In this study, the monocyte/macrophage (Mø) immunophenotype and mesenchymal stromal/stem cell (MSC) differentiation were examined with regard to established and experimental collagen-based biomaterials for MSC entrapment. Important three-way interactions were elucidated between Møs, MSCs, and encapsulating biomatrices, whose combined influences dictated the Mø immunophenotype and MSC multipotency, which are critical for developing effective MSC-biomaterial strategies for promoting tissue regeneration and healing.
Keywords: Cell biology, Multipotential differentiation, Monocyte, Mesenchymal stem cells
Abstract
Bone marrow mesenchymal stromal/stem cell (MSC) encapsulation within a biomatrix could improve cellular delivery and extend survival and residence time over conventional intravenous administration. Although MSCs modulate monocyte/macrophage (Mø) immunophenotypic properties, little is known about how such interactions are influenced when MSCs are entrapped within a biomaterial. Furthermore, the impact of the cell-encapsulating matrix on MSC multipotency and on Møs, which infiltrate biomaterials, remains poorly understood. Here we elucidate this three-way interaction. The Mø immunophenotype and MSC differentiation were examined with regard to established and experimental collagen-based biomaterials for MSC entrapment. Tumor necrosis factor-α secretion was acutely inhibited at 4 days. MSCs cocultured with Møs demonstrated attenuated chondrocyte differentiation, whereas osteoblast differentiation was enhanced. Adipocyte differentiation was considerably enhanced for MSCs entrapped within the gelatin/polyethylene glycol-based matrix. A better understanding of the effect of cell encapsulation on differentiation potency and immunomodulation of MSCs is essential for MSC-based, biomaterial-enabled therapies.
Introduction
In addition to mesenchymal stromal/stem cells' (MSCs') intrinsic regenerative, angiogenic, and tissue-repair properties, MSCs' immunomodulatory effect on innate and adaptive immune cells has been subject to extensive investigation [1]. Although studies have successfully coaxed MSCs down specific cell lineages in vitro, the lack of spatial and differentiation control remains a major confounding factor when they are administered in vivo [2, 3]. Recently, there is growing interest in combining MSCs with biomaterials to enhance their therapeutic potential. Biomaterials that encapsulate MSCs create a unique extracellular microenvironment that influences focal adhesion formation required to prolong MSC survival and function [4]. Biomatrices with tailored mechanical properties, incorporated exogenous growth factors, or specific functional groups conjugated to synthetic polyethylene glycol (PEG) polymer chains have been successfully used to guide MSC fate down specific differentiation pathways [5, 6]. However, assessment of MSC multidifferentiation potential has primarily been limited to MSCs cultured on two-dimensional substrates or has been applied after MSCs have grown out of or been chemically removed from the supporting biomatrix [7–9]. Moreover, little is known regarding monocyte/macrophage (Mø) influence on MSC fate when encapsulated within a three-dimensional biomatrix, which is an important interaction that must be considered when delivering MSCs to the inflammatory milieu of injured or diseased tissues.
Determining the Mø immunophenotype in response to the encapsulated allogeneic MSCs is critical, as it determines whether infiltrating macrophages will promote wound resolution or remain in a proinflammatory state. Møs respond to changes in adsorbed protein on the biomaterial surface, which influences adhesion, apoptosis, or foreign body giant cell formation, as well as secretion of extracellular matrix (ECM) proteins, growth factors, pro- and anti-inflammatory cytokines, matrix metalloproteinases/tissue inhibitor of metalloproteinases, and reactive oxygen species [10]. Depending on the overall phenotypic character, macrophages are generally defined as being classically activated (M1) or alternatively activated (M2). M1 macrophages secrete high amounts of proinflammatory cytokines and efficiently destroy microorganisms, whereas M2 macrophages secrete high levels of interleukin-10 (IL-10) (an anti-inflammatory cytokine), demonstrate high phagocytic activity, promote angiogenesis and tissue remodeling/repair, and modulate the immune system [11]. Dynamic Mø phenotypic switching from a resting M2 state to a proinflammatory M1 state is implicated in acute injury and chronic disease [12–15]. The host foreign body reaction must also be considered, as it could adversely affect the overall biocompatibility of the biomatrix and prevent healing [16, 17]. Here we address this three-way interaction by elucidating the Mø immunophenotype and determining MSC differentiation potential when encapsulated within three-dimensional collagen-based biomaterials.
Materials and Methods
MSC Isolation and Culture
Mesenchymal stem cells were isolated from leftover bone marrow harvest filters from healthy donors after obtaining written consent approved by the University of Wisconsin Hospital and Clinics Regulatory Committee. Bone marrow cells isolated from the filter were washed in phosphate-buffered saline (PBS) (Cellgro Mediatech Inc., Manassas, VA, http://www.cellgro.com), and mononuclear cells were separated using Ficoll-Paque Premium (ρ = 1.073; GE Healthcare, Little Chalfont, U.K., http://www.gehealthcare.com) and a Leucosep tube (Greiner Bio-One, Monroe, NC, http://www.gbo.com/en) according to the manufacturers' protocols. Red blood cells were lysed after incubation in Ammonium-Chloride-Potassium lysis buffer (Invitrogen, Eugene, OR, http://www.invitrogen.com) lysis buffer (3 minutes), and mononuclear cells were plated in T-75-cm2 tissue culture flasks (Techno Plastic Products, Trasadingen, Switzerland, http://www.tpp.ch) (precoated with gelatin; Sigma-Aldrich, St. Louis, MO, http://sigmaaldrich.com) and cultured in α-minimum essential media supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan, UT, http://www.hyclone.com), nonessential amino acids (1×) (Invitrogen), and 4 mM l-glutamine (Invitrogen). Adherent bone marrow-derived MSCs were cultured with medium changes every 3–5 days until 90% confluence was attained. TrypLE cell dissociation enzyme (Invitrogen) was used to harvest (passage 0) MSCs, which were then replated into new T-75-cm2 flasks with subsequent expansion, harvest (using 0.05% trypsin; Invitrogen), and cryopreservation. Isolated MSCs (passage 4) were positive for MSC surface markers CD29-phycoerythrin (PE), CD44-PE, CD54-PE, CD73-PE, CD90-allophycocyanin (APC) (BD Biosciences, San Jose, CA, http://www.bdbiosciences.com), and CD105-APC (eBiosciences Inc., San Diego, CA, http://www.ebiosciences.com) and were negative for hematopoietic markers CD34-fluorescein isothiocyanate (FITC), CD45-PE, and CD31-PE (BD Biosciences) using monoclonal antibodies (with appropriate gating controls) and analyzed using a FACSCalibur flow cytometer with CellQuest acquisition software (BD Biosciences) and FlowJo software (Tree Star, Ashland, OR, http://www.treestar.com) [18]. MSC multidifferentiation potential into adipocytes, chondrocytes, and osteoblasts was tested by culturing in Adipo NHdiff medium (14 days), Chondro NHdiff (14 days), and Osteo NHdiff medium (10 days) in 48-well (BD Biosciences) culture plates (Miltenyi Biotec, Auburn, CA, http://www.miltenyibiotec.com) with successive medium changes every 3 days. Only MSCs (4–6 passages) that met the aforementioned criteria were used for the subsequent coculture studies.
