In this study the utility of the combined application of adipose-derived stem cells (ADSCs) and resected intraperitoneal fatty tissues as a sealant for the pelvic dead space is clarified. The findings suggest that the angiogenic and adipogenic subsets act in coordination with each other and are essential for efficient graft maintenance.
Keywords: Adipose, Adult stem cells, Angiogenesis, Cell transplantation, Endothelial differentiation, Tissue regeneration, Transplantation
Abstract
Adipose-derived stem cells (ADSCs) are a very attractive cell source for regenerative and reconstructive medicine. Although ADSCs have already been used in cardiovascular disease and cosmetic surgery, they have not yet been used in gastroenterological surgery. In this study, we clarified the utility of the combined application of ADSCs and resected intraperitoneal fatty tissues as a sealant for the pelvic dead space that sometimes causes severe and fatal complications in colorectal and gynecological surgeries. In pelvic dead space model mice, mouse ADSCs efficiently maintained transplanted intraperitoneal fatty tissues without any incidence of adhesion to surrounding organs. In vivo and in vitro analyses revealed that transplanted ADSCs differentiated into endothelial cells by expressing the angiogenic factors vascular endothelial growth factor and hepatocyte growth factor. Mouse and human ADSCs contained a CD45−CD34+ subset possessing high colony formation and sphere formation abilities. In addition, the CD45−CD34+ subset consisted of two characteristic subsets: the CD34+CD90+ angiogenic subset and the CD34+CD90− adipogenic subset. Grafts of human ADSCs with fat transplanted into mice were efficiently maintained for more than 12 months without volume reductions. A comparative study of graft maintenance efficacy between cultured human ADSCs and freshly isolated ADSCs indicated that the cultivation of ADSCs decreased their graft maintenance ability. These findings suggested that the angiogenic and adipogenic subsets act in coordination with each other and are essential for efficient graft maintenance.
Introduction
Adipose-derived stem cells (ADSCs) are a very attractive cell source for regenerative and reconstructive medicine because they are technically easy to collect and it is easy to obtain a high number of ADSCs compared with bone marrow stem cells [1–3]. Recently, ADSCs have been applied in functional reconstruction of cardiovascular disease [4] based on their multidifferentiation, angiogenic, and angiogenesis-inducing properties [5–9]. In cosmetic surgery, ADSCs have been also used for augmentation mammoplasty because they can efficiently maintain transplanted fatty tissues [10–12]. In contrast, ADSCs have not yet been well applied in gastroenterological surgery. In this study, we focused on the graft maintenance ability of ADSCs for the reconstruction of pelvic defects caused during gastroenterological surgery and, in particular, pelvic surgery. Insufficient management of the pelvic cavity sometimes induces infection, inflammation, and ileus. In these complications, severe inflammation of the pelvic dead space develops [13, 14], particularly in the large pelvic dead space formed by radical surgery in colorectal and gynecological surgeries. This may result in fetal complications because it causes severe infection, sepsis, and disseminated intravascular coagulation [15, 16]. To prevent the occurrence of complications following pelvic surgery, it is necessary to manage the surgically formed pelvic cavity. To this end, various surgical techniques have been developed, including the use of pedicled flaps and omentopexy [17–21]. However, there are problems associated with these surgical procedures, for example, difficulties in surgical techniques, graft atrophy, high surgical stress, lowered quality of life of the patient, long operation time, and graft length. If resected intraperitoneal fatty tissues are available as a cement for pelvic defects and if the transplant can be efficiently maintained by cotransplantation with ADSCs, it will be a powerful tool for pelvic surgery. In the operative procedures, fatty tissues are very easy to collect and an abundant number of ADSCs can also be easily collected from subcutaneous fat pads. Although a combination of ADSCs with subcutaneous fat has been already applied in breast reconstruction and cosmetic surgery, no study has clarified the availability of resected intraperitoneal fat pads with ADSCs as a cement for pelvic defects.
In this study, we aimed to clarify the utility of ADSCs and resected intraperitoneal fat pads for pelvic cavity repair using a mouse model. We also aimed to clarify the graft maintenance mechanism of ADSCs by in vivo and in vitro analyses. For future clinical applications, using human ADSCs, we aimed to assess the efficiency of transplant maintenance based on the preparation of ADSCs (cultured or freshly isolated).
Materials and Methods
Animals
Wild-type (C57BL/6J) and nude mice (BALB-nu) were obtained from CLEA Japan (Tokyo, Japan, http://www.clea-japan.com). Adult green fluorescent protein (GFP) transgenic mice (C57/6-Tg [CAG-EGFP] C14-Y01-FM131Osb) were a gift from Masaru Okabe (Osaka University, Osaka, Japan). All protocols were approved by the institutional animal care and use committees of Osaka University and Kyushu University.
ADSC Isolation
After the mice were anesthetized with tribromoethanol, adipose tissue was resected from inguinal fat pads, cut into fine pieces, and placed in phosphate-buffered saline (PBS) containing antibiotic/antimycotic agents (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com). Adipose tissue was then placed in Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich) containing 1 mg/ml collagenase II (Sigma-Aldrich) and antibiotic/antimycotic agents and incubated at 37°C for 60 minutes with gentle agitation. The digested tissue was filtered through a sterile 70-μm nylon mesh, centrifuged at 400g for 5 minutes, and resuspended. This washing process was repeated twice.
Human ADSCs were isolated from five human lipoaspirate samples using the same methods described above. All the human samples (remaining lipoaspirate materials for reconstructive surgery) were obtained after informed consent and approval of the ethics boards of Osaka University and Kyushu University.
