This study indicates that human mesenchymal stem cells (hMSCs) can be efficiently transduced using a protamine sulfate concentration of 100 μg/ml at low volumes over 3 days. With the capacity to easily, reliably, and inexpensively transduce hMSCs, it will be possible to efficiently use genetic engineering to add or delete a function, improve or replace an existing one, and gain insight into the biology of these cells through the use of reporter genes.
Keywords: Mesenchymal stem cells, Lentiviral vector, Gene therapy, Transduction, Adult human bone marrow, Adult stem cells, Cellular therapy, Osteoblast
Abstract
Long-term lentiviral transduction of human mesenchymal stem cells (hMSCs) greatly enhances the usefulness of these cells. However, such transduction currently requires the use of polybrene, which severely inhibits hMSC proliferation. In contrast, protamine sulfate at 100 μg/ml doubled transduction efficiencies without affecting proliferation or differentiation potential. Expression levels improved 2.2-fold with the addition of a woodchuck hepatitis post-transcriptional regulatory element. Further improvements in transduction efficiencies could be obtained by a modest increase in viral concentrations through increased viral titers or decreased transduction volumes without changing multiplicity of infection, by transducing over multiple days, or by culturing the cells in fibroblast growth factor-2. Centrifugation improved expression but had no effect on efficiency. Transgene expression was stable over 6 weeks in vitro and in vivo. Donor-to-donor and intradonor variability were observed in primary passage through passage 2 cultures, but not at passage 3. These results provide a better optimized approach for expanded use of hMSCs through genetic manipulation.
Introduction
Mesenchymal stem cells (MSCs) are multipotent adult stem cells that can differentiate into adipocytes, chondrocytes, and osteoblasts [1, 2]. This ability to serve as cell progenitors for different mesenchymal lineages has been used in regenerative medicine to structurally repair injured tissues and restore their lost functions, such as in bone [3] and cartilage repair [4]. In addition, these cells have been found to exert trophic and other local effects that allow them to modulate the immune system, protect surrounding cells from apoptosis, and establish a regenerative microenvironment. The translational potential of these capabilities ranges from treating autoimmune diseases, such as multiple sclerosis [5] and rheumatoid arthritis [6], and other immune disorders such as graft versus host disease [7] to treating ischemic events such as stroke [8], inflammatory disorders such as asthma [9], and other instances of tissue damage such as acute lung injury [10].
The usefulness of MSCs can be greatly expanded through genetic modification of the cells that provide new functions or improve on existing ones. For example, when MSCs were genetically modified to overexpress stromal cell-derived factor-1 [11] or Akt [12] and delivered to mice after a myocardial infarction, the cardiac function significantly improved. MSCs have been genetically modified and used as targeted anticancer drug carriers. For example, when they were modified to secrete interferon-β, survival of mice implanted with either glioma [13] or melanoma [14] significantly increased. MSC homing itself could be improved, as Kumar and Ponnazhagan demonstrated by increasing the expression of α4 integrin [15]. They could also be used to treat genetic diseases for which there currently is no cure [16]. For example, in osteogenesis imperfecta (OI), a disease in which osteoblasts cannot produce either functional or adequate levels of type I collagen [17], MSCs from the patient could be isolated, genetically corrected to produce sufficient amounts of functional collagen, and then re-engrafted into the patient [18]. Finally, genetic modification of MSCs can be a useful tool to track and study normal MSC functions. For example, MSCs have been labeled with lacZ [19] or green fluorescent protein (GFP) [20] in order to study their trafficking behavior.
There are a number of short-term transfection methods available for MSCs. These include viral methods, such as treatment with adenovirus [14, 15, 21], as well as nonviral methods [22] such as liposomes [23] or electroporation [24]. In contrast, methods for long-term/stable genetic modification of MSCs are significantly more limited. One method is to use transgenic mice [25]. However, there are serious limitations with this method, including its restriction to mouse MSCs, which can have different properties from other species, such as human MSCs [26], and the necessity of generating new transgenic mice for every protein under study.
An alternative method is to use lentiviruses to deliver the engineered DNA construct. The current standard protocol for efficient lentiviral transduction of MSCs involves the addition of polybrene during transduction. Unfortunately, as we recently published, polybrene severely inhibits MSC proliferation, even with the addition of fibroblast growth factor-2 (FGF-2), a potent mitogen [27], and without polybrene, transduction efficiency is low [20, 27]. In addition to the increased cost of transducing MSCs, the inhibition of MSC proliferation is problematic for any therapy that requires sufficient cells to repopulate a host, such as the treatment of OI described above. Furthermore, we have some initial indications that polybrene might also affect human MSC (hMSC) differentiation potential in addition to proliferation. For instance, in the study of chondrogenesis in aggregate (pellet) cultures, we observed that pellets made with polybrene-treated hMSCs were smaller and exhibited less cartilage extracellular matrix than untreated controls (unpublished observations). Thus, there is a necessity for an alternative hMSC transduction method that preserves the cells' proliferation and differentiation potential.
