This study describes the isolation, characterization, and differentiation of chromaffin progenitor cells obtained from adult human adrenals. The presence of progenitor cells in the human adrenal medulla is demonstrated and their potential use in regenerative medicine, especially in the treatment of neuroendocrine and neurodegenerative diseases, is revealed.
Keywords: Adult stem cells, Culture, Differentiation, Nestin, Neural differentiation, Progenitor cells, Somatic stem cells
Abstract
Chromaffin cells, sympathetic neurons of the dorsal ganglia, and the intermediate small intensely fluorescent cells derive from a common neural crest progenitor cell. Contrary to the closely related sympathetic nervous system, within the adult adrenal medulla a subpopulation of undifferentiated progenitor cells persists, and recently, we established a method to isolate and differentiate these progenitor cells from adult bovine adrenals. However, no studies have elucidated the existence of adrenal progenitor cells within the human adrenal medulla. Here we describe the isolation, characterization, and differentiation of chromaffin progenitor cells obtained from adult human adrenals. Human chromaffin progenitor cells were cultured in low-attachment conditions for 10–12 days as free-floating spheres in the presence of fibroblast growth factor-2 (FGF-2) and epidermal growth factor. These primary human chromosphere cultures were characterized by the expression of several progenitor markers, including nestin, CD133, Notch1, nerve growth factor receptor, Snai2, Sox9, Sox10, Phox2b, and Ascl1 on the molecular level and of Sox9 on the immunohistochemical level. In opposition, phenylethanolamine N-methyltransferase (PNMT), a marker for differentiated chromaffin cells, significantly decreased after 12 days in culture. Moreover, when plated on poly-l-lysine/laminin-coated slides in the presence of FGF-2, human chromaffin progenitor cells were able to differentiate into two distinct neuron-like cell types, tyrosine hydroxylase (TH)+/β-3-tubulin+ cells and TH−/β-3-tubulin+ cells, and into chromaffin cells (TH+/PNMT+). This study demonstrates the presence of progenitor cells in the human adrenal medulla and reveals their potential use in regenerative medicine, especially in the treatment of neuroendocrine and neurodegenerative diseases.
Introduction
Chromaffin cells, sympathetic neurons of the dorsal ganglia, and the intermediate small intensely fluorescent cells develop from a common neural crest-derived sympathoadrenal (SA) progenitor cell. Along their migratory route, SA progenitors become catecholaminergic, aggregate at the dorsal aorta, and then further migrate to the secondary sympathetic ganglia and the adrenal medulla, where they differentiate into mature sympathetic neurons and chromaffin cells, respectively (reviewed in [1]). In the adrenal medulla, chromaffin cells acquire an endocrine phenotype and specialize in the synthesis and secretion of catecholamines. In addition, chromaffin cells produce and secrete, by exocytosis, a “cocktail” of bioactive substances, including neuropeptides, cytokines, enkephalines, and neurotrophic factors [2, 3]. Despite this endocrine phenotype and in contrast to sympathetic neurons, chromaffin cells are also characterized by their plastic properties, which include the ability to proliferate throughout life and the capacity to acquire a neural-like phenotype in response to neurogenic stimulation [4–9]. These properties promoted the study of chromaffin cells for their potential in the treatment of neurodegenerative diseases and chronic pain [10–15]. Already in 1985, Backlund et al. reported the first autologous transplant of human medullary tissue in patients with Parkinson's disease (PD) [10]. Since that time, a substantial number of groups have reported the use of chromaffin cells for transplantation in PD patients, showing clinical improvements that unfortunately disappeared within 1–2 years after transplantation because of low survival rates of the grafts in the brain (reviewed in [11]).
In the last few years, the research in stem cell biology has opened new perspectives concerning the potential use of adult stem cells from different tissues for cell therapy and regenerative medicine. Recently, we isolated chromaffin progenitor cells from bovine adrenal medulla [16, 17]. Analogous to neural stem cells, these progenitor cells grow in suspension as free-floating spheres, which we named chromospheres, and differentiate into functional neurons. The proliferation and differentiation of these cells into functional neurons might be a promising strategy in the regenerative treatment of neurodegenerative diseases [18–20]. However, until now no studies have elucidated the isolation of chromaffin progenitor cells from the adult human adrenal medulla. Thus, in this study, we aimed to isolate and characterize these cells from adult human adrenal glands as a prerequisite for their future use in transplantation trials.
Materials and Methods
Cell Culture of Human Adrenal Medulla Progenitor Cells
The ethical committees of the University of Dresden and the University of Coimbra approved this study. Human adrenal glands were obtained from kidney transplant donors (Coimbra) and from patients undergoing nephrectomy where the ipsilateral adrenal was removed together with the kidney (Dresden). A cell digest from the medullary tissue was obtained as previously described [21]. Briefly, the glands were cleaned of fat tissue and opened, and the medullae were separated from the majority of the cortex by scraping off the brown interdigited islets of chromaffin cells with a scalpel. Medullary tissue was collected in 0.2% collagenase Type H solution (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) and incubated for 45 minutes at 37°C. The digested tissue was washed twice with culture medium and filtered through a 100-μm strainer. For chromosphere culture, the cell digest was resuspended in Dulbecco's modified Eagle's medium/F-12 with GlutaMAX, 100 U/ml penicillin, and 100 μg/ml streptomycin, and supplemented with 1% B27, 10 ng/ml fibroblast growth factor-2 (FGF-2), 10 ng/ml endothelial growth factor (EGF; all from Invitrogen, Carlsbad, CA, http://www.invitrogen.com), and 5 μg/ml heparin (Sigma-Aldrich). Chromospheres were allowed to grow for 10–12 days in low-attachment flasks at 37°C in a 5% CO2 atmosphere. The medium was changed every 4th day.
RNA Extraction and Reverse Transcription-Polymerase Chain Reaction
Cells were collected and disrupted in RNeasy lysis buffer with 1% β-mercaptoethonal and total RNA was extracted using the RNeasy Plus Mini kit (Qiagen, Hilden, Germany, http://www.qiagen.com) according to the manufacturer's instructions. M-MLV reverse transcriptase, 5× reverse transcriptase buffer, oligo(dT) 15 primer, and RNase inhibitor (Promega, Madison, WI, http://www.promega.com) were used for the reverse transcription (RT) of 1 μg of total RNA. cDNA amplifications were done using specific primer pairs (Table 1) in NH4 reaction buffer with 1.5 mM MgCl2, 250 μM dNTPs, 0.5 μM of each primer, and 2.5 U/20 μl Taq DNA polymerase (Stratec, Berlin, Germany, http://www.stratec.com). Thermal cycler conditions were as follows: denaturation at 94°C for 5 minutes, followed by 40 cycles at 94°C for 15 seconds, optimal annealing temperature (Table 1) for 15 seconds, and 72°C for 15 seconds, with a final extension step at 72°C for 1 minute. The housekeeping gene GAPDH was amplified from each cDNA sample. Polymerase chain reaction (PCR) amplification products were analyzed on 1% agarose gel containing ethidium bromide.