Mouse Model and Cell Transplantation
A pelvic dead space mouse model was established as described below. After anesthetizing the C57BL/6J mice, traditional laparotomy was performed. The greater omentum of mice is diminutive; therefore, peritesticular fat pads were used. To prepare free fat grafts, bilateral peritesticular fat pads were resected and cut into 5-mm pieces. To construct the abdominal dead space, organs were manipulated into the upper abdomen and fixed with suturing, and then resected fat pads were grafted into the constructed abdominal dead space with (n = 20) or without (n = 20) 1 × 106 ADSCs suspended in 1 ml of PBS. Grafts were resected on days 3, 7, 14, and 21 (n = 5 in each arm and on each day) after transplantation and were then weighed.
To trace ADSCs in vivo, ADSCs were isolated from GFP transgenic mice. Following this, 1 × 106 cells of ADSCs from GFP transgenic mice were suspended in 1 ml of PBS and were reinoculated into wild-type C57BL/6J mice with resected and cut peritesticular fat pads (n = 9; sacrificed on days 3, 7, and 21; three mice each).
To assess the cellular characteristics of human ADSCs, 5 × 105 cells of freshly isolated ADSCs or cultured ADSCs were inoculated with 1 ml of lipoaspirate. Human ADSCs were cultured in DMEM with 10% fetal bovine serum (FBS; Thermo Shandon Inc., Pittsburgh, PA, http://www.thermo.com) for 1 month and then used for transplantation. As a control, PBS was mixed with 1 ml of lipoaspirate instead of human ADSCs. The sizes of the transplants were measured 12 months after transplantation and were calculated as follows: size of transplant (mm3) = a × b/2, where a = the long axis and b = the short axis.
Flow Cytometry Analysis
To identify and characterize mouse ADSCs, the following antibodies were used: Brilliant Violet 570-conjugated anti-mouse CD45 (clone 30-F11; BioLegend, San Diego, CA, http://www.biolegend.com), fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD34 (clone RAM34; eBioscience, San Diego, CA, http://www.ebioscience.com), phycoerythrin (PE)-conjugated anti-mouse CD31 (clone 390; eBioscience), and allophycocyanin (APC)-conjugated anti-mouse CD90.2 (clone 53–2.1; eBioscience). To identify and characterize human ADSCs, the following antibodies were used: FITC-conjugated anti-human CD45 (clone HI30; BD Pharmingen, San Diego, CA, http://www.bdbiosciences.com), PE-conjugated anti-human CD31 (clone WM59; BD Pharmingen), and APC-conjugated anti-human CD34 (clone 581; BD Pharmingen). Doublet cells were eliminated using forward scatter-height/forward scatter-width and side scatter-height/side scatter-width. Dead and damaged cells were eliminated with 7-aminoactinomycin D (BD Pharmingen). Isotype controls (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com) were used for each antibody. Fc receptor (FcR) blocking was performed using an FcR blocking reagent (Miltenyi Biotec, Cologne, Germany, http://www.miltenyi-biotec.com). The cells were analyzed and isolated using a fluorescence-activated cell sorter (FACSAria; Becton, Dickinson and Company, Franklin Lakes, NJ, http://www.bd.com), and data were analyzed with Diva software (Becton Dickinson).
In Vitro Assays
To assess angiogenic activity, a tube formation assay was performed using the Clonetics Aortic Endothelial Cell System (Lonza, Walkersville, MD, http://www.lonza.com) and the BD BioCoat Angiogenesis System (BD Biosciences), according to the manufacturers' instructions. Adipocyte differentiation was induced using NH AdipoDiff Medium (Miltenyi Biotec), according to the manufacturer's instructions. For the sphere formation assay, 1 × 105 cells were seeded onto six-well ultralow-attachment culture dishes (Corning Life Sciences, Acton, MA, http://www.corning.com/lifesciences) and cultured in mTeSR1 basal medium (StemCell Technologies, Vancouver, Canada, http://www.stemcell.com). For the colony formation assay, 1 × 105 cells were seeded onto six-well plates and cultured in DMEM with 10% FBS. The cells were cultured at 37°C in a humidified atmosphere containing 5% CO2.
Immunohistochemical Analysis
The grafted fat pads were excised, fixed in formalin, and embedded in paraffin. After blocking, the 4-μm-thick sections were incubated with rat anti-mouse CD31-specific antibody (clone DIA310; Dianova, Hamburg, Germany, http://www.dianova.com) or human von Willebrand factor (vWF)-specific antibody (clone VW28–1; GenWay Biotech, San Diego, CA, http://www.genwaybio.com). Following this, the sections were incubated with horseradish peroxidase-conjugated secondary antibody (Bethyl Inc., Montgomery, TX, http://www.bethyl.com) and then visualized with 0.02% diaminobenzidine (Sigma-Aldrich). After washing, the sections were counterstained with hematoxylin. Control tissue sections without primary antibody were prepared in a similar manner. Ten random microscopic fields (high-power field; ×400) were examined to calculate the number of CD31+ cells. To identify the origin of endothelial cells, GFP-positive cells were assessed using rat anti-CD31 and rabbit anti-GFP specific antibodies (MBL International Corp., Woburn, MA, http://www.mblintl.com). Alexa Fluor 488-conjugated goat anti-rat IgG (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) and Alexa Fluor 594-conjugated goat anti-rabbit IgG (Invitrogen) were used as secondary antibodies. The sections were mounted using anti-fade reagent with 4′,6-diamidino-2-phenylindole (Invitrogen) and then visualized using a fluorescence microscope (BZ-9000; Keyence, Chicago, IL, http://www.keyence.com).
