Abstract
Ginsenoside, one of the active ingredients of Panax ginseng, has a variety of physiologic and pharmacologic effects. The purpose of this study was to explore the effects of ginsenoside Rd (G-Rd) on melastatin type transient receptor potential 7 (TRPM7) channels with respect to the proliferation and survival of AGS and MCF-7 cells (a gastric and a breast cancer cell line, respectively). AGS and MCF-7 cells were treated with different concentrations of G-Rd, and caspase-3 activities, mitochondrial depolarizations, and sub-G1 fractions were analyzed to determine if cell death occurred by apoptosis. In addition, human embryonic kidney (HEK) 293 cells overexpressing TRPM7 channels were used to confirm the role of TRPM7 channels. G-Rd inhibited the proliferation and survival of AGS and MCF-7 cells and enhanced caspase-3 activity, mitochondrial depolarization, and sub-G1 populations. In addition, G-Rd inhibited TRPM7-like currents in AGS and MCF-7 cells and in TRPM7 channel overexpressing HEK 293 cells, as determined by whole cell voltage-clamp recordings. Furthermore, TRPM7 overexpression in HEK 293 cells promoted G-Rd induced cell death. These findings suggest that G-Rd inhibits the proliferation and survival of gastric and breast cancer cells by inhibiting TRPM7 channel activity.
Keywords: Panax ginseng, Ginsenoside Rd, Melastatin type transient receptor potential 7 channel, Gastric cancer, Breast cancer
INTRODUCTION
Ginseng is one of the most popular herbal medicines and has a variety of pharmacological and therapeutic applications. In particular, the ginsenosides have been reported to have anticancer, anti inflammatory, antioxidative, and vasorelaxing properties. The molecular components primarily responsible for the action of ginseng are ginsenosides (ginseng saponins). About 50 different ginsenosides have been isolated and identified in Panax ginseng roots, and ginsenoside Rd (G-Rd) is known to be one of the more active ginsenosides [1]. However, the (patho) physiological activity of G-Rd and its signaling pathways in cancer cells have not been determined.
Gastric cancer and breast cancer are leading causes of mortality in Korea. In previous studies, we found that human gastric cancer cells (AGS) express the melastatin type transient receptor potential 7 (TRPM7) channel and that this channel has a negative effect on cell survival and suggested that it be considered a therapeutic target in gastric cancer [2]. Similarly, Guilbert et al. [3] suggested that TRPM7 has a negative effect on breast cancer cell (MCF) viability.
Transient receptor potential (TRP) melastatin 7 (TRPM7), a member of the TRP channel family, is ubiquitously expressed in the body [4-6] and involved in a number of physiological and pharmacological processes [7-9]. Recently, Kim et al. [10] suggested that Rg3 is involved in gastric cancer apoptosis by influencing TRPM7 channels. Also Rf depolarized the pacemaking activity and inhibited the amplitude in murine small intestine. But, Rf had no effects on TRPM7 channels [11]. Among ginsenosides, the effect of G-Rd on TRPM7 channel with respect to the survival and electrophysiological characteristics of cancer cells is unknown. Therefore, in this study, it was examined for the effects of G-Rd on TRPM7 channels with respect to the survival and proliferation and the electrophysiological characteristics of AGS and MCF-7 cells.
MATERIALS AND METHODS
Drugs
G-Rd was purchased in the AMBO Institute (Seoul, Korea). All other drugs were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cells
The AGS and MCF-7 cell lines were established at the Cancer Research Center, College of Medicine, Seoul National University. The cell lines were cultured in RPMI-1640 (Gibco-BRL, Rockville, MD, USA) supplemented with 10% fetal bovine serum. Cells were grown at 37℃ in a 5% CO2 humidified incubator.
Patch-clamp experiments
Cells were transferred to a small chamber on the stage of an inverted microscope (IX70; Olympus, Tokyo, Japan) and constantly perfused with Tyrode solution containing (mmol/L) KCl 2.8, NaCl 145, CaCl2 2, glucose 10, MgCl2 1.2, and HEPES 10, adjusted to pH 7.4 with NaOH at a rate of 2 to 3 mL/min. The pipette solution contained (mmol/L) Cs-glutamate 145, NaCl 8, Cs-2-bis(2-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid 10, and HEPES-CsOH 10, adjusted to pH 7.2 with CsOH. The conventional whole cell patch-clamp technique was adopted to hold the membrane potential at -60 mV using an Axopatch 1-D patch-clamp amplifier (Axon Instruments, Union City, CA, USA). For data acquisition and the application of command pulses, pCLAMP software ver. 9.2 and Digidata 1322A (Axon Instruments) were used. Data were analyzed using pCLAMP and Microcal Origin ver. 6.0 (Microcal Software, Northampton, MA, USA).
