The study was designed to determine whether diabetes-associated early outgrowth cell (EOC) dysfunction might be attenuated by pharmacological activation of silent information regulator protein 1 (SIRT1), a lysine deacetylase implicated in nutrient-dependent life span extension in mammals. The findings suggest that the impaired angiogenicity of EOCs in diabetes may be a consequence of a reduction in SIRT1 and that its activation may represent a novel therapeutic strategy to augment endothelial repair in this disease.
Keywords: Diabetes, Angiogenesis, Early outgrowth cell, SIRT1, Chemokine
Abstract
Impaired endothelial repair is a key contributor to microvascular rarefaction and consequent end-organ dysfunction in diabetes. Recent studies suggest an important role for bone marrow-derived early outgrowth cells (EOCs) in mediating endothelial repair, but the function of these cells is impaired in diabetes, as in advanced age. We sought to determine whether diabetes-associated EOC dysfunction might be attenuated by pharmacological activation of silent information regulator protein 1 (SIRT1), a lysine deacetylase implicated in nutrient-dependent life span extension in mammals. Despite being cultured in normal (5.5 mM) glucose for 7 days, EOCs from diabetic rats expressed less SIRT1 mRNA, induced less endothelial tube formation in vitro and neovascularization in vivo, and secreted less of the proangiogenic ELR+ CXC chemokines CXCL1, CXCL3, and CXCL5. Ex vivo SIRT1 activation restored EOC chemokine secretion and increased the in vitro and in vivo angiogenic activity of EOC conditioned medium derived from diabetic animals to levels similar to that derived from control animals. These findings suggest a pivotal role for SIRT1 in diabetes-induced EOC dysfunction and that its pharmacologic activation may provide a new strategy for the restoration of EOC-mediated repair mechanisms.
Introduction
Microvascular rarefaction is a characteristic feature in organs affected by long-term diabetes, where it is closely linked to tissue dysfunction. Although initially viewed as a consequence of accelerated endothelial cell death, recent studies have focused on defective endothelial repair as a major contributor, reporting a diminution in the number and angiogenic activity of bone marrow-derived proangiogenic early outgrowth cells (EOCs) in diabetes [1–3].
Like diabetes, aging is also characterized by microvascular loss. Given this similarity, we considered whether silent information regulator protein 1 (SIRT1), a deacetylase implicated both in aging and regulation of angiogenesis [4], might contribute to the dysfunctional angiogenicity of EOCs in diabetes. Here we demonstrate that prolonged, 7-day culture in normal glucose failed to restore the angiogenicity of EOCs derived from diabetic animals. This diabetes-associated defect was accompanied by a marked reduction in EOC SIRT1 expression and a diminished secretion of proangiogenic factors. Pharmacological SIRT1 activation restored diabetic animal EOC production of these proangiogenic factors. Together, these findings suggest a pivotal role for SIRT1 in mediating the defective reparative response to endothelial loss in diabetes.
Materials and Methods
Detailed methods can be found in the supplemental online data.
EOC Culture and Flow Cytometry
EOCs were cultured from bone marrow isolates of diabetic and nondiabetic Fischer 344 rats (Charles River Laboratories, Montreal, QC, Canada, http://www.criver.com) as previously reported [5]. EOCs underwent flow cytometric analysis (MACS Quant; Miltenyi Biotec, Auburn, CA, http://www.miltenyibiotec.com) of the cell surface markers CD34, vascular endothelial growth factor receptor 2 (VEGFR2), and CD133.
Quantitative Reverse Transcription-Polymerase Chain Reaction
Quantitative reverse transcription-polymerase chain reaction was performed on isolated EOC RNA using sequence-specific primers for SIRT1 and expressed relative to RPL13a.
