This study assessed the safety and initial efficacy of umbilical cord-derived mesenchymal stem cell (UC-MSC) transfusions for acute-on-chronic liver failure (ACLF) patients associated with hepatitis B virus (HBV) infection. No significant side effects were observed, and the UC-MSC transfusions significantly increased the survival rates in ACLF patients. It was found that UC-MSC transfusions are safe in the clinic and may serve as a novel therapeutic approach for HBV-associated ACLF patients.
Keywords: Clinical trials, Stem cell transplantation, Umbilical cord, Liver
Abstract
Acute-on-chronic liver failure (ACLF) is a severe, life-threatening complication, and new and efficient therapeutic strategies for liver failure are urgently needed. Mesenchymal stem cell (MSC) transfusions have been shown to reverse fulminant hepatic failure in mice and to improve liver function in patients with end-stage liver diseases. We assessed the safety and initial efficacy of umbilical cord-derived MSC (UC-MSC) transfusions for ACLF patients associated with hepatitis B virus (HBV) infection. A total of 43 ACLF patients were enrolled for this open-labeled and controlled study; 24 patients were treated with UC-MSCs, and 19 patients were treated with saline as controls. UC-MSC therapy was given three times at 4-week intervals. The liver function, adverse events, and survival rates were evaluated during the 48-week or 72-week follow-up period. No significant side effects were observed during the trial. The UC-MSC transfusions significantly increased the survival rates in ACLF patients; reduced the model for end-stage liver disease scores; increased serum albumin, cholinesterase, and prothrombin activity; and increased platelet counts. Serum total bilirubin and alanine aminotransferase levels were significantly decreased after the UC-MSC transfusions. UC-MSC transfusions are safe in the clinic and may serve as a novel therapeutic approach for HBV-associated ACLF patients.
Introduction
Liver failure is a serious clinical syndrome characterized by massive necrosis of hepatocytes due to a variety of acute or chronic injuries that are induced by alcohol consumption, hepatotoxic drugs, autoimmune attack of hepatocytes, or infection with viruses, such as hepatitis B virus (HBV) and hepatitis C virus (HCV) [1–3]. In China, HBV infection accounts for the highest proportion of liver failure cases. In particular, some chronic hepatitis B (CHB) patients may rapidly progress toward acute-on-chronic liver failure (ACLF) [4], which is a severe, life-threatening condition, and liver transplantation is considered the standard treatment for these patients. However, several limitations, such as limited numbers of donors, long waiting lists, high cost, and multiple complications (e.g., rejection, problems associated with the long-term use of immunosuppressants, and perioperative morbidity and mortality) have restricted the use of liver transplantation in many ACLF patients. Therefore, new strategies to delay or prevent disease progression of liver failure are urgently required.
Mesenchymal stem cells (MSCs) are multipotent cells that have self-renewing abilities and the potential to differentiate into various types of cells, including hepatocytes [5, 6]. More importantly, these cells can interact with immune cells, leading to immunomodulation [7, 8]. MSCs appear to be effective in regulating the invoked immune response in settings such as tissue injury, transplantation, and autoimmunity [8, 9]. In particular, MSCs have been used therapeutically in clinical trials for graft-versus-host disease following bone marrow transplantation [10], and they have also been used recently to treat liver diseases in animal models and patients. Several studies have shown that the infusion of bone marrow-derived MSCs (BM-MSCs) can ameliorate liver fibrosis [11, 12] and reverse fulminant hepatic failure in experimental mice [13, 14]. Infusions of autologous BM-MSCs or umbilical cord mesenchymal stem cells (UC-MSCs) have significantly improved liver function in patients with liver cirrhosis [15–18] and liver failure [19], and been found. Although these studies have indicated that MSCs have the potential to improve liver function in patients with chronic liver diseases, their safety and efficacy (in particular whether MSC therapy increases the survival of ACLF patients) remain obscure.
This open-labeled, parallel-controlled, phase I/II trial was aimed at determining the safety and initial efficacy of UC-MSC transfusions in ACLF patients. No significant adverse effects were observed. The UC-MSC transfusions significantly increased the survival of ACLF patients and improved liver functions. These results demonstrate that the UC-MSC transfusions are safe and can serve as a novel therapy for ACLF patients in the clinic.
Patients and Methods
Patients
This open-labeled and controlled study was registered at the NIH's ClinicalTrial.gov site (no. NCT01218464) and was authorized by the General Logistic Ministry of Health, China. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. After approval of the project by the ethics committee of our hospital, 43 ACLF inpatients at our hospital with associated chronic HBV infection between March 3, 2009, and September 3, 2010, were recruited. All patients gave written informed consent in accordance with the institutional review board guidelines for the protection of subjects. Among the 43 participants, 24 were treated with UC-MSCs, and 19 were treated with saline as controls. The baseline data were comparable in the two groups (Table 1). Patients in the treatment group were given UC-MSC transfusions, whereas patients in the control group received saline infusions. All patients received the same conventional treatment throughout the study; for example, all the CHB patients received antiviral therapy by using lamivudine (100 mg daily) treatment. In addition, the ACLF patients with ascites in both MSC-treated and control groups received the same dose of diuretics (40 mg spironolactone daily plus 20 mg of furosemide daily) until the loss of ascites. Patients presenting the following situations were excluded: history of variceal bleeding; recent infection; bleeding tendency, ascites ultrafiltration, and/or dialysis during the last 2 months before enrollment; presence of severe renal, respiratory, or cardiac disease or any detectable tumor by ultrasonography, computed tomography, or magnetic resonance imaging; evidence of extrahepatic biliary diseases (e.g., presence of primary sclerosing cholangitis or dilated common bile duct by ultrasonography); detection of hepatic, portal, or splenic vein thromboses by Doppler ultrasonography; active substance abuse; lack of a supportive family; or unwillingness to sign the informed consent form.
