In this study metformin, an antidiabetic agent, is identified as a therapeutic activator of FOXO3. The study demonstrates that targeting glioma-initiating cells via the AMP-activated protein kinase- FOXO3 axis is a viable therapeutic strategy against glioblastoma, with metformin being the most clinically relevant drug ever reported for targeting of glioma-initiating cells.
Keywords: Cancer, Cell biology, Cell signaling, Glioma
Abstract
Control of the cancer stem/initiating cell population is considered key to realizing the long-term survival of glioblastoma patients. Recently, we demonstrated that FOXO3 activation is sufficient to induce differentiation of glioma-initiating cells having stem-like properties and inhibit their tumor-initiating potential. Here we identified metformin, an antidiabetic agent, as a therapeutic activator of FOXO3. Metformin activated FOXO3 and promoted differentiation of such stem-like glioma-initiating cells into nontumorigenic cells. Furthermore, metformin promoted FOXO3 activation and differentiation via AMP-activated protein kinase (AMPK) activation, which was sensitive to extracellular glucose availability. Importantly, transient, systemic administration of metformin depleted the self-renewing and tumor-initiating cell population within established tumors, inhibited tumor formation by stem-like glioma-initiating cells in the brain, and provided a substantial survival benefit. Our findings demonstrate that targeting glioma-initiating cells via the AMPK-FOXO3 axis is a viable therapeutic strategy against glioblastoma, with metformin being the most clinically relevant drug ever reported for targeting of glioma-initiating cells. Our results also establish a novel, direct link between glucose metabolism and cancer stem/initiating cells.
Introduction
Glioblastoma, the most common primary brain tumor in adults, is a highly aggressive, invasive, and deadly malignancy [1, 2]. Glioblastoma is also one of the human malignancies with the most miserably poor prognosis: even with the best optimal treatment currently available, patients suffering from this devastating disease rarely survive 5 years, and most patients die within 2 years because of recurrence of the tumor that invariably occurs during the course of this disease and that is essentially fatal [3]. To achieve long-term survival and, ultimately, cure of the patients, control of recurrence is therefore of the utmost clinical importance; glioma stem/initiating cells are now considered to play a critical role in this control [4].
Cancer stem/initiating cells are a subpopulation of tumor cells that not only retain self-renewal capacity and potential to differentiate into multiple lineages similarly to normal tissue stem cells but also have the capacity, when transplanted in vivo, to initiate a tumor reproducing the characteristics of the original tumor [5, 6]. The cancer stem cell hypothesis presumes that such cancer stem cells are the “privileged few” that always remain, as the only cells capable of tumor initiation, at the apex of the hierarchical society of tumor cells composing the tumor [5, 6], although this central tenet of the cancer stem cell hypothesis per se is now being challenged and indeed may not be applicable to some cancer types [7]. Nevertheless, a wealth of observations over decades that gave rise to and are in support of the cancer stem cell hypothesis have consistently indicated that the differentiation status of tumor cells is closely associated with their capacity to initiate tumor formation in vivo, with undifferentiated tumor cells having much higher tumor-initiating potential than differentiated ones within a tumor [6, 8]. Such tight association between differentiation and (loss of) tumor-initiating potential strongly suggests that the core mechanism controlling these seemingly disparate cellular properties is shared and that interventions to promote commitment of cancer stem/initiating cells to differentiation would also lead them to a condition where the cancer stem/initiating cells remain deprived of their tumor-initiating potential as stably as in the differentiated status. In line with such an idea, previous reports have demonstrated that promoting differentiation of glioma stem/initiating cells reduces their tumor-initiating potential, underscoring the idea that differentiation therapy is a promising approach to deplete the stem-like, tumor-initiating cell population of glioblastoma and prevent recurrence [9].
On the basis of the premise that elucidation of the molecular mechanism involved in the maintenance and differentiation of cancer stem/initiating cells would, therefore, provide useful clues to control their tumor-initiating potential, we have been dissecting the mechanism using glioma-initiating cells harboring stem-like properties directly derived from glioblastoma tissues. We have demonstrated that the phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathways are required for the maintenance of such stem-like glioma-initiating cells and that inhibition of both pathways induces differentiation and suppresses their tumor-initiating potential much more effectively than single pathway inhibition alone [10–12]. Furthermore, as an explanation for such a cooperative function of the MAPK and PI3K pathways in the maintenance of stem-like glioma-initiating cells, we have recently identified FOXO3 as an integrator of these pathways. We found that FOXO3 is kept inactive through AKT- and ERK-mediated phosphorylation and that loss of phosphorylation at both the AKT and extracellular signal-regulated kinase (ERK) phosphorylation sites efficiently activates FOXO3, culminating in differentiation of stem-like glioma-initiating cells. Importantly, we confirmed that FOXO3 activation is sufficient to induce differentiation and suppress the tumor-initiating potential of stem-like glioma-initiating cells, suggesting that FOXO3 activators could be a potential molecular targeting drug for glioma-initiating cell-directed therapies [13].
Here in this study, we have identified metformin, a biguanide and the most widely used drug for the treatment of type 2 diabetes, as an activator of FOXO3 in stem-like glioma-initiating cells. Metformin activated FOXO3 via AMP-activated protein kinase (AMPK) and thereby induced differentiation of stem-like glioma-initiating cells in vitro, and effectively suppressed their tumor formation in vivo. Together with the fact that metformin has already been used safely in the clinic and that it efficiently penetrates the blood-brain barrier (BBB) and accumulates in the brain parenchyma [14], our findings suggest that metformin is a strong candidate for clinical use as a cancer stem/initiating cell-targeting drug against glioblastoma.
Materials and Methods
Culture and Analyses of Stem-Like Glioma-Initiating Cells
Patient-derived, stem-like glioma-initiating cells used in this study were directly established from glioblastoma tissues in accordance with a protocol approved by the institutional review boards of Yamagata University School of Medicine and National Cancer Center (SJ28P3, #38, GS-Y01, 02) or were kindly provided by Drs. Tomoki Todo and Nobuhito Saito at the University of Tokyo (TGS01, 04). The details of the cells and their culture conditions are described in the supplemental online data.
Sphere formation assay, gene silencing by small interfering RNA (siRNA), immunoblot analysis, subcellular fractionation, and immunofluorescence were conducted essentially as previously described [13], and the details of the methods as well as information on the reagents and antibodies used are given in the supplemental online data.
Mouse Xenograft Models
Subcutaneous and intracranial xenograft models were created by injecting tumor cells suspended in phosphate-buffered saline into the flank region and the corpus striatum, respectively, of 5-week-old male BALB/cAJcl-nu/nu mice. The details of the animal experiments are described in the supplemental online data. All animal experiments were performed under a protocol approved by the Animal Research Committee of Yamagata University.
Statistical Analysis
Data are expressed as means ± SD, and differences were compared using a two-tailed Student's t test. Mouse survival was evaluated by the Kaplan-Meier method and analyzed by using the log-rank test. p values less than .05 were considered statistically significant.