RNA Extraction and Reverse Transcription Polymerase Chain Reaction
Total RNA was isolated using a modified acid guanidinium phenol chloroform procedure. cDNA was synthesized using random hexamer primers and Moloney murine leukemia virus reverse transcriptase (SS-III; Invitrogen). Real-time polymerase chain reaction (PCR) amplification was performed in the LightCycler 480 System (Roche Applied Science, Penzburg, Germany, https://www.roche-applied-science.com) using the LightCycler 480 Probes Master Kit (Roche Applied Science), according to the manufacturer's instructions. To confirm RNA quality, the β-actin gene (ACTB; NM_007393.3) served as an internal control. The PCR primers used for amplification were as follows: vascular endothelial growth factor (VEGF), 5′-gttagagccctggtcctcct-3′ and 5′-cacccaggggttctactgag-3′; hepatocyte growth factor (HGF), 5′-caccccttgggagtattgtg-3′ and 5′-gggacatcagtctcattcacag-3′; and ACTB, 5′-ctaaggccaaccgtgaaaag-3′ and 5′-accagaggcatacagggaca-3′.
Statistical Analysis
Statistical analysis was performed using JMP software v.8.0.1 (SAS Institute Japan Ltd., Tokyo, Japan, http://www.sas.com/offices/asiapacific/japan/). All data are presented as means ± SD, and two-group comparisons were performed using the two-tailed Student's t-test. A p value <.05 was considered statistically significant.
Results
ADSCs Promote Graft Survival in a Mouse Model
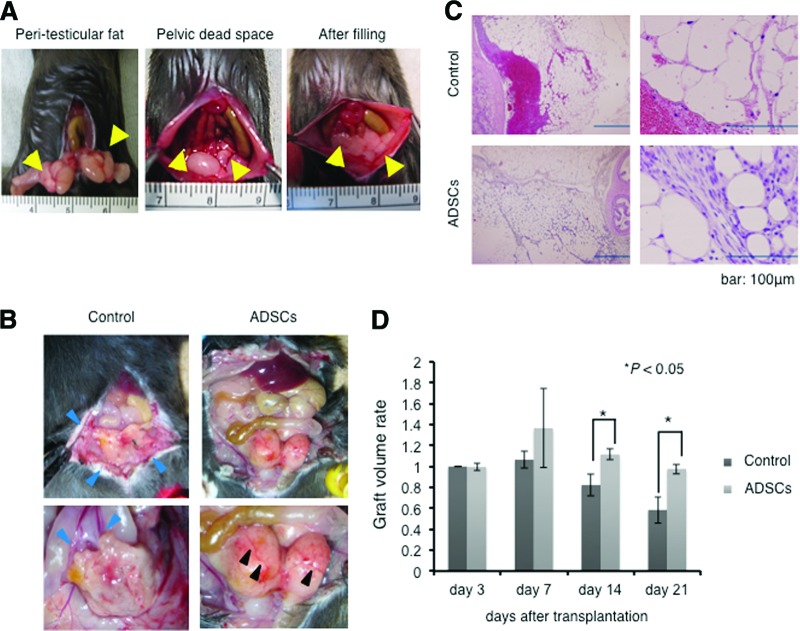
A mouse model was established to assess whether ADSCs possess positive effects on the maintenance of transplanted adipose tissues. In this model, bilateral peritesticular fat pads were resected to construct the pelvic cavity, and the intestinal tract was sutured to the retroperitoneal fascia to prevent it from dropping into the formed cavity. Following this, the resected fat pads were returned into the constructed pelvic defect with or without 1 × 106 cells of ADSCs (n = 20 each) suspended in 1 ml of PBS (Fig. 1A). After 21 days, the mice were sacrificed to assess the transplants. In the control mice, atrophic change of the transplant, hemorrhagic ascites formation in the intraperitoneal space, and adhesion of intraperitoneal tissues were observed by abdominal laparotomy. In contrast to control mice, the grafts in ADSC-treated mice showed a vivid image with surface neovascularization and showed no ascites or adhesion (Fig. 1B). Microscopic analysis of the grafts showed hemorrhagic necrosis with infiltration of inflammatory cells in the control group, whereas healthy fat tissue construction was observed in the grafts of the ADSC-treated group (Fig. 1C).
Figure 1.
ADSCs maintain intraperitoneal fat grafts in the pelvic dead space mouse model. (A): Bilateral peritesticular fat pads (arrowheads in the left panel) were resected, and the intestinal tract was sutured to the retroperitoneal fascia to form the pelvic cavity (arrowheads in the middle panel). The peritesticular fat pads were cut into 5-mm sections and then transplanted into the constructed peritoneal dead space with or without ADSCs (arrowheads in the right panel). (B): Macroscopic findings of pelvis in mice transplanted with graft treated with phosphate-buffered saline (PBS) control or with ADSCs. The graft showed atrophic features with adhesion to the peritoneum and colon (blue arrowheads) in control mice, whereas it showed vivid features with neovascularization (black arrowheads) without adhesion to the peritoneum and colon in ADSC-treated mice. (C): Hematoxylin and eosin staining of resected graft transplanted with PBS control or with ADSCs. The right panels show high-magnification figures. Scale bars = 100 μm. (D): Graft volume rate (resected fat weight/transplanted fat weight) of PBS control and ADSC-treated groups on days 3, 7, 14, and 21 after transplantation. Abbreviation: ADSC, adipose-derived stem cell.
To quantify the engraftment efficacy, the mice were sacrificed on days 3, 7, 14, and 21 after transplantation, and the grafts were then weighed (n = 5 in each arm and on each day). The weight ratios (weight of engraft/weight of implanted fat pad) of the transplants were significantly higher in the ADSC-treated group than in the control group on days 14 and 21 (p < .05) (Fig. 1D).