Table 1.
cDNA amplifications
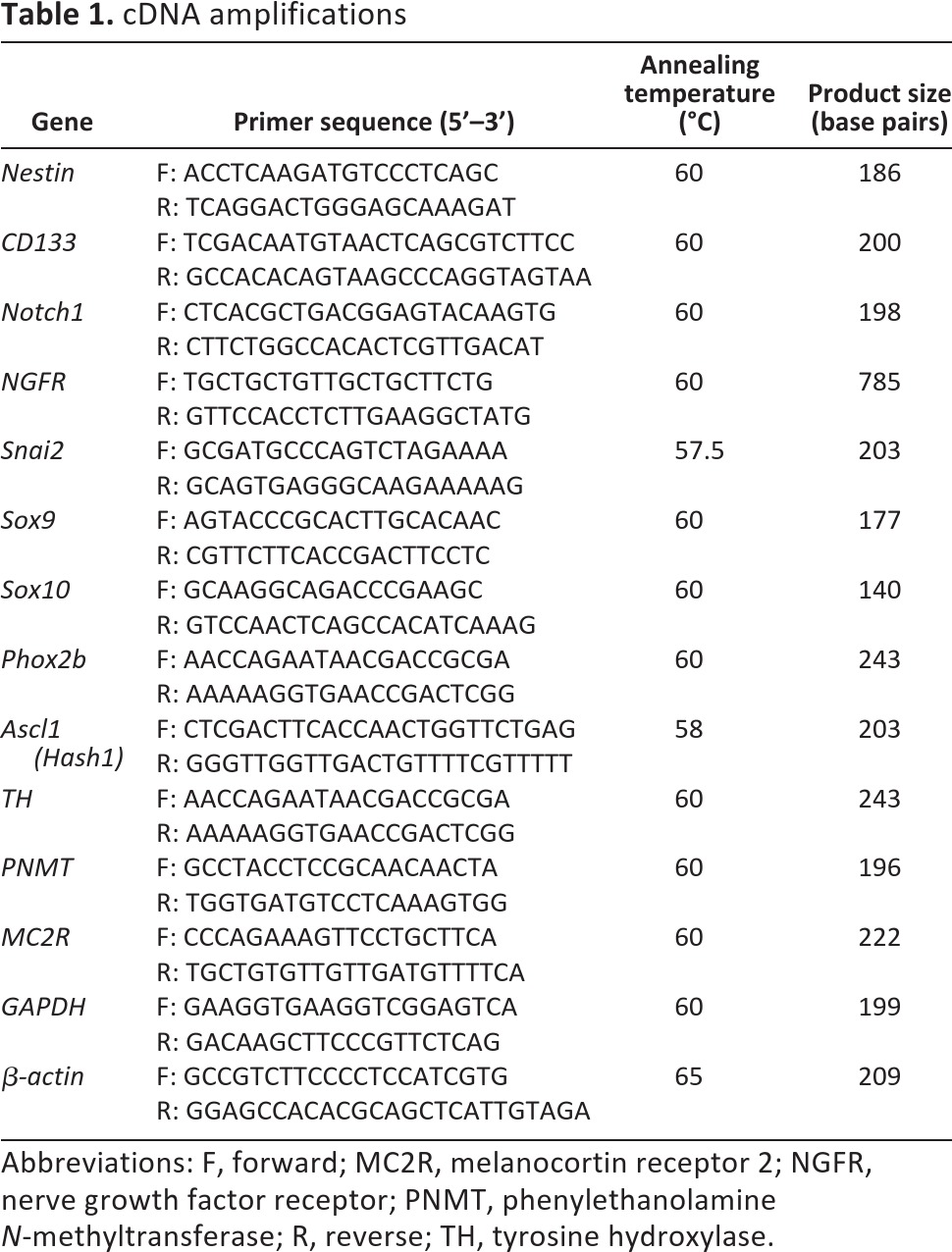
Abbreviations: F, forward; MC2R, melanocortin receptor 2; NGFR, nerve growth factor receptor; PNMT, phenylethanolamine N-methyltransferase; R, reverse; TH, tyrosine hydroxylase.
Real-Time RT-PCR
Expression levels of nestin, phenylethanolamine N-methyltransferase (PNMT), and melanocortin receptor 2 (MC2R) were measured using SYBR Green master mix (Qiagen) in a light cycler (Light Cycler 1.5; Roche Applied Science, Basel, Switzerland, https://www.roche-applied-science.com), as previously described [16, 22]. To generate standard curves for real-time PCR quantification, DNA fragments were amplified, cloned into plasmid vectors (pCRII-TOPO), and transformed into competent Escherichia coli using the TOPO TA Cloning kit (Invitrogen). Plasmids were purified (Maxi Plasmid kit; Qiagen) and their concentration was measured by spectroscopy. A serial dilution of plasmid was used to generate a linear regression standard curve. PCRs were performed in capillaries with initial denaturation at 94°C for 4 minutes; 45–50 cycles of amplification at 94°C for 15 seconds, annealing for 20 seconds, and elongation at 72°C for 15 seconds; and a final extension at 72°C for 4 minutes. The expression level of each gene was calculated by relative quantification to the expression of the reference gene β-actin.
Cell Differentiation
Chromospheres were mechanically dissociated, as described previously for neurosphere cultures [23], plated on slides coated with 50 μg/ml poly-l-lysine (PLL) and 5 μg/ml laminin (both from Sigma-Aldrich), and cultured for 1 additional day to allow cell attachment and expansion. Removing EGF from the medium and increasing the FGF-2 concentration to 20 ng/ml induced cell differentiation. Cells were allowed to differentiate for 6 days at 37°C in 5% CO2 atmosphere. Half of the medium was replaced by fresh medium every 2nd day.
Immunofluorescence Staining
Chromospheres embedded in OCT (Tissue-Tek; Sakura Finetek, Torrance, CA, http://www.sakura.com) were cut into 7-μm cryosections using a cryostat (Leica, Wetzlar, Germany, http://www.leica.com) and collected to Super Frost Plus glass slides (R. Langenbrinck, Emmendingen, Germany, http://www.langenbrinck.com). For immunostaining, cryosections were fixed in 4% paraformaldehyde for 20 minutes at room temperature and incubated in 0.1 M glycine solution for 30 minutes at room temperature. Cells were permeabilized with 1% Triton X-100 for 5 minutes, and nonspecific binding was prevented by incubating cells with blocking solution (3% [wt/vol] fatty acid-free bovine serum albumin supplemented with 0.2% Tween 20) for 1 hour at room temperature. Cryosections were then incubated with the primary antibody rabbit anti-SOX9 (1:200; Abcam, Cambridge, U.K., http://www.abcam.com) overnight at 4°C. Cells were washed three times with phosphate-buffered saline (PBS) and incubated for 1 hour at room temperature with the secondary antibody donkey anti-rabbit Cy5 (1:200; Jackson Immunoresearch Laboratories, West Grove, PA, http://www.jacksonimmuno.com). Nuclei were stained with Hoechst (1 μg/ml; Sigma-Aldrich) for 5 minutes. All antibody solutions were prepared in blocking solution. A negative control, without primary antibody, was performed to check for nonspecific binding and identify background due to cell autofluorescence. Cells were visualized with a fluorescent microscope (PALM Laser microdissection; Carl Zeiss, Jena, Germany, http://www.zeiss.com), coupled to an AxioCam HRc camera (Zeiss).