Table 1.
Baseline characteristics of the acute-on-chronic liver failure patients
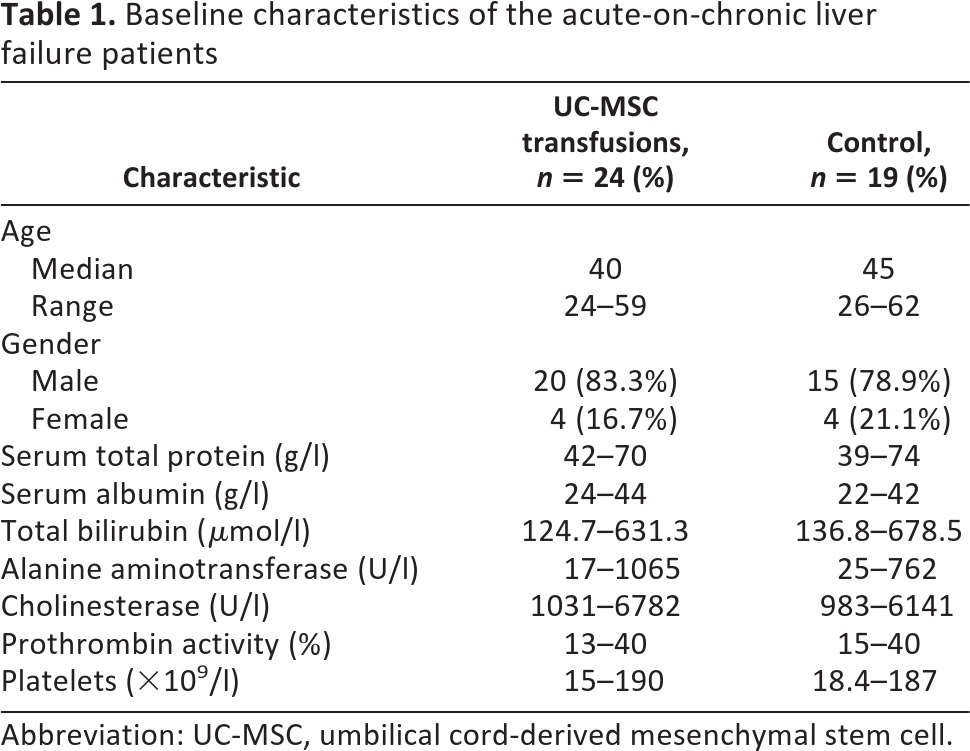
Abbreviation: UC-MSC, umbilical cord-derived mesenchymal stem cell.
Preparation and Identification of UC-MSCs
With the written consent of the maternity patients, fresh human umbilical cords were obtained after birth and collected in cold α-minimal essential medium (α-MEM) (Gibco Invitrogen, Carlsbad, CA, http://www.invitrogen.com). Viruses, including HBV, HCV, HIV, and cytomegalovirus, were monitored in all umbilical cords and prepared UC-MSC samples. Virus-free UC-MSCs were prepared in an approved cGMP-compliant facility according to previous studies [18, 20]. Briefly, the mesenchymal tissues in umbilical cord Wharton's jelly were diced into cubes of approximately 0.5 cm3 and then washed with Hanks' balanced saline solution (Gibco Invitrogen). Then, the tissue pellets were seeded in a tissue culture flask (Corning Enterprises, Corning, NY, http://www.corning.com) in α-MEM supplemented with 10% fetal bovine serum (StemCell Technologies, Vancouver, BC, Canada, http://www.stemcell.com). The medium was replenished every 3 days. After 12–15 days of culture, the adherent cells were cultured to confluence (first passage). Then, the UC-MSCs were cultured and collected between the third and fourth passages for transfusion. For UC-MSC quality control, the cultured cells at the third passage were tested for the expression of phenotypes such as CD31, CD34, CD105, CD45, CD90, CD44, CD73, and human leukocyte antigen (HLA)-DR and were cultured for osteogenesis and adipogenesis differentiation assays.
UC-MSC Transfusions
Approximately 0.5 × 106 UC-MSCs per kilogram of body weight at the fourth passage were infused intravenously through the cubital vein of the arm. Bacteriological tests were performed at every passage and prior to injection. The controls received the same volume of saline. The patients were discharged after 6 hours of observation. Each patient received UC-MSC or saline treatment three times at 4-week intervals (Fig. 1).