ADSCs Promote Angiogenesis
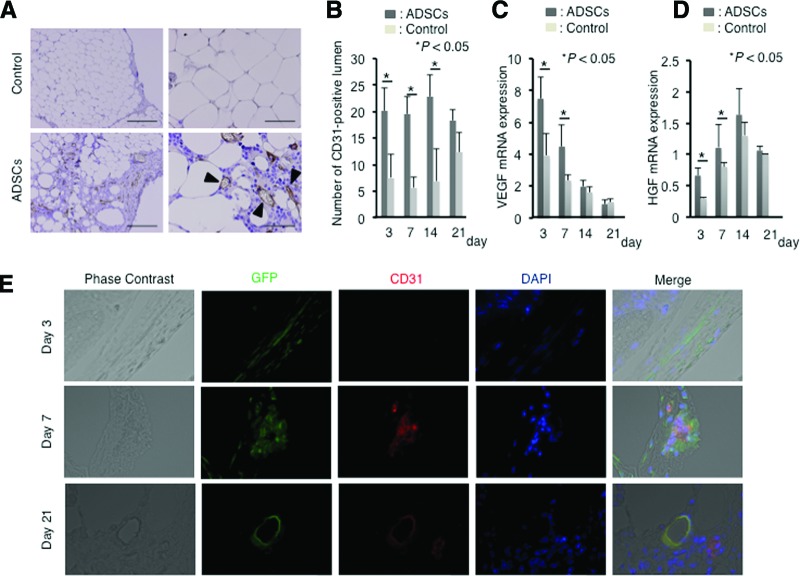
The grafts in the ADSC-treated group showed higher neovascularization; therefore, we focused on angiogenesis to clarify the reason why ADSCs induce positive effects on transplant maintenance. Immunohistochemical analysis for the endothelial marker CD31 was performed to assess graft vascularity. The number of CD31+ lumen formations was obviously higher in the ADSC-treated group (Fig. 2A). For definite assessments, the transitive number of CD31+ lumen formations was counted on days 3, 7, 14, and 21 after transplantation (n = 5 in each arm and on each day). This value was significantly higher in the ADSC-treated group on days 3, 7, and 14 when compared with that in the control group (p < .05); however, it was not significant on day 21 (p = .78) (Fig. 2B). This may be because of the increased relative number of blood vessels for higher atrophy of the grafts in the control group at 21 days after transplantation. To assess angiogenic activity, VEGF expression was measured by quantitative reverse transcription PCR, which showed that VEGF mRNA was significantly higher in the ADSC-treated group on days 3 and 7 after transplantation (p < .05) (Fig. 2C). In addition, expression of HGF, an angiogenic and wound-healing factor, was also significantly higher in the ADSC-treated group on days 3 and 7 (p < .05) compared with that in the control group (Fig. 2D). Expression of interferon-γ, tumor necrosis factor-α, and interleukin-6 did not differ significantly between the groups (data not shown).
Figure 2.
ADSCs differentiate into CD31+ endothelial vascular cells. (A): Immunohistochemical staining for CD31. Sections from ADSC-treated mice contained abundant stromal structures with CD31+ vascular formation (black arrowheads) compared with those from control mice. Scale bars = 100 μm. (B): Numbers of CD31+ capillary structures in sections obtained from control and ADSC-treated mice. Bars indicate ±SD. (C): Semiquantitative analysis of VEGF mRNA expression in resected grafts from control and ADSC-treated mice. Bars indicate ±SD. (D): Semiquantitative analysis of HGF mRNA expression in resected grafts from control and ADSC-treated mice. Bars indicate ±SD. (E): Tracing analysis for GFP-positive cells (green) on days 3, 7, and 21 after transplantation. ADSCs were isolated from GFP transgenic mice. Sections were stained for CD31 (red) and nuclei for DAPI (blue). Abbreviations: ADSC, adipose-derived stem cell; DAPI, 4′,6-diamidino-2-phenylindole; GFP, green fluorescent protein; HGF, hepatocyte growth factor; VEGF, vascular endothelial growth factor.
Next, GFP-positive ADSCs were traced to assess whether ADSCs differentiate into endothelial cells to form vascular structures (n = 9; sacrificed on days 3, 7, and 21; three mice each). In vivo time course analysis of GFP-positive cells revealed that GFP-positive cells formed cellular clusters with CD31 expression on day 7 and then morphologically changed to vascular structures on day 21 (Fig. 2E).
ADSCs Contain Adipogenic and Angiogenic Characterized Cell Fractions
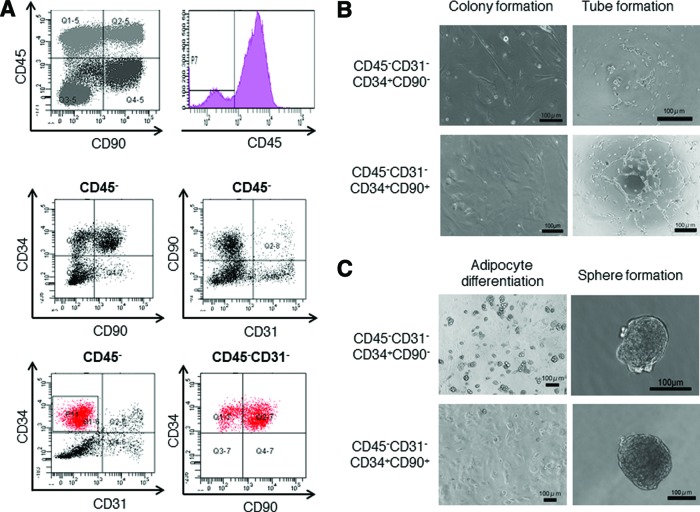
To clarify and characterize the cell fractions that constitute ADSCs, multicolor flow cytometry analysis was performed (Fig. 3A). Analysis with CD45 revealed that ADSCs were divided into CD45+ and CD45− fractions and these were further divided into CD90+ and CD90− fractions. Regardless of CD90 expression, CD45+ cell fractions could not be maintained in vitro (Fig. 3B). CD45− cells were further analyzed with CD34, CD90, and CD31. In the CD45− cell fraction, a small number of CD31+ cells (∼25%) was identified, which were suggested to be endothelial cells. Because CD31+ vascular endothelial cells arose from CD31− GFP+ cells in vitro (Fig. 2E) and the CD31+ cell fraction could not be maintained in vitro (data not shown), the CD31− cell fraction was further analyzed with CD34 and CD90 to clarify the origin of CD31+ cells. CD45− and CD31− cells were constructed by CD34+ and CD34− cell components, and the CD34+ cell component was divided into CD34+CD90+ and CD34+CD90− cell fractions (Fig. 3A). To assess cellular proliferative capacity and angiogenic activity, a colony formation assay and Matrigel (BD Biosciences) tube formation assay were performed in isolated CD45−CD31−CD34+CD90− and CD45−CD31−CD34+CD90+ cell fractions. The colony formation assay revealed that both these cell fractions formed colonies and that there was no significant difference between colony formation activity of the cell fractions (Fig. 3B). Interestingly, in contrast to the CD45−CD31− CD34+CD90− cell fraction, which showed low tube formation activity, the Matrigel tube formation assay revealed high tube formation activity in the CD45−CD31−CD34+ CD90+ cell fraction (Fig. 3B).