Melastatin type transient receptor potential 7 expression in human embryonic kidney 293 cells
Human embryonic kidney (HEK) 293 cells (ATCC, Manassas, VA, USA) were maintained according to the supplier’s recommendations. For transfection, cells were transfected with the Flag-murine LTRPC7/pCDNA4-TO construct and grown on glass coverslips in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum, blasticidin (5 μg/mL), and zeocin (0.4 mg/mL) using the transfection reagent FuGENE 6 (Roche Molecular Biochemicals, Indianapolis, IN, USA) according to the manufacturer’s protocol. TRPM7 expression was induced by adding 1 μg/mL tetracycline.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
AGS or MCF-7 cells were seeded into each well of 12-well culture plates and then cultured in RPMI-1640 supplemented with G-Rd for 72 h. After incubation, 100 μL of (5 mg/mL) 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagent was added to each well, and the plates were placed at 37℃ in an incubator for 3 hours. After aspirating supernatant, 400 μL of dimethyl sulfoxide (Jersey Lab Supply, Livingston, NJ, USA) was added to each well. The yellow formazan product was assayed spectrophotometrically at 570 nm using an enzyme-linked immunosorbent assay plate reader.
Caspase-3 activity assay
Caspase-3 activity was determined using assay kits (BioMol, Plymouth, PA, USA). Absorbance was measured at 405 nm on microplate spectrophotometer at several time-points. A pan-caspase inhibitor zVAD-fmk (Calbiochem, La Jolla, CA, USA) was used to validate the assay method.
Assessment of mitochondrial membrane depolarization
Mitochondrial membrane depolarization was evaluated using JC-1 fluorescence probe according to the manufacturer’s instructions (Molecular Probes, Eugene, OR, USA). Cells were analyzed by flow cytometry using excitation wavelength of 488 nm and 530 or 585 nm bypass emission filters.
Flow cytometric analysis
To analyze cell cycles, cells were labeled with propidium iodide (50 μg/mL) solution containing RNase A (100 μg/mL), and analyzed by flow cytometry (FACScan; Becton Dickinson Immunocytometry Systems, San Jose, CA, USA). Sub-G1 fractions were used as measures of apoptotic cells [12].
Statistical analysis
Data are expressed as means±SEMs. The significances of differences were determined using the Student’s t-test. Statistical significance was accepted for p-values <0.05.
RESULTS
Ginsenoside Rd induced AGS and MCF-7 cell death
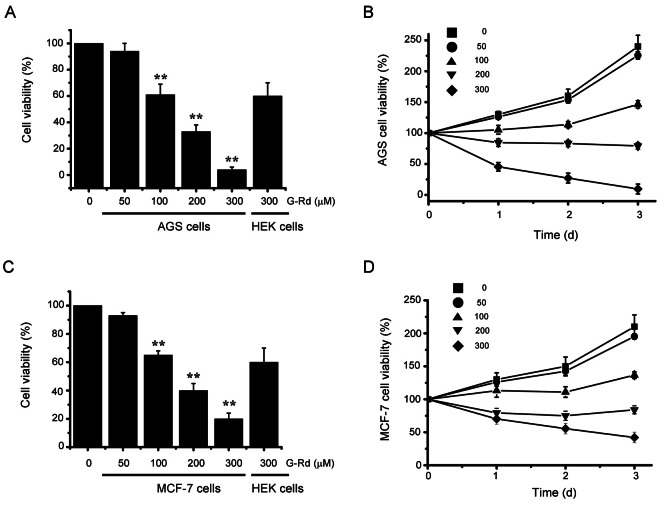
MTT assays were used to ascertain whether G-Rd inhibits AGS and MCF-7 cells. Cell numbers were gradually decreased concentration-dependently by G-Rd, which had an IC50 value of 131.2 μM in AGS cells (Fig. 1A) and of 154.3 μM in MCF-7 cells (Fig. 1C). IC50 values were determined by quantifying cell proliferation over time (Fig. 1B, D). To confirm cell viability, we used a negative control, HEK 293 cells, which are human normal cells. G-Rd 300 μM more increased the cell viability in HEK 293 cells than AGS and MCF-7 cells (Fig. 1A, C).
