This study investigates the feasibility of intranasal administration of neural stem/progenitor cells (NSPCs) as an alternative, noninvasive, and direct passage for the delivery of stem cells to target intracerebral pathologies such as malignant gliomas. Intranasal administration offers the possibility of multiple treatments potentially using tumor-targeting NSPCs with different therapeutic payloads during the disease course.
Keywords: Cell transplantation, Clinical translation, Neural stem cell, Glioma
Abstract
Stem cell-based therapies for neurological disorders, including brain tumors, advance continuously toward clinical trials. Optimized cell delivery to the central nervous system remains a challenge since direct intracerebral injection is an invasive method with low transplantation efficiency. We investigated the feasibility of intranasal administration of neural stem/progenitor cells (NSPCs) as an alternative, noninvasive, and direct passage for the delivery of stem cells to target malignant gliomas. Tumor-targeting and migratory pathways of murine and human NSPCs were investigated by intravital magnetic resonance imaging and in histological time course analyses in the intracerebral U87, NCE-G55T2, and syngenic Gl261 glioblastoma models. Intranasally administered NSPCs displayed a rapid, targeted tumor tropism with significant numbers of NSPCs accumulating specifically at the intracerebral glioma site within 6 hours after intranasal delivery. Histological time series analysis revealed that NSPCs migrated within the first 24 hours mainly via olfactory pathways but also by systemic distribution via the microvasculature of the nasal mucosa. Intranasal application of NSPCs leads to a rapid, targeted migration of cells toward intracerebral gliomas. The directional distribution of cells accumulating intra- and peritumorally makes the intranasal delivery of NSPCs a promising noninvasive and convenient alternative delivery method for the treatment of malignant gliomas with the possibility of multiple dosing regimens.
Introduction
Treatment options for gliomas, which are the most common type of primary brain tumors, are very limited because of their diffuse invasive nature and their capacity to eventually evade current conventional chemo- and radiotherapeutic regimens [1, 2]. Stem cell-based therapies are emerging as a novel and highly promising strategy in various central nervous system (CNS) diseases. The restorative potential and the inherent pathotropism of neural stem and progenitor cells (NSPCs) make them ideal candidates for the targeted delivery of biologically active gene products in many neurological disorders, such as stroke, neurodegenerative disorders, and brain tumors [3]. While advancing continuously toward clinical trials, translational aspects, such as a safe and efficient delivery of NSPCs, become more important and need to be addressed [4].
The surgical implantation of cells directly into the CNS is the most frequently used method of delivery. However, the transplantation efficiency of allogenic cells derived from embryonic, fetal, or adult tissue is known to be very low because of numerous procedure- and host-related factors compromising the survival rate of grafted cells [5–10]. Moreover, it is an invasive method and carries a procedural risk for the patient, making a repeated treatment option difficult. Malignant gliomas are rapidly growing tumors with a grim prognosis. Therefore, sufficient numbers of NSPCs delivering a therapeutic payload to the remaining tumor cells in the invasion zone are needed, ideally early after maximal surgical resection of the main tumor mass [11]. The intravascular administration of stem and progenitor cells as an alternative delivery method leads to enrichment within intracerebral tumors, but the massive systemic distribution of cells increases the risk of accumulation in peripheral organs with loss of cells and possible systemic side effects when using cells as drug delivery vehicles [12–14].
The intranasal delivery of NSPCs as a cell suspension would be a convenient and noninvasive alternative with potential as a chronic treatment option. The intranasal cavity provides a direct passage to the intracerebral compartment along olfactory pathways and has been used for the delivery of drugs directly to the CNS, bypassing the blood-brain barrier and minimizing systemic exposure [15]. Recently, it has been demonstrated that cells are able to enter the naive murine brain after intranasal delivery [16]. Here, we demonstrate that the presence of intracerebral growing gliomas as a paradigm of intraparenchymal pathology results in the rapid and targeted migration of numerous neural stem/progenitor cells via intranasally accessible pathways toward the intracerebral compartment. The high numbers of NSPCs enriching specifically at the site of neuropathology indicate the intranasal delivery of cells as a highly efficient alternative delivery method for stem cell-based CNS therapies.
Materials and Methods
Cell Culture
The murine glioma cell line GL261 (Division of Cancer Treatment and Diagnosis Tumor Repository, National Cancer Institute, Frederick, MD) [8], human fibroblasts, and the human glioblastoma cell lines NCE-G55T2 [17] and U87 (American Type Culture Collection, Manassas, VA, http://www.atcc.org) were cultured in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) or in MEM-α (Invitrogen) for the U87 cell line supplemented with 2 mM l-glutamine, 2 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml fungizone, and 10% fetal calf serum (Invitrogen).
The multipotent NSPC line was established from the frontoparietal brain of a 3-week-old C57BL/6 mouse and characterized as described previously [8]. Enhanced green fluorescent protein-expressing NSPCs (NSPCs-eGFP) were established by retroviral transduction with pMSCV-eGFP using a commercially available kit (MSCV Retroviral Expression System; BD Biosciences, San Diego, CA, http://www.bdbiosciences.com). NSPCs and NSPCs-eGFP were grown as neurospheres in culture medium containing neurobasal medium (Invitrogen) with B27 supplement (20 μl/ml; Invitrogen), GlutaMAX (10 μl/ml; Invitrogen), fibroblast growth factor-2 (20 ng/ml; Peprotech, Rocky Hill, NJ, http://www.peprotech.com), epidermal growth factor (20 ng/ml; Peprotech), and heparin (32 IE/ml; Ratiopharm, Ulm, Germany, http://www.ratiopharm.de). Growth factors and heparin were refreshed twice weekly. Neurospheres were routinely split by mechanical dissociation when they reached 200–500 μm. The multipotent neural stem cell line HB1.F3 was generated by immortalizing cells obtained from fetal human telencephalon cells of 15 weeks of gestation, using a retrovirus encoding the v-myc gene. The HB1.F3 cell line has been extensively characterized in previous studies [18, 19] and was maintained as adherent cultures in DMEM supplemented with 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml fungizone, and 10% fetal calf serum (Invitrogen).