In order to overcome these technical limitations, we comprehensively and systematically addressed various factors that affect viral transduction, including the virus, the cells, and the protocol used, in order to evaluate their impact on both efficiency and transgene expression and their effects on proliferation and differentiation. We found for the first time that the use of high concentrations of protamine sulfate is a viable alternative to polybrene that preserves hMSC proliferation and differentiation capabilities while significantly improving lentiviral transduction. In conjunction with protamine sulfate, efficiency was further improved by modest increases in viral concentrations without the need for ultracentrifugation, via increased viral titers or decreased transduction volumes, or by transducing over multiple days. Expression levels could be enhanced with centrifugation or with the addition of the woodchuck hepatitis post-transcriptional regulatory element (WPRE) at the end of the transgene. Furthermore, we investigated for the first time the possibility of transducing hMSCs grown in the presence of FGF-2.
Materials and Methods
Human MSC Isolation and Culture
Human MSCs were isolated as described previously [28, 29]. An aspirate from the bone marrow was obtained from the posterior superior iliac crest of consenting adult donors (institutional review board-approved protocol from University Hospitals, Cleveland, OH). The aspirate was washed with Dulbecco's modified Eagle's medium, low glucose (DMEM-LG) (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) supplemented with 10% fetal bovine serum (FBS), screened to support hMSC proliferation and differentiation (hMSC medium) [30]. The cells were centrifuged, resuspended in 5 ml of medium, and placed on top of 35 ml of 65% (vol/vol) Percoll gradient. After 15 minutes of centrifugation at 460g, the top 25% of the gradient was transferred to a new tube and washed again. The cells were then seeded at 1.8 × 105 per cm2 and cultured for 2 weeks before passage. The hMSCs were cultured in 10% FBS DMEM-LG with medium changes every 3–4 days. For maintenance cultures, cells were passaged weekly with 0.25% trypsin/4 mM EDTA (Gibco, Grand Island, NY, http://www.invitrogen.com) and seeded at 3–4.5 × 103 per cm2 in 100-mm tissue culture dishes or T175 flasks.
Viral Production
The bicistronic reporter gene constructs pLV-MND-LR (LR) and pLV-MND-LR-WPRE (LR-WPRE) were created by modifying the fl-mrfp-ttk triple fusion reporter vector [31] (gift from Dr. Zhenghong Lee, Case Western Reserve University) containing firefly luciferase (fl), monomeric red fluorescent protein (mrfp), and herpes simplex thymidine kinase (ttk) driven by a modified myeloproliferative sarcoma virus promoter (MND). First, the herpes simplex thymidine kinase (ttk) domain was removed from the triple fusion construct with BamHI to create the pLV-MND-LR plasmid. For the pLV-MND-LR-WPRE construct, WPRE was obtained from the pLV-MND-P140K-2A-GFP plasmid (gift from Dr. Stanton Gerson, Case Western Reserve University) with BamHI and then inserted into the bicistronic vector after the transgenes. Both plasmids were sent for sequencing to ensure the correct insertion and that the nucleotides were in-frame.
To produce the second-generation replication-incompetent lentivirus, near confluent 293T cells were transfected with the reporter plasmid, pCMVΔR8.91 (packaging plasmid), and pMD.G (vesicular stomatitis virus protein G envelope plasmid) at a ratio of 3:3:1 using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) in Opti-MEM culture medium (Gibco). Opti-MEM was replaced with fresh hMSC medium after 12 hours, and viral supernatant was collected after 36 hours and again after another 24 hours at half the volume. The supernatant was sterile filtered, aliquoted, and frozen at −80°C until use. Viral titers were determined with 293T cells by measuring the monomeric red fluorescent protein (mRFP) and ranged from 2.8 × 106 to 7.1 × 106 infectious units (IU)/ml for pLV-MND-LR and from 4.9 × 106 to 15.5 × 106 IU/ml for pLV-MND-LR-WPRE. Viruses were thawed immediately before titer and cell transduction.
Transduction
Transductions were done in six-well plates. Unless otherwise specified, hMSCs were seeded at 1 × 105 cells per well in triplicate at a final volume of 1 ml per well with a multiplicity of infection (MOI) of 5. Protamine sulfate (catalog no. P4020-1G; Sigma-Aldrich) from a 5 mg/ml stock solution (in DMEM-LG, sterile filtered) was added to obtain the desired final concentration. Cells were transduced for 24 hours before being replaced with 1.5 ml per well. Cells cultured from the initial marrow aspirate and then trypsinized and transduced were termed P0 (passage 0). Experiments were repeated with at least two donors.
In the FGF-2 experiments, either hMSCs were cultured in the presence of recombinant human (rh) FGF-2 (final concentration, 10 ng/ml; Peprotech, Rocky Hill, NJ, http://www.peprotech.com) from primary passage or rhFGF-2 was added at the time of transduction and subsequent medium changes. For the centrifugation and the different transduction volume experiments, hMSCs were first seeded at 1.5 ml per well for 24 hours prior to transduction. In the low-volume experiment, the cells were initially transduced at 0.5 ml per well for 8 hours. Subsequently another 0.5 ml (hMSC medium plus protamine sulfate) per well was added for 16 hours before medium change. Volumes for other size dishes are listed in supplemental online Table 1. In the centrifugation experiments, hMSCs were centrifuged for 1 hour in an Allegra 6KR centrifuge (Beckman Coulter, Fullerton, CA, http://www.beckmancoulter.com) with a GH-3.8 rotor and Micro Plus Carriers at 2,000 rpm (730g) and 1.5 ml per well. The centrifuge was run to bring the temperature up to ∼32°C before the experiment.
