In this study, an optimized two-step enzyme digestion protocol was used to strip the surface epithelium from tracheal specimens separate from submucosal gland (SMG) cells, and the basal and duct stem/progenitors were then sorted using fluorescence-activated cell sorting. Sorted stem/progenitor cells were cultured to characterize their self-renewal and differentiation ability. It was found that global inhibition of aldehyde dehydrogenase (ALDH), as well as specific inhibition of the ALDH2 isoform, inhibited self-renewal of both basal and duct cells, thereby producing fewer and smaller spheres.
Keywords: Adult stem cells, Respiratory tract, Stem/progenitor cell, Tissue-specific stem cells, Self-renewal
Abstract
Basal cells and submucosal gland (SMG) duct cells have been isolated and shown to be stem/progenitor cell populations for the murine airway epithelium. However, methods for the isolation of basal and SMG duct cells from human airways have not been defined. We used an optimized two-step enzyme digestion protocol to strip the surface epithelium from tracheal specimens separate from SMG cells, and we then sorted the basal and duct stem/progenitors using fluorescence-activated cell sorting. We used nerve growth factor receptor, as well as a combination of CD166 and CD44, to sort basal cells and also used CD166 to isolate SMG duct cells. Sorted stem/progenitor cells were cultured to characterize their self-renewal and differentiation ability. Both basal and SMG duct cells grew into spheres. Immunostaining of the spheres showed mostly dense spheres with little to no central lumen. The spheres expressed cytokeratins 5 and 14, with some mucus- and serous-secreting cells. The sphere-forming efficiency and the rate of growth of the spheres varied widely between patient samples and correlated with the degree of hyperplasia of the epithelium. We found that only aldehyde dehydrogenase (ALDH)hi basal and duct cells were capable of sphere formation. Global inhibition of ALDH, as well as specific inhibition of the ALDH2 isoform, inhibited self-renewal of both basal and duct cells, thereby producing fewer and smaller spheres. In conclusion, we have developed methods to isolate basal and SMG duct cells from the surface epithelium and SMGs of human tracheas and have developed an in vitro model to characterize their self-renewal and differentiation.
Introduction
The airway epithelium is in direct contact with the environment and hence is constantly exposed to injury from pollution and microbial agents. An efficient mechanism for repair of the airways is therefore an essential part of host defense. Adult stem cells in the airway are critical for repair and regeneration, yet despite their importance, these cells are poorly characterized.
Recently, it has been shown that mouse basal cells (BCs) in the trachea and Clara cells in the bronchi and bronchioles can self-renew and generate differentiated progeny of the surface epithelium (SE) [1, 2]. In the rodent, BCs are confined to the trachea, whereas in the human lung they are present throughout the airways, including the small bronchioles [3]. We showed that mouse submucosal gland (SMG) duct cells are stem/progenitor cells, which are involved in maintaining the SMGs and repairing them and the SE after injury [4]. In mouse lungs, SMGs are restricted to the upper trachea, whereas in human lungs SMGs exist along the airways from the larynx down to the distal part of the main bronchi. Methods for isolation of BCs from human tracheobronchial SE have not been clearly defined, and human SMG duct cells have never been isolated. The ability to isolate stem cell populations from the human airway and culture them is critical for advancing our understanding of airway epithelial repair and regeneration.
Materials and Methods
Human Sample Collection and Digestion
Human large airways were obtained from the National Disease Research Interchange Human Tissue Procurement Project or from discarded, deidentified large airways after lung transplantation. In preliminary studies, samples were cut into 1–2-cm2 pieces and then incubated in 0.15% pronase (Roche, Indianapolis, IN, http://www.roche.com) at 4°C for 4, 8, and 16 hours or in 16 U/ml dispase (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com) at room temperature for 1, 2, 4, and 6 hours. Then samples were examined under a dissecting microscope to confirm detachment of SE before all tissue pieces were fixed and embedded. Tissue sections were stained to examine which enzyme digestion protocol completely removed the SE while leaving SMGs and SMG ducts behind. Airway tissue from which the SE was stripped contained SMGs and ducts, which were minced with scissors to open the SMG compartments and were then incubated overnight in pronase at 4°C to detach the SMGs and ducts from the cartilage and surrounding connective tissue. Cell sheets and clumps collected from SE and SMGs were incubated in 0.25% trypsin/EDTA.