All cells were maintained in tissue culture flasks in 5% CO2/95% air at 37°C in a humidified incubator and were routinely passaged at confluence. For the in vivo experiments, the cells were dispersed with a 0.05% solution of trypsin/EDTA (Invitrogen), washed with phosphate-buffered saline (PBS), and adjusted to the final concentration in PBS.
Cellular Labeling
Cell labeling using the lipophilic tracer DiI (Molecular Probes, Eugene, OR, http://probes.invitrogen.com) was performed for 30 minutes according to the manufacturer's protocol. In vivo tracking of the human neural stem cell line HB1.F3 by magnetic resonance (MR) imaging was enabled by labeling the cells with superparamagnetic iron oxide particles (SPIO) as described previously [20]. Briefly, SPIO (25 mg of Fe per milliliter; Micromod, Rostock, Germany, http://www.micromod.de) were added to poly-l-lysine (750 ng/ml; Sigma-Aldrich, Munich, Germany, http://www.sigmaaldrich.com) and mixed with medium at room temperature for 60 minutes. The medium of cultured HB1.F3 cells was then replaced with the freshly prepared labeling solution and incubated for 24 hours. Cellular internalization of SPIO particles was confirmed prior to intranasal application by Prussian blue staining, and possible cytotoxic effects were excluded by a cell viability assay (data not shown).
In Vivo Studies
All animal experiments were performed in accordance with federal and institutional guidelines and approved by the Institutional Animal Care and Use Committee of the University of Hamburg. Orthotopic glioblastoma xenografts were established in 4- to 6-week-old male NMRI-nu/nu or C57BL/6 mice (Charles River Laboratories, Sulzfeld, Germany, http://www.criver.com). Mice were anesthetized (100 mg/kg ketamine and 5 mg/kg xylazine) and received a stereotactically guided injection of human glioblastoma cells (4.5 × 104 NCE-G55T2 or 2 × 105 U87) or murine glioma cells (1.4 × 105 Gl261) into the right forebrain (2 mm lateral and 1 mm anterior to bregma, at a 2.5 mm depth from the skull surface). Ten days after tumor cell injection mice received DiI-labeled NSPCs, HB1.F3 cells or human fibroblasts, NSPCs-eGFP or SPIO-loaded HB1.F3 cells via intranasal application. Mice without glioma xenografts served as controls and received the cells at the same time point. Intranasal administration of cells was performed without anesthesia. Mice received alternate applications (left and right), twice for each nostril, of 2 μl drops (8 μl total) containing cell suspension at the opening of the nostrils, allowing the animal to sniff the cell suspension into the nasal cavity. At the end of each experiment animals were sacrificed by CO2 inhalation. Brain, spleen, liver, lung, nasal mucosa, and trigeminal nerves were removed, embedded in optimal cutting temperature (OCT) medium, and stored at −80°C until being further processed for histological analysis.
Magnetic Resonance Imaging
Cell migration after intranasal application of SPIO-labeled HB1.F3 cells in intracerebral glioma-bearing mice was evaluated on a 7T MR imaging system (ClinScan; Bruker, Ettlingen, Germany). Mice were anesthetized with 1% isoflurane (Baxter, Munich, Germany, http://www.baxter.com) in oxygen (0.5 l/minute). Respiratory rates were monitored using a small animal vital sign monitor (SA Instruments Inc., Stony Brook, NY, http://www.i4sa.com). Coronal 2D T2 weighted turbo spin echo images were acquired to assess tumor location and size. Sequence parameters were as follows: TE = 39 ms, TR = 2,500 ms, BW = 250 Hz/pixel, turbo factor 7, matrix = 256 × 192, FOV = 20 × 15 mm2, 19 slices, 0.4-mm slice thickness with 0.1-mm gap. Three-dimensional susceptibility weighted imaging (SWI) [21] was acquired in the same orientation to detect clusters of SPIO-labeled stem cells. Sequence parameters were as follows: TE = 7 ms, TR = 50 ms, FA = 15°, BW = 250 Hz/pixel, matrix = 192 × 144 × 72, FOV = 19 × 14 × 7.2 mm3. Images were analyzed on a viewing task card (Syngo MR B15; Siemens, Erlangen, Germany, http://www.medical.siemens.com) running on the control unit of the MR system.
Histological Analyses
Frozen tissue embedded in OCT was cut into serial 5-μm sections and counterstained with hematoxylin and eosin for histological evaluation. Frozen sections were fixed with 4% paraformaldehyde and mounted using Kaiser's glycerine gelatine (Merck & Co., Darmstadt, Germany, http://www.merck.com). The whole brains of tumor-bearing animals and control animals without brain tumors were evaluated for cellular migration and accumulation by analyzing DiI- or eGFP-positive cells in 4′,6-diamidino-2-phenylindole counterstained sections. Hot spots of intratumoral (IT) and peritumoral (PT) localized neural stem cells were quantified by counting the number of DiI- or eGFP-positive cells in five randomly chosen high-power fields (0.1584 mm2 each) using a fluorescence microscope (Axioskop; Carl Zeiss, Göttingen, Germany, http://www.zeiss.com) and imaging software (Axiovision AC 4.1; Zeiss). The PT area was defined as a zone of 100 μm around the tumor border. In contrast to U87 and NCE-G55T2 glioblastoma xenografts, which usually display a distinct tumor border, Gl261 xenografts exhibit a more invasive growth pattern. Therefore, the tumor area in the C57BL/6-Gl261 glioma model was defined as the tumor mass including the maximum extent of the invasion zone. Tumor volumes were calculated by the ellipsoid formula [22]. The total number of migrated NSPCs were estimated by counting DiI-positive cells in 25 randomly chosen high-power fields (0.02 mm3) within the tumor area. SPIO-labeled cells were detected by Prussian blue staining. Briefly, cryosectioned brains were incubated with freshly prepared Perls' reagent (1% potassium ferrocyanide and 3% hydrochloric acid in distilled water) for 60 minutes and counterstained with neutral red.
