Google Scholar]
- 42.Christensen LP. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 43.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 44.Choi KT. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 2008;29:1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 45.Liberti LE, Der Marderosian A. Evaluation of commercial ginseng products. J Pharm Sci. 1978;67:1487–1489. doi: 10.1002/jps.2600671050. [DOI] [PubMed] [Google Scholar]
- 46.Yuan CS, Wang CZ, Wicks SM, Qi LW. Chemical and pharmacological studies of saponins with a focus on American ginseng. J Ginseng Res. 2010;34:160–167. doi: 10.5142/jgr.2010.34.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim DH. Chemical diversity of Panax ginseng, Panax quinquifolium, and Panax notoginseng. J Ginseng Res. 2012;36:1–15. doi: 10.5142/jgr.2012.36.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu S, Zou K, Fushimi H, Cai S, Komatsu K. Comparative study on triterpene saponins of ginseng drugs. Planta Med. 2004;70:666–677. doi: 10.1055/s-2004-827192. [DOI] [PubMed] [Google Scholar]
- 49.Li C, Cai J, Geng J, Li Y, Wang Z, Li R. Purification, characterization and anticancer activity of a polysaccharide from Panax ginseng. Int J Biol Macromol. 2012;51:968–973. doi: 10.1016/j.ijbiomac.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 50.Wang R, Chen P, Jia F, Tang J, Ma F. Optimization of polysaccharides from Panax japonicus C.A. Meyer by RSM and its anti-oxidant activity. Int J Biol Macromol. 2012;50:331–336. doi: 10.1016/j.ijbiomac.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 51.Wang J, Flaisher-Grinberg S, Li S, Liu H, Sun L, Zhou Y, Einat H. Antidepressant-like effects of the active acidic polysaccharide portion of ginseng in mice. J Ethnopharmacol. 2010;132:65–69. doi: 10.1016/j.jep.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 52.Wang J, Li S, Fan Y, Chen Y, Liu D, Cheng H, Gao X, Zhou Y. Anti-fatigue activity of the water-soluble polysaccharides isolated from Panax ginseng C. A. Meyer. J Ethnopharmacol. 2010;130:421–423. doi: 10.1016/j.jep.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 53.Court WE. Ginseng, the genus Panax. CRC Press; Boca Raton: 2000. [Google Scholar]
- 54.Soldati F, Sticher O. HPLC separation and quantitative determination of ginsenosides from Panax ginseng, Panax quinquefolium and from ginseng drug preparations. 2nd communication. Planta Med. 1980;39:348–357. doi: 10.1055/s-2008-1074929. [DOI] [PubMed] [Google Scholar]
- 55.Grandhi A, Mujumdar AM, Patwardhan B. A comparative pharmacological investigation of Ashwagandha and ginseng. J Ethnopharmacol. 1994;44:131–135. doi: 10.1016/0378-8741(94)01119-2. [DOI] [PubMed] [Google Scholar]
- 56.Singh A, Saxena E, Bhutani KK. Adrenocorticosterone alterations in male, albino mice treated with Trichopus zeylanicus, Withania somnifera and Panax ginseng preparations. Phytother Res. 2000;14:122–125. doi: 10.1002/(sici)1099-1573(200003)14:2<122::aid-ptr529>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 57.Jung K, Kim IH, Han D. Effect of medicinal plant extracts on forced swimming capacity in mice. J Ethnopharmacol. 2004;93:75–81. doi: 10.1016/j.jep.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 58.Choi JY, Woo TS, Yoon SY, dela Pena IC, Choi YJ, Ahn HS, Lee YS, Yu GY, Cheong JH. Red ginseng supplementation more effectively alleviates psychological than physical fatigue. J Ginseng Res. 2011;35:331–338. doi: 10.5142/jgr.2011.35.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nocerino E, Amato M, Izzo AA. The aphrodisiac and adaptogenic properties of ginseng. Fitoterapia. 2000;71 Suppl 1:S1–S5. doi: 10.1016/s0367-326x(00)00170-2. [DOI] [PubMed] [Google Scholar]
- 60.Min YK, Chung SH, Lee JS, Kim SS, Shin HD, Lim BV, Shin MC, Jang MH, Kim EH, Kim CJ. Red ginseng inhibits exercise-induced increase in 5-hydroxytryptamine synthesis and tryptophan hydroxylase expression in dorsal raphe of rats. J Pharmacol Sci. 2003;93:218–221. doi: 10.1254/jphs.93.218. [DOI] [PubMed] [Google Scholar]
- 61.Zhao W, Zhang X, Wang W, Zhang L. Experimental study for the anti-fatigue effect of ginseng general ginsenosides P.E. in vivo. Wei Sheng Yan Jiu. 2009;38:184–187. [PubMed] [Google Scholar]