Gelatin/Polyethylene Glycol and Collagen-MSC Encapsulation
The gelatin/polyethylene glycol biomatrix is a covalently cross-linked network composed of PEG (Sigma-Aldrich) and type B gelatin (Sigma-Aldrich) originally developed by our laboratory for wound healing applications and encapsulation of keratinocytes and fibroblasts [19]. Gelatin/polyethylene glycol biomatrices were constructed by adding Irgacure 2959 (0.5%) (BASF, Ludwigshafen, Germany) photoinitiator sterile-filtered in phosphate buffered saline and mixed with Cys-PEG-gelatin to form a 20% (wt/vol) solution. PEG diacrylate (PEGda) was also sterile-filtered with Irgacure 2959 to make a 20% (wt/vol) solution. A 3:1 ratio of Cys-PEG-gelatin to PEGda and a bone marrow-derived MSC suspension were thoroughly mixed to obtain a final concentration of 1 × 106 MSCs per cm3. The precursor solution was pipetted into a glass-bottomed Petri dish (In Vitro Scientific, Sunnyvale, CA, http://www.invitrosci.com) with a circular insert and then polymerized by exposing to UV light (λmax = 365 nm, 100 W/cm2) for 2 minutes. MSC medium (Dulbecco's modified Eagle's medium [DMEM] [Cellgro Mediatech], 10% FBS, 2 mM l-glutamine, and 2 mM nonessential amino acids) was added to the tissue culture Petri dish, and the hydrogels were swollen overnight. Rat-tail collagen (5 mg/ml; BD Biosciences) was mixed with phosphate buffered saline (10×), DMEM, and 1 N NaOH (Sigma-Aldrich) to obtain a neutralized solution. The collagen solution was allowed to nucleate on ice for 35 minutes before adding a cell suspension to obtain a concentration of 1 × 106 MSCs per cm3. The collagen solutions were pipetted into a tissue culture well plate (48-well plate) and allowed to polymerize for 15 minutes (37°C) before MSC medium was added.
Mø Isolation
Venipuncture was performed using a 19-gauge butterfly needle (Fisher Scientific, Fair Lawn, NJ, http://www.fischersci.com) on a registered donor after obtaining written consent approved by the University of Wisconsin Hospital and Clinics Regulatory Committee. Sixty milliliters of whole blood was drawn into a syringe containing 3 ml of sodium citrate (Sigma-Aldrich), diluted in 15 ml of Dulbecco's phosphate-buffered saline (Cellgro Mediatech)/5 mM EDTA (Fisher Scientific) (DPBSE), and centrifuged at 400g for 20 minutes in Leucosep tubes containing Ficoll Paque Premium. The resulting Mø/lymphocyte/platelet band was collected in 15-ml volumes, diluted with 35 ml of DPBSE, and then centrifuged at 150g for 10 minutes. The cell pellet was resuspended in DPBSE and centrifuged again at the previous time and speed two more times. The cell pellet was then resuspended in Iscove's modified Dulbecco's medium (IMDM) (Cellgro Mediatech) (without phenol red) at approximately 1–2 × 106 Mø per milliliter, and then 25 ml of 46% Percoll/IMDM solution (with phenol red) was slowly underlayered using a spinal needle, which formed a bilayer solution that was then centrifuged at 550g for 30 minutes. The Mø band was collected in 15-ml volumes diluted with 35 ml of DPBSE, centrifuged at 400g for 10 minutes, and then resuspended for the coculture studies [20, 21]. The Møs were assessed for purity using CD14-PE (AbD Serotec, Raleigh, NC, http://www.abdserotec.com) and CD45-FITC (BD Biosciences) monoclonal antibodies with appropriate monoculture controls, resulting in ∼80%–90% monocyte purity, with the primary contaminant being lymphocytes using flow cytometry analysis.
MSC-Mø Coculture
MSCs encapsulated in gelatin/polyethylene glycol-based matrices (1 × 106 MSCs per cm3) were transferred to 12-well plate Transwell polyester permeable supports (Corning Inc., Corning, NY, http://www.corning.com/lifesciences) and then supplemented with the normal MSC (Dulbecco's modified Eagle's medium, 10% FBS, 2 mM l-glutamine, 2 mM nonessential amino acids) or the aforementioned differentiation media (same culture conditions for collagen). In the coculture and the Mø-monoculture conditions, Møs were seeded on either gelatin/polyethylene glycol or collagen hydrogels at 1 × 106 Møs per well. Samples from three different whole blood donors and three different primary MSC donors were consistently paired in order to appropriately account for donor-to-donor variability present in quantified protein concentrations of tumor necrosis factor-α (TNF-α), IL-6, IL-10, and IL-12. Supernatant samples were also collected after 1 and 4 days and centrifuged at 10,000g for 10 minutes, and 50 μl of protein sample was subsequently analyzed by Bio-Plex protein detection (Bio-Rad, Hercules, CA, http://www.bio-rad.com) of TNF-α, IL-6, IL-10, and IL-12 following the manufacturer's instructions for all culture conditions. Using the same culture wells, additional medium changes were performed until the differentiation end points were met for subsequent detection.
Differentiation and Detection of Encapsulated MSCs
Encapsulated MSCs cultured in osteogenic media were detected at 10 days, whereas MSCs cultured in standard MSC, adipogenic, and chondrogenic media (Miltenyi Biotec) were detected at 14 days. All components came from Sigma-Aldrich unless stated otherwise. All encapsulated MSCs were fixed in 10% neutral buffered formalin overnight. Adipogenic differentiated MSC hydrogels were washed in PBS, dehydrated in 20% sucrose/PBS solution overnight, and then dehydrated further in a 1:1 ratio of 20% sucrose/PBS and O.C.T. (Optimal Cutting Temperature) for 3 additional days. The adipocyte-differentiated MSC hydrogels were frozen in liquid nitrogen and cryosectioned (10 μm) prior to staining. All other MSC biomatrices were paraffin-embedded after formalin fixation and sectioned (10 μm). For adipogenic detection, sections were refixed in 10% neutral buffered formalin with 1% calcium chloride, rehydrated in double-distilled H2O (ddH2O), and then placed in propylene glycol (50%, 100%), each for 2 minutes. The sections were then stained in a 1% Oil Red O/propylene glycol solution for 40 minutes (40°C) and then subjected to more propylene glycol washes (100%, 80%, 20%), each for 2 minutes with agitation. The sections were rinsed twice in ddH2O, counterstained with Mayer's hematoxylin solution for 1 minute, washed for 5 minutes in running tap water, and coverslipped in aqueous based permanent mounting medium (Sigma-Aldrich). For chondrogenic differentiation, sections were deparaffinized by placing them in successive xylene and ethanol washes (100%, 90%, and 70%, each 10 minutes long) before washing them in ddH2O. The sections were then counterstained with 0.02% Fast Green FCF for 3 minutes and then 1% acetic acid for 30 seconds and 1% Safranin O for 5 minutes. Subsequently, the sections were quickly washed in ddH2O and dehydrated with ethanol and xylene washes (10 dips each) before the sections were mounted. Chondrocyte differentiation was also detected by immunostaining for aggrecan, a proteoglycan highly expressed in cartilage tissue. The sections were deparaffinized in three xylene washes, each for 5 minutes, and hydrated through graded ethanol (EtOH) treatment to ddH2O. The sections were then permeabilized in PBS with 0.1% Triton X-100 (Sigma-Aldrich) for 45 minutes and then blocked with 10% donkey serum for 30 minutes. Mouse anti-human aggrecan primary antibody (Millipore, Billerica, MA, http://www.millipore.com) was incubated overnight in PBS (1:200) containing 1% donkey serum and 0.1% Triton X-100 at 4°C. The sections were subsequently washed three times in PBS for 5 minutes with agitation and then reacted with donkey anti-mouse Alexa Fluor 555 (Invitrogen) in PBS (1:400) for 30 minutes. The sections were similarly washed in PBS, washed in ddH2O for 5 minutes with agitation, and mounted with Prolong Gold Antifade Reagent (Invitrogen) containing 4′,6-diamidino-2-phenylindole (DAPI) [22]. Images were taken with a Nikon fluorescent microscope (Nikon, Melville, NY, http://www.nikon.com) at ×100 magnification. For osteogenic detection, sections were deparaffinized in the same manner as the Safranin O staining and washed in ddH2O. The sections were then stained in 2% alizarin red S solution for 5 minutes, washed in ddH2O, counterstained in Mayer's hematoxylin for 1 minute, and quickly rinsed in tap water. Subsequently, sections were dehydrated in acetone, acetone-xylene (1:1), and xylene (20 dips each) before being coverslipped in CYTO-Seal xylene-based mounting medium (Andwin Scientific, Schaumburg, IL, http://www.andwinsci.com). Osteogenic differentiation was also detected using the von Kossa method for staining minerals. The sections were again deparaffinized and hydrated to ddH2O. The slides were then placed in 5% silver nitrate solution for 1 hour, rinsed four times with ddH2O, and placed in photographic black and white developer solution (Kodak, Rochester, NY, http://www.kodak.com) for 2 minutes. The sections were quickly rinsed in two ddH2O washes, placed in 5% sodium thiosulfate for 5 minutes, and washed in running tap water for 2 minutes. The sections were then counterstained in nuclear fast solution for 5 minutes and again washed in running tap water. The sections were then washed twice in 90% EtOH, 100% EtOH, and xylene each for 2 minutes and similarly coverslipped in CYTO-Seal xylene-based mounting medium (Andwin Scientific). Three images of the stained slides were taken at ×100 magnification with a Leica DM LB microscope (Leica Microsystems, Wetzlar, Germany, http://www.leica.com) for each culture condition.