Statistical Analyses
Differences between experimental groups were calculated by Student's t test or one-way analysis of variance using σ-plot (Systat Software Inc., Germany, http://www.systat.com). All values were calculated as mean ± standard deviation (SD), with a p value of <.05 considered statistically significant.
Results
Neural Stem Cells Target Intracerebral Glioblastoma Xenografts After Intranasal Administration
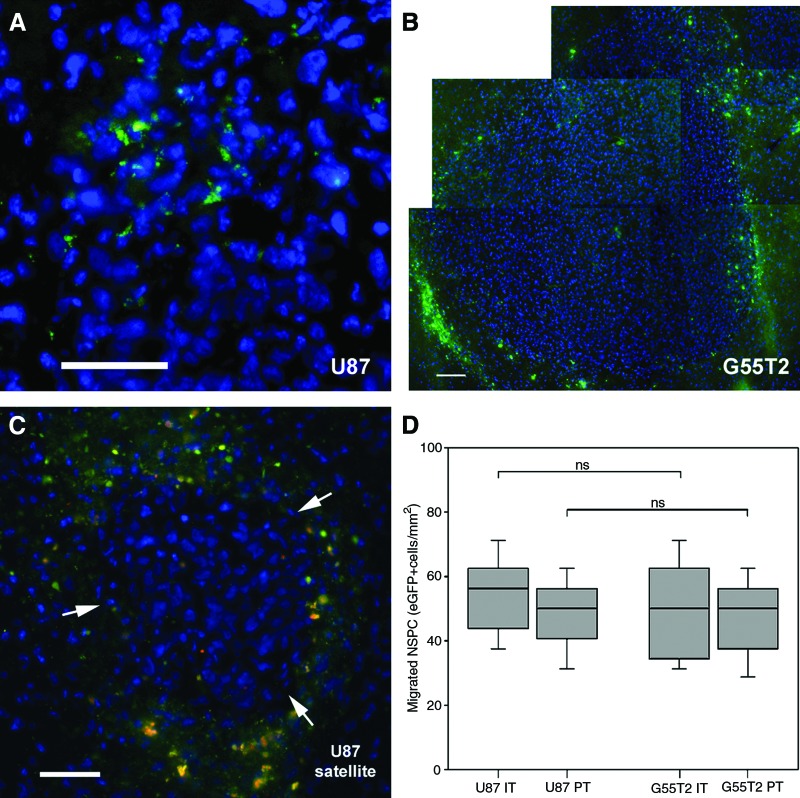
In order to assess whether neural stem/progenitor cells are able to enter the murine brain after intranasal administration and to target growing intracerebral glioma, 3 × 105 eGFP-expressing NSPCs were intranasally applied as a cell suspension 10 days after injection of U87 human glioblastoma cells (n = 5) or NCE-G55T2 human glioblastoma cells (n = 5) into the right forebrain of nude mice. Within 5 days intranasally administered NSPCs-eGFP had entered the brain and were almost exclusively localized in the immediate tumor area of human U87 and NCE-G55T2 glioblastoma xenografts, indicating a directed tumor tropism (Fig. 1A, 1B). Even small tumor satellites distant from the main tumor mass were targeted by intranasally administered NSPCs (Fig. 1C). No cells were observed in the contralateral hemisphere. NSPCs-eGFP were observed to enrich intratumorally and in the immediate peritumoral area with an inhomogeneous distribution pattern. Quantification of enriched areas of NSPCs-eGFP revealed a mean intratumoral accumulation of 54 ± 13 cells per mm2 in U87 tumors and 50 ± 16 cells per mm2 cells in NCE-G55T2 tumors (Fig. 1D). Quantification of the immediate peritumoral zone which was defined as a zone of 100 μm around the distinct tumor border demonstrated a mean of 49 ± 11 cells per mm2 and 46 ± 14 cells per mm2, respectively (Fig. 1D). There were no significant differences in the numbers of tumor-targeting NSPCs between the two different tumor models, indicating that there was no substantial difference in NSPC attraction by U87 and NCE-G55T2 glioblastoma cells. Control animals (n = 5) without intracerebal gliomas received an intranasal administration of 3 × 105 NSPCs-eGFP to assess the rate of intracerebral accumulation in the absence of a neuropathology. Five days after intranasal administration no NSPCs-eGFP were detected in the brains of control animals, indicating that guidance signals from intracerebrally growing brain tumors are needed for the cells to enter the brain and to display a targeted migration.
Figure 1.
Glioma-targeted migration of murine NSPCs after intranasal administration. (A): Intratumoral accumulation of eGFP-expressing NSCP (green) 5 days after intranasal administration in the intracerebral U87 human glioblastoma model. (B): A similar distribution pattern of intranasally applied eGFP-expressing NSPCs was observed in the intracerebral NCE-G55T2 human glioblastoma model. (C): Intranasally applied NSCPs-eGFP enriched in the immediate peritumoral brain parenchyma and surrounded a small U87 glioblastoma satellite (arrows) distant from the main tumor mass. (D): Quantification of intratumoral and peritumoral accumulated NSPCs-eGFP 5 days after intranasal application (box-whisker chart). Scale bars = 50 μm. Abbreviations: eGFP, enhanced green fluorescent protein; IT, intratumoral; ns, not significant; NSPC, neural stem/progenitor cells; PT, peritumoral.