- 62.Filaretov AA, Bogdanova TS, Podvigina TT, Bodganov AI. Role of pituitary-adrenocortical system in body adaptation abilities. Exp Clin Endocrinol. 1988;92:129–136. doi: 10.1055/s-0029-1210793. [DOI] [PubMed] [Google Scholar]
- 63.Wang LC, Lee TF. Effect of ginseng saponins on exercise performance in non-trained rats. Planta Med. 1998;64:130–133. doi: 10.1055/s-2006-957389. [DOI] [PubMed] [Google Scholar]
- 64.Wang LW, Liu XM, Lu GH, Gao NN, Xiao PG. Primary research of pharmacological effects of PEC on mice. Zhongguo Zhong Yao Za Zhi. 2004;29:568–569. 593. [PubMed] [Google Scholar]
- 65.Martinez B, Staba EJ. The physiological effects of Aralia, Panax and Eleutherococcus on exercised rats. Jpn J Pharmacol. 1984;35:79–85. doi: 10.1254/jjp.35.79. [DOI] [PubMed] [Google Scholar]
- 66.Williams MH. Ergogenic and ergolytic substances. Med Sci Sports Exerc. 1992;24(9 Suppl):S344–S348. [PubMed] [Google Scholar]
- 67.Bucci LR. Selected herbals and human exercise performance. Am J Clin Nutr. 2000;72(2 Suppl):624S–636S. doi: 10.1093/ajcn/72.2.624S. [DOI] [PubMed] [Google Scholar]
- 68.Ziemba AW, Chmura J, Kaciuba-Uscilko H, Nazar K, Wisnik P, Gawronski W. Ginseng treatment improves psychomotor performance at rest and during graded exercise in young athletes. Int J Sport Nutr. 1999;9:371–377. doi: 10.1123/ijsn.9.4.371. [DOI] [PubMed] [Google Scholar]
- 69.Pieralisi G, Ripari P, Vecchiet L. Effects of a standardized ginseng extract combined with dimethylaminoethanol bitartrate, vitamins, minerals, and trace elements on physical performance during exercise. Clin Ther. 1991;13:373–382. [PubMed] [Google Scholar]
- 70.Kulaputana O, Thanakomsirichot S, Anomasiri W. Ginseng supplementation does not change lactate threshold and physical performances in physically active Thai men. J Med Assoc Thai. 2007;90:1172–1179. [PubMed] [Google Scholar]
- 71.Forgo I, Kayasseh L, Staub JJ. Effect of a standardized ginseng extract on general well-being, reaction time, lung function and gonadal hormones. Med Welt. 1981;32:751–756. [PubMed] [Google Scholar]
- 72.Forgo I. Effect of drugs on physical exertion and the hormonal system of athletes. 2. MMW Munch Med Wochenschr. 1983;125:822–824. [PubMed] [Google Scholar]
- 73.World Health Organization. Radix Ginseng. In: World Health Organization. WHO monographs on selected medicinal plants. Volume 1. World Health Organization; Geneva: 1999. pp. 168–182. [Google Scholar]
- 74.Von Ardenne M, Klemm W. Measurements of the increase in the difference between the arterial and venous Hb-O2 saturation obtained with daily administration of 200 mg standardized ginseng extract G115 for four weeks. Long-term increase of the O2 transport into the organs and tissues of the organism through biologically active substances. Panminerva Med. 1987;29:143–150. [PubMed] [Google Scholar]
- 75.Wolinsky I, Driskell JA. Nutritional ergogenic aids. CRC Press; Boca Raton: 2004. [Google Scholar]
- 76.Caso Marasco A, Vargas Ruiz R, Salas Villagomez A, Begona Infante C. Double-blind study of a multivitamin complex supplemented with ginseng extract. Drugs Exp Clin Res. 1996;22:323–329. [PubMed] [Google Scholar]
- 77.Lifton B, Otto RM, Wygard J. The effect of ginseng on acute maximal aerobic exercise. Med Sci Sports Exerc. 1997;29(Suppl 5):S249. [Google Scholar]
- 78.Engels HJ, Wirth JC. No ergogenic effects of ginseng (Panax ginseng C.A. Meyer) during graded maximal aerobic exercise. J Am Diet Assoc. 1997;97:1110–1115. doi: 10.1016/S0002-8223(97)00271-X. [DOI] [PubMed] [Google Scholar]
- 79.Allen JD, McLung J, Nelson AG, Welsch M. Ginseng supplementation does not enhance healthy young adults’ peak aerobic exercise performance. J Am Coll Nutr. 1998;17:462–466. doi: 10.1080/07315724.1998.10718795. [DOI] [PubMed] [Google Scholar]
- 80.Kolokouri I, Engels HJ, Cieslak T, Wirth JC. Effect of chronic ginseng supplementation on short duration, supramaximal exercise test performance. Med Sci Sports Exerc. 1999;31(Suppl 5):S117. [Google Scholar]
- 81.Engels HJ, Kolokouri I, Cieslak TJ 2nd, Wirth JC. Effects of ginseng supplementation on supramaximal exercise performance and short-term recovery. J Strength Cond Res. 2001;15:290–295. [PubMed] [Google Scholar]