Results
Metformin Promotes Differentiation of Stem-Like Glioma-Initiating Cells into Nontumorigenic Cells In Vitro
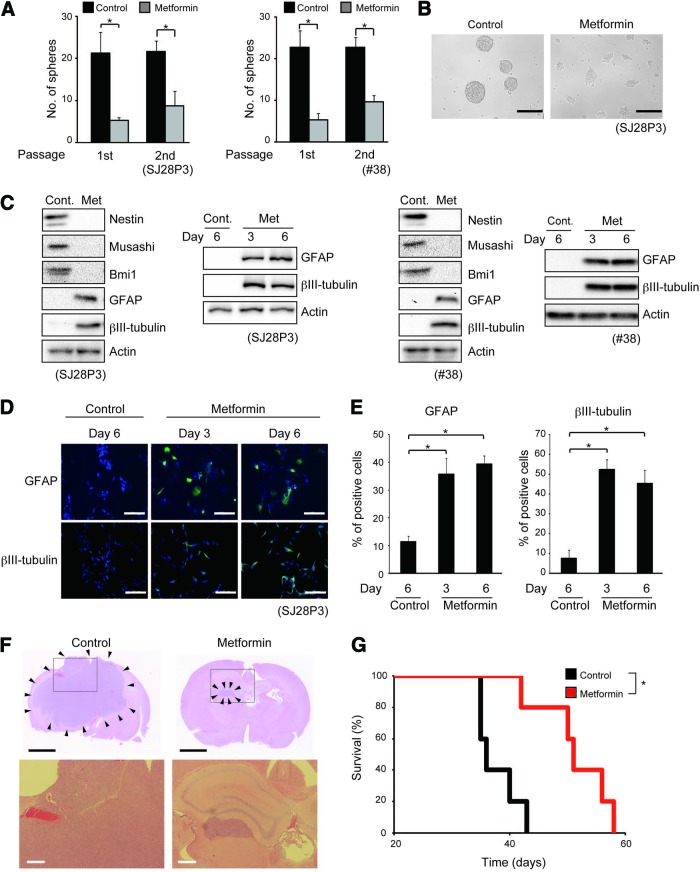
We have recently suggested the possibility that FOXO3-activating agents could promote differentiation/loss of tumor-initiating potential of stem-like glioma-initiating cells and thus contribute to the treatment of glioblastoma [13]. In the course of our search for candidates of such clinically relevant FOXO3 activators, we tested the effect of metformin, an antidiabetic agent that is widely used in the clinic and that has recently been reported to activate FOXO3 [15–17], on stem-like glioma-initiating cells. When stem-like glioma-initiating cells were treated with metformin and examined for their capacity to serially self-renew as spheres in the absence of metformin, metformin treatment resulted in marked inhibition of sphere formation without reducing cellular viability (Fig. 1A, 1B; supplemental online Fig. 1), suggesting that metformin reduces the self-renewal capacity of stem-like glioma-initiating cells. To determine whether the inhibition of sphere-forming capacity actually reflects loss of stem cell properties, we examined the expression of neural stem cell (NSC)/progenitor and differentiation markers. The metformin treatment reduced the expression of nestin, musashi, and Bmi1 (NSC/progenitor markers) and increased that of βIII-tubulin (neural marker) and glial fibrillary acidic protein (GFAP; astrocyte marker) (Fig. 1C). Immunofluorescence analysis also revealed that βIII-tubulin- and GFAP-positive cells increased after the metformin treatment (Fig. 1D, 1E). Thus, metformin inhibits the self-renewal capacity and promotes commitment of stem-like glioma-initiating cells to differentiation.
Figure 1.
Metformin inhibits self-renewal capacity, induces differentiation, and suppresses tumor-initiating potential of stem-like glioma-initiating cells. (A, B): The indicated stem-like glioma-initiating cells were cultured in the presence of either metformin (1 mM) or vehicle control for 3 days and then subjected to sphere formation assay in the absence of metformin. (A): The number of spheres formed (means ± SD from three independent experiments). (B): Representative photomicrographs of spheres formed by SJ28P3 cells. (C): The indicated cells cultured in the presence of either metformin (1 mM) or vehicle control for 3 days (or 6 days where indicated) were subjected to immunoblot analysis of neural stem cell/progenitor (Nestin, Musashi, and Bmi1) and differentiation (GFAP and βIII-tubulin) marker expression. (D, E): SJ28P3 cells cultured in the presence of either metformin (1 mM) or vehicle control for 3 or 6 days were subjected to immunofluorescence analysis for differentiation marker expression. (D): Representative fluorescence images of GFAP and βIII-tubulin expression (green). Nuclei were counterstained with Hoechst 33342 (blue). (E): The proportion of GFAP (left)- and βIII-tubulin (right)-positive cells was determined. The data represent means ± SD from three independent experiments. (F, G): SJ28P3 cells (1 × 104) cultured in the presence of either metformin (1 mM) or vehicle control for 3 days were implanted intracranially into the brain of nude mice. (F): Representative hematoxylin and eosin staining of brain sections from mice sacrificed 30 days after implantation. The lower panels show magnified views of the boxed areas in the corresponding upper panels. The arrowheads in the upper panels indicate tumor areas. (G): Kaplan-Meier plot showing survival of mice (five mice per group) after intracranial implantation. Scale bars = 200 μm (B), 100 μm (D), 2 mm ([F], top), and 200 μm ([F], bottom). *, p < .05. Abbreviations: Cont., control; GFAP, glial fibrillary acidic protein; Met, metformin.
We next determined whether metformin-promoted differentiation of stem-like glioma-initiating cells results in loss of their tumor-initiating potential. When stem-like glioma-initiating cells pretreated in vitro with metformin were implanted into the brains of immunocompromised mice, tumor formation was substantially delayed compared with implantation of their vehicle-pretreated counterparts (Fig. 1F). Consistently, the recipient mice survived significantly longer when they were implanted with metformin-pretreated cells than with vehicle-pretreated cells (Fig. 1G). Together, these results suggested that metformin promotes the differentiation of stem-like glioma-initiating cells and, concurrently, loss of their tumor-initiating potential.
Metformin Promotion of Stem-Like Glioma-Initiating Cell Differentiation Is FOXO3-Dependent
Having shown that metformin promotes differentiation of stem-like glioma-initiating cells, we next asked whether the observed effects of metformin are actually via activation of FOXO3 as we initially surmised. We first determined whether metformin activates FOXO3 in stem-like glioma-initiating cells. Metformin treatment caused nuclear translocation of FOXO3, but not of FOXO1 and FOXO4 (supplemental online Fig. 2A, 2B), accompanied by FOXO3-dependent increase in the expression of p21, the product of a known FOXO3 target gene [18] (supplemental online Fig. 2C). Thus, metformin activates FOXO3 as a transcription factor. We then asked whether the FOXO3 activation is required for the metformin-promoted differentiation. Metformin-induced expression of the differentiation markers GFAP and βIII-tubulin assessed by immunoblot analysis was inhibited by siRNA-mediated knockdown of FOXO3 (supplemental online Fig. 2D). Consistently, an increase in cells positive for the differentiation markers after metformin treatment was effectively blocked by knockdown of FOXO3 (supplemental online Fig. 2E, 2F). Together, these data clearly demonstrate that metformin promotes differentiation of stem-like glioma-initiating cells via activation of FOXO3.