EOC Conditioned Medium Generation and SIRT1 Pharmacologic Manipulation
EOC conditioned medium (CM) was generated by incubating subconfluent healthy or diabetic rat EOCs with serum-free EBM-2 (Lonza, Walkersville, MD, http://www.lonza.com) for 24 hours. EOC SIRT1 activity was modulated by 24-hour treatment with a SIRT1 activator, 0.5 mmol/l SRT1720 (Sirtris, Cambridge, MA, http://www.sirtrispharma.com), or a SIRT1 inhibitor, 2 μmol/l EX527 (Cayman Chemical, Ann Arbor, MI, http://www.caymanchem.com). Following incubation, each drug was washed off prior to CM generation.
Matrigel Angiogenesis Assay
Human umbilical vein endothelial cells (HUVECs) were incubated with EBM-2, EOC CM, and/or the CXCR2 inhibitor SB225002 (Tocris Bioscience, Minneapolis, MN, http://www.tocris.com) and subjected to a Matrigel (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com) tube formation assay as previously described [6].
Mouse Corneal Neovascularization Assay
The eyes of male C57BL/6 mice (20–25 g; Taconic Farms, Hudson, NY, http://www.taconic.com) were injected with 1 μl of (a) phosphate-buffered saline, (b) 200 ng/μl VEGF (Life Technologies, Burlington, ON, Canada, http://www.lifetechnologies.com), (c) CM from healthy EOCs, (d) CM from diabetic EOCs, (e) CM from SRT1720-treated healthy EOCs, or (f) CM from SRT1720-treated diabetic EOCs in a modified mouse corneal neovascularization assay [7].
Cytokine Assay
Cytokine levels in 10× concentrated EOC CM were measured using a membrane blotting assay (R&D Systems Inc., Minneapolis, MN, http://www.rndsystems.com).
Statistical Analysis
All data are shown as means ± SEM. Differences between groups were analyzed by t test or analysis of variance with post hoc Fisher's least significant difference. p < .05 was considered significant.
Results
EOCs Are a Heterogeneous Population of Bone Marrow-Derived Cells Enriched in Hematopoietic and Endothelial Markers