Fluorescence-Activated Cell Sorting of Basal and SMG Duct Cells
Cells from SE were stained with goat anti-CD166 (R&D Systems Inc., Minneapolis, MN, http://www.rndsystems.com) and rat anti-CD44-phycoerythrin or mouse anti-nerve growth factor receptor (anti-NGFR) (BD Biosciences), and cells from SMGs were stained for CD166. For fluorescence-activated cell sorting (FACS) based on aldehyde dehydrogenase (ALDH) expression, cells were stained with the Aldefluor assay kit (StemCell Technologies, Vancouver, BC, Canada, http://www.stemcell.com) in addition to the basal or duct cell markers. Sorting was performed on a FACSAria, and the data were analyzed with FACSDiva software (BD Biosciences).
Immunostaining
Immunostaining was performed as described [4]. The antibodies used were rabbit anti-K5 (Covance, Princeton, NJ, http://www.covance.com), goat anti-CC10, mouse anti-p63 (Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com), rat anti-integrin-α6 (anti-ITGA6), rabbit anti-NGFR, mouse anti-K14 (Abcam, Cambridge, MA, http://www.abcam.com), goat anti-TROP-2 and anti-pIgR (R&D Systems), mouse anti-α-smooth muscle actin, and anti-acetylated β-tubulin (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com).
In Vitro Sphere Cultures
FACS-sorted cells were resuspended in MTEC/Plus medium [5], and mixed 1:1 with growth factor-reduced Matrigel (BD Biosciences). Treatments with the broad-spectrum ALDH inhibitor diethylaminobenzaldehyde (DEAB) (Sigma-Aldrich) at a 100–500 μM concentration and the ALDH2 specific inhibitor Daidzin at 100 μM (Sigma-Aldrich) were given on day 0 or day 7 of culture. The number of spheres per insert was counted on day 7 and/or day 14. After 21 days in culture, spheres were passaged or embedded in Histogel (Richard-Allan Scientific [Thermo Scientific], Kalamazoo, MI, http://www.thermofisher.com) and then in paraffin for sectioning and staining.
Statistical Methods
All experiments were performed with at least three different primary cultures from at least three independent experiments. Significance was evaluated by analysis of variance. Data are presented as mean ± SD.