Figure 3.
Adipose-derived stem cells (ADSCs) constructed from angiogenic and adipogenic subsets. (A): Flow cytometry analysis of mouse ADSCs. CD45− cells were analyzed for CD34, CD90, and CD31. The CD45−CD31−CD34+ subpopulations were further analyzed for CD90. (B): Colony formation and tube formation assays of freshly isolated CD45−CD31−CD34+CD90− and CD45−CD31−CD34+CD90+ mouse ADSC subsets. Scale bars = 100 μm. (C): Adipocyte differentiation and sphere formation activities of freshly isolated ADSC subsets. Scale bars = 100 μm.
To characterize the cellular function of the CD45− CD31−CD34+CD90+ cell fraction, adipose differentiation activity was assessed. Phase contrast microscopy of adipocyte induction showed that the CD45−CD31−CD34+CD90− cell fraction contained a large amount of lipid droplet-like structures compared with the CD45−CD31−CD34+CD90+ cell fraction, which resulted in higher adipose differentiation activity of the CD45−CD31−CD34+CD90− cell fraction (Fig. 3C). Next, to assess stem cell activity, a sphere formation assay was performed. Interestingly, both CD45− CD31−CD34+CD90+ and CD45−CD31− CD34+CD90− cell fractions efficiently formed spheres (Fig. 3C). Taken together, these findings indicate that ADSCs contain at least two characteristic stem cell-like fractions that possess angiogenic and adipogenic properties. These analyses were performed in triplicate from four independently sacrificed mice, and compatible data were obtained.
Human ADSCs Support Transplant Survival
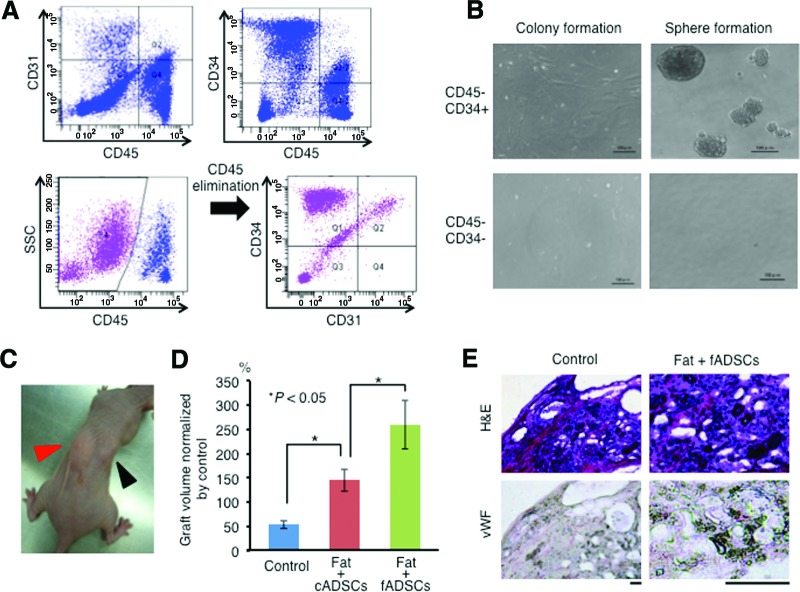
To assess whether human ADSCs share similar properties with mouse ADSCs, human ADSCs were isolated from subcutaneous fat pads and their cellular properties were analyzed. Flow cytometry analysis of five human ADSC samples revealed cell surface marker expression similar to that of mouse ADSCs, which contained CD45+ and CD45− cells. In CD45− cells, the existence of CD34+CD31− (43.40 ± 12.79%), CD34+CD31+ (11.99 ± 5.41%), and CD34−CD45− (25.73 ± 11.52%) cells was confirmed (Fig. 4A). Although the number of CD45−CD34+CD31+ cells was higher in human ADSCs than that in mouse ADSCs (11.99 ± 5.41% in human vs. 4.25 ± 2.41% in mouse), CD45− CD34−CD31+ cells could not actually be identified in human ADSCs (4.12 ± 2.85% in mouse). An in vitro assay indicated that CD45−CD34+ cells but not CD45−CD34− cells formed colonies and spheres (Fig. 4B), suggesting that CD34+ cells in a CD45− fraction possess stem cell activity also in human ADSCs. To assess whether human ADSCs contribute to long-term graft survival, 5 × 105 cells of freshly isolated ADSCs (fADSCs) were added to 1 ml of human lipoaspirate and then transplanted in nude mice (n = 3 from independent patients). PBS was used as a control. The mice inoculated with ADSCs showed efficient engraftment of the transplants with the control mice 6 months after transplantation (Fig. 4C). To assess whether cultured ADSCs (cADSCs) also possess a high maintenance effect on the transplants, each volume of engrafted transplants of fADSCs, cADSCs, and PBS controls was measured 6 months after transplantation. Interestingly, transplant volumes were highly retained in the fADSC-treated mice compared with those in the cADSC-treated and control mice (Fig. 4D). Immunohistochemical staining for human vWF in resected specimens from the control and fADSC-treated mice revealed high angiogenic activity of fADSCs (Fig. 4E).