Flow cytometric analysis of EOCs cultured from rat bone marrow revealed a heterogeneous population enriched in CD34+ cells (Fig. 1A). A significant proportion of EOCs were also positive for VEGFR2 (Fig. 1B) and the progenitor cell surface marker CD133 (Fig. 1C). It was found that 25.3% of EOCs were CD34/VEGFR2 double-positive (Fig. 1D), 16.1% were CD34/CD133 double-positive (Fig. 1E), and 3.5% were CD34/VEGFR2/CD133 triple-positive (Fig. 1F). Determinations of positivity were based on hierarchical gates validated by both appropriate isotype controls and fluorescence minus one controls.
Figure 1.
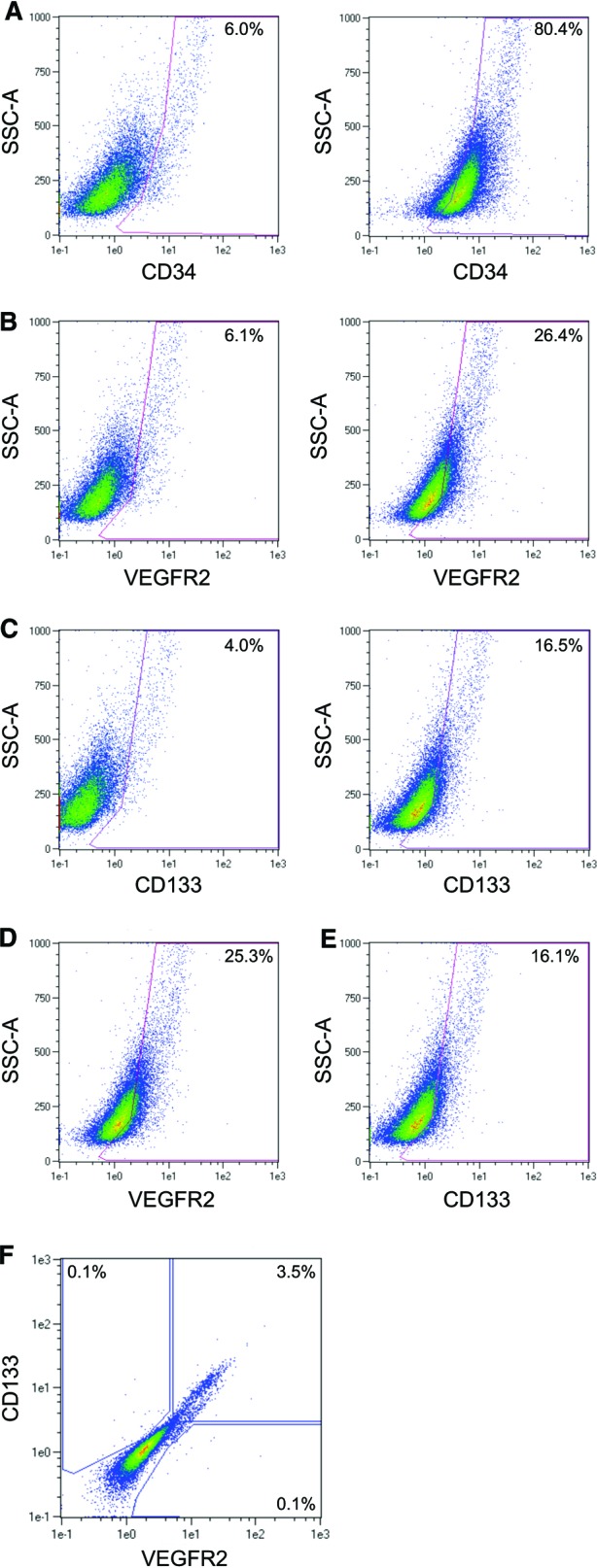
Flow cytometric characterization of early outgrowth cells (EOCs). The surface expression of CD34, CD133, and VEGFR2 on EOCs was measured by fluorescence-activated cell sorting. (A–C): Single-marker staining of single-cell EOCs. Left panels: isotype control antibody; right panels: specific antibody. The percentage of positive cells for a given area is listed on each plot. (A): CD34 staining. (B): VEGFR2 staining. (C): CD133 staining. (D): Single-positive CD34+ cells were gated as shown in (A) and examined for VEGFR2 expression. (E): Single-positive CD34+ cells were gated as shown in (A) and examined for CD133 expression. (F): Single-positive CD34+ cells were gated as shown in (A) and examined for staining of VEGFR2 and CD133. Abbreviations: VEGFR2, vascular endothelial growth factor receptor 2; SSC, side scatter.
Diabetic Rat EOCs Express Lower Levels of SIRT1
One month post-diabetes induction, diabetic rats (n = 10) developed hyperglycemia as compared with healthy controls (n = 8, blood glucose: 32.7 ± 1.0 vs. 6.6 ± 0.5 mmol/l, p < .05). Because both EOCs and SIRT1 are involved in the regulation of angiogenesis, we examined SIRT1 expression in EOCs cultured from diabetic and nondiabetic rats. Although still detectable, SIRT1 mRNA levels were significantly reduced in diabetic rat EOCs as compared with EOCs from healthy controls (0.53 ± 0.08 vs. 1.01 ± 0.08 arbitrary units, p < .05).