Statistical Analysis
Each biomatrix (collagen or gelatin/polyethylene glycol) and culture condition was tested with three different primary MSC and Mø blood donors for independent replicates (n = 3) that were consistently paired for the coculture conditions. One-way analysis of variance and a two-tailed Student's t test was used to compare differences in TNF-α, IL-6, IL-10, and IL-12 expression among Mø monoculture, Mø/MSC coculture, and MSC monoculture conditions for the two separate time points. A p value <.05 was considered statistically significant.
Results
MSC Biomatrix Downregulates Mø Proinflammatory Function
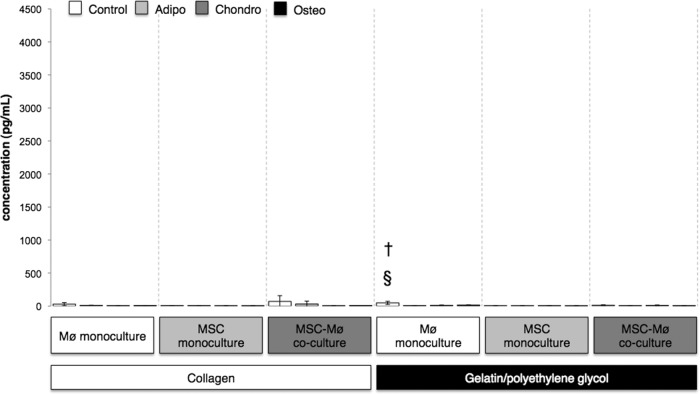
To elucidate the dynamic changes in Mø immunophenotype in response to the biomatrix-MSCs, supernatant samples were collected after 1 and 4 days from each differentiation medium (control, adipocyte, chondrocyte, or osteoblast), biomaterial (collagen or gelatin/polyethylene glycol), and culture condition (MSC monoculture, Mø monoculture, or MSC-Mø coculture). A multiplexed microsphere-based immunoassay was executed for human cytokine detection (human cytokine group I; Bio-Rad) of TNF-α, IL-6, IL-10, and IL-12 [23]. After 4 days of culture, the MSC-Mø coculture cytokine expression profile was generally IL-6high, IL-10high, TNF-αlow, and IL-12low (Figs. 1, 2; supplemental online Figs. 2, 4) for both materials and for all medium types similar to the MSC-educated macrophage immunophenotype described in previous MSC-Mø coculture studies [24]. At day 1, high TNF-α expression (2,000 to 700 pg/ml) was observed for Mø monoculture and Mø-MSC coculture conditions (p < .05) on the gelatin/polyethylene glycol biomaterial (except for adipocyte differentiation media conditions) (Fig. 3); however, by day 4, TNF-α expression (<75 pg/ml) was significantly attenuated for all conditions (Fig. 1). This trend was consistent with previous observations in our laboratory using a semi-interpenetrating network composed of physically entangled polyethylene glycol and gelatin macromolecules [25]. For the Mø monoculture gelatin/polyethylene glycol condition, temporal variations in the type, composition, and concentration of adsorbed protein can contribute to changes in macrophage phenotypic behavior causing lowered TNF-α expression observed at 4 days [26, 27]. MSCs entrapped within gelatin/polyethylene glycol respond to potentially lethal TNF-α levels via a nuclear factor κβ (NF-κB) mechanism that causes several anti-inflammatory factors to be secreted (TNF-α stimulated gene-6 protein, interleukin-1 receptor antagonist, prostaglandin E2, and stanniocalcin-1) that downregulate TNF-α expression and direct adherent macrophages toward an M2 alternatively activated state [28–30]. Indeed, IL-10 concentrations were positively correlated with high TNF-α secretion for Mø-gelatin/polyethylene glycol monoculture and MSC/Mø-gelatin/polyethylene glycol coculture conditions at day 1 (supplemental online Fig. 1), but by day 4 IL-10 slightly decreased (supplemental online Fig. 2), whereas TNF-α expression was significantly attenuated (Fig. 1). Systemic release of TNF-α has been shown to engage macrophages to produce IL-10 among other mitigating factors as a negative feedback mechanism to turn off the NF-κB pathway and inhibit continued proinflammatory cytokine secretion, which may account for the delayed reduction in TNF-α expression [31]. IL-6 expression was variable at 1 day (Fig. 4) and 4 days (Fig. 2), but overall it remained high over the time of culture for almost all conditions and may have also contributed to the time-dependent decrease in TNF-α expression (Fig. 1) caused by the downregulation of the NF-κB pathway in monocyte/macrophages [32, 33].
Figure 1.
Gelatin/polyethylene glycol and gelatin/polyethylene glycol-MSC conditions attenuated tumor necrosis factor-α (TNF-α) expression at day 4. Bars indicate the expression of TNF-α (pg/ml) after 4 days for MSCs encapsulated in collagen or gelatin/polyethylene glycol cultured alone or in the presence of biomaterial-adherent Møs (also under Mø monoculture conditions). Differentiation media were from Miltenyi Biotec. §, Significantly greater than MSC monoculture gelatin/polyethylene glycol; †, significantly greater than Mø-MSC coculture gelatin/polyethylene glycol. Statistical comparisons were made only among the same medium conditions (i.e., Control, Adipo, Chondro, Osteo). Values represent mean ± SD of three wells from three separate donors, which were paired for the MSC-Mø coculture conditions. §, †, p < .05 was considered statistically significant using the two-tailed Student's t test. Control: Standard MSC medium (Dulbecco's modified Eagle's medium, 10% fetal bovine serum, 2 mM l-glutamine, 2 mM nonessential amino acids). Abbreviations: Adipo, Adipo NH differentiation medium; Chondro, Chondro NH differentiation medium; Mø, monocyte/macrophage; MSC, mesenchymal stromal/stem cell; Osteo, Osteo NH differentiation medium.