- 82.Cardinal BJ, Engels HJ. Ginseng does not enhance psychological well-being in healthy, young adults: results of a double-blind, placebo-controlled, randomized clinical trial. J Am Diet Assoc. 2001;101:655–660. doi: 10.1016/S0002-8223(01)00165-1. [DOI] [PubMed] [Google Scholar]
- 83.Kang HY, Kim SH, Lee WJ, Byrne HK. Effects of ginseng ingestion on growth hormone, testosterone, cortisol, and insulin-like growth factor 1 responses to acute resistance exercise. J Strength Cond Res. 2002;16:179–183. doi: 10.1519/1533-4287(2002)016<0179:eogiog>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 84.Kim SH, Park KS, Chang MJ, Sung JH. Effects of Panax ginseng extract on exercise-induced oxidative stress. J Sports Med Phys Fitness. 2005;45:178–182. [PubMed] [Google Scholar]
- 85.Engels HJ, Fahlman MM, Wirth JC. Effects of ginseng on secretory IgA, performance, and recovery from interval exercise. Med Sci Sports Exerc. 2003;35:690–696. doi: 10.1249/01.MSS.0000058363.23986.D2. [DOI] [PubMed] [Google Scholar]
- 86.Ping FW, Keong CC, Bandyopadhyay A. Effects of acute supplementation of Panax ginseng on endurance running in a hot & humid environment. Indian J Med Res. 2011;133:96–102. [PMC free article] [PubMed] [Google Scholar]
- 87.Jung HL, Kwak HE, Kim SS, Kim YC, Lee CD, Byurn HK, Kang HY. Effects of Panax ginseng supplementation on muscle damage and inflammation after uphill treadmill running in humans. Am J Chin Med. 2011;39:441–450. doi: 10.1142/S0192415X11008944. [DOI] [PubMed] [Google Scholar]
- 88.Morris AC, Jacobs I, McLellan TM, Klugerman A, Wang LC, Zamecnik J. No ergogenic effect of ginseng ingestion. Int J Sport Nutr. 1996;6:263–271. doi: 10.1123/ijsn.6.3.263. [DOI] [PubMed] [Google Scholar]
- 89.Biondo PD, Robbins SJ, Walsh JD, McCargar LJ, Harber VJ, Field CJ. A randomized controlled crossover trial of the effect of ginseng consumption on the immune response to moderate exercise in healthy sedentary men. Appl Physiol Nutr Metab. 2008;33:966–975. doi: 10.1139/H08-080. [DOI] [PubMed] [Google Scholar]
- 90.Liang MT, Podolka TD, Chuang WJ. Panax notoginseng supplementation enhances physical performance during endurance exercise. J Strength Cond Res. 2005;19:108–114. doi: 10.1519/00124278-200502000-00019. [DOI] [PubMed] [Google Scholar]
- 91.Saito H, Yoshida Y, Takagi K. Effect of Panax ginseng root on exhaustive exercise in mice. Jpn J Pharmacol. 1974;24:119–127. doi: 10.1254/jjp.24.119. [DOI] [PubMed] [Google Scholar]
- 92.Banerjee U, Izquierdo JA. Antistress and antifatigue properties of Panax ginseng: comparison with piracetam. Acta Physiol Lat Am. 1982;32:277–285. [PubMed] [Google Scholar]
- 93.Dai W, Zhang F, Jia Z, Wei C, Gao P, Lu X, Wu Y, Xu G. Evaluation of the effect of the traditional Chinese medicine tongxinluo or ginseng on excess fatigue rats studied by metabonomics approach based on liquid chromatography-mass spectrometry. Se Pu. 2011;29:1049–1054. [PubMed] [Google Scholar]
- 94.Bentler SE, Hartz AJ, Kuhn EM. Prospective observational study of treatments for unexplained chronic fatigue. J Clin Psychiatry. 2005;66:625–632. doi: 10.4088/jcp.v66n0513. [DOI] [PubMed] [Google Scholar]
- 95.Elam JL, Carpenter JS, Shu XO, Boyapati S, Friedmann-Gilchrist J. Methodological issues in the investigation of ginseng as an intervention for fatigue. Clin Nurse Spec. 2006;20:183–189. doi: 10.1097/00002800-200607000-00007. [DOI] [PubMed] [Google Scholar]
- 96.Barton DL, Soori GS, Bauer BA, Sloan JA, Johnson PA, Figueras C, Duane S, Mattar B, Liu H, Atherton PJ, et al. Pilot study of Panax quinquefolius (American ginseng) to improve cancer-related fatigue: a randomized, double-blind, dose-finding evaluation: NCCTG trial N03CA. Support Care Cancer. 2010;18:179–187. doi: 10.1007/s00520-009-0642-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bahrke MS, Morgan WP. Evaluation of the ergogenic properties of ginseng. Sports Med. 1994;18:229–248. doi: 10.2165/00007256-199418040-00003. [DOI] [PubMed] [Google Scholar]
- 98.Bahrke MS, Morgan WR. Evaluation of the ergogenic properties of ginseng: an update. Sports Med. 2000;29:113–133. doi: 10.2165/00007256-200029020-00004. [DOI] [PubMed] [Google Scholar]