Diabetic Rat EOCs Secrete Factors with Reduced In Vitro Angiogenicity That Is Restored Following SIRT1 Activation
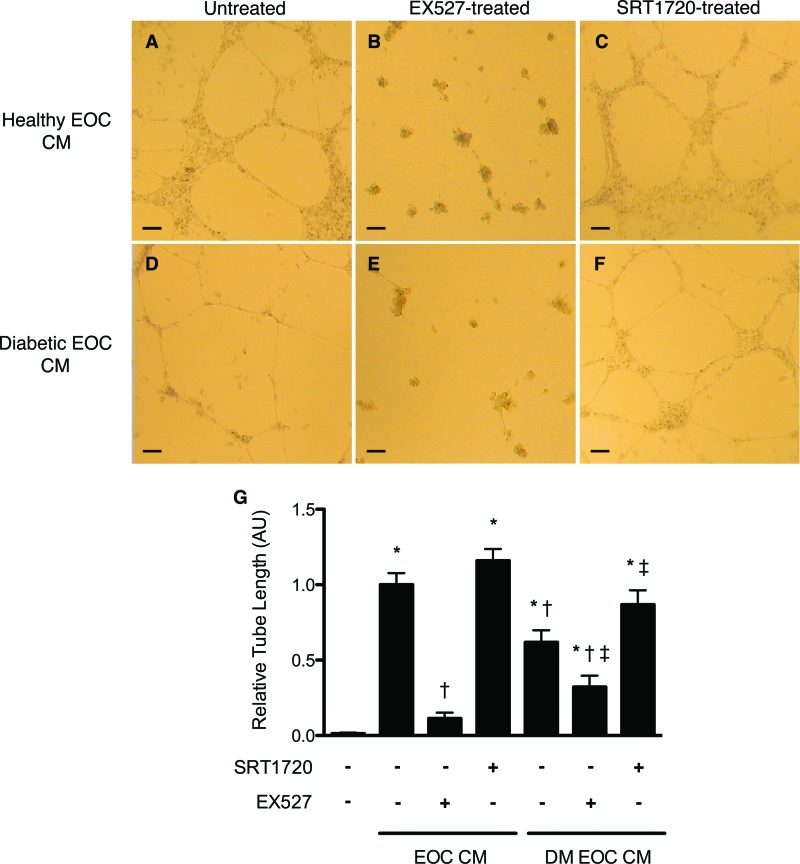
We next examined the angiogenic activity of EOC CM in an in vitro Matrigel assay. Although CM from healthy rat EOCs triggered significant tube formation of endothelial cells seeded in Matrigel, CM from diabetic rats induced a markedly reduced response (Fig. 2). SIRT1 inhibition with EX527 reduced the angiogenic activity of the secretory products of both healthy and diabetic rat EOCs (Fig. 2). SIRT1 activation with SRT1720 restored the angiogenicity of only the diabetic rat EOC CM, whereas SRT1720 had no effect on healthy rat EOC CM activity (Fig. 2).
Figure 2.
Pharmacologic modulation of silent information regulator protein 1 (SIRT1) activity alters early outgrowth cell (EOC) angiogenic activity in vitro. Although healthy rat EOC CM induced a robust endothelial tube formation response of human umbilical vein endothelial cells seeded in Matrigel (A), diabetic rat EOC CM demonstrated diminished angiogenic activity (D). Inhibition of SIRT1 with EX527 reduced EOC CM angiogenicity, regardless of donor diabetes status (B, E). Activation of SIRT1 with SRT1720 increased the angiogenic activity of diabetic, but not healthy, rat EOC CM (C, F). Quantitative analysis of endothelial tube length is shown (G). *, p < .05 versus serum-free medium; †, p < .05 versus healthy rat EOC CM; ‡, p < .05 versus diabetic rat EOC CM. Images were taken at ×4 magnification. Scale bars = 100 μm. Abbreviations: DM EOC CM, conditioned medium from diabetic rat early outgrowth cells; EOC CM, conditioned medium from healthy rat early outgrowth cells.