Figure 4.
Freshly isolated human ADSCs sufficiently maintain fat graft. (A): Flow cytometry analysis of human ADSCs. The CD45− cell fraction was further analyzed for CD31 and CD34. (B): Colony formation and sphere formation abilities of freshly isolated human ADSC subsets (CD45−CD34+ and CD45−CD34− subsets). Scale bars = 100 μm. (C): Mouse fat inoculated with freshly isolated ADSCs (red arrow) and with cultured ADSCs (black arrow). (D): Graft volume of fat with phosphate-buffered saline (control), with cADSCs, and with fADSCs 12 months after transplantation. Data are normalized by the control fat volume. Bars indicate ±SD. (E): H&E staining and immunohistochemical staining for vWF. Scale bars = 100 μm. Abbreviations: ADSC, adipose-derived stem cell; cADSC, cultured adipose-derived stem cell; fADSC, freshly isolated adipose-derived stem cell; H&E, hematoxylin and eosin; SSC, side scatter; vWF, human von Willebrand factor.
Discussion
ADSCs are very attractive biomaterials for tissue engineering. Actually, numerous studies focusing on clinical applications have reported the usability of ADSCs in regenerative and reconstruction medicine [22–27]. In this study, we focused on the use of ADSCs in gastroenterological surgery, and particularly in pelvic surgery, in which omentum flaps have been used to correct pelvic defects [28, 29]. Despite the availability of the greater omentum as a patch and cement to correct tissue defects, it is not sufficient to correct pelvic defects because feeder vessels that supply adequate blood flow to the flap are sometimes too short to reach the pelvic cavity. In addition, the volume of the greater omentum is sometimes not sufficient to correct pelvic defects. It is difficult to use intraperitoneal fatty tissue as a cement for the pelvic dead space; however, if resected intraperitoneal fatty tissue can be maintained efficiently, it will be the most attractive biomaterial for tissue engineering in pelvic reconstruction. During gastroenterological surgery, it is very easy to collect intraperitoneal fatty tissues. In this study, ADSCs efficiently maintained resected intraperitoneal fat pads in vivo. The histopathological findings of isolated grafts from control mice showed necrotic and atrophic changes, suggesting ischemic necrosis. Immunohistochemical analysis of isolated grafts for CD31 showed increased CD31+ capillary formation in grafts with ADSCs. Tracing analysis of GFP+ cells showed that GFP+ cells formed cellular clusters that morphologically changed to capillaries expressing CD31, suggesting that the majority of CD31+ capillaries in grafts were derived from donor mice cells. Immunohistochemical analysis of isolated human xenografts from mice for vWF also indicated that neovessels were derived from donor cells. Although it has been reported that ADSCs promote mobilization of circulating endothelial progenitor cells for neovascularization [30] by synthesis and secretion of multiple cytokines that affect angiogenesis [31, 32], our study indicated that, at least inside the graft, a small subset of ADSCs plays key roles in neovascularization. Actually, ADSCs contain a CD45−CD31−CD34+CD90+ angiogenic subset that possesses sphere formation and tube formation abilities in vitro [33]. In this study, we identified higher VEGF and HGF expression in grafts with ADSCs than that in controls, suggesting that the CD45−CD31−CD34+CD90+ cell fraction produces angiogenic cytokines for tissue repair and graft maintenance [33]. Interestingly, ADSCs also contain a CD45−CD31−CD34+CD90− adipogenic subset that possesses high colony and sphere formation abilities but low tube formation ability. Considering that angiogenic ADSCs promote angiogenesis and that adipogenic ADSCs supply adipocytes, it is suggested that ADSCs maintain transplants by cooperation of angiogenic and adipogenic subsets. In addition, macroscopic findings by laparotomy showed no apparent adhesion in ADSC-treated mice, suggesting that ADSCs suppress inflammatory reactions as reported previously [34–37]. In gastroenterological surgery, intestinal obstruction caused by adhesion after an operative procedure is a major complication that decreases the quality of life of patients, and sometimes, an additional surgery is required to relieve the obstruction. These findings imply the availability of ADSCs for prevention of adhesion in gastroenterological surgery. Of course, for a definitive conclusion, it is necessary to assess immunological factors in a non-immune-deficient animal model.
For the clinical application of ADSCs toward regeneration and reconstruction medicine, it is necessary to perform efficient isolation of ADSCs with high purities and yields. In this study, we used subcutaneous fat pads as a source for isolation of ADSCs because we could not obtain a high number of ADSCs from intraperitoneal fatty tissues. Actually, the number of CD45−CD34+ ADSCs isolated from the greater omentum was about twofold less than that isolated from subcutaneous fatty tissue (data not shown), which is compatible with a previous report [38]. If cultivated ADSCs retain potency in tissue repair equivalent to that possessed by freshly isolated ADSCs, they would be a powerful alternative option to freshly isolated ADSCs because it is very easy to cultivate these cells, increase cell numbers, and prepare cells before clinical application. Unfortunately, cultivated ADSCs showed reduced graft maintenance ability compared with freshly isolated ADSCs in this study. Although CD34 expression reportedly diminishes during culture [39], the cellular characteristics of cultured ADSCs may be somewhat different from those of freshly isolated ADSCs.