- 99.Bahrke MS. Ginseng: a root just like a carrot? J R Soc Med. 1995;88:304. [PMC free article] [PubMed] [Google Scholar]
- 100.Bahrke MS, Morgan WP, Stegner A. Is ginseng an ergogenic aid? Int J Sport Nutr Exerc Metab. 2009;19:298–322. doi: 10.1123/ijsnem.19.3.298. [DOI] [PubMed] [Google Scholar]
- 101.Chen CK, Muhamad AS, Ooi FK. Herbs in exercise and sports. J Physiol Anthropol. 2012;31:4. doi: 10.1186/1880-6805-31-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ferrando A, Vila L, Voces JA, Cabral AC, Alvarez AI, Prieto JG. Effects of ginseng extract on various haematological parameters during aerobic exercise in the rat. Planta Med. 1999;65:288–290. doi: 10.1055/s-2006-960783. [DOI] [PubMed] [Google Scholar]
- 103.Ferrando A, Vila L, Voces JA, Cabral AC, Alvarez AI, Prieto JG. Effects of a standardized Panax ginseng extract on the skeletal muscle of the rat: a comparative study in animals at rest and under exercise. Planta Med. 1999;65:239–244. doi: 10.1055/s-1999-14081. [DOI] [PubMed] [Google Scholar]
- 104.Avakian EV Jr, Evonuk E. Effect of Panax ginseng extract on tissue glycogen and adrenal cholesterol depletion during prolonged exercise. Planta Med. 1979;36:43–48. doi: 10.1055/s-0028-1097238. [DOI] [PubMed] [Google Scholar]
- 105.Avakian EV, Sugimoto RB, Taguchi S, Horvath SM. Effect of Panax ginseng extract on energy metabolism during exercise in rats. Planta Med. 1984;50:151–154. doi: 10.1055/s-2007-969657. [DOI] [PubMed] [Google Scholar]
- 106.Yang Y, Wu T, He K, Fu ZG. Effect of aerobic exercise and ginsenosides on lipid metabolism in diet-induced hyperlipidemia mice. Zhongguo Yao Li Xue Bao. 1999;20:563–565. [PubMed] [Google Scholar]
- 107.Voces J, Cabral de Oliveira AC, Prieto JG, Vila L, Perez AC, Duarte ID, Alvarez AI. Ginseng administration protects skeletal muscle from oxidative stress induced by acute exercise in rats. Braz J Med Biol Res. 2004;37:1863–1871. doi: 10.1590/s0100-879x2004001200012. [DOI] [PubMed] [Google Scholar]
- 108.Cabral de Oliveira AC, Perez AC, Prieto JG, Duarte ID, Alvarez AI. Protection of Panax ginseng in injured muscles after eccentric exercise. J Ethnopharmacol. 2005;97:211–214. doi: 10.1016/j.jep.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 109.Hwang HJ, Kwak YS, Yoon GA, Kang MH, Park JH, Lee BK, Kim SJ, Um SY, Kim YM. Combined effects of swim training and ginseng supplementation on exercise performance time, ROS, lymphocyte proliferation, and DNA damage following exhaustive exercise stress. Int J Vitam Nutr Res. 2007;77:289–296. doi: 10.1024/0300-9831.77.4.289. [DOI] [PubMed] [Google Scholar]
- 110.Yu SH, Huang HY, Korivi M, Hsu MF, Huang CY, Hou CW, Chen CY, Kao CL, Lee RP, Lee SD, et al. Oral Rg1 supplementation strengthens antioxidant defense system against exercise-induced oxidative stress in rat skeletal muscles. J Int Soc Sports Nutr. 2012;9:23. doi: 10.1186/1550-2783-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Korivi M, Hou CW, Huang CY, Lee SD, Hsu MF, Yu SH, Chen CY, Liu YY, Kuo CH. Ginsenoside-Rg1 protects the liver against exhaustive exercise-induced oxidative stress in rats. Evid Based Complement Alternat Med. 2012;2012:932165. doi: 10.1155/2012/932165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang JT, Qu ZW, Liu Y, Deng HL. Preliminary study on antiamnestic mechanism of ginsenoside Rg1 and Rb1. Chin Med J (Engl) 1990;103:932–938. [PubMed] [Google Scholar]
- 113.Benishin CG, Lee R, Wang LC, Liu HJ. Effects of ginsenoside Rb1 on central cholinergic metabolism. Pharmacology. 1991;42:223–229. doi: 10.1159/000138801. [DOI] [PubMed] [Google Scholar]
- 114.Ma TC, Yu QH. Effect of 20(S)-ginsenoside-Rg2 and cyproheptadine on two-way active avoidance learning and memory in rats. Arzneimittelforschung. 1993;43:1049–1052. [PubMed] [Google Scholar]
- 115.Li Z, Guo YY, Wu CF, Li X, Wang JH. Protective effects of pseudoginsenoside-F11 on scopolamine-induced memory impairment in mice and rats. J Pharm Pharmacol. 1999;51:435–440. doi: 10.1211/0022357991772484. [DOI] [PubMed] [Google Scholar]
- 116.Yamazaki M, Hirakura K, Miyaichi Y, Imakura K, Kita M, Chiba K, Mohri T. Effect of polyacetylenes on the neurite outgrowth of neuronal culture cells and scopolamine-induced memory impairment in mice. Biol Pharm Bull. 2001;24:1434–1436. doi: 10.1248/bpb.24.1434. [DOI] [PubMed] [Google Scholar]