Treatment of Diabetic EOCs with a SIRT1 Activator Restores the Angiogenicity of Their Secreted Factors In Vivo
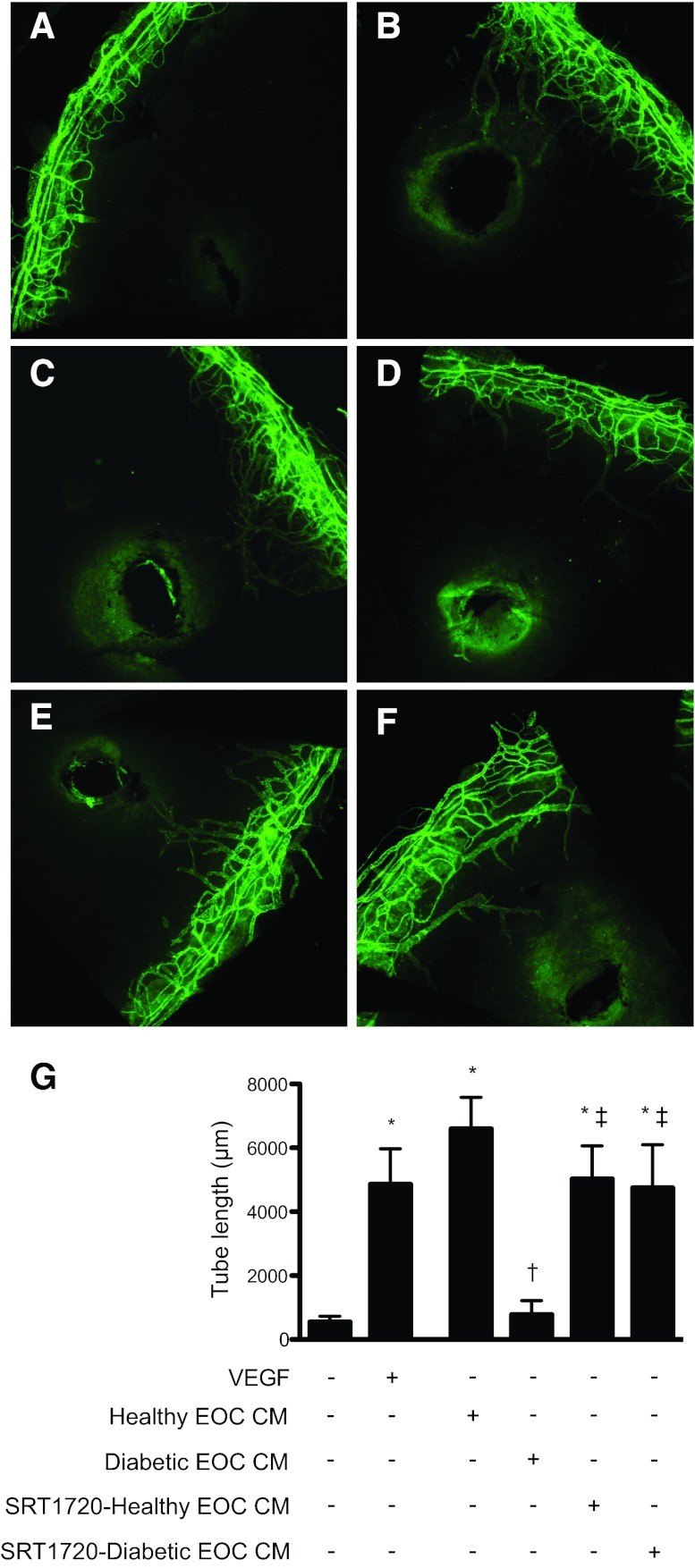
To examine the relevance of these findings in vivo, healthy and diabetic rat EOC CM were injected into the avascular mouse cornea, and the resulting neovascularization was quantified. In line with our in vitro findings, diabetic rat EOC CM induced less neovascularization in the mouse cornea as compared with healthy rat EOC CM (Fig. 3). SIRT1 activation induced by SRT1720 treatment of diabetic rat EOCs significantly increased the angiogenicity of the resulting CM, whereas SRT1720 had no effect on healthy rat EOC CM activity (Fig. 3).
Figure 3.
Silent information regulator protein 1 (SIRT1) activation restores the angiogenic activity of diabetic rat EOC CM in vivo. (A–F): Representative images of corneas harvested from C57BL/6 mice 6 days after injection with 1 μl of phosphate-buffered saline (PBS) (A), 200 ng/μl VEGF (B), CM from healthy rat EOCs (C), CM from diabetic rat EOCs (D), CM from SRT1720-treated healthy rat EOCs (E), or CM from SRT1720-treated diabetic rat EOCs (F). CM was concentrated 40× prior to injection. Six days after injection, the mice were sacrificed, and corneal flat mounts were prepared and stained with an anti-mouse CD31 antibody followed by an Alexa Fluor 488-conjugated secondary antibody (green) to delineate blood vessels. (G): Images were obtained at ×10 magnification, and the cumulative tube lengths of corneal neovessels were quantified. *, p < .05 versus control PBS injection; †, p < .05 versus healthy rat EOC CM injection; ‡, p < .05 versus diabetic rat EOC CM injection. Abbreviations: EOC CM, conditioned medium from healthy rat early outgrowth cells; VEGF, vascular endothelial growth factor.