Figure 1.
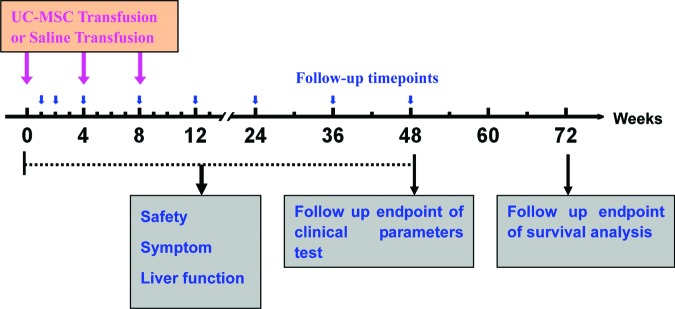
Timeline of UC-MSC treatments in acute-on-chronic liver failure (ACLF) patients. UC-MSC transfusions were given to the ACLF patients three times at the baseline (0-week), 4-week, and 8-week time points. The clinical parameters were tested at the 0-, 1-, 2-, 4-, 8-, 12-, 24-, 36-, and 48-week time points during the follow-up. For survival analysis, the follow-up was prolonged to 72 weeks. Abbreviation: UC-MSC, umbilical cord-derived mesenchymal stem cell.
Trial Design
All participants were followed up for 48 weeks after the first infusion of UC-MSCs or saline, and the clinical parameters were tested. The following liver function tests were performed on weeks 1, 2, 4, 8, 12, 24, 36, and 48: serum alanine aminotransferase (ALT), total bilirubin (TBIL), albumin (ALB), cholinesterase (CHE), prothrombin activity (PTA), platelet count, and the model of end-stage liver disease (MELD) score. Medical histories were taken, and physical examinations were also performed at each visit to record such symptoms as fever, peripheral edema, rash, nausea, and vomiting. All patients were further followed up to 72 weeks for the analysis of survival (Fig. 1).
Statistical Analysis
All data were analyzed using SPSS 13.0 for Windows software (SPSS, Chicago, IL, http://www.spss.com). Multiple comparisons were made among the different groups using the Kruskal-Wallis H nonparametric test. Comparisons between various individuals were performed using the Mann-Whitney U test, whereas comparisons within the same individual were performed using the Wilcoxon matched pairs t test. Actuarial overall survival rates were analyzed by the Kaplan-Meier method, and survival was measured in weeks from diagnosis to death or the last review. For all tests, two-sided p < .05 was considered to be significant.
Results
Characteristics of UC-MSCs
UC-MSCs have the potential for osteogenic and adipogenic differentiation in conditioned medium. These cells highly expressed CD44, CD90, CD73, and CD105 but did not express CD31, CD34, CD45, or HLA-DR, as described in our recent report [18], indicating that these UC-MSCs showed similar characteristics to those of previously reported BM-MSCs.
Safety of UC-MSC Transfusions in ACLF Patients
Since safety is a major concern regarding UC-MSC therapy for ACLF patients, we observed the long-term adverse events during the 48 weeks of follow-up. We monitored uric acid, creatinine, lactate dehydrogenase, and alkaline phosphatase levels before and after the UC-MSC transfusions and found that all parameters were within their respective normal ranges. Two patients developed a self-limiting fever (body temperature, 37°C–38°C) within 2–6 hours after the UC-MSC transfusions but recovered within 12 hours without any additional treatment. In the control group, none of the patients developed a fever. No complications or side effects were found in the UC-MSC-treated patients during the follow-up period. Therefore, the UC-MSC transfusions in the ACLF patients were considered to be safe in the clinic.
UC-MSC Transfusions Reduced the MELD Score for ACLF Patients
The MELD score is generally used to evaluate the prognosis of end-stage liver diseases. We found that the MELD scores were simultaneously decreased in the UC-MSC treatment and control groups. Notably, the baseline MELD scores were similar between the UC-MSC treatment group and the control group (24.05 vs. 26.32, p = .099); however, the MELD scores were decreased more in the UC-MSC-treated patients than the control group at weeks 4, 8, and 12, thus leading to a slight statistical difference between the UC-MSC treatment and control groups (Fig. 2). These data indicate that the UC-MSC treatment may improve the prognosis for the ACLF patients.
Figure 2.
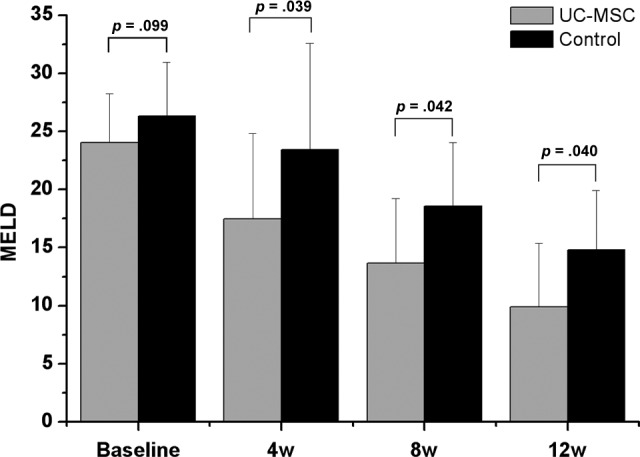
UC-MSC treatment improved model of end-stage liver disease (MELD) scores for acute-on-chronic liver failure patients. The MELD scores were not shown to be markedly different between two groups at baseline. After UC-MSC treatment, the MELD scores were significantly decreased at weeks 4, 8, and 12 as compared with the corresponding control. Abbreviations: UC-MSC, umbilical cord-derived mesenchymal stem cell; w, week.