Results
Characterizing Markers for the Identification and Isolation of Basal and SMG Duct Cells
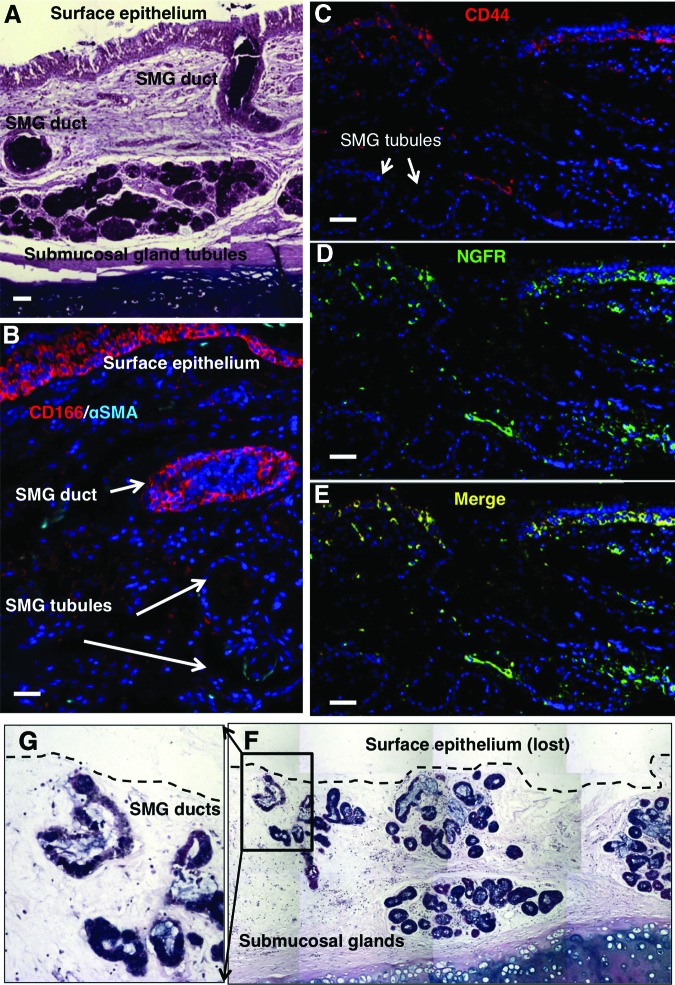
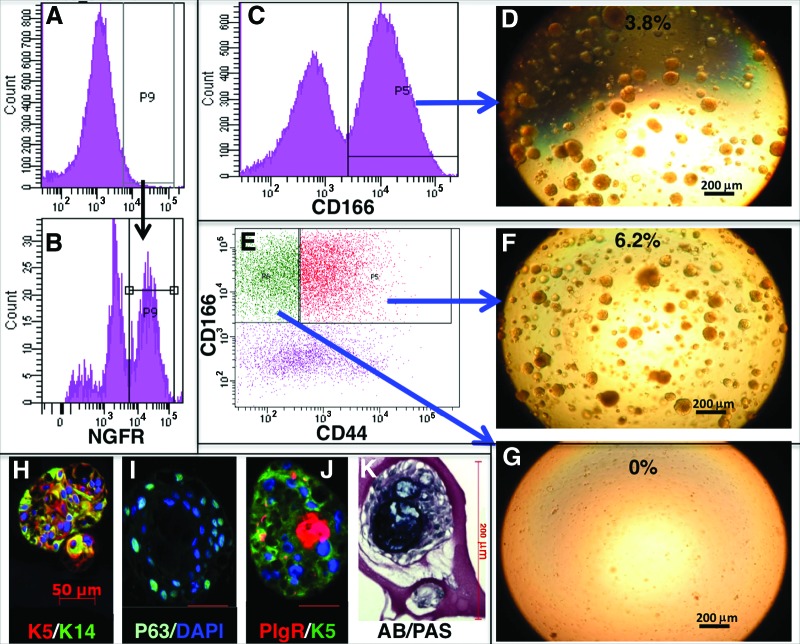
We performed immunofluorescent staining on human tracheal tissue samples to identify specific markers to isolate the basal and SMG duct cells from the human tracheobronchial SE and SMGs, (Fig. 1A–1E). We found that ITGA6 was not restricted to BCs but was also expressed in many non-BCs (data not shown), whereas NGFR expression was confined to BCs (Fig. 1D). We also identified CD44 as a specific marker of airway BCs (Fig. 1C). Coexpression of CD44 and NGFR was seen in all airway BCs (Fig. 1E). On the other hand, TROP-2, the marker that we previously used to isolate mouse SMG duct cells [4], was not expressed robustly enough to be used to sort human SMG duct cells (data not shown). Instead, we identified another marker, CD166, which stained all cells of the SE, as well as the duct cells, but not myoepithelial or tubule cells of SMGs (Fig. 1B). On the basis of the pattern of CD166 expression, we used a two-step enzymatic digestion protocol, similar to the one that we used for mice [4], to completely remove the SE, including all BCs, followed by FACS of the remaining trachea for CD166+ duct cells, thus excluding SMG tubular and nonepithelial stromal cells. We found that 2 hours of dispase digestion completely stripped the SE, leaving behind most of the SMG duct cells (Fig. 1F, 1G). Overnight digestion of stripped tracheal epithelium with pronase resulted in only 5% of the surface epithelial cells expressing NGFR (Fig. 2A), much lower than the 25%–35% expected from the immunostaining (Fig. 1D). We hypothesized that the prolonged dispase enzyme digestion may have digested the NGFR epitopes. Culture of the surface epithelial cells for 48–72 hours at 37°C increased the number of cells expressing NGFR to 30% (Fig. 2B). SMG duct cells were isolated with CD166+ (Fig. 2C), and the BCs were isolated with CD44 and CD166 antibodies or NGFR antibody alone (Fig. 2E).