Differentiated cells were stained using a similar protocol. The primary antibodies and secondary antibodies used were the following: mouse anti-β-3-tubulin (1:500; Covance, Princeton, NJ, http://www.covance.com), rabbit anti-tyrosine hydroxylase (1:1,000; Chemicon, Temecula, CA, http://www.chemicon.com) and rabbit anti-PNMT (1:250; Enzo Life Sciences, Inc., Farmingdale, NY, http://www.enzolifesciences.com), anti-mouse Alexa Fluor 594 (1:200; Invitrogen), goat anti-rabbit Alexa Fluor 488 (1:200; Invitrogen), and donkey anti-rabbit Cy5 (1:200; Jackson Immunoresearch). Differentiated cells were visualized using a laser scanning confocal microscope, LSM 510 META (Zeiss).
Immunostaining of Tissue
Human adrenals were immunostained for tyrosine hydroxylase (TH) (rabbit anti-human, 1:2,000; Chemicon, Temecula, CA, http://www.chemicon.com) and nestin (rabbit anti-human, 1:100; Sigma-Aldrich). Paraformaldehyde-fixed human adrenals were cut in serial section at 4 μm, washed with PBS for 5 minutes at room temperature, post fixed (4% paraformaldehyde for 10 minutes), washed with PBS (three times for 5 minutes at room temperature), incubated with 3% H2O2 (15 minutes at room temperature), washed again, and incubated in blocking solution (1/30 normal goat serum in 1% BSA, 0.3% Triton X-100 in PBS for 1 hour at room temperature). After a 1-hour incubation of serial sections with the primary antibody at room temperature, slides were washed with PBS and incubated with the secondary antibody goat anti-rabbit conjugated to horseradish peroxidase (Bio-Rad, Munich, Germany, http://www.bio-rad.com) in blocking solution (1:250; 1 hour at room temperature), washed, and developed using 3-amino-9-ethylcarbazole (Dako, Hamburg, Germany, http://www.dako.com).
Array-Comparative Genomic Hybridization
Array-comparative genomic hybridization (CGH) was carried out on the SurePrint G3 Human CGH Microarray Kit 2x400K (design ID 021850; Agilent Technologies, Santa Clara, CA, http://www.agilent.com) according to the manufacturer's protocol. Scanning was done on an Agilent microarray scanner, and raw data were processed by Feature Extraction 9.5 (Agilent). Deleted and amplified regions were determined on Agilent's Genomic Workbench Standard Edition 5.0.14. Cell preparations were compared with themselves at different stages of cultivation (day 0 vs. day 12).
Results
Nestin-Positive Cells Are Present in Human Adult Adrenal Medulla
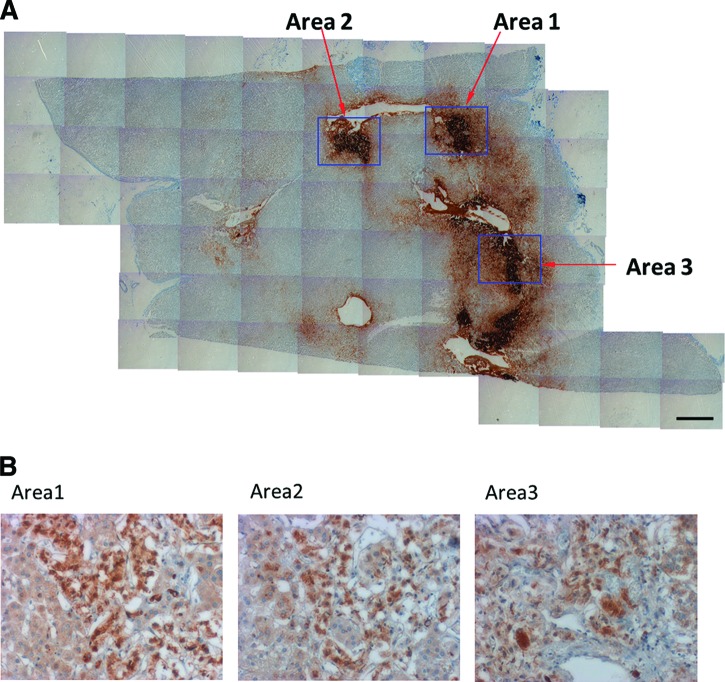
To evaluate the existence of undifferentiated cells within the adult human adrenal medulla, sections of human adrenals were immunostained for nestin, a marker for neural stem cells. Figure 1 shows the presence of nestin-positive cells within the human adrenal medulla, identified by immunoreactivity for TH, the rate-limiting enzyme in catecholamine synthesis.
Figure 1.
Immunohistochemical staining of human adrenal gland. Serial sections of a human adrenal immunostained for tyrosine hydroxylase to identify the adrenal medulla (scale bar = 1 mm) (A) and for nestin (B) revealed nestin+-cells within the adrenal medulla. The three areas identified in (A) are depicted in (B).
Free-Floating Chromospheres Obtained from Human Adult Adrenal Medulla
Recently, we isolated chromaffin progenitor cells from bovine adrenal medulla and established their culture as free-floating spherical colonies, named chromospheres [16]. To investigate whether chromaffin progenitor cells could also be isolated from human adrenal medulla we prepared a cell digest from medullary tissue of human adrenal glands. The digested cells were further cultured in low-attachment conditions, to avoid adherence of the cells to the surface, and in the presence of growth factors (EGF and FGF-2, 10 ng/ml). Under these conditions some of the cells were maintained in suspension, and after 10–12 days free-floating spheres, morphologically similar to bovine chromospheres, could be observed with an average diameter of 42.2 ± 1.1 μm (minimum = 15.8 μm and maximum = 86.9 μm; mean ± SD of 130 spheres from three independent cell culture) (Fig. 2). The number of isolated cells differed among the three glands investigated. At day 12, the total number of spheres obtained was 26,577 per 1,912,000 total isolated chromaffin cells from gland 1; 284,850 per 26,375,000 total isolated chromaffin cells from gland 2; and 88,281 per 7,062,500 total chromaffin cells from gland 3. This corresponds to 1.24 ± 0.155 spheres (day 12) per 100 chromaffin cells (day 0) (mean ± SD from three independent cell culture).
Figure 2.
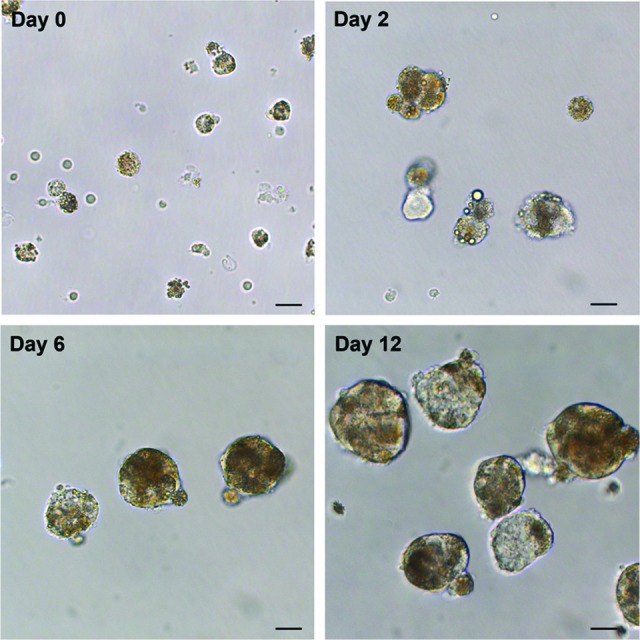
Morphology of human chromospheres. Cells from human adrenal medulla were isolated and cultured in low-attachment conditions in the presence of growth factors (epidermal growth factor and fibroblast growth factor-2; 10 ng/ml). Free-floating spheres were observed after 12 days in culture. Phase-contrast images. Scale bars = 20 μm.