In an additional experiment, we assessed the efficiency of intranasal administration of the human neural stem cell line HB1.F3 to target intracerebral glioma in an immunocompetent orthotopic glioblastoma xenograft model using C57BL/6 mice and the syngenic Gl261 glioma cell line. Three × 105 DiI-labeled HB1.F3 cells were intranasally applied as a cell suspension 10 days after injection of Gl261 murine glioblastoma cells into the right forebrain of nude mice (n = 6). Five days later histological analyses demonstrated a targeted tumor tropism of the human neural stem cell line after intranasal administration (Fig. 2A). Almost no NSPCs were found in other areas of the brain. Quantification of DiI-positive HB1.F3 cells revealed a total of 72,398 ± 7,154 cells within the defined tumor area (32.5 ± 7 mm3), which includes the tumor mass and the adjacent invasion zone of Gl261 glioblastoma xenografts. It was found that 24.1 ± 2% of the intranasally administered human NSPCs were able to enter the intracerebral compartment and migrated toward the glioblastoma xenografts. In control animals (n = 4) which received 3 × 105 DiI-labeled human fibroblasts, no DiI-positive cells were found in the intracerebral compartment (Fig. 2B), indicating that the extensive tumor tropism is a unique capability of stem/progenitor cells. Furthermore, this excludes the possibility that immunocompetent cells such as macrophages or microglia have picked up DiI from dead cells and migrated toward the brain tumors.
Figure 2.
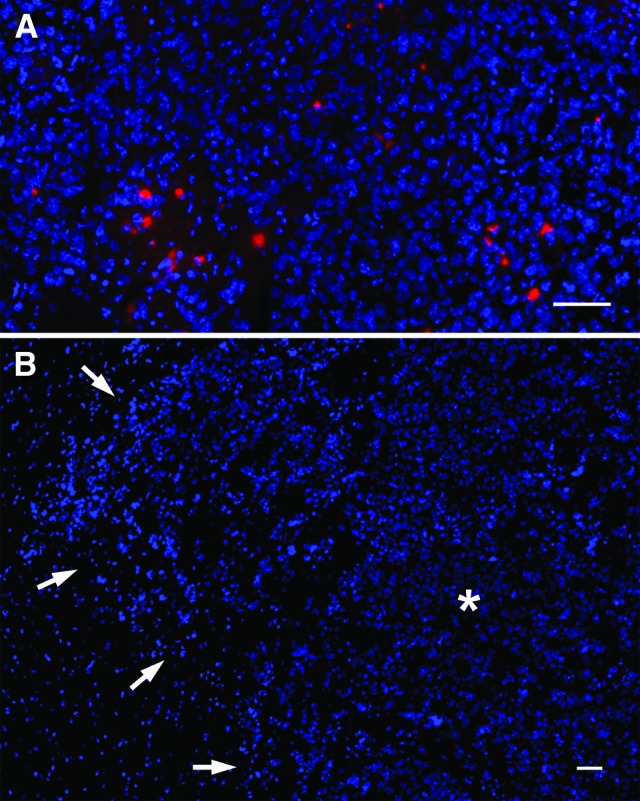
Glioma-targeted migration of intranasally administered human neural stem cells in an immunocompetent glioma model. (A): DiI-labeled HB1.F3 cells (red) enriched throughout murine Gl261 glioma xenografts. (B): In contrast, the intranasal administration of DiI-labeled human fibroblasts did not result in any signal in the tumor area (outlined by arrows; * indicates tumor mass) or other regions of the brain. Scale bars = 50 μm.
In Vivo Tracking of Human Neural Stem Cell Migration by MR Imaging
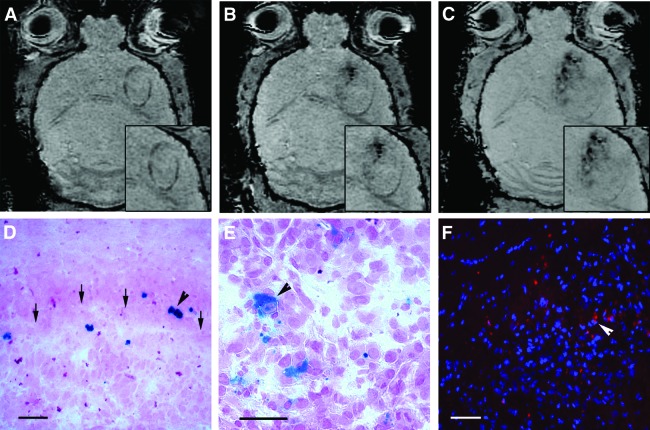
We next investigated possible pathways of intracerebral neural stem cell migration after intranasal delivery by serial MR imaging. The human neural stem cell line HB1.F3 was magnetically labeled with SPIO and 3 × 105 cells were intranasally administered to animals bearing NCE-G55T2 tumors (n = 3) or no tumor as controls (n = 3). Mice were scanned before and immediately after intranasal delivery of SPIO-HB1.F3 cells with a 7T-MRI, followed by MR imaging after 6 hours, 1 day, 3 days, and 5 days. Most sensitive for the MR imaging detection of SPIO-labeled cell clusters were SWI-weighted sequences, but the sensitivity is not sufficient to detect single cells within the brain. Axial cerebral MR images displayed new hypointense signals in the peritumoral and intratumoral area within 3 days after intranasal application of HB1.F3 cells (Fig. 3A, 3B) and further spreading throughout the tumor area (Fig. 3C). Similar signal changes were not observed in other areas of the brain or in brains of control animals without tumor. The frontal distribution pattern is suggestive of a tumor-targeted migration of SPIO-labeled HB1.F3 cells from the olfactory region after intranasal delivery. Histological analysis using Prussian blue staining confirmed the peritumoral and intratumoral presence of SPIO-labeled human neural stem cells in glioma-bearing animals (Fig. 3D, 3E). An additional control experiment confirmed the targeted tumor tropism of HB1.F3 cells toward NCE-G55T2 glioblastoma xenografts after intranasal delivery. A total of 3 × 105 DiI-labeled HB1.F3 cells were intranasally administered 10 days after tumor cell injection (n = 3) or in control animals without tumor (n = 3). Histological analysis 5 days after intranasal delivery revealed a tumor tropism and distribution pattern of the human HB1.F3 cell line in this tumor model similar to the murine NSPC line with a mean intratumoral accumulation of 52 ± 15 cells per mm2 and a mean peritumoral enrichment of 43 ± 12 cells per mm2 (Fig. 3F). No intracerebral cells were detected in controls without tumor up to 5 days after intranasal delivery.