Figure 1.
Isolation of surface epithelium separate from SMG cells. (A): Alcian blue/periodic acid-Schiff (AB/PAS) staining showed the SMGs and their ducts opening onto the surface epithelium. (B): Immunofluorescent staining of tracheal sections showed that CD166 stained all surface epithelial cells and SMG duct cells but not SMG tubules, whereas CD44 (C) and NGFR ([D], merged in [E]) stained only basal cells of surface epithelium and were absent in SMG tubules. (F): AB/PAS staining of tracheas after 2 hours of dispase digestion showed complete detachment of surface epithelium with almost complete preservation of SMGs and ducts. (G): Higher magnification of the inset in (F). Scale bars = 50 μm. Abbreviations: NGFR, nerve growth factor receptor; αSMA, α-smooth muscle actin; SMG, submucosal gland.
Figure 2.
Sorting and in vitro characterization of basal and submucosal gland (SMG) duct cells. (A): After enzyme digestion, NGFR expression was found to be very low. (B): Cells regained NGFR expression after 48–72 hours in culture. (C): Sorting of CD166+ SMG duct cells. (D): Sorted cells produced spheres in Matrigel culture. (E): CD44 separated CD166+ epithelial cells into basal and nonbasal populations. Only basal cells (F) but not nonbasal cells (G) could self-renew into spheres. Spheres expressed K5, K14, and p63 in addition to the serous cell marker pIgR (H–K), and mucus-secreting cells were detected with AB/PAS staining. Abbreviations: AB/PAS, Alcian blue/periodic acid-Schiff; DAPI, 4′,6-diamidino-2-phenylindole; NGFR, nerve growth factor receptor; P, population.
Both Basal and SMG Duct Cells Self-Renew and Differentiate Under In Vitro Culture Conditions
To assess the ability of sorted cell populations to self-renew and differentiate in vitro, they were cultured on Matrigel and observed for sphere formation [4]. Only cultured basal and SMG duct cells were capable of growing into spheres on Matrigel (Fig. 2D, 2F, 2G). The sphere-forming efficiency was, in general, much higher than what we observed in mice, ranging from 0.4% to 12%. At 14 days in culture, spheres were mostly dense balls of cells with little or no central lumen. The size of the spheres varied from 50 to 200 μm in diameter. The rate of growth of the spheres varied from patient to patient. To determine the differentiation ability of the basal and SMG duct cells, we examined expression in the spheres for proteins characteristic of differentiated cell types of the SE and the SMGs and ducts. The spheres from basal and SMG duct cells were morphologically similar and expressed both K5 (basal and duct cell marker) and K14 (duct cell marker) (Fig. 2H). p63 (basal cell marker) was expressed in the outer layer of cells of the spheres (Fig. 2I). A subset of the spheres (17.4 ± 7.3%) also expressed the serous cell marker pIgR (Fig. 2J), and there were spheres that stained for mucus with Alcian blue/periodic acid-Schiff (14.6 ± 6.9%) (Fig. 2K). Neither basal nor SMG duct sphere cells expressed acetylated β-tubulin, Clara cell secretory protein, or α-smooth muscle actin. Both basal and SMG duct cell spheres were passaged up to four times and had the same efficiency of sphere formation, the same morphology, and equivalent differentiation ability.