Fig. 1. Ginsenoside Rd (G-Rd) induced AGS and MCF-7 cell death. (A) AGS cells were incubated with G-Rd at the indicated concentrations for 72 h prior to 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assays. Distilled water was used as the vehicle. Cell viabilities are expressed as percentages versus treatment naïve controls. (B) Time course response to G-Rd. AGS cell viabilities are expressed versus cells treated with vehicle only and harvested at zero time. (C) The MCF-7 cells were incubated with G-Rd at the indicated concentrations for 72 h prior to MTT assays. Distilled water was used as vehicle. Cell viabilities are expressed as percentages versus treatment naïve controls. (D) Time course response to G-Rd. MCF-7 cell viabilities are expressed versus cells treated with vehicle only and harvested at zero time. Results are presented as means±SEMs. HEK 293 cells were used as nagative control. **p<0.01.
Ginsenoside Rd triggered apoptosis in AGS and MCF-7 cells
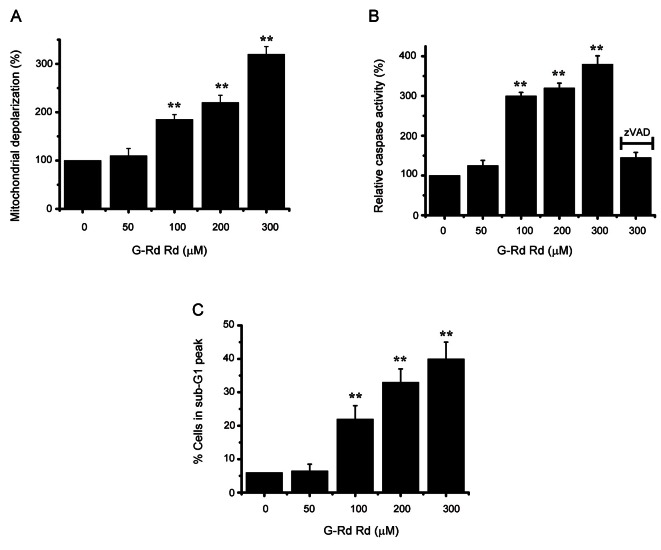
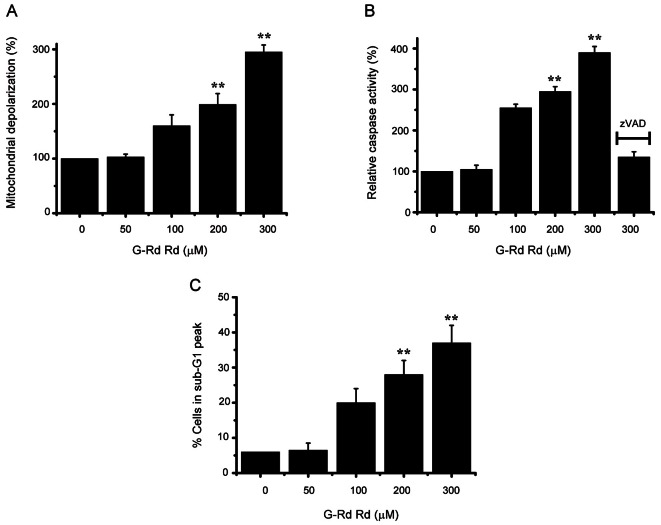
A mitochondrial membrane depolarization assay was used to investigate whether AGS and MCF-7 cell death occurred by apoptosis. G-Rd increased mitochondrial membrane depolarization (an initial event in intrinsic apoptosis signaling) (Figs. 2A and 3A), showing that G-Rd induces apoptosis via intrinsic apoptotic mechanisms. Caspase-3 activation is an indicator of cell death by apoptosis, and therefore, we investigated the effect of G-Rd on caspase-3 enzyme activity in AGS and MCF-7 cells. G-Rd increased caspase-3 enzyme activity, and this was inhibited by zVAD-fmk, a pan-caspase inhibitor (Figs. 2B and 3B). Sub-G1 mode analysis was then used to determine the cell death mode in AGS and MCF-7 cells after incubation with G-Rd [13,14]. After incubation in 300 μM G-Rd, the sub-G1 markedly was increased by 41.2±5.3% in AGS and 37.3±5.1% in MCF-7 cells (Figs. 2C and 3C).
