Abstract
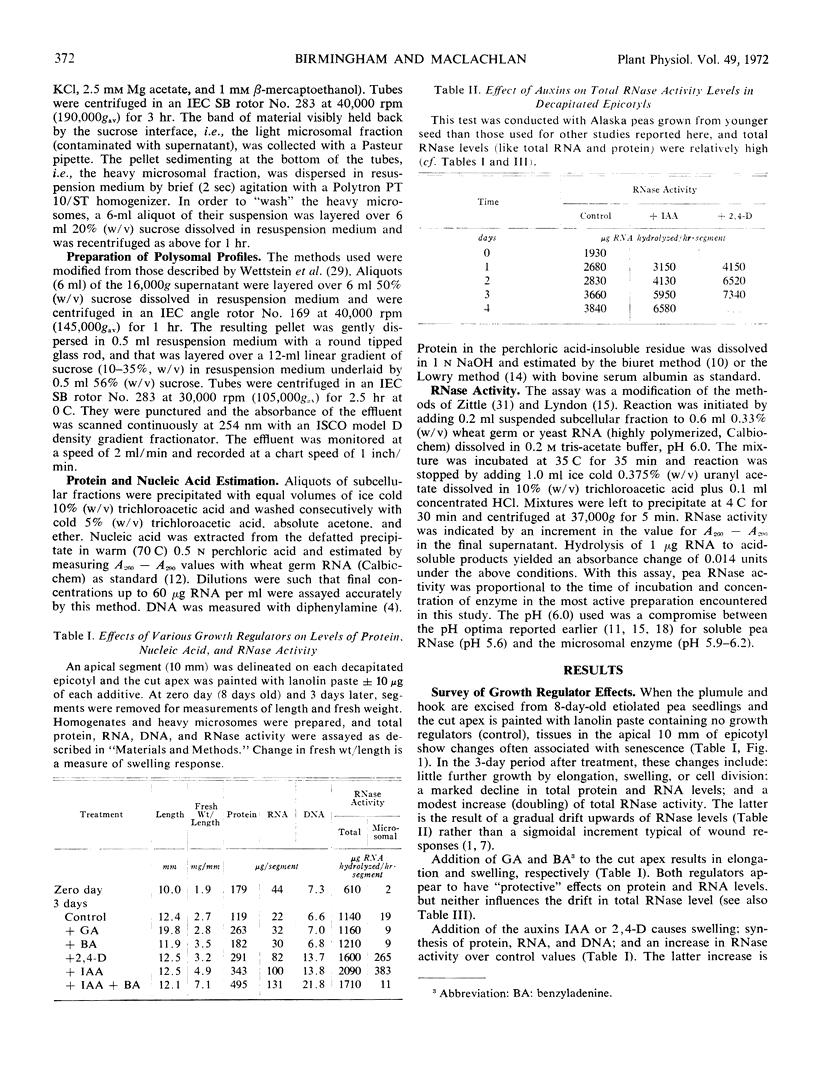
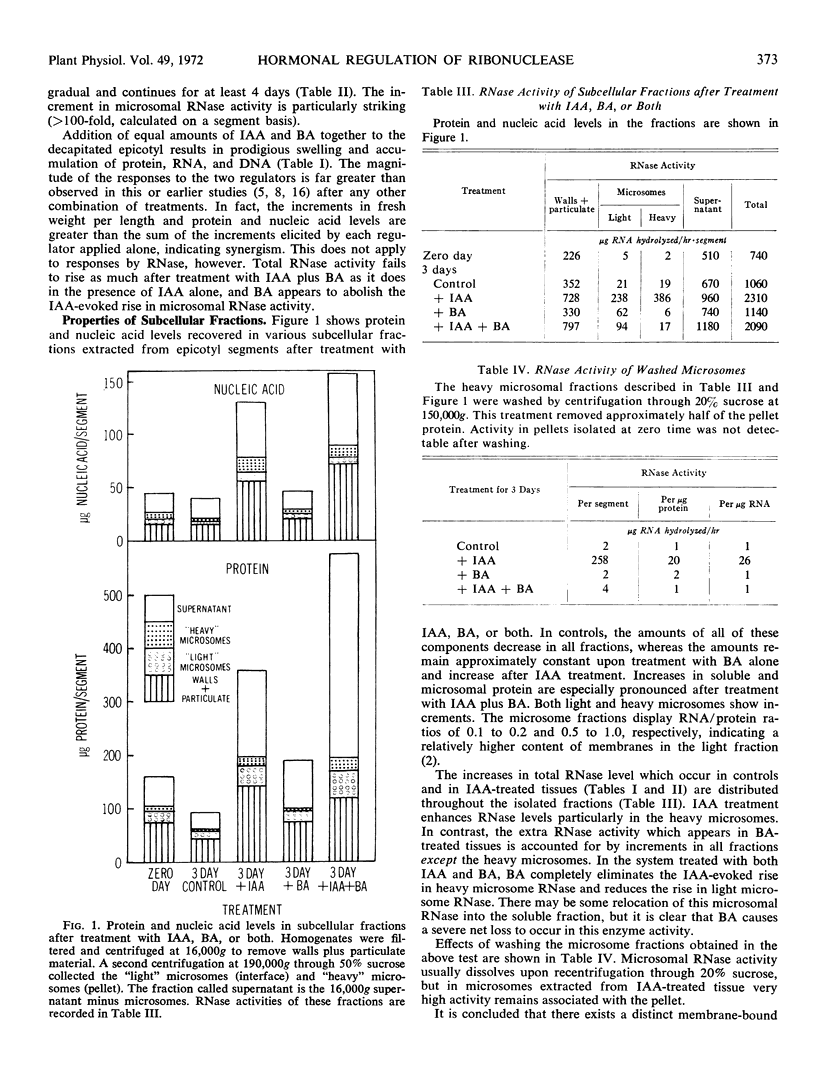
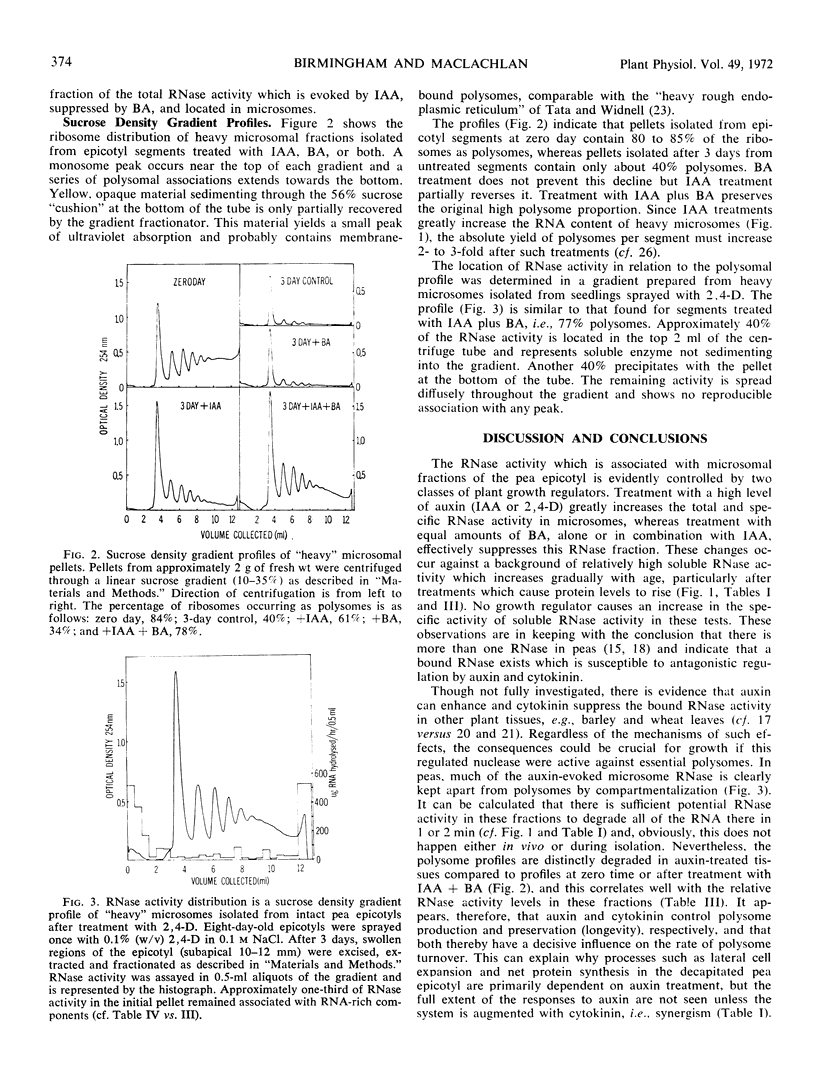
RNase activity was assayed in subcellular fractions of apical regions of Pisum sativum L. var. Alaska epicotyls after seedling decapitation and treatments with various growth regulators. High concentrations of applied indoleacetic acid caused a marked increase to occur in the RNase activity level associated with “heavy” microsomes, e.g., a 20-fold rise per unit RNA or protein in 3 days. This rise could be abolished by treating with the cytokinin benzyladenine along with indoleacetic acid. Nevertheless, indoleacetic acid and benzyladenine acted synergistically in their abilities to evoke swelling and net synthesis of RNA and protein. Polysomal profiles prepared after treatment with indoleacetic acid plus benzyladenine showed less degradation than profiles from any other treatment. It is concluded that auxin generates and cytokinin suppresses the activity of a particular membrane-bound RNase which can control turnover of the auxin-evoked polysomes required for growth in peas. Synergism between the two hormones in this system may be explained by the action of one to increase RNA synthesis and the other to decrease RNA destruction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemendal H., Bont W. S., de Vries M., Benedetti E. L. Isolation and properties of polyribosomes and fragments of the endoplasmic reticulum from rat liver. Biochem J. 1967 Apr;103(1):177–182. doi: 10.1042/bj1030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer E. N., Foster L. B., Sells B. H. A possible role for ribonuclease in the regulation of protein synthesis in normal and hypophysectomized rats. J Biol Chem. 1969 Mar 25;244(6):1389–1392. [PubMed] [Google Scholar]