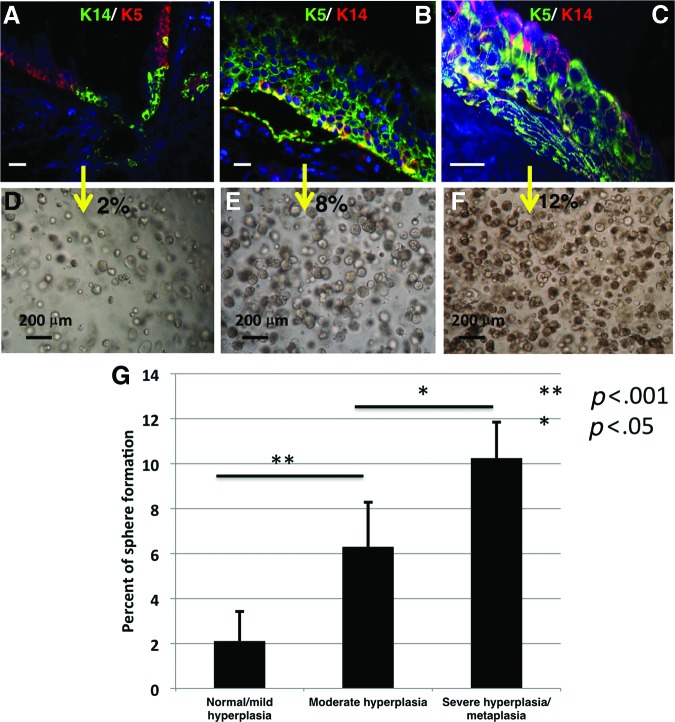
The Correlation Between the Histopathology of the Human Airway Samples and Sphere-Forming Efficiency
The higher sphere forming efficiency of basal and SMG duct human cells compared with the mouse, in addition to the intersample variability, prompted us to try to find a correlation with the histopathology of the airway of the individual samples with sphere forming potential. We found that samples that showed no basal cell hyperplasia to mild basal cell hyperplasia, no goblet cell hyperplasia, no metaplasia, and minimal inflammatory cell infiltration had the lowest incidence of sphere formation (2.1 ± 1.3%) (Fig. 3A, 3D, 3G). On the other hand, samples with extensive basal cell hyperplasia, goblet cell hyperplasia, and metaplasia with inflammatory cell infiltration had the highest incidence of sphere formation (10.2 ± 1.6%) (Fig. 3C, 3F, 3G). Samples with moderate basal cell hyperplasia, goblet cell hyperplasia, no metaplasia, and mild inflammatory cell infiltration had intermediate sphere forming efficiency (6.3 ± 2%) (Fig. 3B, 3E, 3G).
Figure 3.
Effect of epithelial hyperplasia on sphere-forming efficiency. (A–F): Cells sorted from airway epithelium with hyperplasia and metaplasia had a higher incidence of sphere formation compared with cells sorted from normal-appearing epithelium with no K14 expression in basal cells. (G): Bar graph showing the average sphere-forming efficiency from at least three different samples. The sphere-forming efficiency of isolated stem/progenitor cells was higher in epithelium with moderate hyperplasia as compared with normal/mild hyperplasia (p < .001). Stem/progenitor cells isolated from epithelium with severe hyperplasia/metaplasia had greater sphere-forming efficiency than the cells isolated from moderately hyperplastic tissue (p < .05). White scale bars = 50 μm.