To see whether during the time of cultivation the cells acquired genetic aberrations we performed array-CGH. Each cell preparation was compared with itself at different stages of cultivation. Array-CGH analysis did not indicate any loss or gain of genetic material for both preparations tested (day 0 vs. day 12). This analysis was thought to display differences between the samples (supplemental online Fig. 1). Existing copy numbers were not captured with this analysis.
Human Chromospheres Express Different Progenitor Cell Markers
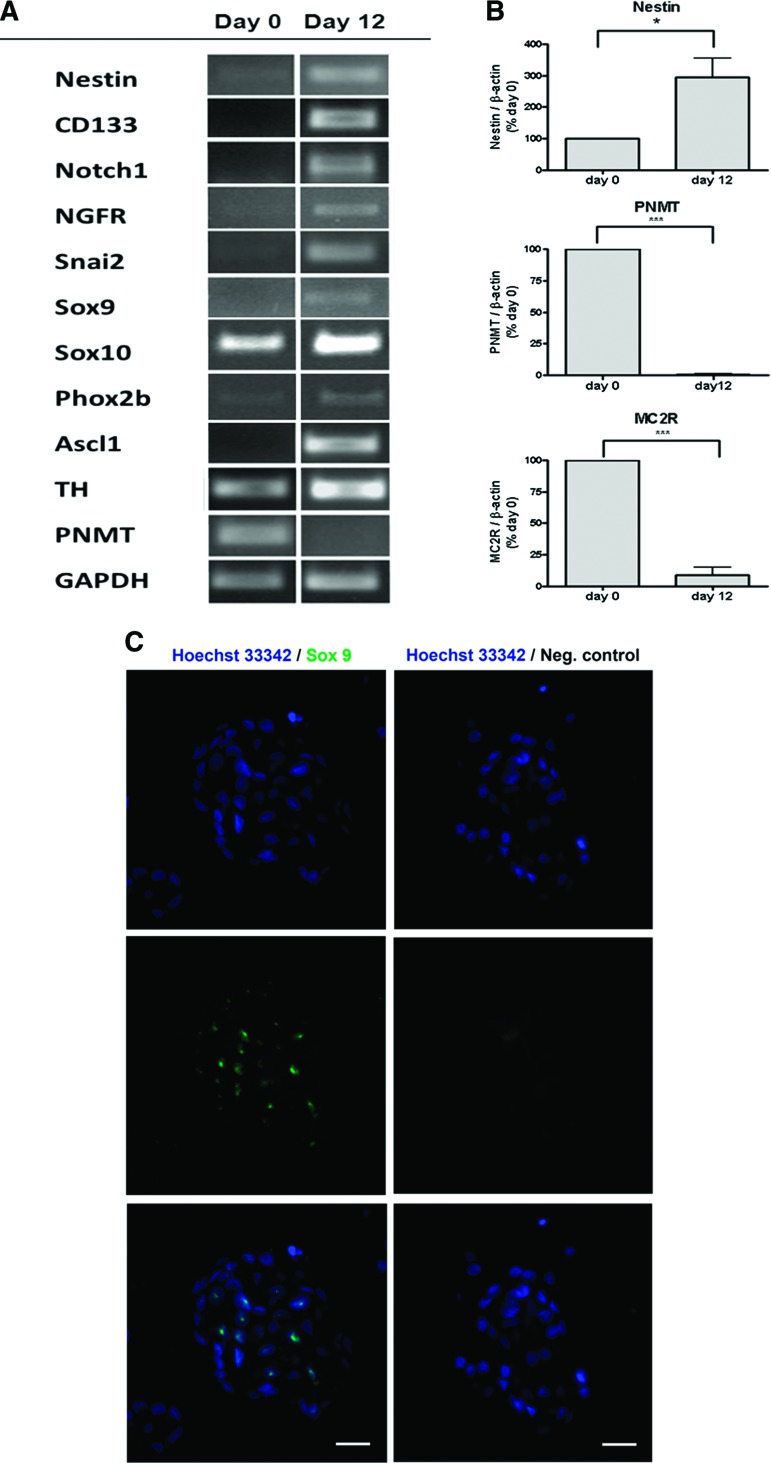
RT-PCR analysis of mRNA expression revealed the expression of several progenitor markers in human chromospheres after 12 days in culture that were not detectable or were present at low levels in primary chromaffin cells (day 0). These included nestin, CD133 (prominin-1), notch1, nerve growth factor receptor (NGFR), Snai2, Sox9, Sox10, Phox2b, and Ascl1 (Hash1). Primary chromaffin cells expressed the chromaffin cell marker PNMT, the enzyme catalyzing the final step of epinephrine synthesis (Fig. 3A). Quantitative real-time PCR revealed a significant effect of sphere formation on PNMT and nestin expression. Expression of PNMT drastically decreased after 12 days in culture; in parallel, nestin expression increased threefold over the time of culture (Fig. 3B). Primary cultures of human chromaffin cells also contain adrenocortical cells as revealed by the expression of MC2R, which was significantly reduced during sphere culture for 12 days (Fig. 3B). Immunofluorescent staining revealed nuclear staining for Sox9 in chromosphere cells, with 46 ± 7% Sox9-positive cells (mean ± SD of 50 spheres from two independent cell cultures); the negative control, where the first antibody was omitted, showed no unspecific staining (Fig. 3C).
Figure 3.
Characterization of human chromospheres. (A): Reverse transcription-polymerase chain reaction (RT-PCR). mRNA levels of neural progenitor cell markers and sympathoadrenal progenitor cell markers were increased in chromosphere cultures (day 12) compared with primary chromaffin cells (day 0). PNMT, a marker for differentiated chromaffin cells, was downregulated in chromospheres (day 12). (B): Quantitative RT-PCR. The expression of the neural stem cell marker nestin was increased after 12 days in culture. In opposition, PNMT expression was drastically reduced when compared with primary chromaffin cells (day 0). Chromospheres (day 12) showed lower adrenocorticotropic hormone receptor (MC2R) expression, a marker for adrenocortical cells, than primary chromaffin cells (day 0). n = 6; *, p < .05; ***, p < .001 (Student's t test for paired samples). (C): Immunofluorescent staining of chromosphere cryosections. Cells expressing Sox9 (green), a neural crest marker, were found in human chromospheres; cell nuclei were stained with Hoechst 33342 (blue). A negative control, without the first antibody, was performed to identify unspecific binding or background due to autofluorescence. Scale bars = 20 μm. Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MC2R, melanocortin receptor 2; Neg., negative; NGFR, nerve growth factor receptor; PNMT, phenylethanolamine N-methyltransferase; TH, tyrosine hydroxylase.