UC-MSC Transfusions Improve Liver Function and Alleviate Liver Damage
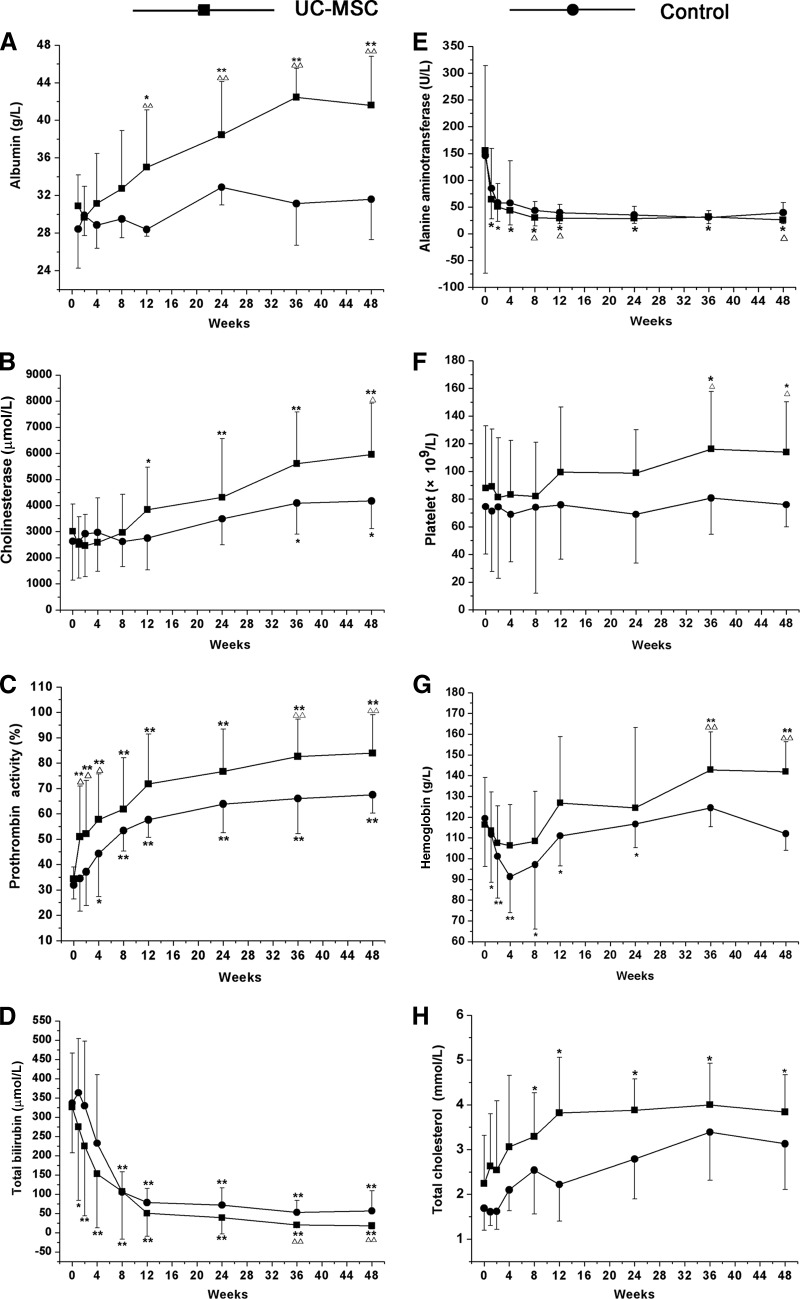
ALB is a major protein produced in liver. In advanced liver disease, the level of the serum ALB is reduced. CHE is an enzyme synthesized by hepatocytes whose activity is decreased when the liver is injured. PTA is a protein made by the liver and is a useful test of liver function, since there is a good correlation between abnormalities of PTA and the degree of liver dysfunction. To investigate the impact of UC-MSCs on liver synthesis functions, ALB, CHE, and PTA levels were analyzed (Fig. 3). At 12 weeks after the first UC-MSC transfusion, the ALB levels increased markedly as compared with the baseline and control groups. By contrast, in the control group, the ALB levels did not show any obvious change during the 48 weeks of follow-up (Fig. 3A). The CHE levels also increased significantly 12 weeks after the first UC-MSC transfusion; this increase occurred earlier than in control groups (where it occurred at week 36) (Fig. 3B). In addition, the PTA levels increased significantly 1 week after the first UC-MSC treatment, and this increase occurred earlier than that in the control (4 weeks after the first treatment) (Fig. 3C). Furthermore, the PTA levels were higher in the UC-MSC treatment group than in the control group at the 2-, 4-, 8-, 36-, and 48-week time points, suggesting that UC-MSC transfusion may improve thrombin function earlier in the ACLF patients.