- 117.Bao HY, Zhang J, Yeo SJ, Myung CS, Kim HM, Kim JM, Park JH, Cho J, Kang JS. Memory enhancing and neuroprotective effects of selected ginsenosides. Arch Pharm Res. 2005;28:335–342. doi: 10.1007/BF02977802. [DOI] [PubMed] [Google Scholar]
- 118.Yang JH, Han SJ, Ryu JH, Jang IS, Kim DH. Ginsenoside Rh2 ameliorates scopolamine-induced learning deficit in mice. Biol Pharm Bull. 2009;32:1710–1715. doi: 10.1248/bpb.32.1710. [DOI] [PubMed] [Google Scholar]
- 119.Wang Q, Sun LH, Jia W, Liu XM, Dang HX, Mai WL, Wang N, Steinmetz A, Wang YQ, Xu CJ. Comparison of ginsenosides Rg1 and Rb1 for their effects on improving scopolamine-induced learning and memory impairment in mice. Phytother Res. 2010;24:1748–1754. doi: 10.1002/ptr.3130. [DOI] [PubMed] [Google Scholar]
- 120.Lasarova MB, Mosharrof AH, Petkov VD, Markovska VL, Petkov VV. Effect of piracetam and of standardized ginseng extract on the electroconvulsive shock-induced memory disturbances in “step-down” passive avoidance. Acta Physiol Pharmacol Bulg. 1987;13:11–17. [PubMed] [Google Scholar]
- 121.Petkov VD, Mosharrof AH. Effects of standardized ginseng extract on learning, memory and physical capabilities. Am J Chin Med. 1987;15:19–29. doi: 10.1142/S0192415X87000047. [DOI] [PubMed] [Google Scholar]
- 122.Zhang L, Zhang JT. Memory facilitation induced by Panax ginseng and pseudoginseng in mice. Zhong Xi Yi Jie He Za Zhi. 1987;7:610–612. [PubMed] [Google Scholar]
- 123.Jaenicke B, Kim EJ, Ahn JW, Lee HS. Effect of Panax ginseng extract on passive avoidance retention in old rats. Arch Pharm Res. 1991;14:25–29. doi: 10.1007/BF02857809. [DOI] [PubMed] [Google Scholar]
- 124.Petkov VD, Cao Y, Todorov I, Lazarova M, Getova D, Stancheva S, Alova L. Behavioral effects of stem-leaves extract from Panax ginseng C.A. Meyer. Acta Physiol Pharmacol Bulg. 1992;18:41–48. [PubMed] [Google Scholar]
- 125.Petkov VD, Kehayov R, Belcheva S, Konstantinova E, Petkov VV, Getova D, Markovska V. Memory effects of standardized extracts of Panax ginseng (G115), Ginkgo biloba (GK 501) and their combination Gincosan (PHL-00701). Planta Med. 1993;59:106–114. doi: 10.1055/s-2006-959623. [DOI] [PubMed] [Google Scholar]
- 126.Nitta H, Matsumoto K, Shimizu M, Ni XH, Watanabe H. Panax ginseng extract improves the performance of aged Fischer 344 rats in radial maze task but not in operant brightness discrimination task. Biol Pharm Bull. 1995;18:1286–1288. doi: 10.1248/bpb.18.1286. [DOI] [PubMed] [Google Scholar]
- 127.Nitta H, Matsumoto K, Shimizu M, Ni XH, Watanabe H. Panax ginseng extract improves the scopolamine-induced disruption of 8-arm radial maze performance in rats. Biol Pharm Bull. 1995;18:1439–1442. doi: 10.1248/bpb.18.1439. [DOI] [PubMed] [Google Scholar]
- 128.Wang A, Cao Y, Wang Y, Zhao R, Liu C. Effects of Chinese ginseng root and stem-leaf saponins on learning, memory and biogenic monoamines of brain in rats. Zhongguo Zhong Yao Za Zhi. 1995;20:493–495. [PubMed] [Google Scholar]
- 129.Zhao R, McDaniel WF. Ginseng improves strategic learning by normal and brain-damaged rats. Neuroreport. 1998;9:1619–1624. doi: 10.1097/00001756-199805110-00066. [DOI] [PubMed] [Google Scholar]
- 130.Jin SH, Park JK, Nam KY, Park SN, Jung NP. Korean red ginseng saponins with low ratios of protopanaxadiol and protopanaxatriol saponin improve scopolamine-induced learning disability and spatial working memory in mice. J Ethnopharmacol. 1999;66:123–129. doi: 10.1016/s0378-8741(98)00190-1. [DOI] [PubMed] [Google Scholar]
- 131.Hsieh MT, Peng WH, Wu CR, Wang WH. The ameliorating effects of the cognitive-enhancing Chinese herbs on scopolamine-induced amnesia in rats. Phytother Res. 2000;14:375–377. doi: 10.1002/1099-1573(200008)14:5<375::aid-ptr593>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 132.Petkov VD, Belcheva S, Petkov VV. Behavioral effects of Ginkgo biloba L., Panax ginseng C.A. Mey. and Gincosan. Am J Chin Med. 2003;31:841–855. doi: 10.1142/S0192415X03001533. [DOI] [PubMed] [Google Scholar]