SIRT1 Activation Restores the Secretion of Proangiogenic ELR+ Chemokines by Diabetic Rat EOCs
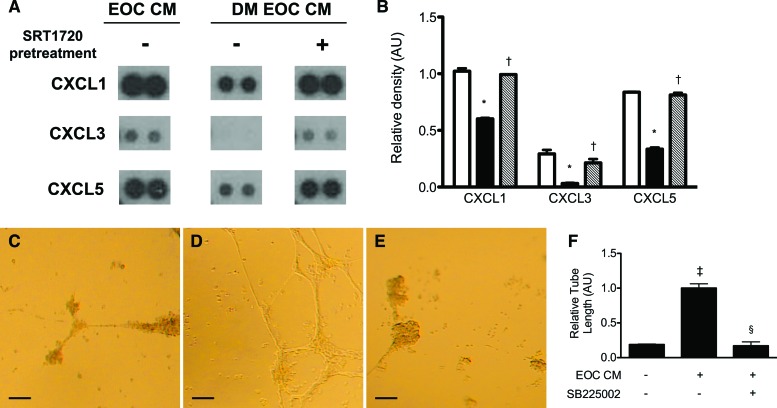
To examine the potential mechanisms by which SIRT1 activation restores diabetic EOC angiogenic activity, we examined the secretion of the proangiogenic ELR+ chemokines CXCL1, CXCL3, and CXCL5, a subfamily of CXC chemokines that exhibit potent proangiogenic activity through activation of the G-protein-coupled receptor CXCR2. When compared with healthy rat EOCs, CM from diabetic rat EOCs contained significantly lower amounts of CXCL1, CXCL3, and CXCL5 (Fig. 4A, 4B). Activation of SIRT1 with SRT1720 restored the secreted levels of these ELR+ chemokines (Fig. 4A, 4B).
Figure 4.
The angiogenic activity of diabetic rat EOC CM is diminished because of a reduction in secretion of proangiogenic ELR+ chemokines. (A): Representative dot blots of EOC CM for the ELR+ chemokines CXCL1, CXCL3, and CXCL5. (B): Quantitative densitometry analysis of the dot blot results. Open bars indicate healthy rat-derived EOC CM. Black bars indicate untreated diabetic rat-derived EOC CM. Cross-hatched bars indicate CM from SRT1720-pretreated diabetic rat-derived EOCs. *, p < .05 versus CM from healthy rat-derived EOCs; †, p < .05 versus untreated diabetic rat-derived EOC CM. (C–F): EOC CM-treated human umbilical vein endothelial cells (HUVECs) were subjected to a Matrigel endothelial tube formation assay, in the presence or absence of SB225002, a selective CXCR2 inhibitor. (C–E): Representative images of endothelial tube formation were taken at ×4 magnification. Scale bars = 100 μm. (C): Serum-free medium-treated HUVECs. (D): Healthy rat-derived EOC CM-treated HUVECs. (E): Healthy rat-derived EOC CM- and SB225002-treated HUVECs. (F): Quantitative analysis of tube length (n = 3). ‡, p < .05 versus serum-free medium; §, p < .05 versus healthy rat-derived EOC CM. Abbreviations: AU, arbitrary units; DM EOC CM, conditioned medium from diabetic rat early outgrowth cells; EOC CM, conditioned medium from healthy rat early outgrowth cells.
The Angiogenic Activity of EOC Secretory Products Is CXCR2-Dependent
The diminished secretion of ELR+ chemokines and the concomitant reduction in angiogenic activity of the diabetic donor EOC secretory product, along with their subsequent restoration with SIRT1 activation, suggested that secreted ELR+ chemokines may play an important role in mediating EOC angiogenic activity. ELR+ chemokines exert their proangiogenic effects primarily through the receptor CXCR2. Pretreatment of HUVECs with SB225002, a selective and potent CXCR2 antagonist, significantly reduced endothelial tube formation induced by healthy rat EOC CM (Fig. 4C–4F).