Metformin Activation of AMPK, a Putative Activator of FOXO3, Is Sensitive to Extracellular Glucose Concentration and Is Closely Correlated with Metformin Effects on Stem-Like Glioma-Initiating Cells
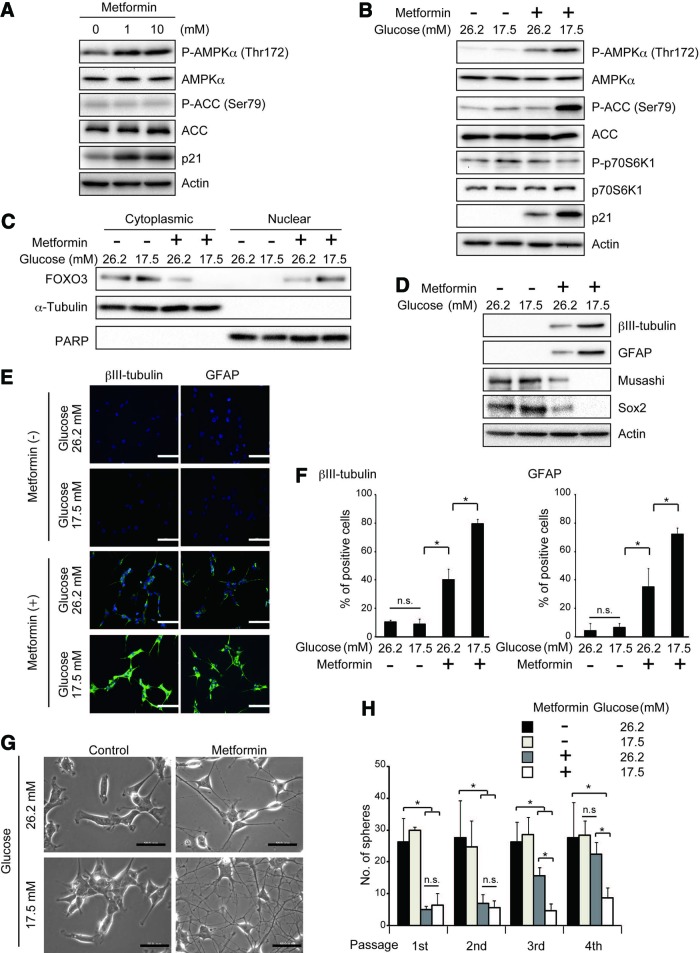
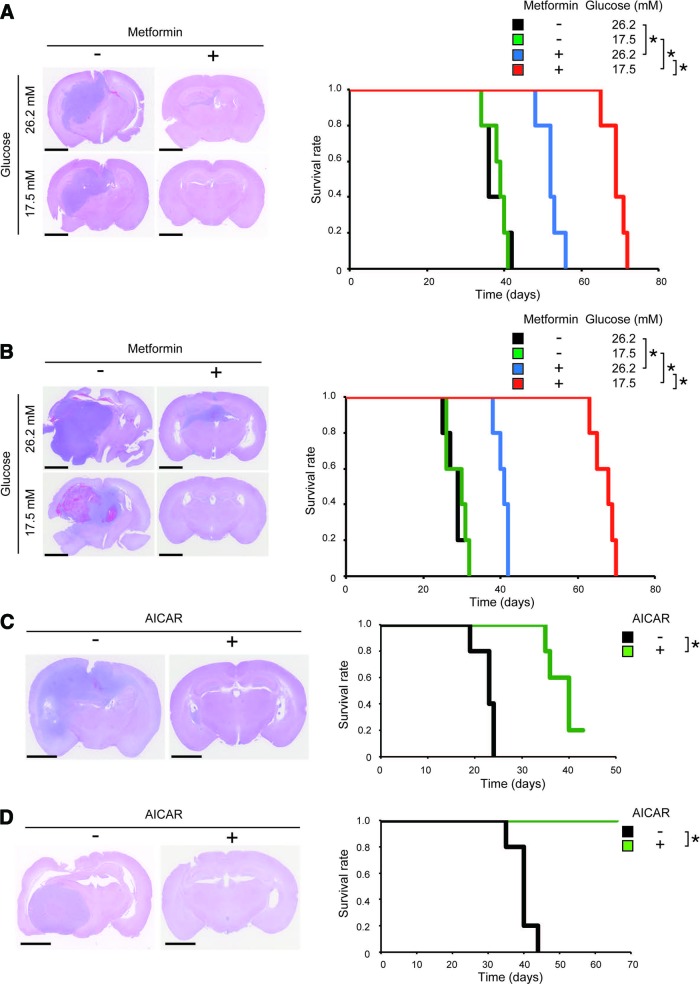
Having shown that metformin promotes differentiation of stem-like glioma-initiating cells in a FOXO3-dependent manner, we next explored the molecular link between metformin and FOXO3. Since previous studies suggested that metformin causes AMPK activation [19–22] and that AMPK may activate FOXO3 either directly or indirectly [23], we examined possible involvement of AMPK in metformin-induced FOXO3 activation in stem-like glioma-initiating cells. To this end, we first evaluated whether metformin treatment activates AMPK by assessing, as an indicator of AMPK activation, the phosphorylation status of Thr172 in the activation loop of the AMPK catalytic subunit α [24]. Metformin treatment (1 mM) caused a significant increase in phosphorylated AMPK (Fig. 2A; supplemental online Fig. 3A), suggesting that metformin activates AMPK in stem-like glioma-initiating cells. However, rather unexpectedly, phosphorylation of acetyl-CoA carboxylase (ACC), a well-established substrate of AMPK, remained unchanged. Higher concentrations of metformin failed to increase phosphorylation of either ACC or AMPK, only to reduce cellular viability (supplemental online Fig. 1). Since previous studies suggested that glucose concentration of the culture medium is a critical determinant of AMPK activation [25, 26], and given that the stem-like glioma-initiating cells are maintained at a much higher glucose concentration (26.2 mM) than physiological concentrations in glioblastoma tissues (consistently lower than 5 mM) [27], we tested the effect of lowering the glucose concentration of the culture medium (Fig. 2B; supplemental online Fig. 3B). Strikingly, when cells cultured in the stem cell culture medium without glucose supplement (glucose concentration, 17.5 mM) were treated with 1 mM metformin, AMPK phosphorylation was accompanied by an apparent increase in ACC phosphorylation and was much more pronounced than when cells cultured in the stem cell culture medium with glucose supplement (glucose concentration, 26.2 mM) were similarly treated. Consistent with the increase in phosphorylated AMPK, we now observed robust phosphorylation of ACC, indicating that metformin activates AMPK far more efficiently at the lower glucose concentration (17.5 mM) than at the higher glucose concentration (26.2 mM). Of note, lowering the glucose concentration of the culture medium per se did not affect the basal activity of AMPK (i.e., phosphorylation of AMPK and ACC) (Fig. 2B; supplemental online Fig. 3B). We also observed that, importantly, metformin activation of FOXO3 is augmented at the lower glucose concentration as revealed by increased nuclear FOXO3 (Fig. 2C; supplemental online Fig. 3C) and p21 expression (Fig. 2B; supplemental online Fig. 3B), in parallel with the enhanced activation of AMPK. We then determined whether such difference of AMPK activation efficiency at different glucose concentrations influences the effect of metformin on the stem cell properties of stem-like glioma-initiating cells. In close correlation with the enhanced activation of AMPK, loss of stem cell marker expression and induction of differentiation marker expression after metformin treatment were considerably promoted at the lower glucose concentration (17.5 mM) (Fig. 2D; supplemental online Fig. 3D). A much larger proportion of cells underwent differentiation upon metformin treatment at the lower glucose concentration than at the higher glucose concentration, as indicated by the increase in GFAP- and βIII-tubulin-positive cells (Fig. 2E, 2F; supplemental online Fig. 3E, 3F), as well as by the increase in cells with extended cellular processes characteristic of differentiation (Fig. 2G; supplemental online Fig. 3G). Consistent with these findings, we observed a sharp decrease in the proportion of cells positive for stem cell markers upon metformin treatment at the lower glucose concentration (supplemental online Fig. 4). The result of serial sphere formation assay also revealed that cells treated with metformin at the lower glucose concentration remained incapable of sphere formation over serial passages, whereas those treated at the higher glucose concentration recovered their ability to form spheres after repeated passages (Fig. 2H; supplemental online Fig. 3H). In sum, these results indicate that the glucose concentration of the culture medium is a critical factor determining metformin activation of AMPK and FOXO3 and that the level of AMPK activation is closely correlated with metformin-promoted differentiation of stem-like glioma-initiating cells.
Figure 2.
AMPK activation by metformin is sensitive to extracellular glucose concentration and is closely correlated with the effects of metformin on stem-like glioma-initiating cells. (A): SJ28P3 cells cultured in the presence of the indicated concentrations of metformin for 3 days under the conventional stem cell culture condition (glucose concentration, 26.2 mM) were subjected to immunoblot analysis of the indicated proteins. (B–H): SJ28P3 cells were cultured with or without metformin (1 mM) for 3 days at the indicated glucose concentrations. The cells were then subjected to immunoblot analysis of the whole (B, D) and fractionated (C) cell lysates, to immunofluorescence analysis (E, F), to observation under a phase contrast microscope (G), or to sphere formation assay (H). Note that the exposure time for each protein is different from that of the corresponding protein in Figure 1C. The data in (F) and (H) represent means ± SD from three independent experiments. Scale bars = 100 μm (E, F) and 50 μm (G). *, p < .05. Abbreviations: ACC, acetyl-CoA carboxylase; AMPK, AMP-activated protein kinase; GFAP, glial fibrillary acidic protein; n.s., not statistically significant; PARP, poly(ADP-ribose) polymerase.