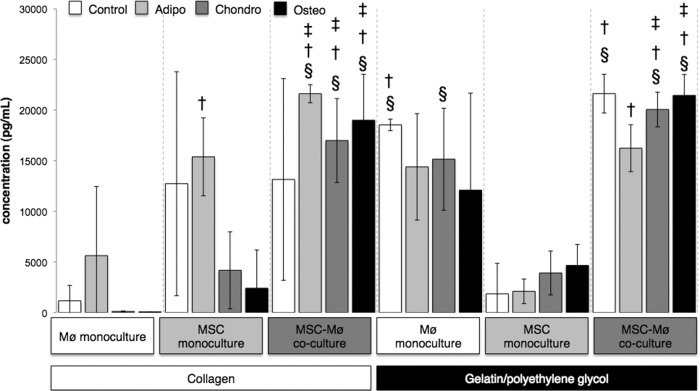
Figure 2.
Interleukin-6 (IL-6) expression varied greatly among culture conditions at day 4. Bars indicate the expression of IL-6 (pg/ml) after 4 days for MSCs encapsulated in collagen or gelatin/polyethylene glycol cultured alone or in the presence of biomaterial-adherent Møs (also under Mø monoculture conditions). Differentiation media were from Miltenyi Biotec. Open bars (Control): Standard MSC medium (Dulbecco's modified Eagle's medium, 10% fetal bovine serum, 2 mM l-glutamine, 2 mM nonessential amino acids). §, Significantly greater than Mø monoculture collagen; †, significantly greater than Mø monoculture gelatin/polyethylene glycol; ‡, significantly greater than MSC monoculture gelatin/polyethylene glycol. Light gray bars (Adipo): §, Significantly greater than Mø monoculture collagen; †, significantly greater than MSC monoculture gelatin/polyethylene glycol. Dark gray bars (Chondro): §, Significantly greater than Mø monoculture collagen; †, significantly greater than MSC monoculture collagen; ‡, significantly greater than Mø monoculture gelatin/polyethylene glycol; *, significantly greater than MSC monoculture gelatin/polyethylene glycol. Black bars (Osteo): §, Significantly greater than Mø monoculture collagen; †, significantly greater than MSC monoculture collagen; ‡, significantly greater than MSC monoculture gelatin/polyethylene glycol. Statistical comparisons were made only among the same medium conditions (i.e., Control, Adipo, Chondro, Osteo). Values represent mean ± SD of three wells from three separate donors, which were paired for the MSC-Mø coculture conditions. §, †, ‡, *, p < .05 was considered statistically significant using the two-tailed Student's t test. Abbreviations: Adipo, Adipo NH differentiation medium; Chondro, Chondro NH differentiation medium; Mø, monocyte/macrophage; MSC, mesenchymal stromal/stem cell; Osteo, Osteo NH differentiation medium.
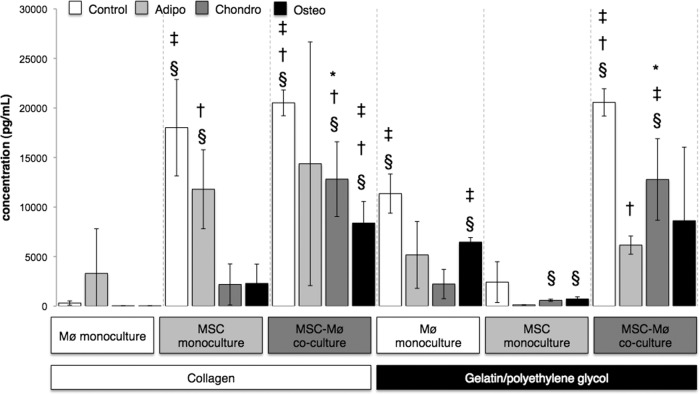
Figure 3.
Gelatin/polyethylene glycol enhanced tumor necrosis factor-α (TNF-α) expression at day 1. Bars indicate the expression of TNF-α (pg/ml) after 1 day for MSCs encapsulated in collagen or gelatin/polyethylene glycol cultured alone or in the presence of biomaterial adherent Møs (also under Mø monoculture conditions). Differentiation media were from Miltenyi Biotec. Open bars (Control): Standard MSC medium (Dulbecco's modified Eagle's medium, 10% fetal bovine serum, 2 mM l-glutamine, 2 mM nonessential amino acids). §, Significantly greater than Mø monoculture collagen; †, significantly greater than MSC monoculture collagen; ‡, significantly greater than MSC-Mø coculture collagen; *, significantly greater than MSC monoculture gelatin/polyethylene glycol. Statistical comparisons were made only among the same medium conditions (i.e., Control, Adipo, Chondro, Osteo). Values represent mean ± SD of three wells from three separate donors, which were paired for the MSC-Mø coculture conditions. §, †, ‡, *, p < .05 was considered statistically significant using the two-tailed Student's t test. Abbreviations: Adipo, Adipo NH differentiation medium; Chondro, Chondro NH differentiation medium; Mø, monocyte/macrophage; MSC, mesenchymal stromal/stem cell; Osteo, Osteo NH differentiation medium.
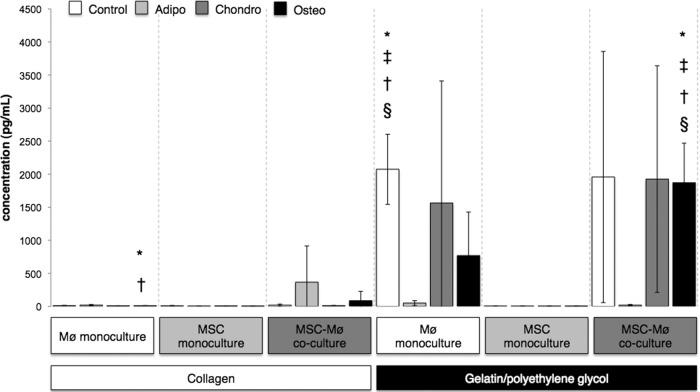
Figure 4.
Interleukin-6 (IL-6) expression varied greatly among culture conditions at day 1. Bars indicate the expression of IL-6 (pg/ml) after 1 day for MSCs encapsulated in collagen or gelatin/polyethylene glycol cultured alone or in the presence of biomaterial-adherent Møs (also under Mø monoculture conditions). Differentiation media were from Miltenyi Biotec. Open bars (Control): Standard MSC medium (Dulbecco's modified Eagle's medium, 10% fetal bovine serum, 2 mM l-glutamine, 2 mM nonessential amino acids). §, Significantly greater than Mø monoculture collagen; †, significantly greater than MSC monoculture gelatin/polyethylene glycol. Light gray bars (Adipo): §, Significantly greater than Mø monoculture collagen; †, significantly greater than MSC monoculture gelatin/polyethylene glycol; ‡, significantly greater than Mø monoculture gelatin/polyethylene glycol. Dark gray bars (Chondro): §, Significantly greater than Mø monoculture collagen; †, significantly greater than MSC monoculture collagen; ‡, significantly greater than MSC monoculture gelatin/polyethylene glycol. Black bars (Osteo): §, Significantly greater than Mø monoculture collagen; †, significantly greater than MSC monoculture collagen; ‡, significantly greater than MSC monoculture gelatin/polyethylene glycol. Statistical comparisons were made only among the same medium conditions (i.e., Control, Adipo, Chondro, Osteo). Values represent mean ± SD of three wells from three separate donors, which were paired for the MSC-Mø coculture conditions. §, †, ‡, *, p < .05 was considered statistically significant using the two-tailed Student's t test. Abbreviations: Adipo, Adipo NH differentiation medium; Chondro, Chondro NH differentiation medium; Mø, monocyte/macrophage; MSC, mesenchymal stromal/stem cell; Osteo, Osteo NH differentiation medium.