Human Chromaffin Progenitor Cells Differentiate into Neuron-Like Cells
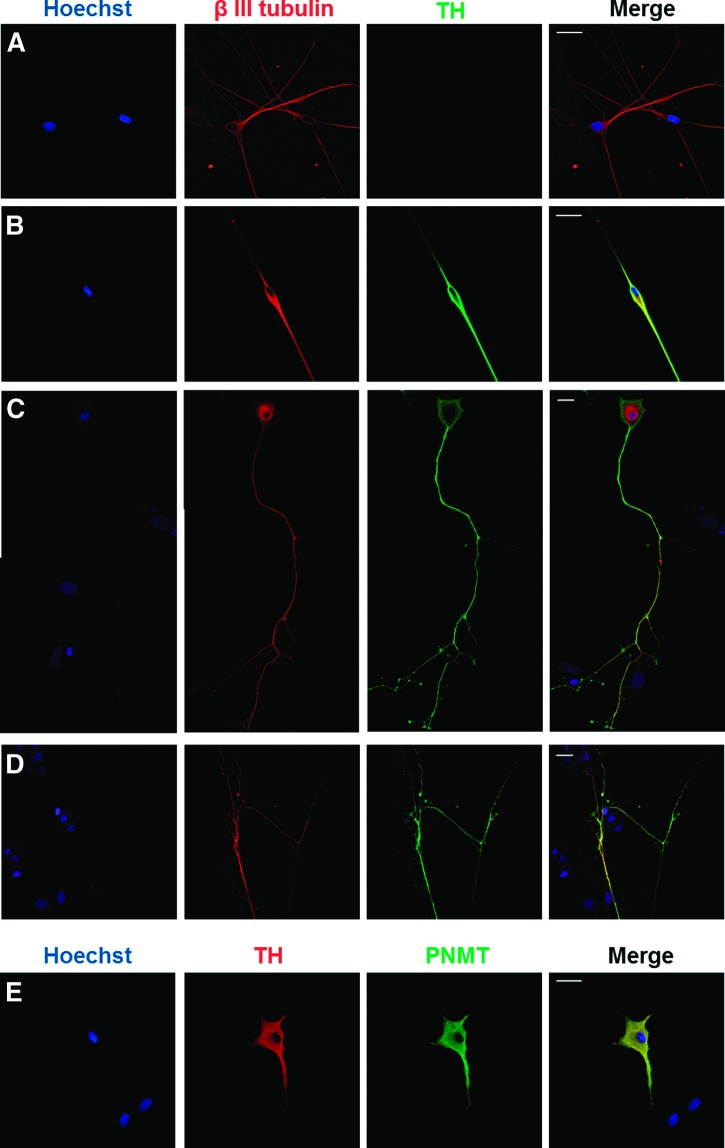
To characterize the differentiation potential of isolated human chromaffin progenitor cells into neuronal cells, chromospheres were dissociated and cells were plated onto PLL/laminin-coated slides. Over the following 24 hours the cells adhered to the surface; then EGF was removed from the medium and the FGF-2 concentration was increased to 20 ng/ml, inducing cell differentiation. After 2 days, the development of neurites was observed; 6 days after plating some cells revealed long neurites (Fig. 4C). The differentiating cells were characterized by immunofluorescent staining for the early neuronal marker β-3-tubulin and the catecholaminergic marker TH. Double staining revealed the presence of neuron-like cells with a catecholaminergic phenotype, β-3-tubulin+/TH+ (Fig. 4B–4D). Noncatecholaminergic neuronal cells, β-3-tubulin+/TH−, could also be observed (Fig. 4A). As expected, some of the cells differentiated into chromaffin cells, as shown by the immunoreactivity to PNMT and TH (Fig. 4E).
Figure 4.
Immunofluorescent staining of differentiated chromosphere cells. Different types of neuron-like cells were obtained after plating the dissociated chromosphere cells on poly-l-lysine/laminin-coated slides in a medium supplemented with fibroblast growth factor-2 (20 ng/ml): TH−/β-3-tubulin+ cells (A) and TH+/β-3-tubulin+ cells (B–D). Differentiated chromaffin cells (TH+/PNMT+) were also present in culture (E). Nuclei: Hoechst 33342 (blue). Scale bars = 20 μm. Abbreviations: PNMT, phenylethanolamine N-methyltransferase; TH, tyrosine hydroxylase.
Discussion
In the present study, human chromaffin progenitor cells were isolated from adult human adrenals by adapting a method previously established by our group for bovine adrenals [16]. Similar to chromaffin progenitor cells from bovine [16, 17, 19], free-floating spherical colonies (chromospheres) were enriched in human chromaffin progenitor cells when cultured in low-attachment conditions and in the presence of EGF and FGF-2. During chromosphere formation, levels of nestin mRNA, a marker for neural progenitor cells, were significantly increased. In contrast, the selective culture conditions led to a dramatic reduction of the expression of PNMT, a marker of mature chromaffin cells. This drastic decrease of PNMT expression after 12 days suggests a selective enrichment of the progenitor cell population existing within human adrenals. In fact, the majority of cells obtained from primary cultures of adrenal medullae are differentiated chromaffin cells, which express PNMT and require adherent conditions to survive. Furthermore, the first unpublished data from a transgenic mouse model where green fluorescent protein is expressed under the regulation of the nestin promoter [24] indicate that the nestin-expressing progenitor cells selectively proliferate under these culture conditions (M.F. Rubin de Celis and M. Ehrhart-Bornstein, unpublished data). Chromospheres from adult human adrenals also show similarities to PNMT-negative progenitor cell-enriched spheres previously observed in primary cultures of human fetal chromaffin cells [25].
The “stemness” of human chromosphere cells is indicated by the expression of several progenitor markers. In contrast to primary chromaffin cells, chromosphere cells expressed genetic markers for neural stem cells (nestin, CD133, NGFR, and Snai2). These cells also expressed Sox9, a member of the SoxE subgroup with an important role in embryonic migration and differentiation of neural crest derivatives [26, 27]. In addition, Sox9 has been identified as a common marker for multiple tissue-specific progenitors [28] and neural stem cells in the brain [29]. Sox9 also induces the expression of Sox10 in neural crest cells, which in turn is required for the specifications and survival of chromaffin precursors [30, 31]. Sox10 expression is downregulated in adult adrenal medulla, and its importance is restricted to the early stages of adrenal medulla development [32–35]. The increase in Sox10 expression in human chromospheres is therefore in agreement with an enrichment in chromaffin progenitor cells.
The sympathoadrenal development is regulated by a complex network of interacting transcription factors that are activated to specify both neural and chromaffin cell lineages. The pro-neural gene Mash1, or Hash1 in humans, is the mammalian homolog of the Drosophila achaete-scute complex encoding the helix-loop-helix-type transcription factor Acsl1, which is expressed in the majority of sympathoadrenal progenitors during embryogenesis and is a key factor in the development of chromaffin cells [36, 37]. It is also present in developing brain [38, 39] preceding neural differentiation [40] and recently was shown to contribute to the conversion of human fibroblasts into dopaminergic neurons [41]. In rodents, Mash1 expression depends on the expression of Phox2b, another transcription factor expressed in all central and peripheral noradrenergic neurons, which is essential for SA development and for the very early steps of chromaffin cell and sympathetic neurons differentiation [37, 42–44]. In accordance, both Acsl1 and Phox2b were upregulated in human chromospheres.