Fig. 2. Ginsenoside Rd (G-Rd) triggered AGS cell apoptosis. (A) Mitochondrial membrane depolarizations are expressed as percentages versus untreated cells. (B) Cells were cultured with G-Rd at the indicated concentrations for 24 h prior to caspase assays. Caspase activities of untreated cells were set at 100%. The pan-caspase inhibitor zVAD-fmk (zVAD, 20 μM) was used to validate the analytical method employed. (C) Sub-G1 peak measured by FACScan. Results are means±SEMs of three independent experiments. **p<0.01.
Fig. 3. Ginsenoside Rd (G-Rd) triggered MCF-7 cell apoptosis. (A) Mitochondrial membrane depolarizations are expressed as percentages versus untreated cells. (B) Cells were cultured with G-Rd at the indicated concentrations for 24 h prior to caspase assays. Caspase activities of untreated cells were set at 100%. The pan-caspase inhibitor zVAD-fmk (zVAD, 20 μM) was used to validate the analytical method employed. (C) Sub-G1 peak measured by FACScan. Results are means±SEMs of three independent experiments. **p<0.01.
Involvement of melastatin type transient receptor potential 7 in cell death
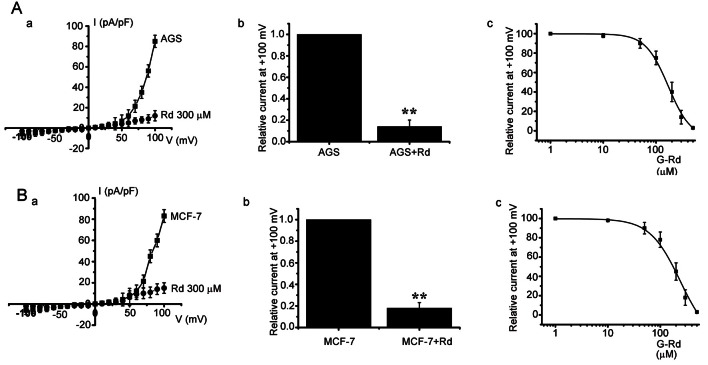
It has been suggested that TRPM7 is required for cell proliferation and survival [5], and Guilbert et al. [3] suggested that TRPM7 is required for MCF-7 cell proliferation. Also, it has been suggested gastric cancer AGS cells express TRPM7 channel and that inhibition of this channel induces cell death [2]. Thus, the effects of G-Rd on TRPM7 channels were investigated, and whole cell patch-clamp recordings were obtained to check the electrophysiological effects of G-Rd on TRPM7-like currents in AGS and MCF-7 cells. In MTT assay and so on, G-Rd 300 μM showed the very good effects on AGS and MCF-7 cells. Therefore, we used G-Rd 300 μM in electrophysiological studies. A voltage ramp was applied from -100 to +100 mV. Subsequently, small inward currents were observed at negative potentials and larger outward currents at positive potentials, showing outward-rectifying cation currents (n=6; Fig. 4Aa, Ba).
Fig. 4. Inhibition of melastatin type transient receptor potential 7 (TRPM7)-like currents by ginsenoside Rd (G-Rd) in AGS and MCF-7 cells. (A) Effect of G-Rd on TRPM7-like currents in AGS cells. I–V curves (a) and a summary bar graph (b) in the absence (■) or presence (●) of G-Rd. (c) Concentration-dependent inhibition of TRPM7-like current by G-Rd in AGS cells. The estimated median inhibitory concentration value was 170 μM. The saturation concentration might be 500 μM. (B) Effect of G-Rd on TRPM7-like currents in MCF-7 cells. I–V curves (a) and a summary bar graph (b) in the absence (■) or presence (●) of G-Rd. (c) Concentration-dependent inhibition of TRPM7-like current by G-Rd in MCF-7 cells. The estimated median inhibitory concentration value was 178 μM. The saturation concentration might be 500 μM. **p<0.01.