Figure 3.
In vivo magnetic resonance (MR) imaging of tumor tropism of human neural stem cells after intranasal delivery. (A): Axial section of a susceptibility-weighted MR image prior to intranasal delivery of superparamagnetic iron oxide particle (SPIO)-labeled HB1.F3 cells displaying an NCE-G55T2 human glioblastoma xenograft in the right forebrain (insets: magnified tumor area). (B): Three days after intranasal delivery hypointensive signals (arrow) in the tumoral area indicated a tumor-targeted migration of SPIO-labeled HB1.F3 cell clusters. (C): Five days after intranasal delivery the hypointensive signals (SPIO-labeled HB1.F3 cell clusters) had spread over the tumoral area. (D): Prussian blue staining confirmed MR imaging results demonstrating that SPIO-labeled HB1.F3 cells (blue, arrowhead) accumulated at the tumor border (arrows). Scale bar = 50 μm. (E): Prussian blue staining of SPIO-labeled HB1.F3 cells (blue, arrowhead) within the glioma mass. Scale bar = 20 μm. (F): Tumor tropism of human neural stem cells after intranasal administration was confirmed by intratumoral enrichment of intranasally delivered DiI-labeled HB1.F3 cells (arrowhead). Scale bar = 50 μm.
Distribution and Migration Pathways of Neural Stem Cells After Intranasal Administration
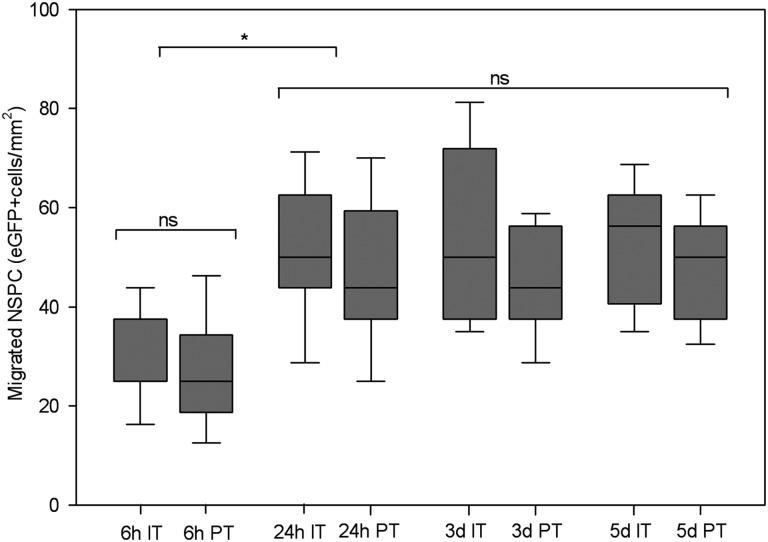
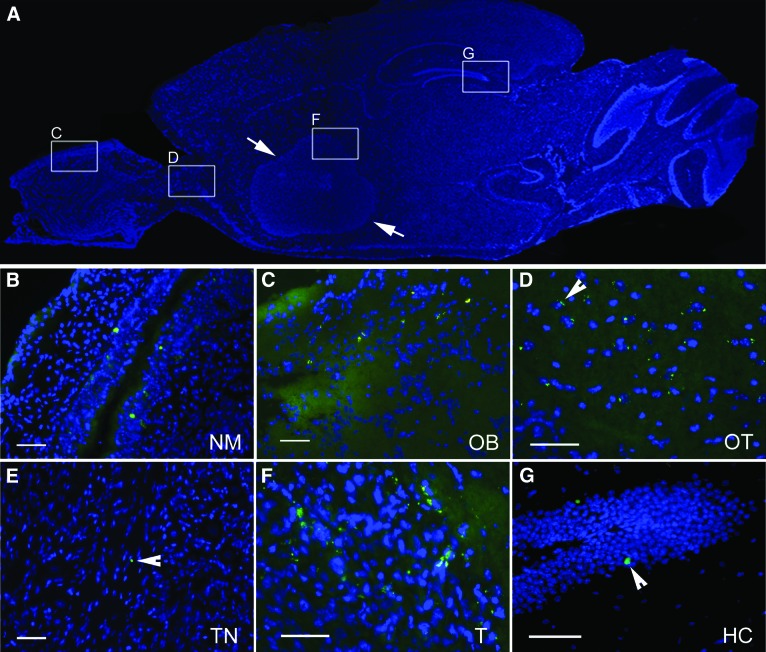
In order to assess the spatial and temporal dynamics of neural stem cell distribution and to determine the pathways to the intracerebral compartment after intranasal administration, we performed a histological time course analysis. Ten days after NCE-G55T2 glioblastoma cell injection into the right forebrain, all animals (n = 20) received an intranasal application of 3 × 105 eGFP-expressing NSPCs. Groups of mice (n = 5 each) were sacrificed 6 hours, 24 hours, 3 days, and 5 days after intranasal delivery of NSPCs. To analyze the local and systemic distribution pattern the nasal mucosa, brain, and trigeminal nerves and the peripheral organs spleen, liver, and lungs were removed and serial sections of the whole organs histologically evaluated. Histological analysis confirmed that intranasal delivery of NSPCs resulted in a targeted migration of NSPCs toward intracerebral glioma as described above. Already within 6 hours, NSPCs appeared in orthotopic growing gliomas with a mean intratumoral and peritumoral density of 32 ± 11 cells per mm2 and 27 ± 11 cells per mm2, respectively (Fig. 4). After 24 hours, enrichment of NSPCs was significantly increased with a mean intratumoral and peritumoral density of 51 ± 15 and 47 ± 16 cells per mm2, respectively (p < .001) (Fig. 4). In total, 43,210 ± 6,835 NSPCs enriched within the defined intracerebral tumor area (6.8 ± 1 mm3), demonstrating that 14.4 ± 2% of the intranasally administered NSPCs were able to enter the intracerebral compartment and displayed a targeted tumor tropism. We observed no significant increase of tumoral NSPC accumulation during the later time points, indicating that the majority of targeted migration occurred in the first 24 hours (Fig. 4). NSPCs infiltrated the nasal mucosa (Fig. 5A, 5B) after intranasal delivery and seemed to enter the brain via olfactory nerve pathways, as single NSPCs were observed at first in the olfactory bulb within 6 hours (Fig. 5C) and then in the olfactory tract within 24 hours (Fig. 5D). In contrast, single eGFP-expressing NSPCs were identified only rarely in the trigeminal nerve tissue after 24 hours (Fig. 5E), suggesting that this