Although the trophic factors required for the differential specifications of sympathetic neurons or chromaffin cells remain to be elucidated, it was suggested that FGF-2 would be a critical factor for the neuronal fate in SA cells, promoting cell proliferation and neurite outgrowth and inducing dependence on nerve growth factor (NGF), which is subsequently responsible for neuron maturation and survival [45, 46]. Accordingly, we induced the differentiation of chromaffin progenitor cells by promoting cell adherence in the presence of FGF-2. In these conditions, chromaffin progenitor cells developed long neurites and differentiated into two different neuronal phenotypes: catecholaminergic neuron-like cells, characterized by the presence of the early neuronal marker β-3-tubulin and the catecholaminergic marker TH, and noncatecholaminergic neuron-like cells, which were immunoreactive for β-3-tubulin but not for TH. As expected, some of the progenitor cells differentiated into chromaffin cells, which were characterized by the presence of the enzymes TH and PNMT. Although chromaffin progenitors were successfully differentiated into neuronal cells, the percentage of differentiation proved to be lower than the expected. In fact, evidence suggests that FGF-2 might be required only for the early proliferative stages of neuronal development, maintaining the proliferative pool of neural stem cells [47, 48], whereas other factors would be responsible for the maturation and survival of differentiated neurons [49–53].
Thus, modifications of the differentiation protocol by additional factors, such as NGF, bone morphogenic proteins, leukemia inhibitory factor, or retinoic acid, could be an alternative to increase the percentage of neuron-like cells. For example, we recently established protocols to differentiate bovine chromaffin progenitor cells into electrophysiological functional neurons with increased capacity to synthesize and secrete dopamine by treating the cells with retinoic and ascorbic acid [17].
The knowledge of the mechanisms and signaling pathways involved in chromaffin progenitor proliferation and differentiation could also be crucial for the establishment of appropriate in vitro culture conditions leading to increased yield and survival of differentiated cells, allowing further use in cell transplantation. In this context, the observed expression of Notch1 in human chromaffin progenitor cells is of interest. Different studies demonstrated the importance of the Notch pathway during neurogenesis [54, 55]. In fact, we could show that the downstream Notch effectors Hes1/5 are downregulated during neuronal differentiation of bovine chromaffin progenitor cells [17], indicating that the maintenance of bovine and human chromaffin progenitors in an undifferentiated state and potentially their neural differentiation are also mediated via the Notch pathway.
Unfortunately, the human adrenal gland is characterized by intense intermingling of medullary and cortical cells [56], making the separation of the two tissues a difficult task. Although adrenocortical cells were clearly reduced during sphere culture, as shown by the decrease in the expression of the adrenocortical cell marker MC2R, protocols need to be established to entirely remove these cells from progenitor cultures for their therapeutic use in the future.
Despite this, the potential of these progenitor cells to acquire both neuronal and chromaffin cell phenotypes is unquestionable, making them an interesting new cell source for cell-based therapies. The currently available cell sources for cell transplantation therapies in the field of neurodegenerative diseases include neural stem cells (NSCs), mesenchymal stem cells, embryonic stem cells (ESCs), and induced pluripotent stem cells. However, the limited availability and the safety and ethical issues associated with the use of the majority of these cells are limitations to their use [57, 58]. Chromaffin progenitor cells seem to be a promising cell source due to the potential use in autologous transplantations avoiding immune rejection and the ethical and political controversies related to the use of other cell sources, such as ESCs or NSCs [19, 20]. Moreover, the restricted growth and differentiation potential and also the chromosomal stability of these progenitor cells make them a safer option.
Nevertheless, the interest in these cells is far from being limited to their use in cell transplantation. Chromaffin progenitor cells might be related to stress-induced adrenal gland hyperplasia [59–61]. Chronic stress induces several alterations in the adrenal medulla, which adapts to enhanced catecholamine synthesis by increasing adrenal size and expression of catecholamine-synthetizing enzymes [62]. Moreover, chronic stress induces an upregulation of several transcription factors, including Mash-1 [63], which, as mentioned above, is essential for SA cell differentiation.
Conclusion
This study proves the existence of chromaffin progenitor cells in the human adrenal medulla and demonstrates that they can be isolated from adult adrenal glands. These cells open new perspectives and challenges in the field of regenerative medicine, especially regarding their potential use in the treatment of neurodegenerative and neuroendocrine diseases. Moreover, these cells might also contribute to a better understanding of adrenal medulla pathologies and dysfunctions, such as stress-induced adrenal medulla hyperplasia.
See www.StemCellsTM.com for supporting information available online.
Acknowledgments
We are grateful to Dr. Volker Janitzky and Dr. Torsten Weirich (Department of Urology, Pirna General Hospital, Pirna, Germany) for generously providing adrenal tissue specimens. This work was supported by Fundação para a Ciência e a Tecnologia, Portugal (FEDER, COMPETE: PTDC/SAU-NEU/108110/2008, SFRH/BPD/31547/2006, SFRH/BD/44664/2008), the Deutsche Forschungsgemeinschaft (SFB 655 “From cells to tissues,” KFO 252 “Microenvironment of the Adrenal in Health and Disease” [EH161/5-1]), and the Center for Regenerative Therapies, Dresden, Germany.