Discussion
The integrity of the endothelium plays an important role in the prevention of diabetic vascular complications. Because diabetes is characterized by increased endothelial cell apoptosis, reparative mechanisms are critical in preventing end-organ injury. Unfortunately, these too are impaired in diabetes where a reduction in EOC angiogenic activity is a consistent feature [1–3]. Here we show not only that SIRT1 expression is reduced in diabetic animal EOCs but also that their in vitro and in vivo angiogenic activity can be restored by ex vivo pharmacological priming with a SIRT1 activator.
EOCs are a heterogeneous population of bone marrow-derived cells that, as demonstrated in the present study, express both hematopoietic and endothelial markers and exhibit potent angiogenic activity [8]. Detailed examination of these cells, however, has failed to establish specific identifying markers for these cells [9], and as such we and others have adopted a functional definition based on culture conditions and duration [5, 8, 10]. EOCs defined by this culture method have been shown to exert their beneficial vascular effects by paracrine mechanisms [11], and reports suggest that diabetes adversely affects this paracrine angiogenic activity. Indeed, Loomans et al. [1] demonstrated that although EOC CM from healthy donors induced HUVECs to form tubes in an in vitro angiogenesis assay, the activity of EOC CM from individuals with type 1 diabetes was substantially impaired. In the current study, we found that CM derived from EOCs of diabetic rats was similarly less angiogenic in both in vitro and in vivo settings, and their EOCs expressed less SIRT1 mRNA. Consistent with the concept of metabolic memory, neither the angiogenic dysfunction nor the reduction in SIRT1 mRNA could be reversed by prolonged culture in a normal glucose environment.
When compared with animal studies, clinical trials of cell therapy have shown only modest benefits [12]. Importantly, human trials have largely used autologous bone marrow-derived progenitor cells that are adversely affected by aging and chronic illnesses, contrasting with the syngeneic cells from young healthy animals used in experimental studies. To overcome the effects of disease and aging, various strategies to enhance cell-based therapy efficacy have been studied, including gene therapy approaches and ex vivo “priming ” with proangiogenic stimuli such as SDF-1 [13] or hypoxia [14, 15]. The present study extends this concept by demonstrating that incubating dysfunctional EOCs from diabetic animals with small molecule SIRT1 modulators can alter their secretory output and restore their angiogenic activity.
Numerous studies have focused on the role of SIRT1 in modulating the ability of immature and mature endothelial cells to form vascular structures [4, 16]. In contrast, the present study examines the ability of the secretory products of EOCs to induce angiogenic activity in differentiated endothelial cells, focusing on the angiogenic chemokines that EOCs produce. Among these are the ELR+ CXC chemokines that possess a common primary structure, consisting of two amino-terminal cysteine residues that are separated by a nonconserved amino acid (CXC), flanked by a glutamate-leucine-arginine (ELR) tripeptide sequence [17]. In the present study, we noted that the expression of three such ELR+ CXC chemokines, CXCL1, CXCL3, and CXCL5, was reduced in the EOC CM from diabetic rats and restored to normal by incubating these dysfunctional EOCs with SRT1720. Moreover, consistent with the proangiogenic role of these cytokines, we further noted that an antagonist of CXCR2, the cognate receptor for CXC ELR+ chemokines, attenuated the ability of EOC CM to promote endothelial cell proliferation. Together, these findings suggest that the impaired angiogenicity of bone marrow-derived EOCs in diabetes may be a consequence of a reduction in SIRT1-mediated secretion of CXC ELR+ chemokines.
Conclusion
Our findings suggest that the impaired angiogenicity of EOCs in diabetes may be a consequence of a reduction in SIRT1 and that its activation may represent a novel therapeutic strategy to augment endothelial repair in this disease.