Figure 3.
Impact of UC-MSC transfusions on liver function. The levels of albumin, cholinesterase, prothrombin activity, total bilirubin, alanine aminotransferase, platelet count, hemoglobin, and total cholesterol were tested at the 0-, 1-, 2-, 4-, 8-, 12-, 24-, 36-, and 48-week time points. *, p < .05, **, p < .01 compared with baseline; ▵, p < .05, ▵▵, p < .01 compared with the control in the corresponding time point. Abbreviation: UC-MSC, umbilical cord-derived mesenchymal stem cell.
ALT is an enzyme that is located in liver cells; it leaks out and makes its way into the general circulation when liver cells are injured. This is thought to be a specific indicator of liver inflammation. TBIL, which is a breakdown product of heme, may be elevated in liver or biliary tract diseases. In this study, the effects of the UC-MSC transfusions on alleviating liver damage were also observed through analyzing ALT and TBIL levels. The TBIL levels significantly decreased 1 week after the first UC-MSC transfusion. This decrease occurred earlier than in the controls (8 weeks after the first treatment) (Fig. 3D). In addition, there was a significant difference between the UC-MSC treatment and saline-treated controls at the 36- and 48-week time points. After UC-MSC treatment, the ALT levels decreased significantly compared with the baseline throughout the 48 weeks of follow-up (Fig. 3E). In the controls, no significant difference was found compared with the baseline. Interestingly, the number of platelets increased gradually after UC-MSC treatment, becoming significantly higher than the baseline and control at the 36- and 48-week time points (Fig. 3F). The hemoglobin level in the UC-MSC treatment group also gradually increased and was higher than baseline and the corresponding controls at the 36- and 48-week time points (Fig. 3G). By contrast, the hemoglobin level decreased in the controls during 24 weeks of follow-up and then gradually became elevated, with no marked differences compared with the baseline at the 36- and 48-week time points. The levels of total cholesterol were increased at 8 weeks after the first UC-MSC transfusion (Fig. 3H). These results indicated that the UC-MSC treatments potentially improved the liver synthesis functions and alleviated liver damage.
UC-MSC Transfusions May Promote Hepatocyte Proliferation
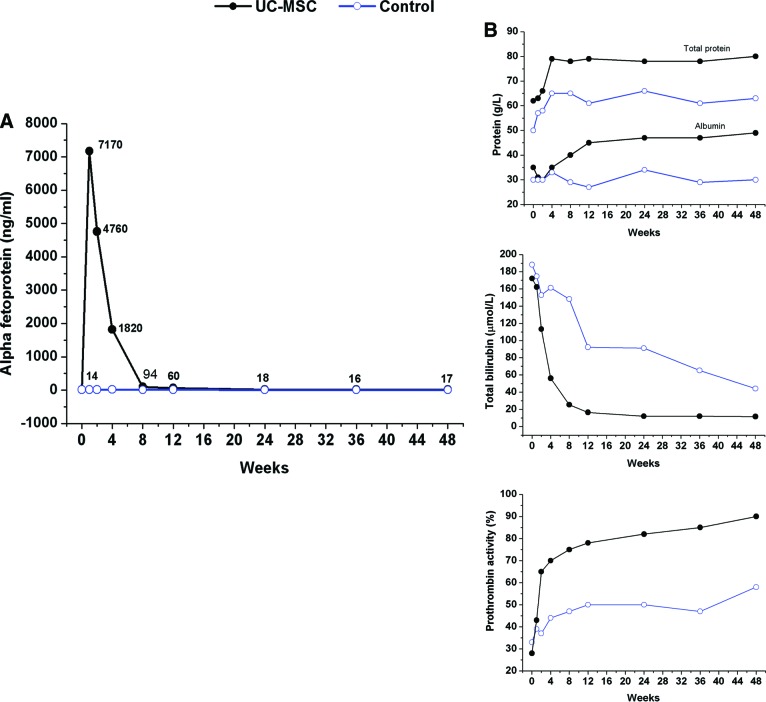
Importantly, the UC-MSC treatment was shown to potentially improve hepatocyte proliferation by the detection of serum α-fetoprotein (α-FP) levels in the participants. One ACLF patient at the middle stage displayed the following test results at baseline: TBIL, 172.1 μmol/l; PTA, 28%; and α-FP, 14 ng/ml. Surprisingly, the level of α-FP increased from 14 ng/ml at the baseline to 7,170 ng/ml 1 week after the first UC-MSC treatment, and it remained at a high level during the subsequent 8 weeks of follow-up (Fig. 4). No supporting evidence of hepatocellular carcinoma (HCC) was found. By contrast, no obvious change in the α-FP level was found in control group. Accordingly, the levels of total protein, ALB, and PTA were also increased, and the TBIL decreased significantly as compared with baseline after UC-MSC transfusion.