pathway is additionally used by the cells to enter the brain but is of minor relevance. The majority of NSPCs displayed a targeted tumor tropism and enriched in the tumoral area (Fig. 5F), but single NSPCs were also observed in the hippocampal area (Fig. 5G) and in the subventricular zone (data not shown), which are known neural stem cell niches [23]. When analyzing the peripheral organs we found no eGFP-expressing NSPCs in lung or liver tissue at all time points, but a patchy distribution of NSPCs was detected in the spleen after 6 hours (data not shown). This finding points toward an additional systemic distribution of NSPCs after intranasal delivery via the microvasculature of the highly vascularized nasal mucosa. The fact that NSPCs appeared in the olfactory bulb and not in the olfactory tract 6 hours after intranasal delivery but were already present at the tumor site indicates that within the first 6 hours NSPCs reached the brain tumor area mainly by pathways other than intrinsic brain parenchymal routes.
Figure 4.
Time course analysis of intratumoral and peritumoral accumulation in the intracerebral NCE-G55T2 glioblastoma model after intranasal delivery of eGFP-expressing NSPCs. NSPCs were identified within 6 hours after intranasal application enriching at the tumor site, with cell numbers increasing significantly after 24 hours and remaining at this level for the following days. No significant numbers of cells were found in nontumorous regions of the brain. *, p < .001. Abbreviations: eGFP, enhanced green fluorescent protein; IT, intratumoral; ns, not significant; NSPC, neural stem/progenitor cells; PT, peritumoral.
Figure 5.
Pathways of neural stem/progenitor cell (NSPC) migration in the intracerebral NCE-G55T2 glioblastoma model after intranasal administration of enhanced green fluorescent protein (eGFP)-expressing NSPCs. (A): Schematic overview of a sagittal sectioned brain of a glioma-bearing animal (arrows: tumor border). (B): Infiltration of the nasal mucosa by eGFP-expressing NSPCs (green). (C): eGFP-expressing NSPCs in the olfactory bulb 6 hours after intranasal delivery. (D): After 24 hours migrating NSPCs were detected in the olfactory tract. (E): Rarely, single NSPCs (arrowhead) were observed in the trigeminal nerve. (F): Intratumoral enrichment of NSPCs in a human glioblastoma xenograft near the tumor border. (G): Few single NSPCs (arrowhead) were localized in the hippocampus. Scale bars = 50 μm. Abbreviations: HC, hippocampus; NM, nasal mucosa; OB, olfactory bulb; OT, olfactory tract; T, tumor; TN, trigeminal nerve.
Discussion
This is the first report demonstrating that murine and human NSPCs are able to enter the intracerebral compartment after intranasal delivery as a cell suspension and to display a rapid and targeted migration toward intracerebral brain tumors in three orthotopic glioma models. The intranasal delivery resulted in a stable accumulation of high numbers of NSPCs specifically at the tumor site. Up to 24% of intranasally administered NSPCs were able to enter the intracerebral compartment and to enrich within the tumor area. NSPCs were detected at the intracerebral tumor site within 6 hours after intranasal delivery with a further significant increase of NSPC numbers within 24 hours. Our study provides proof of concept that the noninvasive intranasal passage of NSPCs to target intracerebral gliomas is a highly attractive and efficient alternative method of cell administration for stem cell-based therapies in brain tumors.