IL-12 expression was consistently low (6–10 pg/ml) at both 1 day (supplemental online Fig. 3) and 4 days (supplemental online Fig. 4) for all culture conditions. The minimal IL-12 expression in both Mø monoculture and MSC-Mø coculture indicates the lack of immunogenicity of the biomatrix or the MSC biomatrix, as high IL-12 expression is a marker for monocyte maturation into dendritic cells upon antigen stimulation. The high IL-6 concentration observed for all culture conditions (Figs. 2, 4) most likely contributed to low IL-12 expression (supplemental online Figs. 3, 4) because of the inhibitory effect that IL-6 has on dendritic cell maturation, which biases monocyte differentiation toward a macrophage phenotype [34].
Adherent Møs and Biomatrix Regulate MSC Multipotency
Collagen or gelatin/polyethylene glycol encapsulated MSCs were exposed to established adipocyte (Adipo NHdiff) or chondrocyte (Chondro NHdiff) differentiation media for 14 days and osteoblast (Osteo NHdiff) differentiation media for 10 days (Miltenyi Biotec). MSC biomatrices were sectioned and detected for adipocyte differentiation using Oil Red O staining (lipid vacuoles), chondrocyte differentiation using Safranin O (glycosaminoglycans) and aggrecan immunostaining, and osteoblast differentiation using alizarin red S and von Kossa staining (calcium deposits). For chondrocyte detection, collagen and gelatin/polyethylene glycol encapsulated cocultured MSCs stained less strongly for Safranin O and demonstrated more extensive FCF green counterstaining (cytoplasmic stain), indicating partial inhibition of chondrocyte differentiation mediated by the presence of adherent Møs (Fig. 5A). Inhibition of chondrogenesis in the coculture was more readily apparent for the gelatin/polyethylene glycol condition, whereas for collagen coculture Safranin O expression displayed a more intermediate differentiation phenotype (purple cytoplasm). This observation is attributed to inherent differences between the gelatin/polyethylene glycol and collagen biomatrices, with three-dimensional collagen hydrogels being better able to support MSC chondrogenesis because of collagen's similarity to the ECM of native cartilage with elevated aggrecan and glycosaminoglycan expression when cultured with medium supplemented with transforming growth factor β (TGF-β) [35]. Similarly, aggrecan expression was more attenuated for the coculture conditions; however, greater differences in expression were observed for the gelatin/polyethylene glycol conditions (Fig. 5B). Greater fluorescent DAPI/aggrecan colocalization was observed for the gelatin/polyethylene glycol conditions, whereas for the collagen conditions aggrecan was less restricted to the pericellular space of the encapsulated MSCs, indicating active degradation and remodeling processes allowing aggrecan to diffuse within the collagen biomatrix [36].
Figure 5.
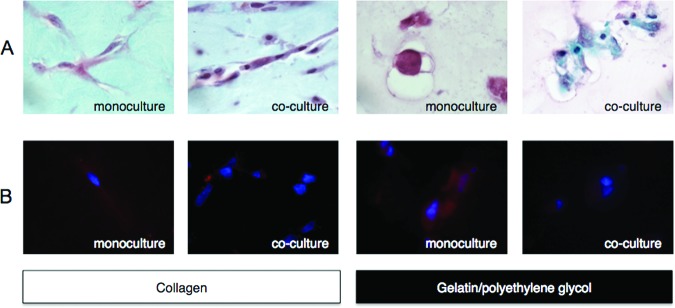
Monocytes/macrophages (Møs) attenuated MSC differentiation into chondrocytes. Collagen or gelatin/polyethylene glycol encapsulated MSCs were cultured alone or in the presence of biomaterial-adherent Møs. (A): MSCs cultured in chondrocyte differentiation medium for 14 days were detected with Safranin O and counterstained with FCF Green. (B): Chondrocyte differentiation was detected by aggrecan immunostaining (Alexa Fluor 555) and counterstained with 4′,6-diamidino-2-phenylindole. Representative images were taken at a magnification of ×100.
For osteoblast differentiation, collagen and gelatin/polyethylene glycol encapsulated cocultured MSCs demonstrated higher alizarin red S and von Kossa staining with less hematoxylin and Nuclear Fast Red counterstaining, respectively (Fig. 6A, 6B). The differences in calcium mineralization were less striking, however, for the monoculture and coculture gelatin/polyethylene glycol conditions. The enhanced mineralization observed is most likely due to the presence of cell-adhesion sites, the degradability of adjacent gelatin chains, and the overall porosity and stiffness of the gelatin/polyethylene glycol biomatrix, which suppresses MSC proliferation and promotes cell aggregation, further enhancing MSC differentiation down the osteoblast phenotype [19, 37–40].
Figure 6.
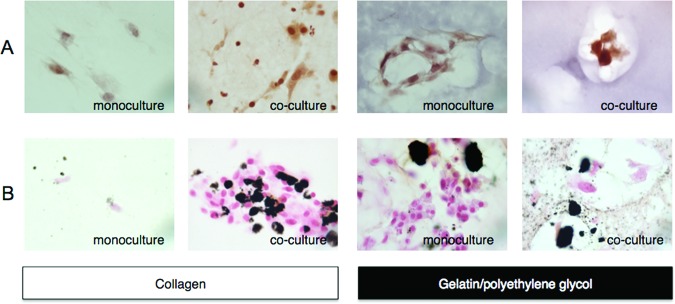
Monocytes/macrophages (Møs) enhanced MSC differentiation into osteoblasts. Collagen or gelatin/polyethylene glycol encapsulated MSCs were cultured alone or in the presence of biomaterial-adherent Møs. (A): MSCs cultured in osteoblast differentiation medium for 10 days were detected with alizarin red S and counterstained with Mayer's hematoxylin. (B): Osteoblast differentiation was also detected by von Kossa staining and counterstained with Nuclear Fast Red. Representative images were taken at a magnification of ×100.
In contrast to chondrocyte and osteoblast differentiation, adipocyte differentiation of MSCs did not appear to be significantly influenced by the presence of adherent macrophages in coculture (Fig. 7). The gelatin/polyethylene glycol biomatrix, however, more strongly drove MSC differentiation down the adipocyte phenotype, as demonstrated by elevated Oil Red O staining, whereas collagen encapsulated MSCs displayed smaller lipid vacuoles and stained more strongly with hematoxylin (cytoplasmic stain). The gelatin/polyethylene glycol biomatrix was particularly adipogenic as both material components have individually been shown to upregulate peroxisome proliferator-activated receptor γ expression and promote adipogenesis when supplemented with basic fibroblast growth factor. The polyethylene glycol chains also maintained the overall hydrogel structure and caused MSCs to adopt a rounded morphology that promoted adipocyte differentiation via downregulation of the RhoA-Rho-associated protein kinase signaling pathway [41]. IL-10 and IL-12 expression is described in supplemental online Figures 1–4.
Figure 7.