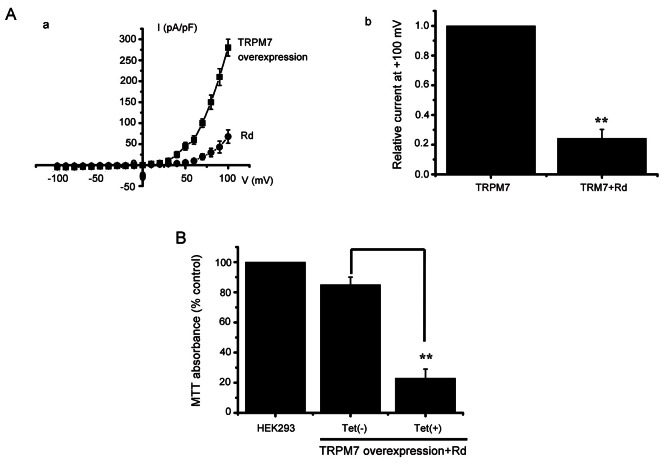
In the presence of G-Rd, the amplitudes of TRPM7-like currents were inhibited by 86.1±5.1% outwardly and by 53.1±3.3% inwardly in AGS cells and by 82.2±3.2% outwardly and 66.2±3.1% inwardly in MCF-7 cells (n=6; Fig. 4Ab, Bb). In addition, similar results were obtained in HEK 293 cells overexpressing TRPM7 (n=6, Fig. 5Aa). In the presence of G-Rd, the amplitudes of TRPM7 currents were inhibited by 76.2±3.1% outwardly and by 63.2±3.4% inwardly in TRPM7 overexpressing HEK 293 cells (n=6, Fig. 5Ab).
Fig. 5. Effects of ginsenoside Rd (G-Rd) on melastatin type transient receptor potential 7 (TRPM7) channel overexpression in human embryonic kidney (HEK) 293 cells. (A) Effect of G-Rd on TRPM7 currents in HEK 293 cells. I–V curves (a) and a summary bar graph (b) in the absence (■) or presence (●) of G-Rd. (B) TRPM7 cells were treated or not treated with tetracycline for 1 d. Cells were incubated with G-Rd, followed by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. **p<0.01.
We investigated the dose dependent curve. The median inhibitory concentration was 170 μM (Fig. 4Ac) and 178 μM (Fig. 4Bc). The AGS and MCF-7 saturation concentration might be 500 μM (Fig. 4Ac, Bc). For further evidence of the effect of G-Rd on TRPM7 channel, the relation between TRPM7 channel level and G-Rd mediated cell death was investigated using HEK 293 cells with tetracycline [Tet(+)]-inducible TRPM7 channel expression [5,7]. In these TRPM7 channel overexpressing cells, treatment with G-Rd caused cell death (n=5, Fig. 5B), showing that TRPM7 channel upregulation increases the rate of G-Rd induced cell death. Taken together, these results show G-Rd regulates TRPM7 channels, and that these channels play vital roles in the proliferation and survival of AGS and MCF-7 cells.
DISCUSSION
TRP channels constitute a superfamily of proteins that form a diverse group of Ca2+-permeable nonselective cation channels. Family members fall into three categories, that is, the classic, vanilloid, and melastatin types. TRPM7 is a ubiquitously expressed ion channel of the melastatin type [4,15-17]. It produces outward currents at positive potentials from +40 to +110 mV and very small inward currents at negative potentials between −120 to −50 mV [5,6,9,18-21]. TRPM7 has multiple cellular and physiological functions, which include cell proliferation and viability [5,21,22], cellular Mg2+ homeostasis [9,22], anoxic cell death [23], neural transmission [24], cellular adhesion [25], and gastrointestinal pacemaking activity [26]. Wykes et al. [27] concluded that TRPM7 channels importantly affect human mast cell viability, whereas Jiang et al. [7] found that TRPM7 channels affect the proliferation of human head and neck cancer cells. Abed et al. [28] showed that TRPM7 is involved in human osteoblast cell growth, and Guilbert et al. [3] demonstrated that TRPM7 is required for breast cancer cell survival. Similarly, in a previous study, we found that TRPM7 is involved in the gastric cancer cell survival and proliferation [2]. In line with these previously studies, the present study shows that G-Rd induces apoptosis in human gastric and breast cancer cells and suggests that this may be due to the inhibition of TRPM7 channel activity.