In 2009 Danielyan et al. [16] observed for the first time that the intranasal administration of cells led to the intracerebral appearance of a few cells in various areas of the normal murine brain. This effect was slightly enhanced by pretreatment of the nasal cavity with hyaluronidase. In contrast, we were able to observe high numbers of NSPCs entering the brain and enriching at the intracerebral tumor site without hyaluronidase pretreatment. Our results demonstrate that the effect of entering the intracerebral compartment after intranasal delivery was tumor-induced since no NSPCs were identified in the brains of control animals without intracerebral glioma. It is likely that guidance signals, such as chemotactic factors, released by the tumor itself and the adjacent reactive brain parenchyma are responsible for the rapid and targeted tumor tropism of NSPCs from the nasal mucosa [19, 24]. The brain parenchyma is known to be partially cleared from molecules, interstitial fluid, and cerebrospinal fluid (CSF) by drainage via perivascular spaces and nerve fibers connecting the CNS and the nasal mucosa [25, 26], therefore potentially providing intranasally applied cells with the necessary guidance signals from a neuropathology such as a brain tumor. These passages also serve as direct entry pathways of intranasally applied drugs to the CNS [15].
Our study is in line with recent results showing that intranasally administered mesenchymal stem cell were able to enter the brain in murine Parkinson disease [27] and neonate ischemic brain damage models [28]. However, the routes and migratory dynamics of cells entering the intracerebral compartment from the nasal cavity are hardly defined. Our analysis of NSPC distribution over time revealed two major migration routes involved in the tumor tropism of NSPCs after intranasal delivery in an intracerebral glioma model. (a) Many NSPCs entered the brain via olfactory nerve pathways and displayed intraparenchymal migration, which was demonstrated by detecting NSPCs in the olfactory epithelium and the olfactory bulb and later on in the olfactory tract. This resulted in a further significant increase of NSPC numbers at the tumor site 24 hours after intranasal delivery. (b) The fact that a significant number of NSPCs reached the intracerebral tumor site already within 6 hours after intranasal delivery points toward an additional migration route. The early detection of NSPCs in the spleen indicates that NSPCs distribute systemically via the highly vascularized nasal mucosa and could potentially achieve peri- and intratumoral entrance by the tumor-associated angiogenic microvasculature [24]. Another possibility of early NSPC accumulation at the tumor site is the migration through the lamina cribosa via perivascular and perineural channels with access to the CSF. However, in our study we did not observe NSPCs in the subarachnoidal space or within the parenchyma of the surface-near cortex, making this possibility unlikely.
Intracerebrally transplanted NSPCs were shown previously to track gliomas and brain metastasis and to elicit significant antitumor effects when delivering a therapeutic payload in preclinical brain tumor models [11]. Encouraged by these results the first clinical phase I study for patients with recurrent glioblastoma (NCT01172964) has recently been launched using the human HB1.F3 neural stem cell line to deliver the prodrug-converting enzyme cytosindeaminase, which converts the nontoxic prodrug 5-fluorocytosine to the chemotherapeutic drug 5-fluorouracil. Here, we provide the first data that this human neural stem cell line (HB1.F3) can potentially be applied via intranasal application as a cell suspension. As in other previous major gene therapy trials, in the current clinical phase I study (NCT01172964) for recurrent glioblastoma, the HB1.F3 cell suspension is delivered directly to the resection cavity by multiple injections immediately after surgical removal of the tumor mass in order to eradicate the remaining invaded glioma cells, thus preventing tumor recurrence. Apart from being an invasive method with no option of repeated cell delivery, intracerebral injection into the resection cavity poses a mechanical problem of cell fixation within the resection cavity/wall [8] whereas additional local inflammatory responses of the surgical wound at this early time point presumably reduce the number of surviving cells further. Ideally, alternative noninvasive strategies with minimal risks for the patient, high transplantation efficiency, and the possibility of a chronic treatment option are needed. Systemic intravascular application of cells could be another option, but major obstacles would be the entrapment and elimination of cells in peripheral organs and the risk of vascular and pulmonary embolization [10, 14, 29]. Although we detected NSPCs in the spleen after intranasal delivery indicating a systemic distribution we did not observe any NSPCs in lung or liver tissue at any time point. Therefore, our data indicate that the intranasal delivery of NSPCs is a safe and efficient alternative to the surgical injection or intravascular administration.
Conclusion
Here, we introduce the concept of intranasal application of NSPCs as a noninvasive and effective alternative delivery method for stem cell-based therapies in brain tumors. This offers the possibility of multiple treatments potentially using tumor-targeting NSPCs with different therapeutic payloads during the disease course. Our study expands the armamentarium of potentially clinically relevant methods to deliver cells to the diseased CNS in many neurological disorders.
Acknowledgments
This work was supported by grants of the Deutsche Forschungsgemeinschaft (Schm 1631/3-1, SE 2052/1-1), Hamburger Krebshilfe e.V., and the Stiftung zur Bekämpfung neuroviraler Erkrankungen to N.O.S.