Acknowledgments
This study was supported by grants from the Canadian Institutes of Health Research (CCT 83030, MOP 97912) and the Physicians' Services Incorporated Foundation (PSI 09-42). D.A.Y. was supported by a KRESCENT postdoctoral fellowship and is currently supported by a Canadian Institutes of Health Research fellowship. A.A. is a Canadian Diabetes Association Clinician Scientist. J.M.S. is the Toronto General and Western Hospital Foundation Glaucoma Research Chair. R.E.G. is the Canadian Research Chair in Diabetes Complications, and this research was supported in part by the Canada Research Chair Program.
Author Contributions
D.A.Y.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; Y.Z., K.T., C.S., L.C., and X.G.: collection and/or assembly of data, data analysis and interpretation; A.A.: conception and design, data analysis and interpretation, provision of study materials; J.M.S.: conception and design, collection and/or assembly of data, provision of study materials; R.E.G.: conception and design, financial support, data analysis and interpretation, manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Loomans CJ, de Koning EJ, Staal FJ, et al. Endothelial progenitor cell dysfunction: A novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 2.Segal MS, Shah R, Afzal A, et al. Nitric oxide cytoskeletal-induced alterations reverse the endothelial progenitor cell migratory defect associated with diabetes. Diabetes. 2006;55:102–109. [PubMed] [Google Scholar]
- 3.Fadini GP, Miorin M, Facco M, et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 4.Potente M, Ghaeni L, Baldessari D, et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–2658. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuen DA, Connelly KA, Advani A, et al. Culture-modified bone marrow cells attenuate cardiac and renal injury in a chronic kidney disease rat model via a novel antifibrotic mechanism. PLoS One. 2010;5:e9543. doi: 10.1371/journal.pone.0009543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skog J, Wurdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers MS, Birsner AE, D'Amato RJ. The mouse cornea micropocket angiogenesis assay. Nat Protoc. 2007;2:2545–2550. doi: 10.1038/nprot.2007.368. [DOI] [PubMed] [Google Scholar]
- 8.Hur J, Yoon CH, Kim HS, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 9.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Yuen DA, Advani A, et al. Early-outgrowth bone marrow cells attenuate renal injury and dysfunction via an antioxidant effect in a mouse model of type 2 diabetes. Diabetes. 2012;61:2114–2125. doi: 10.2337/db11-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urbich C, Aicher A, Heeschen C, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Rosenzweig A. Cardiac cell therapy: Mixed results from mixed cells. N Engl J Med. 2006;355:1274–1277. doi: 10.1056/NEJMe068172. [DOI] [PubMed] [Google Scholar]
- 13.Zemani F, Silvestre JS, Fauvel-Lafeve F, et al. Ex vivo priming of endothelial progenitor cells with SDF-1 before transplantation could increase their proangiogenic potential. Arterioscler Thromb Vasc Biol. 2008;28:644–650. doi: 10.1161/ATVBAHA.107.160044. [DOI] [PubMed] [Google Scholar]
- 14.Di Santo S, Yang Z, Wyler von Ballmoos M, et al. Novel cell-free strategy for therapeutic angiogenesis: In vitro generated conditioned medium can replace progenitor cell transplantation. PLoS One. 2009;4:e5643. doi: 10.1371/journal.pone.0005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurtner GC, Chang E. “Priming” endothelial progenitor cells: A new strategy to improve cell based therapeutics. Arterioscler Thromb Vasc Biol. 2008;28:1034–1035. doi: 10.1161/ATVBAHA.108.163246. [DOI] [PubMed] [Google Scholar]
- 16.Zhou S, Chen HZ, Wan YZ, et al. Repression of P66Shc expression by SIRT1 contributes to the prevention of hyperglycemia-induced endothelial dysfunction. Circ Res. 2011;109:639–648. doi: 10.1161/CIRCRESAHA.111.243592. [DOI] [PubMed] [Google Scholar]
- 17.Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of neovascularization. Arterioscler Thromb Vasc Biol. 2008;28:1928–1936. doi: 10.1161/ATVBAHA.108.162925. [DOI] [PMC free article] [PubMed] [Google Scholar]