- DIENER T. O. Virus infection and other factors affecting ribonuclease activity of plant leaves. Virology. 1961 Jun;14:177–189. doi: 10.1016/0042-6822(61)90193-3. [DOI] [PubMed] [Google Scholar]
- Davies E., Maclachlan G. A. Effects of indoleacetic acid on intracellular distribution of beta-glucanase activities in the pea epicotyl. Arch Biochem Biophys. 1968 Dec;128(3):595–600. doi: 10.1016/0003-9861(68)90068-4. [DOI] [PubMed] [Google Scholar]
- Davies E., Maclachlan G. A. Generation of cellulase activity during protein synthesis by pea microsomes in vitro. Arch Biochem Biophys. 1969 Feb;129(2):581–587. doi: 10.1016/0003-9861(69)90217-3. [DOI] [PubMed] [Google Scholar]
- Fan D. F., Maclachlan G. A. Massive synthesis of ribonucleic Acid and cellulase in the pea epicotyl in response to indoleacetic Acid, with and without concurrent cell division. Plant Physiol. 1967 Aug;42(8):1114–1122. doi: 10.1104/pp.42.8.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filner P., Varner J. E., Wray J. L. Environmental or developmental changes cause many enzyme activities of higher plants to rise or fall. Science. 1969 Jul 25;165(3891):358–367. doi: 10.1126/science.165.3891.358. [DOI] [PubMed] [Google Scholar]
- HOLDEN M., PIRIE N. W. The partial purification of leaf ribonuclease. Biochem J. 1955 May;60(1):39–46. doi: 10.1042/bj0600039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lyndon R. F. Intracellular distribution of ribonuclease activity in pea roots. Biochim Biophys Acta. 1966 Jan 11;113(1):110–119. doi: 10.1016/s0926-6593(66)80126-1. [DOI] [PubMed] [Google Scholar]