Author Contributions
M.M.S. and K.-F.C.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; V.V.: collection and assembly of data, manuscript writing; J.R.-S.: conception and design, data analysis and interpretation; W.K. and V.C.: collection and assembly of data; K.H.: collection and assembly of data, data analysis and interpretation; C.A.B. and A.M.: provision of study material; E.S.: data analysis and interpretation; S.R.B.: conception and design, financial support; C.C.: conception and design, financial support, data analysis and interpretation, manuscript writing; M.E.-B.: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Huber K, Kalcheim C, Unsicker K. The development of the chromaffin cell lineage from the neural crest. Auton Neurosci. 2009;151:10–16. doi: 10.1016/j.autneu.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 2.Cavadas C, Silva AP, Mosimann F, et al. NPY regulates catecholamine secretion from human adrenal chromaffin cells. J Clin Endocrinol Metab. 2001;86:5956–5963. doi: 10.1210/jcem.86.12.8091. [DOI] [PubMed] [Google Scholar]
- 3.Crivellato E, Nico B, Ribatti D. The chromaffin vesicle: Advances in understanding the composition of a versatile, multifunctional secretory organelle. Anat Rec (Hoboken) 2008;291:1587–1602. doi: 10.1002/ar.20763. [DOI] [PubMed] [Google Scholar]
- 4.Doupe AJ, Landis SC, Patterson PH. Environmental influences in the development of neural crest derivatives: Glucocorticoids, growth factors, and chromaffin cell plasticity. J Neurosci. 1985;5:2119–2142. doi: 10.1523/JNEUROSCI.05-08-02119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sicard F, Ehrhart-Bornstein M, Corbeil D, et al. Age-dependent regulation of chromaffin cell proliferation by growth factors, dehydroepiandrosterone (DHEA), and DHEA sulfate. Proc Natl Acad Sci USA. 2007;104:2007–2012. doi: 10.1073/pnas.0610898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tischler AS, Ruzicka LA, Donahue SR, et al. Chromaffin cell proliferation in the adult rat adrenal medulla. Int J Dev Neurosci. 1989;7:439–448. doi: 10.1016/0736-5748(89)90004-x. [DOI] [PubMed] [Google Scholar]
- 7.Tischler AS, Ruzicka LA, Riseberg JC. Immunocytochemical analysis of chromaffin cell proliferation in vitro. J Histochem Cytochem. 1992;40:1043–1045. doi: 10.1177/40.7.1351491. [DOI] [PubMed] [Google Scholar]
- 8.Unsicker K, Krisch B, Otten U, et al. Nerve growth factor-induced fiber outgrowth from isolated rat adrenal chromaffin cells: Impairment by glucocorticoids. Proc Natl Acad Sci USA. 1978;75:3498–3502. doi: 10.1073/pnas.75.7.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verhofstad AA. Kinetics of adrenal medullary cells. J Anat. 1993;183:315–326. [PMC free article] [PubMed] [Google Scholar]
- 10.Backlund EO, Granberg PO, Hamberger B, et al. Transplantation of adrenal medullary tissue to striatum in parkinsonism. First clinical trials. J Neurosurg. 1985;62:169–173. doi: 10.3171/jns.1985.62.2.0169. [DOI] [PubMed] [Google Scholar]
- 11.Drucker-Colín R, Verdugo-Diaz L. Cell transplantation for Parkinson's disease: Present status. Cell Mol Neurobiol. 2004;24:301–316. doi: 10.1023/B:CEMN.0000022764.94760.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francis NJ, Landis SC. Cellular and molecular determinants of sympathetic neuron development. Annu Rev Neurosci. 1999;22:541–566. doi: 10.1146/annurev.neuro.22.1.541. [DOI] [PubMed] [Google Scholar]
- 13.Jozan S, Aziza J, Chatelin S, et al. Human fetal chromaffin cells: A potential tool for cell pain therapy. Exp Neurol. 2007;205:525–535. doi: 10.1016/j.expneurol.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Lazorthes Y, Sagen J, Sallerin B, et al. Human chromaffin cell graft into the CSF for cancer pain management: A prospective phase II clinical study. Pain. 2000;87:19–32. doi: 10.1016/S0304-3959(00)00263-3. [DOI] [PubMed] [Google Scholar]
- 15.Madrazo I, Drucker-Colin R, Diaz V, et al. Open microsurgical autograft of adrenal medulla to the right caudate nucleus in two patients with intractable Parkinson's disease. N Engl J Med. 1987;316:831–834. doi: 10.1056/NEJM198704023161402. [DOI] [PubMed] [Google Scholar]
- 16.Chung KF, Sicard F, Vukicevic V, et al. Isolation of neural crest derived chromaffin progenitors from adult adrenal medulla. Stem Cells. 2009;27:2602–2613. doi: 10.1002/stem.180. [DOI] [PubMed] [Google Scholar]
- 17.Vukicevic V, Schmid J, Hermann A, et al. Differentiation of chromaffin progenitor cells to dopaminergic neurons. Cell Transplant. 2012 doi: 10.3727/096368912X638874. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Bornstein SR, Ehrhart-Bornstein M, Androutsellis-Theotokis A, et al. Chromaffin cells: The peripheral brain. Mol Psychiatry. 2012;17:354–358. doi: 10.1038/mp.2011.176. [DOI] [PubMed] [Google Scholar]
- 19.Ehrhart-Bornstein M, Chung KF, Vukicevic V, et al. Is there a role for chromaffin progenitor cells in neurodegenerative diseases? Mol Psychiatry. 2009;14:1–4. doi: 10.1038/mp.2008.114. [DOI] [PubMed] [Google Scholar]
- 20.Ehrhart-Bornstein M, Vukicevic V, Chung KF, et al. Chromaffin progenitor cells from the adrenal medulla. Cell Mol Neurobiol. 2010;30:1417–1423. doi: 10.1007/s10571-010-9571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosmaninho-Salgado J, Araujo IM, Alvaro AR, et al. Regulation of catecholamine release and tyrosine hydroxylase in human adrenal chromaffin cells by interleukin-1beta: Role of neuropeptide Y and nitric oxide. J Neurochem. 2009;109:911–922. doi: 10.1111/j.1471-4159.2009.06023.x. [DOI] [PubMed] [Google Scholar]
- 22.Chung KF, Qin N, Androutsellis-Theotokis A, et al. Effects of dehydroepiandrosterone on proliferation and differentiation of chromaffin progenitor cells. Mol Cell Endocrinol. 2011;336:141–148. doi: 10.1016/j.mce.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 23.Tropepe V, Sibilia M, Ciruna BG, et al. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol. 1999;208:166–188. doi: 10.1006/dbio.1998.9192. [DOI] [PubMed] [Google Scholar]
- 24.Androutsellis-Theotokis A, Rubin de Celis MF, Ehrhart-Bornstein M, et al. Common features between chromaffin and neural progenitor cells. Mol Psychiatry. 2012;17:351. doi: 10.1038/mp.2012.18. [DOI] [PubMed] [Google Scholar]
- 25.Zhou H, Aziza J, Sol JC, et al. Cell therapy of pain: Characterization of human fetal chromaffin cells at early adrenal medulla development. Exp Neurol. 2006;198:370–381. doi: 10.1016/j.expneurol.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Cheung M, Briscoe J. Neural crest development is regulated by the transcription factor Sox9. Development. 2003;130:5681–5693. doi: 10.1242/dev.00808. [DOI] [PubMed] [Google Scholar]
- 27.Hong CS, Saint-Jeannet JP. Sox proteins and neural crest development. Semin Cell Dev Biol. 2005;16:694–703. doi: 10.1016/j.semcdb.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Akiyama H, Kim JE, Nakashima K, et al. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci USA. 2005;102:14665–14670. doi: 10.1073/pnas.0504750102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sottile V, Li M, Scotting PJ. Stem cell marker expression in the Bergmann glia population of the adult mouse brain. Brain Res. 2006;1099:8–17. doi: 10.1016/j.brainres.2006.04.127. [DOI] [PubMed] [Google Scholar]