ALDH Expression Enriches for Airway Epithelial Stem/Progenitor Cells of the SE and SMG Duct
On the basis of several recent studies that showed that high expression of ALDH enriched for adult stem cell populations in many tissues both in mouse and human [6], we hypothesized that high ALDH activity in basal and SMG duct cells would enrich for the progenitor/stem cells within these populations. We sorted the human basal and duct cells into ALDHhi and ALDHlo populations and cultured both populations on Matrigel. We found that only ALDHhi basal and duct cells formed spheres, whereas ALDHlo cells rarely formed spheres (Fig. 4A–4C). To examine the role of ALDH in progenitor/stem cell function, FACS-sorted cells were treated with the broad-spectrum ALDH inhibitor DEAB, either at the start of culture or at day 7, after spheres had already formed. We also treated cells with the specific ALDH2 inhibitor Daidzin to examine the specific role of this isoform of ALDH. Treating cells at day 0 resulted in formation of fewer and smaller spheres (Fig. 4D–4F), whereas treatment at day 7 resulted in smaller spheres with no effect on differentiation (data not shown). These data show that one or more specific ALDH isoforms, including ALDH2, are essential for progenitor/stem cell self-renewal without affecting their differentiation ability.
Figure 4.
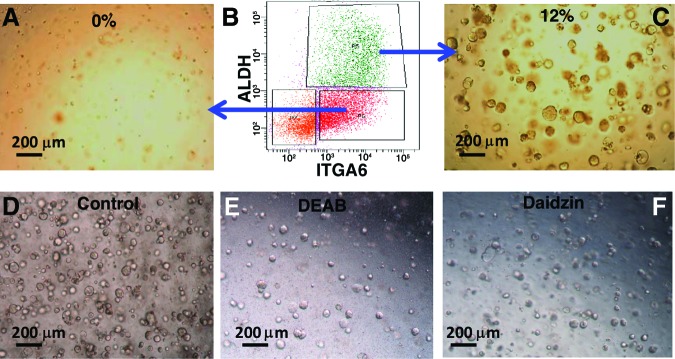
The role of ALDH in self-renewal of basal and submucosal gland duct cells. (A–C): ALDHhi-expressing cells were enriched for the sphere-forming population of cells from both basal and duct cells. (D–F): Treatment of sorted cells with a broad-spectrum ALDH inhibitor (DEAB) or specific ALDH2 inhibitor (Daidzin) markedly reduced sphere formation. Abbreviations: ALDH, aldehyde dehydrogenase; DEAB, diethylaminobenzaldehyde; ITGA6, integrin-α6; P, population.
Discussion
These results are important because isolation of human airway basal and duct stem cells and the ability to grow them in a reproducible in vitro sphere assay enable us to study pathways that control airway epithelial proliferation and differentiation. This culture system of adult stem/progenitor cells especially lends itself to high-throughput drug screening for the discovery of new therapies for airway diseases. Ultimately, it is possible that these adult stem cells may be used for stem cell-based therapies for airway diseases, especially in the setting of severe airway injury with airway epithelial sloughing, such as may occur after burns or severe viral infections. The upregulation of NGFR expression on the basal and duct cells after 48–72 hours in culture may reflect the re-expression of this surface epitope after enzymatic digestion, but it may also reflect the proliferation of NGFR-expressing cells in the culture.
Differential proliferative characteristics of normal versus hyperplastic epithelium have been described before for the airways [7] and other organs [8]. This is the first time that differences in proliferation of stem/progenitor cells have been associated with differences in the self-renewal capacity of the stem cells of the hyperplastic epithelial tissue. We speculate that the correlation between the airway histology and sphere-forming efficiency reflects a change in the airway stem/progenitor cell response to injury and therefore provides a novel model system to study the accompanying alterations in airway repair and regeneration.