Conclusion
In this study, we clarified the availability of ADSCs in gastroenterological surgery, and particularly in pelvic surgery. Of course, it is necessary to assess whether ADSCs affect the frequency of cancer recurrence after radical surgery [40–42]. Previous findings have revealed that the expression of reactive oxygen species in ADSCs is only slightly retained [43], suggesting that ADSCs possess chemo-radio resistance [44, 45]. Grafts with ADSCs can be expected to prevent surrounding tissues from radiation damage as a biomaterial spacing device in case of radiotherapy for rectal cancer recurrence. Nonetheless, for clinical application of ADSCs in pelvic surgery for colorectal cancer, there are several problems that must be solved, and further studies will be required.
Acknowledgments
This work was supported by a Grant-in-Aid for Challenging Exploratory Research (21659301) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, and by the Takeda Science Foundation.
Author Contributions
H.T.: conception and design, provision of study material or patients, data analysis and interpretation, collection and/or assembly of data, manuscript writing; N.H.: conception and design, provision of study material or patients, data analysis and interpretation, collection and/or assembly of data, manuscript writing, final approval of manuscript; S.N.: provision of study material or patients; S.M.: collection and/or assembly of data; Y.S., T.M., and J.N.: financial support; I.T., H.Y., and K.M.: administrative support; H.I., Y.D., and M.M.: final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 2.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 4.Qayyum AA, Haack-Sørensen M, Mathiasen AB, et al. Adipose-derived mesenchymal stromal cells for chronic myocardial ischemia (MyStromalCell Trial): Study design. Regen Med. 2012;7:421–428. doi: 10.2217/rme.12.17. [DOI] [PubMed] [Google Scholar]
- 5.Casteilla L, Planat-Bénard V, Dehez S, et al. Endothelial and cardiac regeneration from adipose tissues. Methods Mol Biol. 2011;702:269–287. doi: 10.1007/978-1-61737-960-4_20. [DOI] [PubMed] [Google Scholar]
- 6.Planat-Benard V, Silvestre JS, Cousin B, et al. Plasticity of human adipose lineage cells toward endothelial cells: Physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 7.Miranville A, Heeschen C, Sengenès C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 8.Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 9.Martínez-Estrada OM, Munoz-Santos Y, Julve J, et al. Human adipose tissue as a source of Flk-1+ cells: New method of differentiation and expansion. Cardiovasc Res. 2005;65:328–333. doi: 10.1016/j.cardiores.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto D, Sato K, Gonda K, et al. Cell-assisted lipotransfer: Supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006;12:3375–3382. doi: 10.1089/ten.2006.12.3375. [DOI] [PubMed] [Google Scholar]
- 11.Pérez-Cano R, Vranckx JJ, Lasso JM, et al. Prospective trial of adipose-derived regenerative cell (ADRC)-enriched fat grafting for partial mastectomy defects: The RESTORE-2 trial. Eur J Surg Oncol. 2012;38:382–389. doi: 10.1016/j.ejso.2012.02.178. [DOI] [PubMed] [Google Scholar]
- 12.Kamakura T, Ito K. Autologous cell-enriched fat grafting for breast augmentation. Aesthetic Plast Surg. 2011;35:1022–1030. doi: 10.1007/s00266-011-9727-7. [DOI] [PubMed] [Google Scholar]
- 13.Uemura M, Ikeda M, Sekimoto M, et al. Prevention of severe pelvic abscess formation following extended radical surgery for locally recurrent rectal cancer. Ann Surg Oncol. 2009;16:2204–2210. doi: 10.1245/s10434-009-0505-6. [DOI] [PubMed] [Google Scholar]
- 14.Temple WJ, Ketcham AS. Sacral resection for control of pelvic tumors. Am J Surg. 1992;163:370–374. doi: 10.1016/0002-9610(92)90035-p. [DOI] [PubMed] [Google Scholar]
- 15.Jimenez RE, Shoup M, Cohen AM, et al. Contemporary outcomes of total pelvic exenteration in the treatment of colorectal cancer. Dis Colon Rectum. 2003;46:1619–1625. doi: 10.1007/BF02660766. [DOI] [PubMed] [Google Scholar]
- 16.Moriya Y, Akasu T, Fujita S, et al. Total pelvic exenteration with distal sacrectomy for fixed recurrent rectal cancer in the pelvis. Dis Colon Rectum. 2004;47:2047–2054. doi: 10.1007/s10350-004-0714-9. [DOI] [PubMed] [Google Scholar]
- 17.Radice E, Nelson H, Mercill S, et al. Primary myocutaneous flap closure following resection of locally advanced pelvic malignancies. Br J Surg. 1999;86:349–354. doi: 10.1046/j.1365-2168.1999.01044.x. [DOI] [PubMed] [Google Scholar]
- 18.Smith HO, Genesen MC, Runowicz CD, et al. The rectus abdominis myocutaneous flap: Modifications, complications, and sexual function. Cancer. 1998;83:510–520. doi: 10.1002/(sici)1097-0142(19980801)83:3<510::aid-cncr20>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 19.Chessin DB, Hartley J, Cohen AM, et al. Rectus flap reconstruction decreases perineal wound complications after pelvic chemoradiation and surgery: A cohort study. Ann Surg Oncol. 2005;12:104–110. doi: 10.1245/ASO.2005.03.100. [DOI] [PubMed] [Google Scholar]
- 20.Bell SW, Dehni N, Chaouat M, et al. Primary rectus abdominis myocutaneous flap for repair of perineal and vaginal defects after extended abdominoperineal resection. Br J Surg. 2005;92:482–486. doi: 10.1002/bjs.4857. [DOI] [PubMed] [Google Scholar]
- 21.Buchel EW, Finical S, Johnson C. Pelvic reconstruction using vertical rectus abdominis musculocutaneous flaps. Ann Plast Surg. 2004;52:22–26. doi: 10.1097/01.sap.0000099820.10065.2a. [DOI] [PubMed] [Google Scholar]