Pivotal Role for the AMPK-FOXO3 Axis in Metformin-Promoted Differentiation of Stem-Like Glioma-Initiating Cells
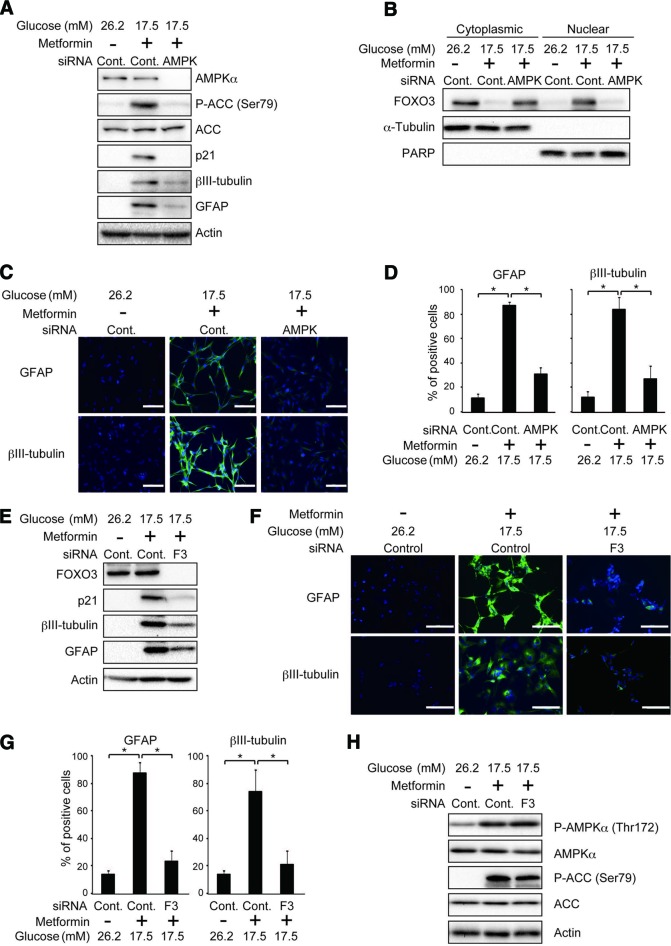
Prompted by the close association among the activation status of AMPK, FOXO3 activation, and differentiation, we next asked whether the AMPK activation plays a causal role in metformin-promoted FOXO3 activation and consequently differentiation of stem-like glioma-initiating cells. Under the experimental condition whereby knockdown of the AMPKα catalytic subunit effectively prevented metformin-induced phosphorylation of ACC (Fig. 3A; supplemental online Fig. 5A), FOXO3 activation (expression of nuclear FOXO3 and p21) by metformin was almost completely blocked (Fig. 3A, 3B; supplemental online Fig. 5A, 5B), and the differentiation-promoting effect of metformin was substantially compromised (Fig. 3A, 3C, 3D; supplemental online Fig, 5A, 5C, 5D). To definitively determine whether AMPK contributes to metformin-promoted differentiation through its function as a kinase, we also tested the effect of compound C, a cell-permeable pharmacological inhibitor of AMPK that inhibits the kinase activity of AMPK in ATP-competitive manner. Compound C was as effective as knockdown-mediated inhibition of AMPK in preventing metformin-induced ACC phosphorylation (supplemental online Fig. 6A, 6E), FOXO3 activation (supplemental online Fig. 6A, 6B, 6E, 6F), and differentiation (supplemental online Fig. 6A, 6C–6E, 6G, 6H). To formally exclude the imaginable possibility that FOXO3 acts upstream of AMPK in the metformin-elicited intracellular signaling culminating in differentiation, we examined the effect of FOXO3 inhibition on AMPK activation. FOXO3 knockdown, which did inhibit metformin-induced differentiation (Fig. 3E–3G; supplemental online Fig. 5E–5G), had no appreciable effect on metformin-induced activation of AMPK (Fig. 3H; supplemental online Fig. 5H). Thus, collectively, the data indicate that AMPK-dependent activation of FOXO3 (the AMPK-FOXO3 axis) plays an essential role in metformin-promoted differentiation of stem-like glioma-initiating cells.
Figure 3.
Pivotal role for the AMPK-FOXO3 axis in metformin-promoted differentiation of stem-like glioma-initiating cells. (A–D): SJ28P3 cells cultured at the indicated glucose concentrations were transfected with a control small interfering RNA (siRNA) (Cont.) or with siRNAs against AMPKα (AMPK) and treated, 10 hours after transfection, with or without metformin (1 mM) for 3 days. The cells were then subjected to immunoblot analysis of the whole (A) and fractionated (B) cell lysates or to immunofluorescence analysis (C, D). Representative fluorescence images (C) and the proportion of cells positive for the indicated markers (D) are shown. (E–H): SJ28P3 cells cultured at the indicated glucose concentrations were transfected with a control siRNA (Cont.) or with an siRNA against FOXO3 (F3) and treated, 10 hours after transfection, with or without metformin (1 mM) for 3 days. The cells were then subjected to immunoblot (E, H) or to immunofluorescence analysis (F, G). Representative fluorescence images (F) and the proportion of cells positive for the indicated markers (G) are shown. The data in (D) and (G) represent means ± SD from three independent experiments. Scale bars = 100 μm. *, p < .05. Abbreviations: ACC, acetyl-CoA carboxylase; AMPK, AMP-activated protein kinase; Cont., control; F3, FOXO3; GFAP, glial fibrillary acidic protein; PARP, poly(ADP-ribose) polymerase; siRNA, small interfering RNA.
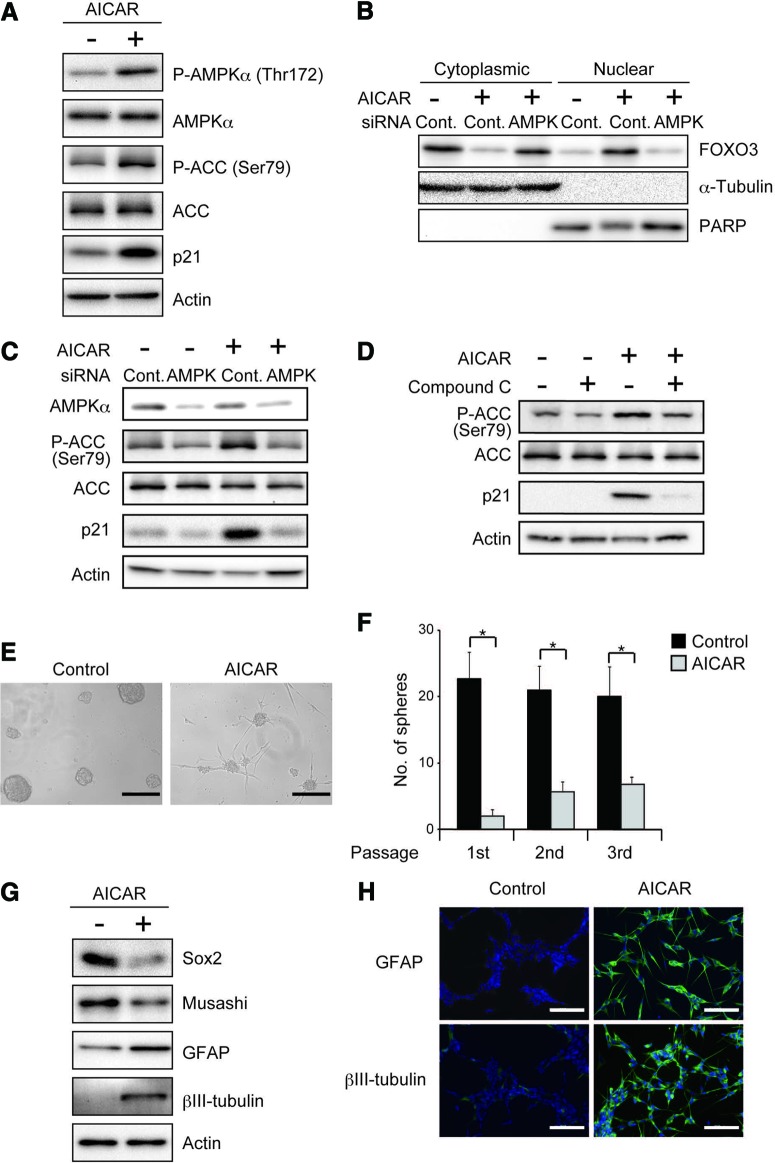
Previously, we demonstrated that FOXO3 activation is sufficient to promote differentiation of stem-like glioma-initiating cells. We therefore wished to ask in this study whether, similarly, AMPK activation is not only required for differentiation promoted by metformin but also sufficient by itself to commit them to differentiation even in the absence of metformin treatment. In an attempt to test this point, we took advantage of 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR), a cell-permeable precursor to ZMP (AICAR monophosphate), an AMP mimetic that binds to the AMPKγ subunits and thus activates AMPK directly [28]. As expected, AICAR treatment of stem-like glioma-initiating cells caused activation of AMPK as indicated by increased phosphorylation of AMPK and ACC (Fig. 4A; supplemental online Fig. 7A) and was also sufficient to induce the expression of nuclear FOXO3 (Fig. 4B; supplemental online Fig. 7B) and its target gene product p21 (Fig. 4A; supplemental online Fig. 7A), indicating that AICAR activates FOXO3. Importantly, AICAR activation of FOXO3 as assessed by its nuclear translocation and p21 expression was impaired when AMPK was inhibited either genetically by siRNA-mediated knockdown or pharmacologically by use of compound C, indicating that AICAR does activate FOXO3 via AMPK (Fig. 4B–4D; supplemental online Fig. 7B–7D). Consistent with its ability to activate FOXO3, AICAR inhibited sphere formation (Fig. 4E, 4F; supplemental online Fig. 7E, 7F) and promoted differentiation (Fig. 4G, 4H; supplemental online Fig. 7G, 7H). Thus, these results of AICAR experiments lend strong support to the idea that AMPK activation is sufficient to promote commitment of stem-like glioma-initiating cells to differentiation via activation of FOXO3.