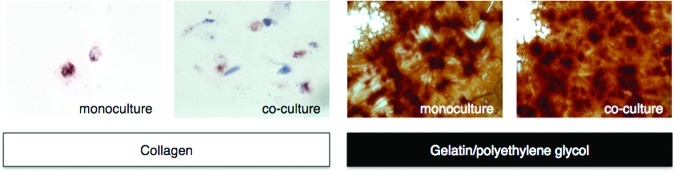
MSC differentiation into adipocytes was enhanced for the gelatin/polyethylene glycol biomatrix. Collagen or gelatin/polyethylene glycol encapsulated MSCs were cultured alone or in the presence of biomaterial-adherent monocytes/macrophages. MSCs cultured in adipocyte differentiation medium for 14 days were detected with Oil Red O and counterstained with Mayer's hematoxylin. Representative images were taken at a magnification of ×100.
Discussion
ECM-mimic biomaterials that incorporate MSCs are attractive platforms for tissue engineering as they provide a supportive three-dimensional microenvironment that could fill critical tissue defects, contain cell adhesive sequences that support MSC viability and function, and promote cell infiltration for ECM deposition and remodeling. Inclusion of localized growth factor concentrations and matrix metalloproteinase (MMP) cleavable peptide sequences within the biomaterial can also stimulate angiogenesis or MSC differentiation within the construct and promote tissue regeneration [42, 43]. Type I collagen is an ECM component commonly used for MSC encapsulation because of its low toxicity, weak immunogenicity, and favorable tissue remodeling properties. Purely ECM-based materials such as collagen are limited in their applicability as a stable MSC-encapsulating matrix for tissue regeneration because of their high batch-to-batch variability, poor mechanical properties, and significant degradation in vivo that often results in contraction or complete clearance of the cell-encapsulating construct [44, 45]. To overcome these challenges, a biomatrix containing gelatin (type B) and polyethylene glycol was developed. This strategy improved the overall mechanical stability and delayed gelatin degradation within the construct while presenting both MMP cleavable and cell adhesive peptide sequences such as arginine-glycine-aspartic acid, required for remodeling and focal adhesion formation of encapsulated cells, respectively [19]. Development of ECM-mimic biomaterials that properly couple the mechanical and biochemical characteristics of native MSC niches could better maintain MSC stemness and function long-term. This approach would allow MSCs to exert their immunomodulatory effects on infiltrating immune cells, promote tissue regeneration, and minimize fibrosis [46]. The behavior of infiltrating Møs typically present in the inflammatory microenvironment where MSCs would be delivered must not be overlooked, as this cell type can contribute to chronic inflammation and to foreign body reactions that lead to biomaterial failure and can influence MSC multipotency and function in vivo. Collagen and gelatin/polyethylene glycol-based matrices were selected to elucidate the Mø immunophenotype and encapsulated MSC multidifferentiation because of their favorable characteristics, mentioned above, for maintaining MSC viability and function.
The specific macrophage immunophenotype induced via interaction with the collagen or gelatin/polyethylene glycol biomatrix or the biomatrix-MSC combination was characterized as IL-6high, IL-10high, TNF-αlow, and IL-12low (Figs. 1, 2; supplemental online Figs. 2, 4). This macrophage immunophenotype is favorable for wound resolution, as IL-6 is critical for the progression of normal wound healing processes, whereas IL-10, among other factors, downregulates inflammation associated with excessive TNF-α and IL-12 expression [24, 47]. Similarly, encapsulation of MSCs within a biomatrix that maintains MSC survival and function long-term is expected to reprogram infiltrating macrophages toward an anti-inflammatory phenotype that promotes overall wound healing processes in vivo [48, 49]. The initial burst of TNF-α expression (Fig. 3) followed by a significant attenuation (Fig. 1) observed for gelatin/polyethylene glycol-adherent macrophages could be considered prohealing because TNF-α also stimulates macrophage growth factor production, which contributes to wound resolution. If TNF-α concentrations remain high long-term, re-epithelialization is inhibited and further ECM degradation prevents cell migration and collagen deposition, which is typically observed in chronic nonhealing wounds [50, 51]. When compared with local concentrations of TNF-α in chronic wound fluid of nonhealing diabetic ulcers (4,734 pg/ml), the in vitro concentrations of TNF-α observed in this study were significantly lower for all conditions, whereas the marked decrease in TNF-α expression by 4 days suggested that Møs are being actively reprogrammed toward a prohealing phenotype [52]. Overall, the time-dependent decrease in TNF-α expression for gelatin/polyethylene glycol adherent Møs in monoculture and coculture conditions illustrates both material- and MSC-mediated influences on the Mø immunophenotype. The lack of TNF-α expression for macrophage monoculture and MSC-macrophage coculture conditions may be due to the presence of additional factors included in the adipogenic medium. Dexamethasone, 3-isobutyl-1-methylxanthine, and indomethacin are commonly used to promote MSC differentiation into adipocytes; however, the exact concentrations of these factors are not disclosed because of supplier proprietary limitations (Miltenyi Biotec). For example, dexamethasone is a glucocorticoid with both anti-inflammatory and immunosuppressant properties that may have influenced macrophage behavior and inhibited TNF-α expression. Delineating the contribution of dexamethasone toward macrophage phenotypic behavior is difficult, however, because of the presence of additional factors commonly included in the adipogenic medium. Conversely, dexamethasone is often used in MSC-chondrocyte and -osteoblast differentiation media, which demonstrated elevated TNF-α expression at day 1 in contrast to MSC-adipocyte differentiation medium for macrophage monoculture or MSC-macrophage coculture in the presence of the gelatin/polyethylene glycol biomatrix [53].
The combined effect of the Mø immunophenotype and the biomatrix on MSC multipotency has not been thoroughly studied and warranted further investigation, as both can influence MSC fate when encapsulated and delivered to inflamed tissue damaged by trauma or disease. Variable differentiation was observed when encapsulated in collagen or the gelatin/polyethylene glycol biomatrix in coculture or monoculture. In addition to the material-dependent effect contributed by the collagen or gelatin/polyethylene glycol biomatrices, the expression of TNF-α and IL-6 also influences MSC multidifferentiation potential. For example, stimulation of the NF-κB pathway in MSCs by TNF-α was previously shown to prevent chondrogenesis in a dose-dependent manner by downregulating the expression of the TGF-β and SOX9 transcription factors, which effectively blocked aggrecan, collagen, and glycosaminoglycan secretion [54]. Synovial MSCs extracted from the joints of rheumatoid arthritis and osteoarthritis patients also displayed a decreased capacity to proliferate and differentiate into chondrocytes that was negatively correlated with the extent of inflammation as evaluated by the visual analog score [55, 56]. Mø secretion of TNF-α within injured or diseased joints represents a major obstacle toward MSC biomatrix therapies for cartilage regeneration, as MSC differentiation was attenuated for coculture conditions (Fig. 5A, 5B). The less significant differences observed for cocultured versus monocultured MSCs within the collagen as opposed to gelatin/polyethylene glycol illustrate the material-dependent influence on MSC-chondrocyte differentiation and suggest that collagen biomatrices are more chondrogenic and are a more appropriate biomaterial for MSC-directed cartilage repair and regeneration.