P. ginseng is a famous traditional oriental herb, which has been used to treat a wide variety of diseases in Asian for thousands of years. The main molecular components contributive to the pharmacologic effects of P. ginseng are ginsenosides. At present, more than 50 ginsenosides have been identified, among which, G-Rd is one of the most active and efficient ingredients [29,30]. There are many papers about the relation of G-Rd and TRPM7 channels. A number of glutamate receptor-independent non-selective cation channels, such as TRPM7 play important roles in ischemic stroke. For example, TRPM7 channel activity was enhanced in oxygen/glucose deprivation-treated cortical neurons and TRPM7 knockdown by RNA interference in cultured neurons and hippocampal delayed anoxic cell death [23,31]. Also, G-Rd had significant neuroprotective effects. For example, G-Rd can protect oxygen-glucose deprivation-injured cultured hippocampal neurons [32] and reduce the infarct size in rats after middle cerebral artery occlusion [33-36]. Moreover, G-Rd was efficient and safe for the treatment of acute ischemic stroke [37]. Zhang et al. [38] suggested that G-Rd and TRPM7 are involved in neuroprotection following cerebral ischemia. However, this paper did describe the effects of Rd on TRPM7 electrophysiological responses or on the relation between TRPM7 cell viability. Among the functions of G-Rd, the effect of G-Rd on TRPM7 channel with respect to the survival and electrophysiological characteristics of cancer cells is unknown. Therefore, in this study, we showed that the effects of G-Rd on TRPM7 channels with respect to the survival and proliferation and the electrophysiological characteristics of AGS and MCF-7 cells.
An anticancer compound isolated from P. ginseng has an effect on human pancreatic tumours [39]. Ginsenoside Rh2 induces apoptosis and paraptosis-like cell death in colorectal cancer cells by activation of p53 [40]. Ginsenoside Rg3 inhibits colorectal tumour growth by down-regulation of Wnt/β-catenin signalling [41]. These anticancer ginsenosides provide reason for further development of this compound as a chemotherapeutic agent.
In this study, G-Rd suppressed the survival and proliferation of AGS and MCF-7 cells. In addition, G-Rd increased caspase-3 activity, mitochondrial depolarization, and sub-G1 fractions, but inhibited TRPM7-like currents in AGS and MCF-7 cells and in TRPM7 overexpressing HEK 293 cells in whole-cell voltage-clamp recordings. Furthermore, the overexpression of TRPM7 in mammalian cells increased G-Rd induced cell death.
Taken together, our findings suggest that G-Rd inhibits the proliferation and survival of gastric and breast cancer cells, and that G-Rd-induced apoptosis is due to the inhibition of TRPM7. Thus, our data suggest G-Rd importantly affects the proliferation and survival in gastric and breast cancer cell by influencing TRPM7 channels.
Acknowledgments
This research was supported by the Basic Science Research Program of the Korean National Research Foundation (NRF) funded by the Ministry of Education, Science and Technology (Grant no. 2010-0021347).
References
- 1.Kim HS, Parajuli SP, Yeum CH, Park JS, Jeong HS, So I, Kim KW, Jun JY, Choi S. Effects of ginseng total saponins on pacemaker currents of interstitial cells of Cajal from the small intestine of mice. Biol Pharm Bull. 2007;30:2037–2042. doi: 10.1248/bpb.30.2037. [DOI] [PubMed] [Google Scholar]
- 2.Kim BJ, Park EJ, Lee JH, Jeon JH, Kim SJ, So I. Suppression of transient receptor potential melastatin 7 channel induces cell death in gastric cancer. Cancer Sci. 2008;99:2502–2509. doi: 10.1111/j.1349-7006.2008.00982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guilbert A, Gautier M, Dhennin-Duthille I, Haren N, Sevestre H, Ouadid-Ahidouch H. Evidence that TRPM7 is required for breast cancer cell proliferation. Am J Physiol Cell Physiol. 2009;297:C493–C502. doi: 10.1152/ajpcell.00624.2008. [DOI] [PubMed] [Google Scholar]
- 4.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 5.Nadler MJ, Hermosura MC, Inabe K, Perraud AL, Zhu Q, Stokes AJ, Kurosaki T, Kinet JP, Penner R, Scharenberg AM, et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 6.Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–1047. doi: 10.1126/science.1058519. [DOI] [PubMed] [Google Scholar]