Figure 4.
AMPK activation by AICAR is sufficient to activate FOXO3, inhibit self-renewal capacity, and induce differentiation of stem-like glioma-initiating cells. (A): SJ28P3 cells cultured in the presence or absence of AICAR (1 mM) for 3 days under the conventional stem cell culture condition (glucose concentration, 26.2 mM) were subjected to immunoblot analysis for the indicated protein expression. (B, C): SJ28P3 cells transfected with a control siRNA (Cont.) or with siRNAs against AMPKα (AMPK) were treated, 10 hours after transfection, with or without AICAR (1 mM) for 3 days. The cells were then subjected to immunoblot analyses of the fractionated (B) and whole (C) cell lysates. (D): SJ28P3 cells treated with or without AICAR (1 mM) in the presence or absence of compound C (10 μM) for 3 days were subjected to immunoblot analysis of the indicated proteins. (E–H): SJ28P3 cells cultured in the presence or absence of AICAR (1 mM) for 3 days were subjected to sphere formation assay (E, F), immunoblot analysis (G), and immunofluorescence staining (H). Representative photomicrographs of the spheres formed in the primary sphere formation assay (E) and the number of spheres formed (F) are shown. The data in (F) represent means ± SD from three independent experiments. Scale bars = 200 μm. *, p < .05. Abbreviations: ACC, acetyl-CoA carboxylase; AICAR, 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside; AMPK, AMP-activated protein kinase; Cont., control; GFAP, glial fibrillary acidic protein; PARP, poly(ADP-ribose) polymerase; siRNA, small interfering RNA.
Because previous studies have shown that metformin may inhibit mammalian target of rapamycin (mTOR) dependently and independently of AMPK [29, 30] and that mTOR inhibition could affect FOXO3 [31], we also investigated the possibility that metformin activates FOXO3 via mTOR inhibition. However, the phosphorylation of p70 S6 kinase, a representative substrate of mTOR, remained unchanged even after the metformin treatment (Fig. 2B; supplemental online Fig. 3B), arguing against the involvement of mTOR in metformin activation of FOXO3. Altogether, the data demonstrate that the AMPK-FOXO3 axis plays a pivotal role in metformin-promoted differentiation of stem-like glioma-initiating cells.
The AMPK-FOXO3 Axis Negatively Regulates the Tumor-Initiating Potential of Stem-Like Glioma-Initiating Cells
The pivotal role of the AMPK-FOXO3 axis demonstrated in the control of differentiation of stem-like glioma-initiating cells strongly suggested that the axis also has a key role in the control of their tumor-initiating potential. We therefore evaluated next the impact of activating the AMPK-FOXO3 axis on the tumor-initiating potential of stem-like glioma-initiating cells.
As shown earlier, metformin activates the AMPK-FOXO3 axis in stem-like glioma-initiating cells, which is further augmented at the lower glucose concentration (17.5 mM) than at the higher glucose concentration (26.2 mM) (Fig. 2B, 2C; supplemental online Fig. 3B, 3C). To determine, first, whether activation of the AMPK-FOXO3 axis is associated with loss of tumor-initiating potential, we subjected stem-like glioma-initiating cells to metformin treatment in vitro at the higher and lower glucose concentrations and then assessed their tumor-initiating potential in the intracranial xenograft analysis. The results clearly indicated that survival of the tumor-bearing mice is extended in good association with the preimplantation activation status of the AMPK-FOXO3 axis of the implanted cells (Fig. 5A, 5B), suggesting that the axis negatively regulates the tumor-initiating potential of the cells. Then, to definitively establish that the activation of the AMPK-FOXO3 axis deprives stem-like glioma-initiating cells of their tumor-initiating potential, we treated the cells with AICAR in vitro to directly activate the AMPK-FOXO3 axis (Fig. 4A, 4B; supplemental online Fig. 7A, 7B) and then subjected them to the intracranial xenograft analysis. The AICAR treatment substantially delayed or even prevented tumor formation by stem-like glioma-initiating cells (Fig. 5C, 5D), suggesting that activation of the AMPK-FOXO3 axis may be sufficient to inhibit their tumor-initiating potential. Thus, the data clearly demonstrate that the AMPK-FOXO3 axis is a negative regulator of the tumor-initiating potential and also suggest that the axis may be at least in part responsible for the inhibitory effect of metformin on tumor initiation by stem-like glioma-initiating cells.
Figure 5.
Inhibition of tumor-initiating potential of stem-like glioma-initiating cells by metformin and AICAR as activators of AMP-activated protein kinase. (A, B): SJ28P3 (A) or #38 (B) cells (1 × 104) cultured at the indicted glucose concentrations and treated with or without metformin (1 mM) for 3 days were implanted orthotopically into the brain of nude mice. (C, D): SJ28P3 (C) or #38 (D) cells (1 × 104) cultured under the conventional stem cell culture condition (glucose concentration, 26.2 mM) and treated with or without AICAR (1 mM) for 3 days were implanted orthotopically into the brain of nude mice. Left: Representative hematoxylin and eosin staining of brain sections from mice sacrificed 30 days after implantation. Right: Kaplan-Meier plots showing survival of mice (five mice per group) after implantation. Scale bars = 2 mm. *, p < .05. Abbreviation: AICAR, 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside.
Systemically Administered Metformin Depletes the Self-Renewing and Tumor-Initiating Cell Population Within Established Tumors
The results of our in vitro analyses demonstrated that metformin effectively promotes commitment of stem-like glioma-initiating cells to differentiation accompanied by loss of tumor-initiating potential, at least in part through activation of the AMPK-FOXO3 axis. We then asked, with an intention to explore the therapeutic potential of metformin in glioblastoma treatment, whether metformin effectively inhibits tumor initiation by stem-like glioma-initiating cells in vivo as well.