Author Contributions
M.R.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; M.D. and J.S.: collection and assembly of data, data analysis and interpretation, final approval of manuscript; H.M.: collection and assembly of data, administrative support; J.F. and M.W.: provision of study material, financial support, final approval of manuscript; S.U.K.: provision of study material, final approval of manuscript; N.O.S.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, financial support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Stummer W, Reulen HJ, Meinel T, et al. Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery. 2008;62:564–576. doi: 10.1227/01.neu.0000317304.31579.17. discussion 564–576. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.Müller FJ, Snyder EY, Loring JF. Gene therapy: Can neural stem cells deliver? Nat Rev Neurosci. 2006;7:75–84. doi: 10.1038/nrn1829. [DOI] [PubMed] [Google Scholar]
- 4.Aboody K, Capela A, Niazi N, et al. Translating stem cell studies to the clinic for CNS repair: Current state of the art and the need for a Rosetta Stone. Neuron. 2011;70:597–613. doi: 10.1016/j.neuron.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Coyne TM, Marcus AJ, Woodbury D, et al. Marrow stromal cells transplanted to the adult brain are rejected by an inflammatory response and transfer donor labels to host neurons and glia. Stem Cells. 2006;24:2483–2492. doi: 10.1634/stemcells.2006-0174. [DOI] [PubMed] [Google Scholar]
- 6.Coyne TM, Marcus AJ, Reynolds K, et al. Disparate host response and donor survival after the transplantation of mesenchymal or neuroectodermal cells to the intact rodent brain. Transplantation. 2007;84:1507–1516. doi: 10.1097/01.tp.0000288185.09601.4d. [DOI] [PubMed] [Google Scholar]
- 7.Tatard VM, Menei P, Benoit JP, et al. Combining polymeric devices and stem cells for the treatment of neurological disorders: A promising therapeutic approach. Curr Drug Targets. 2005;6:81–96. doi: 10.2174/1389450053344885. [DOI] [PubMed] [Google Scholar]
- 8.Hansen K, Muller FJ, Messing M, et al. A 3-dimensional extracellular matrix as a delivery system for the transplantation of glioma-targeting neural stem/progenitor cells. Neuro Oncol. 2010;12:645–654. doi: 10.1093/neuonc/noq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sortwell CE. Strategies for the augmentation of grafted dopamine neuron survival. Front Biosci. 2003;8:s522–s532. doi: 10.2741/1096. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi Y, Tsuji O, Kumagai G, et al. Comparative study of methods for administering neural stem/progenitor cells to treat spinal cord injury in mice. Cell Transplant. 2011;20:727–739. doi: 10.3727/096368910X536554. [DOI] [PubMed] [Google Scholar]
- 11.Aboody KS, Najbauer J, Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008;15:739–752. doi: 10.1038/gt.2008.41. [DOI] [PubMed] [Google Scholar]
- 12.Brown AB, Yang W, Schmidt NO, et al. Intravascular delivery of neural stem cell lines to target intracranial and extracranial tumors of neural and non-neural origin. Hum Gene Ther. 2003;14:1777–1785. doi: 10.1089/104303403322611782. [DOI] [PubMed] [Google Scholar]
- 13.Pendharkar AV, Chua JY, Andres RH, et al. Biodistribution of neural stem cells after intravascular therapy for hypoxic-ischemia. Stroke. 2010;41:2064–2070. doi: 10.1161/STROKEAHA.109.575993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reekmans KP, Praet J, De Vocht N, et al. Clinical potential of intravenous neural stem cell delivery for treatment of neuroinflammatory disease in mice? Cell Transplant. 2011;20:851–869. doi: 10.3727/096368910X543411. [DOI] [PubMed] [Google Scholar]
- 15.Dhuria SV, Hanson LR, Frey WH., 2nd Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J Pharm Sci. 2010;99:1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- 16.Danielyan L, Schafer R, von Ameln-Mayerhofer A, et al. Intranasal delivery of cells to the brain. Eur J Cell Biol. 2009;88:315–324. doi: 10.1016/j.ejcb.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Kunkel P, Ulbricht U, Bohlen P, et al. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 2001;61:6624–6628. [PubMed] [Google Scholar]
- 18.Kim SU, Nagai A, Nakagawa E, et al. Production and characterization of immortal human neural stem cell line with multipotent differentiation property. Methods Mol Biol. 2008;438:103–121. doi: 10.1007/978-1-59745-133-8_10. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt NO, Przylecki W, Yang W, et al. Brain tumor tropism of transplanted human neural stem cells is induced by vascular endothelial growth factor. Neoplasia. 2005;7:623–629. doi: 10.1593/neo.04781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank JA, Miller BR, Arbab AS, et al. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003;228:480–487. doi: 10.1148/radiol.2281020638. [DOI] [PubMed] [Google Scholar]
- 21.Reichenbach JR, Haacke EM. High-resolution BOLD venographic imaging: A window into brain function. NMR Biomed. 2001;14:453–467. doi: 10.1002/nbm.722. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt KF, Ziu M, Schmidt NO, et al. Volume reconstruction techniques improve the correlation between histological and in vivo tumor volume measurements in mouse models of human gliomas. J Neurooncol. 2004;68:207–215. doi: 10.1023/b:neon.0000033364.43142.bf. [DOI] [PubMed] [Google Scholar]
- 23.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353:811–822. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt NO, Koeder D, Messing M, et al. Vascular endothelial growth factor-stimulated cerebral microvascular endothelial cells mediate the recruitment of neural stem cells to the neurovascular niche. Brain Res. 2009;1268:24–37. doi: 10.1016/j.brainres.2009.02.065. [DOI] [PubMed] [Google Scholar]
- 25.Zhang ET, Richards HK, Kida S, et al. Directional and compartmentalised drainage of interstitial fluid and cerebrospinal fluid from the rat brain. Acta Neuropathol. 1992;83:233–239. doi: 10.1007/BF00296784. [DOI] [PubMed] [Google Scholar]
- 26.Carare RO, Bernardes-Silva M, Newman TA, et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: Significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol. 2008;34:131–144. doi: 10.1111/j.1365-2990.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 27.Danielyan L, Schafer R, von Ameln-Mayerhofer A, et al. Therapeutic efficacy of intranasally delivered mesenchymal stem cells in a rat model of Parkinson disease. Rejuvenation Res. 2011;14:3–16. doi: 10.1089/rej.2010.1130. [DOI] [PubMed] [Google Scholar]
- 28.van Velthoven CT, Kavelaars A, van Bel F, et al. Nasal administration of stem cells: A promising novel route to treat neonatal ischemic brain damage. Pediatr Res. 2010;68:419–422. doi: 10.1203/PDR.0b013e3181f1c289. [DOI] [PubMed] [Google Scholar]
- 29.Walczak P, Zhang J, Gilad AA, et al. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008;39:1569–1574. doi: 10.1161/STROKEAHA.107.502047. [DOI] [PMC free article] [PubMed] [Google Scholar]