Figure 4.
UC-MSC transfusions improved serum α-fetoprotein (α-FP) level in one acute-on-chronic liver failure patient. (A): The level of α-FP increased after the UC-MSC transfusions (black line) but remained stable in a representative control case (blue line). (B): The levels of serum total protein, albumin, and prothrombin activity increased and total bilirubin decreased significantly compared with baseline after the UC-MSC transfusion during the follow-up period. Abbreviation: UC-MSC, umbilical cord-derived mesenchymal stem cell.
UC-MSC Treatments May Help the Recovery of ACLF Patients
For the survival analysis, the enrolled ACLF patients were followed up for 72 weeks; 87.5% (21 of 24) patients in the UC-MSC treatment group and 89.5% (17 of 19) patients in the control group were followed up for 72 weeks. The survival curve for these participants during the 72 weeks is shown in Figure 5. During the first 12 weeks of follow-up, 5 patients (20.8%) in UC-MSC treatment group and 11 patients (47.4%) in control group died. The results showed that the UC-MSC treatments helped the recovery of ACLF patients as compared with the saline control (p = .015).
Figure 5.
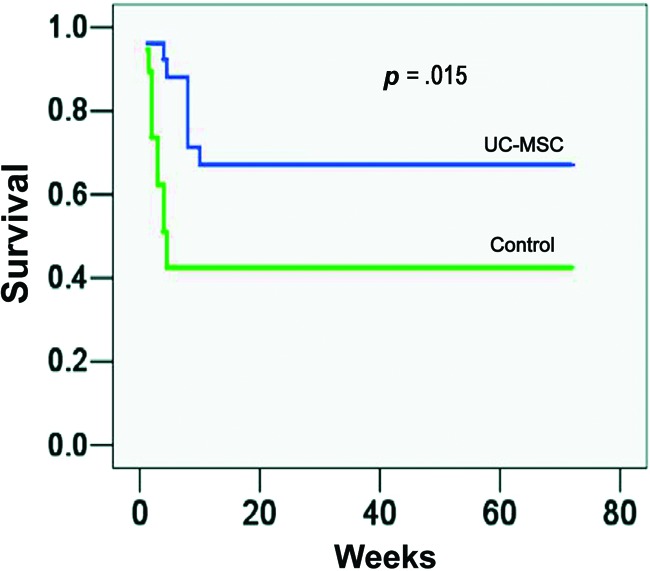
UC-MSC treatments improved the survival of acute-on-chronic liver failure (ACLF) patients. Patients in the treatment group were given UC-MSC transfusions three times at 4-week intervals, whereas patients in the control group received saline infusions. All patients received the same conventional treatment throughout the study. The UC-MSC treatment increased the survival rate for ACLF patients compared with the saline-treated controls. Abbreviation: UC-MSC, umbilical cord-derived mesenchymal stem cell.
Discussion
Stem cell-based therapies are emerging as a novel alternative for the treatment of end-stage liver diseases [21]. In particular, BM-MSC transfusions have been demonstrated to be safe and feasible in clinical use for the treatment of chronic liver cirrhosis and liver failure [15–17, 19]. Our recent trial has shown that UC-MSC treatment improves liver function and ascites in decompensated liver cirrhosis patients [18]; however, it remains obscure whether UC-MSC treatment exerts therapeutic effects on ACLF patients.
Our findings indicate that UC-MSC transfusion through the peripheral vein is safe and feasible in ACLF patients. Compared with BM-MSCs, UC-MSCs may be the better choice for treatment of liver disease. One main reason is that the proliferative abilities of BM-MSCs from patients with chronic HBV infection are deficient [22], whereas UC-MSCs can be obtained from discarded umbilical cords and can be produced on a larger scale. It has been reported that the infusion of human UC-MSCs can improve liver fibrosis in rats and human [18, 23, 24]. This study indicated that the UC-MSC treatments significantly enhanced general liver function as indicated by increased liver functional markers, including ALB, CHE, and PTA levels. In particular, the treated ACLF patients exhibited a decreased MELD score and increased survival rate, and no significant side events were found, indicating the potential therapeutic effects on ACLF.
The mechanisms underlying the improvement of liver function by UC-MSC treatment remain obscure. One possibility is that the UC-MSCs may differentiate into hepatocytes in vivo. Our study indicated a significant increase of α-FP level at 1 week after the first UC-MSC treatment in one ACLF patient. In this patient, the quick increase of α-FP was followed by the improvement of general liver function, indicated by a subsequent increase in the liver synthesis functional marker ALB and PTA and a simultaneous decrease in the liver injury marker TBIL. Furthermore, no supporting evidence of HCC was found. These findings, in combination with several previous studies in which MSCs have the potential to differentiate into hepatocytes in vitro and in vivo [25, 26], suggested a possibility of differentiation of transfused UC-MSCs into hepatocytes in vivo in the patients. Indeed, UC-MSCs can also secrete high levels of hepatic growth factor [18], which may facilitate hepatocyte proliferation [27]. However, we should be cautious in interpreting the observations in this patient. Only one ACLF patient displayed an increase α-FP after UC-MSC treatment. In addition, the rescue of ACLF in such a short period may suggest that differentiation of donor cells into hepatocytes was not the primary cause of the condition. This hypothesis is also supported by several other animal studies, which have shown that only a few (1%–3%) transplanted donor cells have the ability to differentiate into hepatocytes [13, 27, 28]. Notably, although differentiation of some of the transplanted UC-MSCs into hepatocytes was possible, the rapidity of ACLF recovery may suggest that UC-MSC-mediated therapeutic effects may be involved several indirect mechanisms other than functional complementation from direct differentiation.