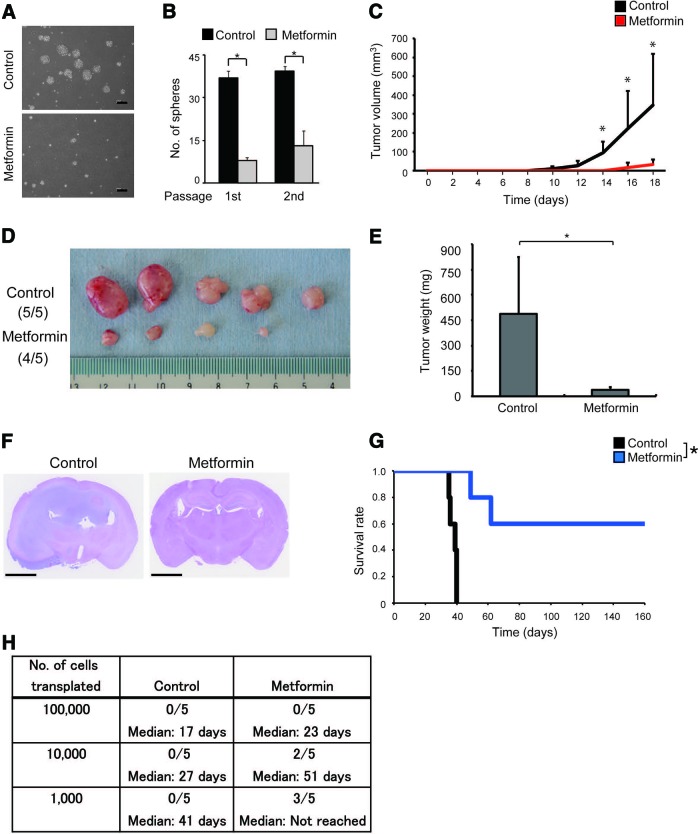
To evaluate such in vivo effectiveness of metformin and demonstrate in the most straightforward and unequivocal manner that metformin targets stem-like glioma-initiating cells in vivo and differentiates them into cells incapable of self-renewal and tumor initiation, we treated tumor-bearing mice with systemic administration of metformin for a defined period and then examined the impact of the short-term in vivo metformin treatment on the self-renewing and tumor-initiating cell population within the tumors. We generated subcutaneous tumors in nude mice by implanting stem-like glioma-initiating cells, and when the tumor diameter reached 8–9 mm, the tumor-bearing mice received daily intraperitoneal injections of metformin (500 mg/kg) or the vehicle control for 10 consecutive days. Comparison of the tumor volumes before and after the metformin treatment indicated that the metformin treatment has only marginal inhibitory effect on the growth of the tumors in vivo (supplemental online Fig. 8). After completion of the 10-day treatment, the tumors were excised, dissociated, and subjected to sphere formation assay to assess the abundance of the stem cell population with self-renewal capacity within the tumors. Compared with control-treated tumors, tumors treated in vivo with metformin yielded significantly smaller numbers of tumorspheres (Fig. 6A, 6B). At the same time, we also subjected the dissociated tumor cells to xenograft analyses. Subcutaneous transplantation of tumor cells treated in vivo with the vehicle control led to rapid formation of secondary tumors. In stark contrast, the in vivo metformin treatment remarkably delayed or even prevented secondary tumor formation by the subcutaneously transplanted tumor cells (Fig. 6C–6E). Similarly, whereas intracranial transplantation of the control-treated tumor cells invariably led to massive brain tumor formation and eventual death of the recipient mice, mice receiving tumor cells treated in vivo with metformin in general survived much longer, and some were still alive without any signs of brain tumor burden even after an extended observation period (160 days after transplantation) (Fig. 6F–6H). Altogether, these data clearly demonstrate that metformin treatment depletes the self-renewing and tumor-initiating cell population within established tumors in vivo.
Figure 6.
Transient, systemic administration of metformin depletes the self-renewing and tumor-initiating cell population from established tumors. (A, B): Nude mice implanted subcutaneously with SJ28P3 cells were, after tumor formation, randomized into control and metformin treatment groups and received intraperitoneal injection of vehicle control and metformin (500 mg/kg per day, once daily for 10 days), respectively. On the next day of the final drug treatment, the mice were sacrificed, and dissociated tumor cells (1 × 104 viable cells) were subjected to sphere formation assay (A, B). Representative phase-contrast micrographs of primary spheres (A) and the number of spheres formed (B) are shown. The data in (B) represent means ± SD from three independent cultures derived from three independent tumors. *, p < .05. (C–E): Alternatively, the dissociated tumor cells (1 × 106 viable cells per mouse) were transplanted subcutaneously into the right flank of nude mice (five mice per group). Tumor volume was measured at the indicated time points (C), and at the end of the observation period (18 days after transplantation), the secondary tumors derived from control-treated (tumors formed in five of five mice) and metformin-treated (tumors formed in four of five mice) primary tumors were excised, photographed (D) and evaluated for their weight (E). (F–H): Serial dilutions of the dissociated tumor cells (1 × 105, 1 × 104, or 1 × 103 viable cells) derived from control- and metformin-treated primary tumors were also transplanted intracranially into nude mice. (F): Representative hematoxylin and eosin staining of brain sections from mice receiving transplantation of cells (1 × 104) from primary tumors treated with metformin or vehicle control (sacrificed at 27 days after transplantation). (G): The survival of mice (five mice per group) receiving transplantation of control- or metformin-treated primary tumor cells (1 × 103) was evaluated by Kaplan-Meier analysis. (H): A table showing survival of mice at 160 days after intracranial transplantation of control- or metformin-treated primary tumor cells (upper rows) and the median survival time (lower rows). Scale bars = 200 μm (A) and 2 mm (F).
Systemically Administered Metformin Effectively Suppresses Tumor Formation by Stem-Like Glioma-Initiating Cells Implanted in the Brain Parenchyma and Prolongs Survival of Recipient Mice
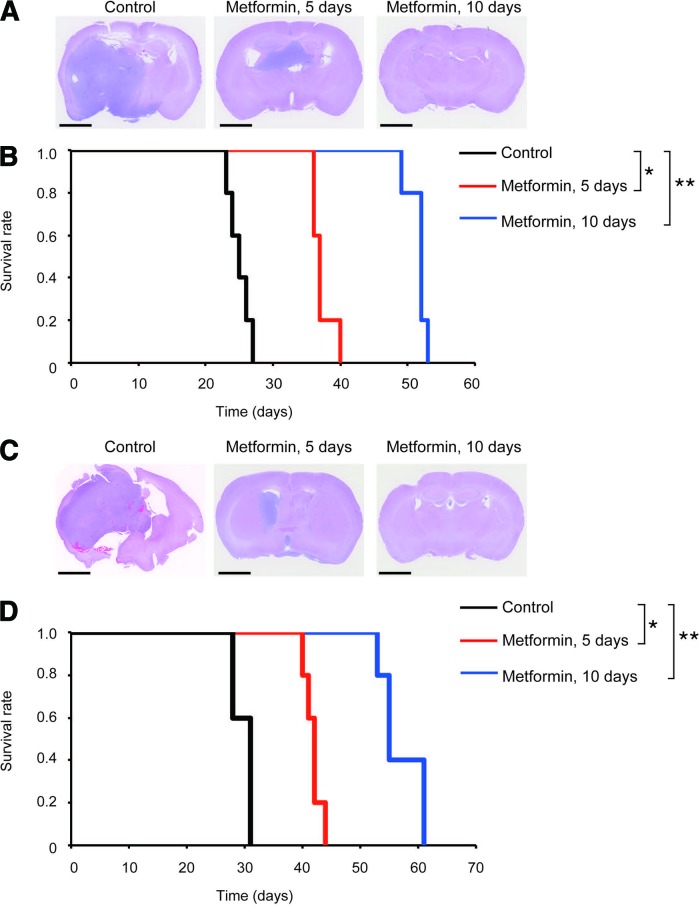
Since glioblastomas are highly invasive in nature, it is essential that therapeutic agents targeting glioma-initiating cells do reach tumor cells that deeply infiltrate the brain and are therefore protected by the intact BBB. Given the well-documented property of metformin to penetrate the BBB and efficiently accumulate in the brain parenchyma after systemic administration [14], we next tested the effect of systemically administered metformin against stem-like glioma-initiating cells in the brain parenchyma. To correctly evaluate whether metformin promotes “commitment” of the cells to differentiation into nontumorigenic cells, it is desired that metformin treatment be initiated as early as possible after implantation of stem-like glioma-initiating cells before they produce nonstem progenies and be discontinued after a defined period (because, otherwise, it is impossible to discern whether the observed antitumor effect of metformin is due to its expected effect or simply to reversible proliferation block). We therefore, in the initial treatment protocol, started metformin treatment (500 mg/kg per day, intraperitoneal injection) on the next day of implantation, and metformin was administered for 5 consecutive days thereafter and then discontinued. When recipient mice with intracranially implanted stem-like glioma-initiating cells were treated under this treatment protocol, brain tumor formation was retarded, with the survival of the mice treated with metformin being extended by approximately 2 weeks compared with the control mice that invariably succumbed to brain tumors within 1 month (Fig. 7). Since mice tolerated this 5-day treatment protocol quite well, we modified the protocol so that metformin was administered for 10 consecutive days. Mice again tolerated this modified metformin treatment protocol quite well, and strikingly, the 10-day protocol successfully suppressed brain tumor formation and extended mouse survival by approximately 1 month, almost doubling the survival time (Fig. 7). These results clearly demonstrate that metformin effectively targets stem-like glioma-initiating cells in the brain parenchyma and also suggest that metformin inhibits brain tumor formation by depletion of stem-like glioma-initiating cells rather than by simply blocking their proliferation.