- 133.Kurimoto H, Nishijo H, Uwano T, Yamaguchi H, Zhong YM, Kawanishi K, Ono T. Effects of nonsaponin fraction of red ginseng on learning deficits in aged rats. Physiol Behav. 2004;82:345–355. doi: 10.1016/j.physbeh.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 134.Lee B, Park J, Kwon S, Park MW, Oh SM, Yeom MJ, Shim I, Lee HJ, Hahm DH. Effect of wild ginseng on scopolamine-induced acetylcholine depletion in the rat hippocampus. J Pharm Pharmacol. 2010;62:263–271. doi: 10.1211/jpp.62.02.0015. [DOI] [PubMed] [Google Scholar]
- 135.Sanghavi CR, Barhate SA, Mahajan MS, Mohan M, Kasture SB. Korean ginseng extract attenuates reserpine-induced orofacial dyskinesia and improves cognitive dysfunction in rats. Nat Prod Res. 2011;25:704–715. doi: 10.1080/14786410802583031. [DOI] [PubMed] [Google Scholar]
- 136.Sloley BD, Pang PK, Huang BH, Ba F, Li FL, Benishin CG, Greenshaw AJ, Shan JJ. American ginseng extract reduces scopolamine-induced amnesia in a spatial learning task. J Psychiatry Neurosci. 1999;24:442–452. [PMC free article] [PubMed] [Google Scholar]
- 137.Zhong YM, Nishijo H, Uwano T, Tamura R, Kawanishi K, Ono T. Red ginseng ameliorated place navigation deficits in young rats with hippocampal lesions and aged rats. Physiol Behav. 2000;69:511–525. doi: 10.1016/s0031-9384(00)00206-7. [DOI] [PubMed] [Google Scholar]
- 138.Wang LC, Wang B, Ng SY, Lee TF. Effects of ginseng saponins on beta-amyloid-induced amnesia in rats. J Ethnopharmacol. 2006;103:103–108. doi: 10.1016/j.jep.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 139.Chatterjee M, Singh S, Kumari R, Verma AK, Palit G. Evaluation of the antipsychotic potential of Panax quinquefolium in ketamine induced experimental psychosis model in mice. Neurochem Res. 2012;37:759–770. doi: 10.1007/s11064-011-0670-4. [DOI] [PubMed] [Google Scholar]
- 140.Chuang CM, Hsieh CL, Lin HY, Lin JG. Panax notoginseng Burk attenuates impairment of learning and memory functions and increases ED1, BDNF and beta-secretase immunoreactive cells in chronic stage ischemia-reperfusion injured rats. Am J Chin Med. 2008;36:685–693. doi: 10.1142/S0192415X08006156. [DOI] [PubMed] [Google Scholar]
- 141.Zhong ZG, Lv L, Chai LM, Wu DP, Zhang WY, Huang JL, Gang YW, Li F, Zu B. Effect of Panax notoginseng saponins on APP gene transcription in the brain tissue of SAMP8. Zhong Yao Cai. 2011;34:77–80. [PubMed] [Google Scholar]
- 142.Saito H, Tsuchiya M, Naka S, Takagi K. Effects of Panax ginseng root on conditioned avoidance response in rats. Jpn J Pharmacol. 1977;27:509–516. doi: 10.1254/jjp.27.509. [DOI] [PubMed] [Google Scholar]
- 143.Takagi K, Saito H, Tsuchiya M. Pharmacological studies of Panax ginseng root: pharmacological properties of a crude saponin fraction. Jpn J Pharmacol. 1972;22:339–346. doi: 10.1254/jjp.22.339. [DOI] [PubMed] [Google Scholar]
- 144.Takagi K, Saito H, Nabata H. Pharmacological studies of Panax ginseng root: estimation of pharmacological actions of Panax ginseng root. Jpn J Pharmacol. 1972;22:245–249. doi: 10.1254/jjp.22.245. [DOI] [PubMed] [Google Scholar]
- 145.Wang XY, Chen J, Zhang JT. Effect of ginsenoside Rg1 on learning and memory impairment induced by beta-amyloid peptide(25-35) and its mechanism of action. Yao Xue Xue Bao. 2001;36:1–4. [PubMed] [Google Scholar]
- 146.Wee JJ, Park KM, Chung AS. Biological activities of ginseng and its application to human health. In: Benzie IF, Wachtel-Galor S, eds. Herbal medicine: biomolecular and clinical aspects. 2nd ed. CRC Press; Boca Raton: 2011. pp. 157–174. [Google Scholar]
- 147.Liu L, Hoang-Gia T, Wu H, Lee MR, Gu L, Wang C, Yun BS, Wang Q, Ye S, Sung CK. Ginsenoside Rb1 improves spatial learning and memory by regulation of cell genesis in the hippocampal subregions of rats. Brain Res. 2011;1382:147–154. doi: 10.1016/j.brainres.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 148.Kochmareva LL. The effect of Schizandra chinensis and ginseng on processes of concentration. In: Lazarev NV, ed. Materials for the study of ginseng and Schizandra. V. L. Komarov’s Far East Branch of USSR Academy of Science; Leningrad: 1958. pp. 12–17. [Google Scholar]
- 149.Medvedev MA. Effect of ginseng and eleutherococcus on working parameters of radio-telegraph operators. In: Brekhman II, Belikov IF, Kurentsova GE, eds. Materials of studies of ginseng and other medicinal plants of Far East. Primorye Publishing Press; Vladivostok: 1963. pp. 237–239. [Google Scholar]