- 22.Ma L, Yang Y, Sikka SC, et al. Adipose tissue-derived stem cell-seeded small intestinal submucosa for tunica albuginea grafting and reconstruction. Proc Natl Acad Sci USA. 2012;109:2090–2095. doi: 10.1073/pnas.1113810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu GB, Cheng YX, Feng YK, et al. Adipose-derived stem cells promote peripheral nerve repair. Arch Med Sci. 2011;7:592–596. doi: 10.5114/aoms.2011.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmadi N, Razavi S, Kazemi M, et al. Stability of neural differentiation in human adipose derived stem cells by two induction protocols. Tissue Cell. 2012;44:87–94. doi: 10.1016/j.tice.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Tzouvelekis A, Koliakos G, Ntolios P, et al. Stem cell therapy for idiopathic pulmonary fibrosis: A protocol proposal. J Transl Med. 2011;9:182. doi: 10.1186/1479-5876-9-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Fang JS, Wang W. Stem cell therapy for idiopathic pulmonary fibrosis: A protocol proposal. Transplantation of Schwann cells differentiated from adipose-derived stem cells modifies reactive gliosis after contusion brain injury in rats. J Int Med Res. 2011;39:1344–1357. doi: 10.1177/147323001103900421. [DOI] [PubMed] [Google Scholar]
- 27.Zhu Y, Liu T, Ye H, et al. Enhancement of adipose-derived stem cell differentiation in scaffolds with IGF-I gene impregnation under dynamic microenvironment. Stem Cells Dev. 2010;19:1547–1556. doi: 10.1089/scd.2010.0054. [DOI] [PubMed] [Google Scholar]
- 28.Kim TH, Kim DY, Jung KH, et al. The role of omental flap transposition in patients with locoregional recurrent rectal cancer treated with reirradiation. J Surg Oncol. 2010;102:789–795. doi: 10.1002/jso.21737. [DOI] [PubMed] [Google Scholar]
- 29.O'Leary DP. Use of the greater omentum in colorectal surgery. Dis Colon Rectum. 1999;42:533–539. doi: 10.1007/BF02234183. [DOI] [PubMed] [Google Scholar]
- 30.Kondo K, Shintani S, Shibata R, et al. Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:61–66. doi: 10.1161/ATVBAHA.108.166496. [DOI] [PubMed] [Google Scholar]
- 31.Boomsma RA, Geenen DL. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One. 2012;7:e35685. doi: 10.1371/journal.pone.0035685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsiao ST, Asgari A, Lokmic Z, et al. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev. 2012;21:2189–2203. doi: 10.1089/scd.2011.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Francesco F, Tirino V, Desiderio V, et al. Human CD34/CD90 ASCs are capable of growing as sphere clusters, producing high levels of VEGF and forming capillaries. PLoS One. 2009;4:e6537. doi: 10.1371/journal.pone.0006537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006;36:2566–2573. doi: 10.1002/eji.200636416. [DOI] [PubMed] [Google Scholar]
- 35.Cui L, Yin S, Liu W, et al. Expanded adipose-derived stem cells suppress mixed lymphocyte reaction by secretion of prostaglandin E2. Tissue Eng. 2007;13:1185–1195. doi: 10.1089/ten.2006.0315. [DOI] [PubMed] [Google Scholar]
- 36.Calderon D, Planat-Benard V, Bellamy V, et al. Immune response to human embryonic stem cell-derived cardiac progenitors and adipose-derived stromal cells. J Cell Mol Med. 2012;16:1544–1552. doi: 10.1111/j.1582-4934.2011.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Technau A, Froelich K, Hagen R, et al. Adipose tissue-derived stem cells show both immunogenic and immunosuppressive properties after chondrogenic differentiation. Cytotherapy. 2011;13:310–317. doi: 10.3109/14653249.2010.504769. [DOI] [PubMed] [Google Scholar]
- 38.Toyoda M, Matsubara Y, Lin K, et al. Characterization and comparison of adipose tissue-derived cells from human subcutaneous and omental adipose tissues. Cell Biochem Funct. 2009;27:440–447. doi: 10.1002/cbf.1591. [DOI] [PubMed] [Google Scholar]
- 39.Taha MF, Hedayati V. Isolation, identification and multipotential differentiation of mouse adipose tissue-derived stem cells. Tissue Cell. 2010;42:211–216. doi: 10.1016/j.tice.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Han ZP, Zhang SS, et al. Effects of inflammatory factors on mesenchymal stem cells and their role in the promotion of tumor angiogenesis in colon cancer. J Biol Chem. 2011;286:25007–25015. doi: 10.1074/jbc.M110.213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pearl RA, Leedham SJ, Pacifico MD. The safety of autologous fat transfer in breast cancer: Lessons from stem cell biology. J Plast Reconstr Aesthet Surg. 2012;65:283–288. doi: 10.1016/j.bjps.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zimmerlin L, Donnenberg AD, Rubin JP, et al. Regenerative therapy and cancer: In vitro and in vivo studies of the interaction between adipose-derived stem cells and breast cancer cells from clinical isolates. Tissue Eng Part A. 2011;17:93–106. doi: 10.1089/ten.tea.2010.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carrière A, Ebrahimian TG, Dehez S, et al. Preconditioning by mitochondrial reactive oxygen species improves the proangiogenic potential of adipose-derived cells-based therapy. Arterioscler Thromb Vasc Biol. 2009;29:1093–1099. doi: 10.1161/ATVBAHA.109.188318. [DOI] [PubMed] [Google Scholar]
- 44.Riley P. Free radicals in biology: Oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 45.Girdhani S, Bhosle SM, Thulsidas SA, et al. Potential of radiosensitizing agents in cancer chemo-radiotherapy. J Cancer Res Ther. 2005;1:129–131. doi: 10.4103/0973-1482.19585. [DOI] [PubMed] [Google Scholar]