- 7.Jiang J, Li MH, Inoue K, Chu XP, Seeds J, Xiong ZG. Transient receptor potential melastatin 7-like current in human head and neck carcinoma cells: role in cell proliferation. Cancer Res. 2007;67:10929–10938. doi: 10.1158/0008-5472.CAN-07-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin J, Desai BN, Navarro B, Donovan A, Andrews NC, Clapham DE. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+homeostasis. Science. 2008;322:756–760. doi: 10.1126/science.1163493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitz C, Perraud AL, Johnson CO, Inabe K, Smith MK, Penner R, Kurosaki T, Fleig A, Scharenberg AM. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell. 2003;114:191–200. doi: 10.1016/s0092-8674(03)00556-7. [DOI] [PubMed] [Google Scholar]
- 10.Kim BJ, Nah SY, Jeon JH, So I, Kim SJ. Transient receptor potential melastatin 7 channels are involved in ginsenoside Rg3-induced apoptosis in gastric cancer cells. Basic Clin Pharmacol Toxicol. 2011;109:233–239. doi: 10.1111/j.1742-7843.2011.00706.x. [DOI] [PubMed] [Google Scholar]
- 11.Han S, Kim JS, Jung BK, Han SE, Nam JH, Kwon YK, Nah SY, Kim BJ. Effects of ginsenoside on pacemaker potentials of cultured interstitial cells of Cajal clusters from the small intestine of mice. Mol Cells. 2012;33:243–249. doi: 10.1007/s10059-012-2204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 13.Wang BJ, Won SJ, Yu ZR, Su CL. Free radical scavenging and apoptotic effects of Cordyceps sinensis fractionated by supercritical carbon dioxide. Food Chem Toxicol. 2005;43:543–552. doi: 10.1016/j.fct.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Hotz MA, Gong J, Traganos F, Darzynkiewicz Z. Flow cytometric detection of apoptosis: comparison of the assays of in situ DNA degradation and chromatin changes. Cytometry. 1994;15:237–244. doi: 10.1002/cyto.990150309. [DOI] [PubMed] [Google Scholar]
- 15.Vermes I, Haanen C, Reutelingsperger C. Flow cytometry of apoptotic cell death. J Immunol Methods. 2000;243:167–190. doi: 10.1016/s0022-1759(00)00233-7. [DOI] [PubMed] [Google Scholar]
- 16.Fleig A, Penner R. Emerging roles of TRPM channels. Novartis Found Symp. 2004;258:248–258. [PubMed] [Google Scholar]
- 17.Harteneck C, Plant TD, Schultz G. From worm to man: three subfamilies of TRP channels. Trends Neurosci. 2000;23:159–166. doi: 10.1016/s0166-2236(99)01532-5. [DOI] [PubMed] [Google Scholar]
- 18.Montell C. Physiology, phylogeny, and functions of the TRP superfamily of cation channels. Sci STKE. 2001;2001:re1. doi: 10.1126/stke.2001.90.re1. [DOI] [PubMed] [Google Scholar]
- 19.Monteilh-Zoller MK, Hermosura MC, Nadler MJ, Scharenberg AM, Penner R, Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol. 2003;121:49–60. doi: 10.1085/jgp.20028740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82:429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- 21.Hanano T, Hara Y, Shi J, Morita H, Umebayashi C, Mori E, Sumimoto H, Ito Y, Mori Y, Inoue R. Involvement of TRPM7 in cell growth as a spontaneously activated Ca2+ entry pathway in human retinoblastoma cells. J Pharmacol Sci. 2004;95:403–419. doi: 10.1254/jphs.fp0040273. [DOI] [PubMed] [Google Scholar]
- 22.He Y, Yao G, Savoia C, Touyz RM. Transient receptor potential melastatin 7 ion channels regulate magnesium homeostasis in vascular smooth muscle cells: role of angiotensin II. Circ Res. 2005;96:207–215. doi: 10.1161/01.RES.0000152967.88472.3e. [DOI] [PubMed] [Google Scholar]
- 23.Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- 24.Krapivinsky G, Mochida S, Krapivinsky L, Cibulsky SM, Clapham DE. The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron. 2006;52:485–496. doi: 10.1016/j.neuron.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 25.Su D, May JM, Koury MJ, Asard H. Human erythrocyte membranes contain a cytochrome b561 that may be involved in extracellular ascorbate recycling. J Biol Chem. 2006;281:39852–39859. doi: 10.1074/jbc.M606543200. [DOI] [PubMed] [Google Scholar]