- 30.Kapur RP. Early death of neural crest cells is responsible for total enteric aganglionosis in Sox10(Dom)/Sox10(Dom) mouse embryos. Pediatr Dev Pathol. 1999;2:559–569. doi: 10.1007/s100249900162. [DOI] [PubMed] [Google Scholar]
- 31.Reiprich S, Stolt CC, Schreiner S, et al. SoxE proteins are differentially required in mouse adrenal gland development. Mol Biol Cell. 2008;19:1575–1586. doi: 10.1091/mbc.E07-08-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deal KK, Cantrell VA, Chandler RL, et al. Distant regulatory elements in a Sox10-beta GEO BAC transgene are required for expression of Sox10 in the enteric nervous system and other neural crest-derived tissues. Dev Dyn. 2006;235:1413–1432. doi: 10.1002/dvdy.20769. [DOI] [PubMed] [Google Scholar]
- 33.Kelsh RN. Sorting out Sox10 functions in neural crest development. Bioessays. 2006;28:788–798. doi: 10.1002/bies.20445. [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Lo L, Dormand E, et al. SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron. 2003;38:17–31. doi: 10.1016/s0896-6273(03)00163-6. [DOI] [PubMed] [Google Scholar]
- 35.Kuhlbrodt K, Herbarth B, Sock E, et al. Sox10, a novel transcriptional modulator in glial cells. J Neurosci. 1998;18:237–250. doi: 10.1523/JNEUROSCI.18-01-00237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guillemot F, Lo LC, Johnson JE, et al. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–476. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- 37.Huber K, Bruhl B, Guillemot F, et al. Development of chromaffin cells depends on MASH1 function. Development. 2002;129:4729–4738. doi: 10.1242/dev.129.20.4729. [DOI] [PubMed] [Google Scholar]
- 38.Johnson JE, Birren SJ, Anderson DJ. Two rat homologues of Drosophila achaete-scute specifically expressed in neuronal precursors. Nature. 1990;346:858–861. doi: 10.1038/346858a0. [DOI] [PubMed] [Google Scholar]
- 39.Lo LC, Johnson JE, Wuenschell CW, et al. Mammalian achaete-scute homolog 1 is transiently expressed by spatially restricted subsets of early neuroepithelial and neural crest cells. Genes Dev. 1991;5:1524–1537. doi: 10.1101/gad.5.9.1524. [DOI] [PubMed] [Google Scholar]
- 40.Torii M, Matsuzaki F, Osumi N, et al. Transcription factors Mash-1 and Prox-1 delineate early steps in differentiation of neural stem cells in the developing central nervous system. Development. 1999;126:443–456. doi: 10.1242/dev.126.3.443. [DOI] [PubMed] [Google Scholar]
- 41.Pfisterer U, Kirkeby A, Torper O, et al. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci USA. 2011;108:10343–10348. doi: 10.1073/pnas.1105135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huber K. The sympathoadrenal cell lineage: Specification, diversification, and new perspectives. Dev Biol. 2006;298:335–343. doi: 10.1016/j.ydbio.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 43.Huber K, Karch N, Ernsberger U, et al. The role of Phox2B in chromaffin cell development. Dev Biol. 2005;279:501–508. doi: 10.1016/j.ydbio.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Morigushi T, Lim K, Engel J. Transcription factor networks specify sympathetic and adrenal chromaffin cell differentiation. Funct Dev Embryol. 2007;1:130–135. [Google Scholar]
- 45.Anderson DJ. Molecular control of cell fate in the neural crest: The sympathoadrenal lineage. Annu Rev Neurosci. 1993;16:129–158. doi: 10.1146/annurev.ne.16.030193.001021. [DOI] [PubMed] [Google Scholar]
- 46.Stemple DL, Mahanthappa NK, Anderson DJ. Basic FGF induces neuronal differentiation, cell division, and NGF dependence in chromaffin cells: A sequence of events in sympathetic development. Neuron. 1988;1:517–525. doi: 10.1016/0896-6273(88)90182-1. [DOI] [PubMed] [Google Scholar]
- 47.Namaka MP, Sawchuk M, MacDonald SC, et al. Neurogenesis in postnatal mouse dorsal root ganglia. Exp Neurol. 2001;172:60–69. doi: 10.1006/exnr.2001.7761. [DOI] [PubMed] [Google Scholar]
- 48.Villegas SN, Canham M, Brickman JM. FGF signalling as a mediator of lineage transitions–evidence from embryonic stem cell differentiation. J Cell Biochem. 2010;110:10–20. doi: 10.1002/jcb.22536. [DOI] [PubMed] [Google Scholar]
- 49.Anderson DJ. Cell fate determination in the peripheral nervous system: The sympathoadrenal progenitor. J Neurobiol. 1993;24:185–198. doi: 10.1002/neu.480240206. [DOI] [PubMed] [Google Scholar]
- 50.Stavridis MP, Collins BJ, Storey KG. Retinoic acid orchestrates fibroblast growth factor signalling to drive embryonic stem cell differentiation. Development. 2010;137:881–890. doi: 10.1242/dev.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen H, Tung YC, Li B, et al. Trophic factors counteract elevated FGF-2-induced inhibition of adult neurogenesis. Neurobiol Aging. 2007;28:1148–1162. doi: 10.1016/j.neurobiolaging.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 52.Murphy M, Reid K, Ford M, et al. FGF2 regulates proliferation of neural crest cells, with subsequent neuronal differentiation regulated by LIF or related factors. Development. 1994;120:3519–3528. doi: 10.1242/dev.120.12.3519. [DOI] [PubMed] [Google Scholar]
- 53.Denham M, Dottori M. Signals involved in neural differentiation of human embryonic stem cells. Neurosignals. 2009;17:234–241. doi: 10.1159/000231890. [DOI] [PubMed] [Google Scholar]
- 54.Androutsellis-Theotokis A, Leker RR, Soldner F, et al. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- 55.Yoon K, Gaiano N. Notch signaling in the mammalian central nervous system: Insights from mouse mutants. Nat Neurosci. 2005;8:709–715. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- 56.Ehrhart-Bornstein M, Hinson JP, Bornstein SR, et al. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev. 1998;19:101–143. doi: 10.1210/edrv.19.2.0326. [DOI] [PubMed] [Google Scholar]
- 57.Hipp J, Atala A. Sources of stem cells for regenerative medicine. Stem Cell Rev. 2008;4:3–11. doi: 10.1007/s12015-008-9010-8. [DOI] [PubMed] [Google Scholar]
- 58.Dantuma E, Merchant S, Sugaya K. Stem cells for the treatment of neurodegenerative diseases. Stem Cell Res Ther. 2010;1:37. doi: 10.1186/scrt37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scaria KS, Premalatha LS. Cold induced adrenal weight and volume changes in white rats. Indian J Exp Biol. 1967;5:256–257. [PubMed] [Google Scholar]
- 60.Ulrich-Lai YM, Figueiredo HF, Ostrander MM, et al. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab. 2006;291:E965–E973. doi: 10.1152/ajpendo.00070.2006. [DOI] [PubMed] [Google Scholar]
- 61.Wolman M, Cervos-Navarro J, Sampaolo S, et al. Pathological changes in organs of rats chronically exposed to hypoxia. Development of pulmonary lipidosis. Histol Histopathol. 1993;8:247–255. [PubMed] [Google Scholar]
- 62.Kvetnansky R, Sabban EL, Palkovits M. Catecholaminergic systems in stress: Structural and molecular genetic approaches. Physiol Rev. 2009;89:535–606. doi: 10.1152/physrev.00042.2006. [DOI] [PubMed] [Google Scholar]
- 63.Liu X, Serova L, Kvetnansky R, et al. Identifying the stress transcriptome in the adrenal medulla following acute and repeated immobilization. Ann NY Acad Sci. 2008;1148:1–28. doi: 10.1196/annals.1410.082. [DOI] [PMC free article] [PubMed] [Google Scholar]