More pronounced osteoblast differentiation was observed for cocultured MSCs (Fig. 6A, 6B) and could also be attributed to early TNF-α expression (Fig. 3) from adherent Møs. Previous studies indicate that TNF-α stimulation of the NF-κB pathway enhances activation of TAZ, RUNX2, and Osterix transcription factors involved in osteoblast differentiation, which resulted in increased matrix mineralization; alkaline phosphatase activity; and osteocalcin, osteopontin, and bone morphogenetic protein-2 expression [57, 58]. IL-6 is a pleiotropic cytokine that can have both pro- or anti-inflammatory activities depending on the tissue type, concentration, and exposure time. When MSC toll-like receptors (TLR-2, TLR-3) were stimulated, the activated NF-κB pathway committed MSCs to an immunosuppressive phenotype that secreted more IL-6 and impeded MSC differentiation into osteoblasts, adipocytes, or chondrocytes [59, 60]. Overproduction of IL-6 is also strongly implicated in chronic inflammatory diseases, such as rheumatoid arthritis, and promotes osteoclast activation, thereby preventing MSC-mediated cartilage regeneration and contributing to bone resorption respectively [61–63]. In contrast to MSC-chondrocyte differentiation, the presence of adherent Møs appeared to enhance MSC-osteoblast differentiation, demonstrating an Mø-dependent effect on MSC multipotency (Fig. 6A, 6B). The less striking differences observed for cocultured versus monocultured MSCs within gelatin/polyethylene glycol as compared with collagen biomatrices exhibits the material-dependent influence on MSC-osteoblast differentiation. These results also suggest that the gelatin/polyethylene biomatrix may also be a better material for filling a critical bone defect that encourages MSC-mediated ossification and functional bone regeneration.
Very little TNF-α expression was observed for collagen or gelatin/polyethylene glycol biomatrices for adipocyte differentiation conditions in both monoculture and coculture (Figs. 1, 3), and IL-6 expression (Figs. 2, 4) did not appear to correlate with adipocyte differentiation (Fig. 7). The lack of significant differences in Oil Red O staining between monoculture and coculture conditions suggests that the presence of adherent Møs did not have a major influence on adipocyte differentiation, in stark contrast to MSC-chondrocyte and -osteoblast differentiation. MSCs encapsulated within gelatin/polyethylene glycol biomatrices had greatly enhanced adipocyte differentiation compared with collagen, indicating the material-dependent influence on MSC differentiation most likely mediated by maintenance of MSCs in a rounded cell morphology that favored adipogenesis [41].
Conclusion
This investigation demonstrated encapsulated MSC multipotency, to varying degrees, for two different biomatrices that could potentially be extrapolated to other materials and alternate MSC differentiation pathways. The presence of adherent Møs that reacted to both to the material and encapsulated MSCs had an impact on MSC multipotency as shown with chondrocyte and osteoblast differentiation within the material and correlated with early Mø proinflammatory cytokine expression of TNF-α. The gelatin/polyethylene glycol biomatrix particularly enhanced MSC differentiation into adipocytes, and MSC-adipocyte differentiation appeared to be independent of the presence of adherent Møs. Adherent Møs developed a prohealing immunophenotype characterized as TNF-αlow, IL-6high, IL-10high, and IL-12low by 4 days (Figs. 1, 2; supplemental online Figs. 2, 4); however, the gelatin/polyethylene glycol biomatrix induced high expression of TNF-α at 1 day (Fig. 3), indicating a temporal material-dependent effect on the Mø immunophenotype. Overall, this study elucidated important three-way interactions between Møs, MSCs, and encapsulating biomatrices, whose combined influences dictated the Mø immunophenotype and MSC multipotency, which are critical for developing effective MSC-biomaterial strategies for promoting tissue regeneration and healing.
Acknowledgments
We thank J. Hardin, B. Gray, and R. Sullivan for technical assistance involving bone marrow MSC-biomaterial histological processing and differentiation staining. We also thank Kedi Xu and Yao Fu for immunocytochemistry staining and gelatin/polyethylene glycol synthesis assistance, respectively. This research was supported in part by the University of Wisconsin–Madison School of Pharmacy and NIH Grant EB6613. D.A.C. and W.J.K. are currently affiliated with the School of Pharmacy, University of Wisconsin–Madison, Madison, Wisconsin.
Author Contributions
D.A.C.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; P.H.: conception and design, provision of study materials or patients, manuscript writing (editing), final approval of manuscript; W.J.K.: conception and design, financial support, administrative support, provision of study materials, manuscript writing (editing), final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Jackson WM, Nesti LJ, Tuan RS. Concise review: Clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Translational Medicine. 2012;1:44–50. doi: 10.5966/sctm.2011-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JS, Hong JM, Moon GJ, et al. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099–1106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- 3.Freyman T, Polin G, Osman H, et al. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 4.Wang C, Varshney RR, Wang D-A. Therapeutic cell delivery and fate control in hydrogel and hydrogel hybrids. Adv Drug Deliv Rev. 2010;62:699–710. doi: 10.1016/j.addr.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Park JS, Yang HN, Woo DG, et al. The promotion of chondrogenesis, osteogenesis, and adipogenesis of human mesenchymal stem cells by multiple growth factors incorporated into nanosphere-coated microspheres. Biomaterials. 2011;32:28–38. doi: 10.1016/j.biomaterials.2010.08.088. [DOI] [PubMed] [Google Scholar]
- 6.Benoit DS, Schwartz MP, Durney AR, et al. Small functional groups for controlled differentiation of hydrogel encapsulated human mesenchymal stem cells. Nat Mater. 2008;7:816–823. doi: 10.1038/nmat2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rustad KC, Wong VW, Sorkin M, et al. Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials. 2012;33:80–90. doi: 10.1016/j.biomaterials.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan BP, Hui TY, Yeung CW, et al. Self-assembled collagen-human mesenchymal stem cell microspheres for regenerative medicine. Biomaterials. 2007;28:4652–4666. doi: 10.1016/j.biomaterials.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 9.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433–441. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantovani A, Sica A, Sozzani S, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Lumeng CN, DelProposto JB, Westcott DJ, et al. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophages subtypes. Diabetes. 2008;57:3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol. 2008;130:147–158. doi: 10.1016/j.ijcard.2008.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi N, de Jager VCL, Glück A, et al. The molecular signature of oxidative metabolism and the mode of macrophage activation determine the shift from acute to chronic disease in experimental arthritis: Critical role of interleukin-12p40. Arthritis Rheum. 2008;58:3471–3484. doi: 10.1002/art.23956. [DOI] [PubMed] [Google Scholar]
- 16.Franz S, Rammelt S, Scharnweber D, et al. Immune responses to implants: A review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011;32:6692–6709. doi: 10.1016/j.biomaterials.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 17.Brown BN, Valentin JE, Stewart-Akers AM, et al. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30:1482–1491. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trivedi P, Hematti P. Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Exp Hematol. 2008;36:350–359. doi: 10.1016/j.exphem.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu Y, Xu K, Xiaoxiang Z, et al. 3D cell entrapment in crosslinked thiolated gelatin-polyethylene glycol diacrylate hydrogels. Biomaterials. 2012;33:48–58. doi: 10.1016/j.biomaterials.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seager Danciger J, Lutz M, Hama S, et al. Method for large-scale isolation, culture and cryopreservation of human monocytes suitable for chemotaxis, cellular adhesion assays macrophage and dendritic cell differentiation. J Immunol Methods. 2004;288:123–134. doi: 10.1016/j.jim.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Waldeck H, Wang X, Joyce E, et al. Active leukocyte detachment and apoptosis/necrosis on PEG hydrogels and the implication in the host inflammatory response. Biomaterials. 2012;33:29–37. doi: 10.1016/j.biomaterials.2011.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spagnoli A, Longobardi L, O'Rear L. Cartilage disorders: Potential therapeutic use of mesenchymal stem cells. Endocr Dev. 2005;9:17–30. doi: 10.1159/000085719. [DOI] [PubMed] [Google Scholar]