Figure 7.
Prevention of brain tumor formation by stem-like glioma-initiating cells implanted in the brain parenchyma via transient, systemic administration of metformin. (A–D): Nude mice implanted intracranially with SJ28P3 (A, B) or #38 (C, D) cells (1 × 104) underwent transient, systemic administration (intraperitoneal delivery, once daily for 5 or 10 consecutive days) of metformin (500 mg/kg per day) or vehicle control, which started on the next day of intracranial implantation. (A, C): Representative hematoxylin and eosin staining of brain sections from mice sacrificed at 25 days after implantation. (B, D): Survival of mice was evaluated by Kaplan-Meier analysis. Scale bars = 2 mm (A, C). *, p < .05; **, p < .01.
Discussion
In the course of our search for therapeutic FOXO3-activating agents that induce differentiation and inhibit tumor-initiating potential of stem-like glioma-initiating cells, we identified metformin as one such agent and also found that AMPK plays a pivotal role in metformin-promoted FOXO3 activation and differentiation of stem-like glioma-initiating cells. In addition to the two patient-derived cells used in the main analyses of the present study, we tested the effect of metformin on three other patient-derived stem-like glioma-initiating cells and confirmed that metformin efficiently activates AMPK and induces differentiation in all of them (supplemental online Fig. 9). Thus, the susceptibility to the metformin effect and the role of the AMPK-FOXO3 axis in the control of differentiation/tumor-initiating potential might be common to stem-like glioma-initiating cells in general, which is a great advantage when metformin and/or AMPK-FOXO3-targeting drugs are considered for clinical application.
Most importantly, we have demonstrated in this study that systemic administration of metformin for a defined, short-term period is sufficient to successfully deplete the tumor-initiating cell population within established tumors and to suppress brain tumor formation by stem-like glioma-initiating cells in the brain so effectively as to provide substantial survival benefit. Thus, to the best of our knowledge, metformin is the first drug capable of depleting stem-like glioma-initiating cells in vivo in a therapeutically significant manner (i.e., with significant survival benefit) via systemic administration. Although we did not achieve a “cure” in the orthotopic xenograft treatment model (Fig. 7) in this study, this might have been possible if we had extended the treatment period, decreased the initial tumor burden (i.e., the number of stem-like glioma-initiating cells at the time of treatment initiation), or both. Indeed, such factors (treatment period and initial tumor burden) are expected to become critical factors that may influence the therapeutic impact of metformin when it is used in clinic. Together with the fact that metformin easily penetrates the BBB and accumulates in the brain parenchyma [14], as well as with the fact that metformin has been widely and safely used in clinic as an antidiabetic drug [32, 33], our findings imply that metformin is by far closer to the clinic than any other drugs reported to date that target stem-like glioma-initiating cells. Of note, although this is the first study to demonstrate the ability of metformin to differentiate stem-like glioma-initiating cells into nontumorigenic cells, there is an in vitro study reporting that metformin either inhibits cellular proliferation or induces apoptosis of serum-cultured glioma cell lines in cell density-dependent manner [34], which suggests that metformin may have a growth inhibitory effect on bulk glioblastoma cells as well. Consistent with this report, we observed that systemic administration of metformin does retard, albeit modestly, the growth of established tumors (supplemental online Fig. 8). Thus, our results may also be the first evidence that metformin has a growth inhibitory effect on bulk glioblastoma in vivo.
Compared with glioblastoma, the antitumor effect of metformin has been studied and documented in somewhat more detail in a number of human cancers, most typically breast cancer [32, 33]. The inhibitory effect of metformin on cancer stem cells of breast, prostate, and lung cancers has also been reported [35, 36]. Nevertheless, the molecular pathway(s) mediating such antitumor effect of metformin still remains ill-defined. Although AMPK appears to be a common, key molecule for the antitumor effect of metformin in most instances, a number of downstream molecules/pathways have been proposed, of which AMPK-mediated downregulation of mTOR (the AMPK-mTOR axis) is considered to play a major role [32, 33]. Here in this study, we have shown that, instead of the well-known AMPK-mTOR axis, AMPK-mediated activation of FOXO3 plays a predominant role in metformin-mediated promotion of stem-like glioma-initiating cell differentiation into nontumorigenic cells. Apparently, this is the first demonstration that the AMPK-FOXO3 axis has a role in the antitumor effect of metformin. It remains unknown at this moment whether the role of this AMPK-FOXO3 axis in the antitumor effect of metformin is unique to glioblastoma or to cancer stem cells, but future investigations will clarify this point and delineate the role of this axis in other human cancers as well as in stem and nonstem cancer cells.
In addition to the therapeutic impact of metformin, we have also shed the very first light in this study on AMPK as a critical molecule involved in the control of differentiation and tumor-initiating potential of stem-like glioma-initiating cells. Of note, the activity of AMPK (and, similarly, FOXO3) was required for metformin-promoted differentiation of stem-like glioma-initiating cells but not for loss of stem cell marker expression and self-renewal capacity caused by metformin (not shown). However, AMPK activation by the direct activator AICAR was sufficient to inhibit stem cell marker expression/self-renewal capacity and induce differentiation. Thus, the findings suggested that (a) metformin may cause initial loss of stem cell marker expression/self-renewal capacity independently of the AMPK-FOXO3 axis, and (b) the activation of the AMPK-FOXO3 axis may promote full differentiation of such “intermediate” cells and also ensure that they will not regain stem cell properties. On the other hand, it remains to be shown whether AMPK is required for the inhibitory effect of metformin on the tumor-initiating potential of stem-like glioma-initiating cells. However, our results indicated that AMPK activation, similarly to differentiation, is sufficient to inhibit their tumor-initiating potential, quite in line with our previous observation that FOXO3 activation is sufficient to inhibit it. Thus, although our study does not necessarily preclude activation of another, parallel signaling pathway(s) by metformin that negatively regulates the tumor-initiating potential of stem-like glioma-initiating cells in conjunction with the AMPK-FOXO3 axis, our findings clearly indicate that, once activated, the AMPK-FOXO3 axis has a dominant role in the inhibition of the tumor-initiating potential of stem-like glioma-initiating cells and is therefore a potential target of cancer stem/initiating cell-directed glioblastoma therapy.
Quite intriguingly, our identification of AMPK, a key sensor and regulator of cellular metabolism [24], as a critical regulator of differentiation and tumor-initiating potential of stem-like glioma-initiating cells suggests a novel molecular link between glioblastoma cell biology and cellular metabolism, which is apparently subject to the environmental milieu surrounding the tumor cells. Indeed, we observed that the effect of metformin was substantially compromised against cells cultured at a higher glucose concentration (26.2 mM) where AMPK activation was restrained. We also noted that stem-like glioma-initiating cells cannot be maintained for a long time when the glucose concentration of the culture medium was reduced from 26.2 to 17.5 mM (unpublished observation). These observations point to the possibility that glucose metabolism could have a significant impact on the therapeutic response and/or recurrence of glioblastoma, given the expected role of stem-like glioma-initiating cells in these processes.