ALDH has been found to enrich for progenitor cells in many normal tissues, including bone marrow, neural tissue, and breast tissue, as well as cancer stem cells [6, 9]. Our data suggest that ALDH has a functional role in airway epithelial stem/progenitor cell self-renewal. The Aldefluor assay detects many isoforms of ALDH, including ALDH1A1, ALDH1A2, and ALDH2, and so from our studies it is not possible to say which isoform is most involved in airway epithelial stem/progenitor cell self-renewal. ALDH isoforms function in many important physiologic and pharmacologic processes in the airways, including playing a role in cell proliferation and drug resistance [10]. They play a major role in metabolizing aldehydes, which arise endogenously and are also present in the environment (e.g., aldehydes are present in cigarette smoke). We speculate that the higher levels of certain ALDH isoforms in airway epithelial stem cells may facilitate normal repair and regeneration of the airway epithelium but may also promote carcinogenesis.
Conclusion
We have developed a method for the isolation and culture of human airway stem/progenitor cells. Important differences between human and mouse airways make it critical to develop these methods to study human airway epithelial repair and regeneration. In addition, sphere-forming efficiency is greater and more variable in humans and correlates with the phenotype of the airway epithelium. These findings allow the successful isolation and culture of human airway epithelial stem/progenitor cells to identify mechanisms and ultimately develop new therapies for airway diseases.
Acknowledgments
We thank Rita Kern and John Belperio for assistance with tissue collection and Richard Chiou for technical assistance. This work was supported by California Institute for Regenerative Medicine Grant RN2-00904-1, NIH Grant R01-HL094561, American Thoracic Society/COPD Foundation Grant ATS-06-065, the Concern Foundation, the UCLA Jonsson Comprehensive Cancer Center Thoracic Oncology Program/Lung Cancer Specialized Programs of Research Excellence, the University of California Cancer Research Coordinating Committee, and the Gwynne Hazen Cherry Memorial Laboratories (to B.N.G.).
Author Contributions
A.E.H. and B.N.G.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; V.L.H. and D.W.N.: collection and/or assembly of data, data analysis and interpretation; D.O.D., J.L.G., A.T.O., Y.S.A., and B.B.: collection and/or assembly of data.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Rawlins EL, Okubo T, Xue Y, et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rock JR, Onaitis MW, Rawlins EL, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: A perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hegab AE, Ha VL, Gilbert JL, et al. Novel stem/progenitor cell population from murine tracheal submucosal gland ducts with multipotent regenerative potential. Stem Cells. 2011;29:1283–1293. doi: 10.1002/stem.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.You Y, Richer EJ, Huang T, et al. Growth and differentiation of mouse tracheal epithelial cells: Selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1315–L1321. doi: 10.1152/ajplung.00169.2002. [DOI] [PubMed] [Google Scholar]
- 6.Balber AE. Concise review: Aldehyde dehydrogenase bright stem and progenitor cell populations from normal tissues: Characteristics, activities, and emerging uses in regenerative medicine. Stem Cells. 2011;29:570–575. doi: 10.1002/stem.613. [DOI] [PubMed] [Google Scholar]
- 7.Sajjan U, Keshavjee S, Forstner J. Responses of well-differentiated airway epithelial cell cultures from healthy donors and patients with cystic fibrosis to Burkholderia cenocepacia infection. Infect Immun. 2004;72:4188–4199. doi: 10.1128/IAI.72.7.4188-4199.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonkhoff H, Stein U, Remberger K. The proliferative function of basal cells in the normal and hyperplastic human prostate. Prostate. 1994;24:114–118. doi: 10.1002/pros.2990240303. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan JP, Spinola M, Dodge M, et al. Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on notch signaling. Cancer Res. 2010;70:9937–9948. doi: 10.1158/0008-5472.CAN-10-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreb JS, Ucar D, Han S, et al. The enzymatic activity of human aldehyde dehydrogenases 1A2 and 2 (ALDH1A2 and ALDH2) is detected by Aldefluor, inhibited by diethylaminobenzaldehyde and has significant effects on cell proliferation and drug resistance. Chem Biol Interact. 2012;195:52–60. doi: 10.1016/j.cbi.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]