Another possible explanation is that the UC-MSCs largely enhanced the resident hepatocyte function. Several previous reports have shown that MSCs have the capacity to secrete various bioactive molecules, such as some growth factors and cytokines [29–31]. Such effects can also conceivably enhance endogenous cell proliferation and revascularization of the liver, as shown in an animal model [12]. The contribution of paracrine effects is further supported by a recent study in which MSC-derived conditioned medium (MSC-CM) treatment can induce selective emigration of homed leukocytes out of the injured liver, thus modulating the immune response to liver injury [11]. This observation demonstrates the therapeutic benefit of using MSC-derived biomolecules for treatment of acute liver injury. In addition, MSC-CM treatment can also prevent hepatocyte apoptosis and inhibit bile duct duplication [11]. Indeed, the immunosuppressive properties of MSCs have already been implicated in human disease [7]. MSCs can act through multiple mechanisms to coordinate a dynamic, integrated response to liver injury, and additional studies to examine these multicellular effects are warranted. Further investigation is also needed to determine whether these direct or indirect effects of UC-MSCs, as well as the temporal sequence of histological changes, are the primary targets of UC-MSCs or merely secondary effects.
Notably, the present study was conducted on larger groups relative to previous studies [15–17], and the control and treatment groups were homogeneous in the etiology of liver disease and their baseline clinical characteristics (Table 1). Therefore, this design, at least to some extent, provides more convincing data related to safety and efficacy of UC-MSCs in the treatment of ACLF patients. Similar to our recent study [18], several other limitations were also present in this study. First, we did not track the infused UC-MSCs in patients in vivo. Second, we did not document the histological alterations in the liver in the studied patients because liver biopsies carry a high risk in ACLF patients. These points will be considered in further studies. The present study also highlights several key issues that will need to be considered in the design of future clinical studies, such as the optimal type of stem cells that will be infused, the minimum effective number of the cells, and the best route of administration. We have proposed a randomized control trial of UC-MSC treatment, which may further ascertain the efficacy of UC-MSC treatment in ACLF patients.
Conclusion
In conclusion, our findings demonstrate that UC-MSC transfusion via a peripheral vein is well tolerable and, at least in part, effective in the treatment of ACLF. Partial improvements in liver function and decreases of MELD scores in some ACLF patients are promising. Although we identified the reduction of mortality rates in this study, the protocol for treatment with UC-MSCs should be further refined, and its efficacy should be further assessed in randomized trials with a large cohort study.
Acknowledgments
This study was supported by grants from the Chinese High Tech Research and Development (863) Program (No. 2011AA020104).
Author Contributions
M.S.: conception and design, collection and/or assembly of data, manuscript writing, final approval of manuscript; Z. Zhang: conception and design, collection and/or assembly of data, manuscript writing; R.X.: conception and design, collection and/or assembly of data; H.L., J.F., Z. Zou, A.Z., J.S., L.C., S.L., W.H., and H.G.: collection and/or assembly of data, provision of study material or patients; L.J. and Z.L.: collection and/or assembly of data; F.-S.W.: conception and design, financial support, collection and/or assembly of data, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Bernuau J, Rueff B, Benhamou JP. Fulminant and subfulminant liver failure: Definitions and causes. Semin Liver Dis. 1986;6:97–106. doi: 10.1055/s-2008-1040593. [DOI] [PubMed] [Google Scholar]
- 2.Farci P, Alter HJ, Shimoda A, et al. Hepatitis C virus-associated fulminant hepatic failure. N Engl J Med. 1996;335:631–634. doi: 10.1056/NEJM199608293350904. [DOI] [PubMed] [Google Scholar]
- 3.Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006;35:731–739. doi: 10.1056/NEJMra052270. [DOI] [PubMed] [Google Scholar]
- 4.Sarin SK, Kumar A, Almeida JA, et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific Association for the study of the liver (APASL) Hepatol Int. 2009;3:269–282. doi: 10.1007/s12072-008-9106-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 6.Lee KD, Kuo TK, Whang-Peng J, et al. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275–1284. doi: 10.1002/hep.20469. [DOI] [PubMed] [Google Scholar]