- 23.Kellar KL, Douglass JP. Multiplexed microsphere-based flow cytometric immunoassays for human cytokines. J Immunol Methods. 2003;279:277–285. doi: 10.1016/s0022-1759(03)00248-5. [DOI] [PubMed] [Google Scholar]
- 24.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: A novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung SA, Kao WJ. Fibroblast regulated monocyte response to ECM-derived matrix: The effects on monocyte adhesion and the production of inflammatory, matrix remodeling, and growth factor proteins. J Biomed Mater Res A. 2009;89:841–853. doi: 10.1002/jbm.a.32431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Schmidt DR, Joyce EJ, et al. Application of MS-based proteomics to study serum protein adsorption/absorption and complement C3 Activation on poly(ethylene glycol) hydrogels. J Biomater Sci Polym Ed. 2011;22:1343–1362. doi: 10.1163/092050610X508400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt D, Joyce EJ, Kao WJ. Fetal bovine serum xenoproteins modulate human monocyte adhesion and protein release on biomaterials in vitro. Acta Biomater. 2011;7:515–525. doi: 10.1016/j.actbio.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): Role as guardians of inflammation. Mol Ther. 2012;20:14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maggini J, Mirkin G, Bognanni I, et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang QZ, Su WR, Shi SH, et al. Human gingiva-derived mesenchymal stem cell elicit polarization of M2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy: Review of a new approach. Pharmacol Rev. 2003;55:241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 32.Tilg H, Trehu E, Atkins MB, et al. Interluekin-6 (IL-6) as an anti-inflammatory cytokine: Induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83:113–118. [PubMed] [Google Scholar]
- 33.Jones SA. Directing transition from innate to acquired immunity: Defining a role for IL-6. J Immunol. 2005;175:3463–3468. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- 34.Yagi H, Soto-Guiterrez A, Parekkadan B, et al. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010;19:667–679. doi: 10.3727/096368910X508762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bosnakovski D, Mizuno M, Kim G, et al. Chondrogeneic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: Influence of collagen type II extracellular matrix chondrogenesis. Biotechnol Bioeng. 2006;93:1152–1163. doi: 10.1002/bit.20828. [DOI] [PubMed] [Google Scholar]
- 36.Dudhia J. Aggrecan, aging and assembly in articular cartilage. Cell Mol Life Sci. 2005;62:2241–2256. doi: 10.1007/s00018-005-5217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang F, Williams CG, Wang D-A, et al. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cell. Biomaterials. 2005;26:5991–5998. doi: 10.1016/j.biomaterials.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 38.Dadsetan M, Hefferan TE, Szatkowski JP, et al. Effect of hydrogel porosity on marrow stromal cell phenotypic expression. Biomaterials. 2008;29:2193–2202. doi: 10.1016/j.biomaterials.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Hayashi K, Tabata Y. Preparation of stem cell aggregates with gelatin microspheres to enhance biological functions. Acta Biomater. 2011;7:2797–2803. doi: 10.1016/j.actbio.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 41.Flynn L, Woodhouse KA. Adipose tissue engineering with cells in engineered matrices. Organogenesis. 2008;4:228–235. doi: 10.4161/org.4.4.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 43.Tomizawa Y. Clinical benefits and risk analysis of topical hemostats: A review. J Artif Organs. 2005;8:137–142. doi: 10.1007/s10047-005-0296-x. [DOI] [PubMed] [Google Scholar]
- 44.Dawson E, Mapili G, Erickson K, et al. Biomaterials for stem cell differentiation. Adv Drug Deliv Rev. 2008;60:215–228. doi: 10.1016/j.addr.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 45.Wallace DG, Rosenblatt J. Collagen gel systems for sustained delivery and tissue engineering. Adv Drug Deliv Rev. 2003;55:1631–1649. doi: 10.1016/j.addr.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanson SE, King SN, Kim J, et al. The effect of mesenchymal stromal cell–hyaluronic acid hydrogel constructs on immunophenotype of macrophages. Tissue Eng Part A. 2011;17:2463–2471. doi: 10.1089/ten.tea.2010.0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heo SC, Jeon ES, Lee IH, et al. Tumor necrosis factor-α-activated human adipose tissue-derived mesenchymal stem cell accelerate cutaneous wound healing through paracrine mechanisms. J Invest Dermatol. 2011;131:1559–1567. doi: 10.1038/jid.2011.64. [DOI] [PubMed] [Google Scholar]
- 49.Chen L, Tredget EE, Wu PYG, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barrientos S, Stojadinovic O, Golinko MS, et al. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 51.Ashcroft GS, Jeong M-J, Ashworth JJ, et al. Tumor necrosis factor-alpha (TNF-α) is a therapeutic target for impaired cutaneous wound healing. Wound Repair Regen. 2012;20:38–49. doi: 10.1111/j.1524-475X.2011.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trengove NJ, Bielefeldt-Ohmann H, Stacey MC. Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound Repair Regen. 2000;8:13–25. doi: 10.1046/j.1524-475x.2000.00013.x. [DOI] [PubMed] [Google Scholar]
- 53.Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based engineering. Arthritis Res Ther. 2003;5:32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wehling N, Palmer GD, Pilapil C, et al. Interluekin-1β and tumor necrosis factor α inhibit chondrogenesis by human mesenchymal stem cells through NF-κB-dependent pathways. Arthritis Rheum. 2009;60:801–812. doi: 10.1002/art.24352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones E, Churchman M, English A, et al. Mesenchymal stem cells in rheumatoid synovium: Enumeration and functional assessment in relation to synovial inflammation level. Ann Rheum Dis. 2010;69:450–457. doi: 10.1136/ard.2008.106435. [DOI] [PubMed] [Google Scholar]
- 56.Murphy JM, Dixon K, Beck S, et al. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002;46:704–713. doi: 10.1002/art.10118. [DOI] [PubMed] [Google Scholar]
- 57.Hess K, Ushmorov A, Fiedler J, et al. TNF-α promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-κB pathway. Bone. 2009;45:367–376. doi: 10.1016/j.bone.2009.04.252. [DOI] [PubMed] [Google Scholar]
- 58.Cho HH, Shin KK, Kim YJ, et al. NF-κB activation stimulates osteogenic differentiation of mesenchymal stem cells derived from human adipose tissue by increasing TAZ expression. J Cell Physiol. 2010;223:168–177. doi: 10.1002/jcp.22024. [DOI] [PubMed] [Google Scholar]
- 59.Waterman RS, Tomchuck SL, Henkle SL, et al. A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS One. 2010;5:e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pevsner-Fischer M, Morad V, Cohen-Sfady M, et al. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 2007;109:1422–1432. doi: 10.1182/blood-2006-06-028704. [DOI] [PubMed] [Google Scholar]
- 61.Nishimoto N, Kishimoto T. Inhibition of IL-6 for the treatment of inflammatory diseases. Curr Opin Pharmacol. 2004;4:386–391. doi: 10.1016/j.coph.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 62.Blanchard F, Duplomb L, Baud'huin M, et al. The dual role of IL-6-type cytokines on bone remodeling and bone tumors. Cytokine Growth Factor Rev. 2009;20:19–28. doi: 10.1016/j.cytogfr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 63.Scheller J, Chalaris A, Schmidt-Arras D, et al. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochem Biophys Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]