- 150.Popov IM, Goldwag WJ. A review of the properties and clinical effects of ginseng. Am J Chin Med (Gard City NY) 1973;1:263–270. doi: 10.1142/s0192415x73000280. [DOI] [PubMed] [Google Scholar]
- 151.Hallstrom C, Fulder S, Carruthers M. Effects of ginseng on the performance of nurses on night duty. Am J Chin Med. 1978;6:277–282. [Google Scholar]
- 152.Kennedy DO, Scholey AB, Wesnes KA. Dose dependent changes in cognitive performance and mood following acute administration of ginseng to healthy young volunteers. Nutr Neurosci. 2001;4:295–310. doi: 10.1080/1028415x.2001.11747370. [DOI] [PubMed] [Google Scholar]
- 153.Kennedy DO, Jackson PA, Elliott JM, Scholey AB, Robertson BC, Greer J, Tiplady B, Buchanan T, Haskell CF. Cognitive and mood effects of 8 weeks’ supplementation with 400 mg or 1000 mg of the omega-3 essential fatty acid docosahexaenoic acid (DHA) in healthy children aged 10-12 years. Nutr Neurosci. 2009;12:48–56. doi: 10.1179/147683009X388887. [DOI] [PubMed] [Google Scholar]
- 154.Reay JL, Scholey AB, Kennedy DO. Panax ginseng (G115) improves aspects of working memory performance and subjective ratings of calmness in healthy young adults. Hum Psychopharmacol. 2010;25:462–471. doi: 10.1002/hup.1138. [DOI] [PubMed] [Google Scholar]
- 155.Reay JL, Scholey AB, Milne A, Fenwick J, Kennedy DO. Panax ginseng has no effect on indices of glucose regulation following acute or chronic ingestion in healthy volunteers. Br J Nutr. 2009;101:1673–1678. doi: 10.1017/S0007114508123418. [DOI] [PubMed] [Google Scholar]
- 156.Kennedy DO, Scholey AB. Ginseng: potential for the enhancement of cognitive performance and mood. Pharmacol Biochem Behav. 2003;75:687–700. doi: 10.1016/s0091-3057(03)00126-6. [DOI] [PubMed] [Google Scholar]
- 157.Kennedy DO, Scholey AB, Wesnes KA. Differential, dose dependent changes in cognitive performance following acute administration of a Ginkgo biloba/Panax ginseng combination to healthy young volunteers. Nutr Neurosci. 2001;4:399–412. doi: 10.1080/1028415x.2001.11747376. [DOI] [PubMed] [Google Scholar]
- 158.Kennedy DO, Scholey AB, Wesnes KA. Modulation of cognition and mood following administration of single doses of Ginkgo biloba, ginseng, and a ginkgo/ginseng combination to healthy young adults. Physiol Behav. 2002;75:739–751. doi: 10.1016/s0031-9384(02)00665-0. [DOI] [PubMed] [Google Scholar]
- 159.Reay JL, Kennedy DO, Scholey AB. Single doses of Panax ginseng (G115) reduce blood glucose levels and improve cognitive performance during sustained mental activity. J Psychopharmacol. 2005;19:357–365. doi: 10.1177/0269881105053286. [DOI] [PubMed] [Google Scholar]
- 160.Reay JL, Kennedy DO, Scholey AB. Effects of Panax ginseng, consumed with and without glucose, on blood glucose levels and cognitive performance during sustained ‘mentally demanding’ tasks. J Psychopharmacol. 2006;20:771–781. doi: 10.1177/0269881106061516. [DOI] [PubMed] [Google Scholar]
- 161.Simon W, Kirchdorfer AM, Dahse G. Efficiency control of a ginseng containing geriatric drug by means of the Kraepelin method. Med Monatsschr. 1977;31:39–41. [PubMed] [Google Scholar]
- 162.Hobbs C. The ginseng: a user’s guide. Botanica Press; Santa Cruz: 1996. [Google Scholar]
- 163.D’Angelo L, Grimaldi R, Caravaggi M, Marcoli M, Perucca E, Lecchini S, Frigo GM, Crema A. A double-blind, placebo-controlled clinical study on the effect of a standardized ginseng extract on psychomotor performance in healthy volunteers. J Ethnopharmacol. 1986;16:15–22. doi: 10.1016/0378-8741(86)90063-2. [DOI] [PubMed] [Google Scholar]
- 164.Zhao XZ. Antisenility effect of ginseng-rhizome saponin. Zhong Xi Yi Jie He Za Zhi. 1990;10:586–589. [PubMed] [Google Scholar]
- 165.Wiklund I, Karlberg J, Lund B. A double-blind comparison of the effect on quality of life of a combination of vital substances including standardized ginseng G115 and placebo. Curr Ther Res. 1994;55:32–42. [Google Scholar]
- 166.Sorensen H, Sonne J. A doubled-masked study of the effects of ginseng on cognitive functions. Curr Ther Res. 1996;57:959–968. [Google Scholar]
- 167.Lee ST, Chu K, Sim JY, Heo JH, Kim M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22:222–226. doi: 10.1097/WAD.0b013e31816c92e6. [DOI] [PubMed] [Google Scholar]