- 26.Kim BJ, Lim HH, Yang DK, Jun JY, Chang IY, Park CS, So I, Stanfield PR, Kim KW. Melastatin-type transient receptor potential channel 7 is required for intestinal pacemaking activity. Gastroenterology. 2005;129:1504–1517. doi: 10.1053/j.gastro.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Wykes RC, Lee M, Duffy SM, Yang W, Seward EP, Bradding P. Functional transient receptor potential melastatin 7 channels are critical for human mast cell survival. J Immunol. 2007;179:4045–4052. doi: 10.4049/jimmunol.179.6.4045. [DOI] [PubMed] [Google Scholar]
- 28.Abed E, Moreau R. Importance of melastatin-like transient receptor potential 7 and cations (magnesium, calcium) in human osteoblast-like cell proliferation. Cell Prolif. 2007;40:849–865. doi: 10.1111/j.1365-2184.2007.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong H, Cui CH, Kim JK, Jin FX, Kim SC, Im WT. Enzymatic biotransformation of ginsenoside Rb1 and gypenoside XVII into ginsenosides Rd and F2 by recombinant β-glucosidase from Flavobacterium johnsoniae. J Ginseng Res. 2012;36:418–424. doi: 10.5142/jgr.2012.36.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quan LH, Piao JY, Min JW, Kim HB, Kim SR, Yang DU, Yang DC. Biotransformation of ginsenoside Rb1 to prosapogenins, gypenoside XVII, ginsenoside Rd, ginsenoside F2, and compound K by Leuconostoc mesenteroides DC102. J Ginseng Res. 2011;35:344–351. doi: 10.5142/jgr.2011.35.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun HS, Jackson MF, Martin LJ, Jansen K, Teves L, Cui H, Kiyonaka S, Mori Y, Jones M, Forder JP, et al. Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat Neurosci. 2009;12:1300–1307. doi: 10.1038/nn.2395. [DOI] [PubMed] [Google Scholar]
- 32.Ye R, Li N, Han J, Kong X, Cao R, Rao Z, Zhao G. Neuroprotective effects of ginsenoside Rd against oxygen-glucose deprivation in cultured hippocampal neurons. Neurosci Res. 2009;64:306–310. doi: 10.1016/j.neures.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Li N, Liu B, Dluzen DE, Jin Y. Protective effects of ginsenoside Rg2 against glutamate-induced neurotoxicity in PC12 cells. J Ethnopharmacol. 2007;111:458–463. doi: 10.1016/j.jep.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 34.Wu J, Jeong HK, Bulin SE, Kwon SW, Park JH, Bezprozvanny I. Ginsenosides protect striatal neurons in a cellular model of Huntington’s disease. J Neurosci Res. 2009;87:1904–1912. doi: 10.1002/jnr.22017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye R, Zhang X, Kong X, Han J, Yang Q, Zhang Y, Chen Y, Li P, Liu J, Shi M, et al. Ginsenoside Rd attenuates mitochondrial dysfunction and sequential apoptosis after transient focal ischemia. Neuroscience. 2011;178:169–180. doi: 10.1016/j.neuroscience.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Ye R, Yang Q, Kong X, Han J, Zhang X, Zhang Y, Li P, Liu J, Shi M, Xiong L, et al. Ginsenoside Rd attenuates early oxidative damage and sequential inflammatory response after transient focal ischemia in rats. Neurochem Int. 2011;58:391–398. doi: 10.1016/j.neuint.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Xia J, Wang L, Song Y, Yang J, Yan Y, Ren H, Zhao G. Efficacy and safety of ginsenoside-Rd for acute ischaemic stroke: a randomized, double-blind, placebo-controlled, phase II multicenter trial. Eur J Neurol. 2009;16:569–575. doi: 10.1111/j.1468-1331.2009.02534.x. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Zhou L, Zhang X, Bai J, Shi M, Zhao G. Ginsenoside-Rd attenuates TRPM7 and ASIC1a but promotes ASIC2a expression in rats after focal cerebral ischemia. Neurol Sci. 2012;33:1125–1131. doi: 10.1007/s10072-011-0916-6. [DOI] [PubMed] [Google Scholar]
- 39.Hao M, Wang W, Zhao Y, Zhang R, Wang H. Pharmacokinetics and tissue distribution of 25-hydroxyprotopanaxadiol, an anti-cancer compound isolated from Panax ginseng, in athymic mice bearing xenografts of human pancreatic tumors. Eur J Drug Metab Pharmacokinet. 2011;35:109–113. doi: 10.1007/s13318-010-0022-9. [DOI] [PubMed] [Google Scholar]
- 40.Li B, Zhao J, Wang CZ, Searle J, He TC, Yuan CS, Du W. Ginsenoside Rh2 induces apoptosis and paraptosis-like cell death in colorectal cancer cells through activation of p53. Cancer Lett. 2011;301:185–192. doi: 10.1016/j.canlet.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He BC, Gao JL, Luo X, Luo J, Shen J, Wang L, Zhou Q, Wang YT, Luu HH, Haydon RC, et al. Ginsenoside Rg3 inhibits colorectal tumor growth through the down-regulation of Wnt/β-catenin signaling. Int J Oncol. 2011;38:437–445. doi: 10.3892/ijo.2010.858. [DOI] [PubMed] [Google Scholar]