- Nudel U., Bamberger E. S. Kinetin inhibition of h-uracil and C-leucine incorporation by tobacco cells in suspension culture. Plant Physiol. 1971 Mar;47(3):400–403. doi: 10.1104/pp.47.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava B. I. Increase in chromatin associated nuclease ctivity of excised barley leaves during senescence and its suppression by kinetin. Biochem Biophys Res Commun. 1968 Aug 13;32(3):533–538. doi: 10.1016/0006-291x(68)90695-5. [DOI] [PubMed] [Google Scholar]
- Tata J. R. Hormonal regulation of growth and protein synthesis. Nature. 1968 Jul 27;219(5152):331–337. doi: 10.1038/219331a0. [DOI] [PubMed] [Google Scholar]
- Tata J. R., Widnell C. C. Ribonucleic acid synthesis during the early action of thyroid hormones. Biochem J. 1966 Feb;98(2):604–620. doi: 10.1042/bj0980604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins G. M., Gelehrter T. D., Granner D., Martin D., Jr, Samuels H. H., Thompson E. B. Control of specific gene expression in higher organisms. Expression of mammalian genes may be controlled by repressors acting on the translation of messenger RNA. Science. 1969 Dec 19;166(3912):1474–1480. doi: 10.1126/science.166.3912.1474. [DOI] [PubMed] [Google Scholar]
- WETTSTEIN F. O., STAEHELIN T., NOLL H. Ribosomal aggregate engaged in protein synthesis: characterization of the ergosome. Nature. 1963 Feb 2;197:430–435. doi: 10.1038/197430a0. [DOI] [PubMed] [Google Scholar]
- Walters T. L., Loring H. S. Enzymes of nucleic acid metabolism from mung bean sprouts. I. Fractionation and concentration of phosphomonoesterase, ribonucleases M1 and M2, 3'-nucleotidase, and deoxyribonuclease. J Biol Chem. 1966 Jun 25;241(12):2870–2875. [PubMed] [Google Scholar]
- Wilson C. M. Plant nucleases. I. Separation and purification of two ribonucleases and one nuclease from corn. Plant Physiol. 1968 Sep;43(9):1332–1338. doi: 10.1104/pp.43.9.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]