- 168.Heo JH, Lee ST, Oh MJ, Park HJ, Shim JY, Chu K, Kim M. Improvement of cognitive deficit in Alzheimer’s disease patients by long term treatment with Korean red ginseng. J Ginseng Res. 2011;35:457–461. doi: 10.5142/jgr.2011.35.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yeo HB, Yoon HK, Lee HJ, Kang SG, Jung KY, Kim L. Effects of Korean red ginseng on cognitive and motor function: a double-blind, randomized, placebo-controlled trial. J Ginseng Res. 2012;36:190–197. doi: 10.5142/jgr.2012.36.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Scholey A, Ossoukhova A, Owen L, Ibarra A, Pipingas A, He K, Roller M, Stough C. Effects of American ginseng (Panax quinquefolius) on neurocognitive function: an acute, randomised, double-blind, placebo-controlled, crossover study. Psychopharmacology (Berl) 2010;212:345–356. doi: 10.1007/s00213-010-1964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chepurnov SA, Suleimanova EM, Guliaev MV, Abbasova KR, Pirogov IuA, Chepurnova NE. Neuroprotection in epilepsy. Usp Fiziol Nauk. 2012;43:55–71. [PubMed] [Google Scholar]
- 172.Perry E, Howes MJ. Medicinal plants and dementia therapy: herbal hopes for brain aging? CNS Neurosci Ther. 2011;17:683–698. doi: 10.1111/j.1755-5949.2010.00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jesky R, Hailong C. Are herbal compounds the next frontier for alleviating learning and memory impairments? An integrative look at memory, dementia and the promising therapeutics of traditional chinese medicines. Phytother Res. 2011;25:1105–1118. doi: 10.1002/ptr.3388. [DOI] [PubMed] [Google Scholar]
- 174.Geng J, Dong J, Ni H, Lee MS, Wu T, Jiang K, Wang G, Zhou AL, Malouf R. Ginseng for cognition. Cochrane Database Syst Rev. 2010;(12):CD007769. doi: 10.1002/14651858.CD007769.pub2. [DOI] [PubMed] [Google Scholar]
- 175.Radad K, Moldzio R, Rausch WD. Ginsenosides and their CNS targets. CNS Neurosci Ther. 2011;17:761–768. doi: 10.1111/j.1755-5949.2010.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chu SF, Zhang JT. New achievements in ginseng research and its future prospects. Chin J Integr Med. 2009;15:403–408. doi: 10.1007/s11655-009-0403-6. [DOI] [PubMed] [Google Scholar]
- 177.Liu Y, Li X, Yuan HF. Progress of research on effects of ginsenoside Rg1 in promoting capability of learning and memory. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2006;26:956–960. [PubMed] [Google Scholar]
- 178.Zhang JT. Nootropic mechanisms of ginsenoside Rg1: influence on neuronal plasticity and neurogenesis. Yao Xue Xue Bao. 2005;40:385–388. [PubMed] [Google Scholar]
- 179.Cheng Y, Shen LH, Zhang JT. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin. 2005;26:143–149. doi: 10.1111/j.1745-7254.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- 180.Nishijo H, Uwano T, Zhong YM, Ono T. Proof of the mysterious efficacy of ginseng: basic and clinical trials: effects of red ginseng on learning and memory deficits in an animal model of amnesia. J Pharmacol Sci. 2004;95:145–152. doi: 10.1254/jphs.fmj04001x3. [DOI] [PubMed] [Google Scholar]
- 181.Lee MR, Yun BS, In OH, Sung CK. Comparative study of Korean white, red, and black ginseng extract on cholinesterase inhibitory activity and cholinergic function. J Ginseng Res. 2011;35:421–428. doi: 10.5142/jgr.2011.35.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Salim KN, McEwen BS, Chao HM. Ginsenoside Rb1 regulates ChAT, NGF and trkA mRNA expression in the rat brain. Brain Res Mol Brain Res. 1997;47:177–182. doi: 10.1016/s0169-328x(97)00042-9. [DOI] [PubMed] [Google Scholar]
- 183.Chang Y, Huang WJ, Tien LT, Wang SJ. Ginsenosides Rg1 and Rb1 enhance glutamate release through activation of protein kinase A in rat cerebrocortical nerve terminals (synaptosomes). Eur J Pharmacol. 2008;578:28–36. doi: 10.1016/j.ejphar.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 184.Chang Y, Wang SJ. Ginsenoside Rg1 and Rb1 enhance glutamate exocytosis from rat cortical nerve terminals by affecting vesicle mobilization through the activation of protein kinase C. Eur J Pharmacol. 2008;590:74–79. doi: 10.1016/j.ejphar.2008.05.032. [DOI] [PubMed] [Google Scholar]