Conclusion
We have demonstrated for the first time in this study that metformin, as a FOXO3 activator, is a viable therapeutic agent for cancer stem/initiating cell-directed glioblastoma therapy and that, upon systemic administration, it crosses the BBB and prevents brain tumor formation by stem-like glioma-initiating cells. The key role of the AMPK-FOXO3 axis in the control of stem-like glioma-initiating cells demonstrated in this study gives rise to the novel and intriguing idea that interventions to modulate cellular metabolism could contribute to the prevention and therapy of glioblastoma.
See www.StemCellsTM.com for supporting information available online.
Acknowledgments
We thank Drs. Tomoki Todo and Nobuhito Saito at the University of Tokyo for generously providing us with the TGS cells, and we also thank Dr. Motoo Nagane at Kyorin University for valuable advice on xenograft experiments. We thank members of our laboratory for fruitful discussions and technical advice, and we thank Dr. Tomoko Kagawa for her continuous support/encouragement. This work was supported by Grants-in-Aid for Scientific Research, for Challenging Exploratory Research, and for Young Scientists from the Ministry of Education, Culture, Sports, Science and Technology of Japan; by a Grant-in-Aid from the Global COE Program of the Japan Society for the Promotion of Science; by the National Cancer Center Research and Development Fund (23-A-20); and by a grant from the Japan Brain Foundation.
Author Contributions
A.S.: concept and design, collection and assembly of data, data analysis and interpretation, manuscript writing; J.S.: concept and design, collection and assembly of data, data analysis and interpretation; M.O.: data analysis and interpretation; E.W., S. Seino, and K. Shibuya: collection and assembly of data; K. Suzuki: provision of study material or patients; Y.N., S. Shibui, and T.K.: provision of study material or patients, data analysis and interpretation; C.K.: concept and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Tabatabai G, Weller M. Glioblastoma stem cells. Cell Tissue Res. 2011;343:459–465. doi: 10.1007/s00441-010-1123-0. [DOI] [PubMed] [Google Scholar]
- 3.Lathia JD, Venere M, Rao MS, et al. Seeing is believing: Are cancer stem cells the Loch Ness monster of tumor biology? Stem Cell Rev. 2011;7:227–237. doi: 10.1007/s12015-010-9194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neman J, Jandial R. Decreasing glioma recurrence through adjuvant cancer stem cell inhibition. Biologics. 2010;4:157–162. doi: 10.2147/btt.s9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 6.Clevers H. The cancer stem cell: Premises, promises and challenges. Nat Med. 2011;17:313–319. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- 7.Shackleton M, Quintana E, Fearon ER, et al. Heterogeneity in cancer: Cancer stem cells versus clonal evolution. Cell. 2009;138:822–829. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 8.Frank NY, Schatton T, Frank MH. The therapeutic promise of the cancer stem cell concept. J Clin Invest. 2010;120:41–50. doi: 10.1172/JCI41004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng L, Bao S, Rich JN. Potential therapeutic implications of cancer stem cells in glioblastoma. Biochem Pharmacol. 2010;80:654–665. doi: 10.1016/j.bcp.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sunayama J, Sato A, Matsuda K, et al. Dual blocking of mTor and PI3K elicits a prodifferentiation effect on glioblastoma stem-like cells. Neuro Oncol. 2010;12:1205–1219. doi: 10.1093/neuonc/noq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sunayama J, Matsuda K, Sato A, et al. Crosstalk between the PI3K/mTOR and MEK/ERK pathways involved in the maintenance of self-renewal and tumorigenicity of glioblastoma stem-like cells. Stem Cells. 2010;28:1930–1939. doi: 10.1002/stem.521. [DOI] [PubMed] [Google Scholar]
- 12.Sato A, Sunayama J, Matsuda K, et al. MEK-ERK signaling dictates DNA-repair gene MGMT expression and temozolomide resistance of stem-like glioblastoma cells via the MDM2–p53 axis. Stem Cells. 2011;29:1942–1951. doi: 10.1002/stem.753. [DOI] [PubMed] [Google Scholar]
- 13.Sunayama J, Sato A, Matsuda K, et al. FoxO3a functions as a key integrator of cellular signals that control glioblastoma stem-like cell differentiation and tumorigenicity. Stem Cells. 2011;29:1327–1337. doi: 10.1002/stem.696. [DOI] [PubMed] [Google Scholar]
- 14.L⧹abuzek K, Suchy D, Gabryel B, et al. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol Rep. 2010;62:956–965. doi: 10.1016/s1734-1140(10)70357-1. [DOI] [PubMed] [Google Scholar]
- 15.Cantó C, Gerhart-Hines Z, Feige JN, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zakikhani M, Dowling R, Fantus IG, et al. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66:10269–10273. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]
- 17.Hou X, Song J, Li XN, et al. Metformin reduces intracellular reactive oxygen species levels by upregulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway. Biochem Biophys Res Commun. 2010;396:199–205. doi: 10.1016/j.bbrc.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Seoane J, Le HV, Shen L, et al. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117:211–223. doi: 10.1016/s0092-8674(04)00298-3. [DOI] [PubMed] [Google Scholar]
- 19.Fryer LG, Parbu-Patel A, Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 20.Hawley SA, Gadalla AE, Olsen GS, et al. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 2002;51:2420–2425. doi: 10.2337/diabetes.51.8.2420. [DOI] [PubMed] [Google Scholar]
- 21.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw RJ, Lamia KA, Vasquez D, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiacchiera F, Simone C. The AMPK-FoxO3A axis as a target for cancer treatment. Cell Cycle. 2010;9:1091–1096. doi: 10.4161/cc.9.6.11035. [DOI] [PubMed] [Google Scholar]
- 24.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salt IP, Johnson G, Ashcroft SJ, et al. AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic beta cells, and may regulate insulin release. Biochem J. 1998;335:533–539. doi: 10.1042/bj3350533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Itani SI, Saha AK, Kurowski TG, et al. Glucose autoregulates its uptake in skeletal muscle: Involvement of AMP-activated protein kinase. Diabetes. 2003;52:1635–1640. doi: 10.2337/diabetes.52.7.1635. [DOI] [PubMed] [Google Scholar]
- 27.Marcus HJ, Carpenter KL, Price SJ, et al. In vivo assessment of high-grade glioma biochemistry using microdialysis: A study of energy-related molecules, growth factors and cytokines. J Neurooncol. 2010;97:11–23. doi: 10.1007/s11060-009-9990-5. [DOI] [PubMed] [Google Scholar]
- 28.Hawley SA, Ross FA, Chevtzoff C, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalender A, Selvaraj A, Kim SY, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dowling RJ, Zakikhani M, Fantus IG, et al. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 31.Dormond O, Madsen JC, Briscoe DM. The effects of mTOR-Akt interactions on anti-apoptotic signaling in vascular endothelial cells. J Biol Chem. 2007;282:23679–23686. doi: 10.1074/jbc.M700563200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aljada A, Mousa SA. Metformin and neoplasia: Implications and indications. Pharmacol Ther. 2012;133:108–115. doi: 10.1016/j.pharmthera.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Ben Sahra I, Le Marchand-Brustel Y, Tanti JF, et al. Metformin in cancer therapy: A new perspective for an old antidiabetic drug? Mol Cancer Ther. 2010;9:1092–1099. doi: 10.1158/1535-7163.MCT-09-1186. [DOI] [PubMed] [Google Scholar]
- 34.Isakovic A, Harhaji L, Stevanovic D, et al. Dual antiglioma action of metformin: Cell cycle arrest and mitochondria-dependent apoptosis. Cell Mol Life Sci. 2007;64:1290–1302. doi: 10.1007/s00018-007-7080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirsch HA, Iliopoulos D, Tsichlis PN, et al. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–7511. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iliopoulos D, Hirsch HA, Struhl K. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res. 2011;71:3196–3201. doi: 10.1158/0008-5472.CAN-10-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]