- 7.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 8.Shi M, Liu ZW, Wang FS. Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clin Exp Immunol. 2011;164:1–8. doi: 10.1111/j.1365-2249.2011.04327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singer NG, Caplan AI. Mesenchymal stem cells: Mechanisms of inflammation. Annu Rev Pathol. 2011;6:457–478. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- 10.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 11.Rabani V, Shahsavani M, Gharavi M, et al. Mesenchymal stem cell infusion therapy in a carbon tetrachloride-induced liver fibrosis model affects matrix metalloproteinase expression. Cell Biol Int. 2010;34:601–605. doi: 10.1042/CBI20090386. [DOI] [PubMed] [Google Scholar]
- 12.Chang YJ, Liu JW, Lin PC, et al. Mesenchymal stem cells facilitate recovery from chemically induced liver damage and decrease liver fibrosis. Life Sci. 2009;85:517–525. doi: 10.1016/j.lfs.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Kuo TK, Hung SP, Chuang CH, et al. Stem cell therapy for liver disease: Parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology. 2008;134:2111–2121. doi: 10.1053/j.gastro.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Poll D, Parekkadan B, Cho CH, et al. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology. 2008;47:1634–1643. doi: 10.1002/hep.22236. [DOI] [PubMed] [Google Scholar]
- 15.Terai S, Ishikawa T, Omori K, et al. Improved liver function in patients with liver cirrhosis after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24:2292–2298. doi: 10.1634/stemcells.2005-0542. [DOI] [PubMed] [Google Scholar]
- 16.Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, et al. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med. 2007;10:459–466. [PubMed] [Google Scholar]
- 17.Kharaziha P, Hellström PM, Noorinayer B, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: A phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009;21:1199–1205. doi: 10.1097/MEG.0b013e32832a1f6c. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z, Lin H, Shi M, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012;27(suppl 2):112–120. doi: 10.1111/j.1440-1746.2011.07024.x. [DOI] [PubMed] [Google Scholar]
- 19.Peng L, Xie DY, Lin BL, et al. Autologous bone mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: Short-term and long-term outcomes. Hepatology. 2011;54:820–828. doi: 10.1002/hep.24434. [DOI] [PubMed] [Google Scholar]
- 20.Lu LL, Liu YJ, Yang SG, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91:1017–1026. [PubMed] [Google Scholar]
- 21.Garg V, Garg H, Khan A, et al. Granulocyte-colony stimulating factor mobilizes CD34+ cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology. 2012;142:505–512. doi: 10.1053/j.gastro.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 22.Zhong YS, Lin N, Deng MH, et al. deficient proliferation of bone marrow-derived mesenchymal stem cells in patients with chronic hepatitis B viral infections and cirrhosis of the liver. Dig Dis Sci. 2010;55:438–445. doi: 10.1007/s10620-009-0733-4. [DOI] [PubMed] [Google Scholar]
- 23.Jung KH, Shin HP, Lee S, et al. Effect of human umbilical cord blood-derived mesenchymal stem cells in a cirrhotic rat model. Liver Int. 2009;29:898–909. doi: 10.1111/j.1478-3231.2009.02031.x. [DOI] [PubMed] [Google Scholar]
- 24.Tsai PC, Fu TW, Chen YM, et al. The therapeutic potential of human umbilical mesenchymal stem cells from Wharton's jelly in the treatment of rat liver fibrosis. Liver Transpl. 2009;15:484–495. doi: 10.1002/lt.21715. [DOI] [PubMed] [Google Scholar]
- 25.Hong SH, Gang EJ, Jeong JA, et al. In vitro differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocyte-like cells. Biochem Biophys Res Commun. 2005;330:1153–1161. doi: 10.1016/j.bbrc.2005.03.086. [DOI] [PubMed] [Google Scholar]
- 26.Aurich I, Mueller LP, Aurich H, et al. Functional integration of hepatocytes derived from human mesenchymal stem cells into mouse livers. Gut. 2007;56:405–415. doi: 10.1136/gut.2005.090050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu Y, Lu L, Qian X, et al. Antifibrotic effect of hepatocyte growth factor-expressing mesenchymal stem cells in small-for-size liver transplant rats. Stem Cells Dev. 2010;19:903–914. doi: 10.1089/scd.2009.0254. [DOI] [PubMed] [Google Scholar]
- 28.Sato Y, Araki H, Kato J, et al. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756–763. doi: 10.1182/blood-2005-02-0572. [DOI] [PubMed] [Google Scholar]
- 29.Friedman R, Betancur M, Boissel L, et al. Umbilical cord mesenchymal stem cells: Adjuvants for human cell transplantation. Biol Blood Marrow Transplant. 2007;13:1477–1486. doi: 10.1016/j.bbmt.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 30.Schinköthe T, Bloch W, Schmidt A. In vitro secreting profile of human mesenchymal stem cells. Stem Cells Dev. 2008;17:199–206. doi: 10.1089/scd.2007.0175. [DOI] [PubMed] [Google Scholar]
- 31.Fong CY, Gauthaman K, Cheyyatraivendran S, et al. Human umbilical cord Wharton's jelly stem cells and its conditioned medium support hematopoietic stem cell expansion ex vivo. J Cell Biochem. 2012;113:658–668. doi: 10.1002/jcb.23395. [DOI] [PubMed] [Google Scholar]