Three days after the last round of transduction, the efficiency was measured by detecting the mRFP on a BD LSR II (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com) (excitation: 561, emission: 610/20 band pass filter with a 600LP in front) as shown in supplemental online Figure 1. The positive cells were gated using a 525 nm versus 610 nm scatter plot in order to account for the autofluorescence from hMSCs. Expression levels were calculated from the geometric mean of the gated population. Figures show the results of triplicate wells from one representative experiment.
Proliferation Analysis
Trypsinized cells were washed, and then resuspended at 2 × 104 cells per milliliter. For the protamine sulfate experiment, the cell suspension was additionally supplemented with protamine sulfate to obtain final concentrations of 0, 50, 100, and 200 μg/ml. The cells were seeded into 96-well plates at 1 × 103 cells per 50 μl per well in triplicate and cultured at 37°C, 5% CO2. After 24 hours and 7 days, the medium was removed and the plate was placed in the −80°C freezer until the day of analysis. The CyQUANT assay (Molecular Probes, Eugene, OR, http://probes.invitrogen.com) was then performed on the wells according to manufacturer instructions.
Differentiation Assays
Human MSCs were transduced at P0 and cultured for an additional 4 days after the start of transduction. The cells were then sorted on the iCyt Reflection flow cytometer (excitation: 561 nm, emission: 615/30 band pass filter) with a 100-μm tip. The 30% brightest and dimmest cells were collected and cultured for an additional week before the start of the differentiation assays. The assays were performed as described previously [29].
To assess the chondrogenic potential of the hMSCs, 2.5 × 105 cells were resuspended in chondrogenic medium (1% ITS+, 10−7 M dexamethasone, 1 mM sodium pyruvate, 37.5 μg/ml ascorbic acid-2 phosphate, and 10 ng/ml transforming growth factor-β1; Peprotech) and seeded into V-bottomed polypropylene 96-well plates. The plates were centrifuged at 500g for 5 minutes and cultured at 37°C for 21 days with medium changes every 2–3 days. The pellets were then fixed in 10% formalin, embedded, sectioned, and stained with toluidine blue.
The adipogenic assay was performed by seeding 2.0 × 105 cells per well into six-well plates in adipogenic medium (DMEM-HG with 1 μM indomethacin, 500 μM 3-isobutyl-1-methylxanthine, 10−6 M dexamethasone, and 10 μg/ml insulin). Medium was changed twice a week. After 21 days, the cells were fixed with 4% formaldehyde. The cells were permeabilized with 0.01% digitonin for 20 minutes and blocked with 1% bovine serum albumin. The cells were then stained with mouse monoclonal anti-adipophilin antibody (catalog no. 610102; 1:10; Progen Biotechnik, Heidelberg, Germany, http://www.progen.de) followed by a fluorescein-conjugated goat anti-mouse secondary antibody (1:1,000; Cappel/MP Biomedicals, Solon, OH, http://www.mpbio.com).
To test whether the transduced hMSCs could be detected in vivo and whether they can differentiate into osteoblasts, the cells were loaded into porous calcium phosphate ceramic cubes coated with fibronectin and implanted subcutaneously on the dorsal surface of CB17 SCID mice. (Animal experiments were approved by the Institutional Animal Care and Use Committee of Case Western Reserve University, Cleveland, OH.) After 6 weeks, the mice were x-rayed, the bioluminescence was measured, and the cubes were harvested. Bioluminescent imaging was done with the Xenogen IVIS Imaging 200 Series system (Caliper Life Sciences, Hopkinton, MA, http://www.caliperls.com). The mice were injected with 200 μl of 12.5 mg/ml luciferin substrate (Biosynth, Itasca, IL, http://www.biosynth.com) intraperitoneally, and a 10-second exposure was taken 5 minutes later. The harvested ceramics were fixed, decalcified, paraffin-embedded, sectioned, and stained with Mallory-Heidenhain. Sections were semiquantitatively analyzed as described previously with a ceramic cube score based on the percentage of positive pores [32].
Statistical Analysis
Unless otherwise stated, significance was assessed by analysis of variance (ANOVA) followed by a Tukey's multiple comparison test. For Figure 2F, a one-sample t test was used to test the null hypothesis that the mean was 1.0. For Figure 3A, a two-way ANOVA showed significant difference between cells frozen and thawed and cells in continuous culture. A Bonferroni post test showed significant difference between the conditions in P1 and P3 (p < .001). For Figure 3B, a repeated measures linear mixed model using Tukey-Kramer adjustment for multiple comparisons was used. When multiple comparisons were used, adjusted p values are reported.
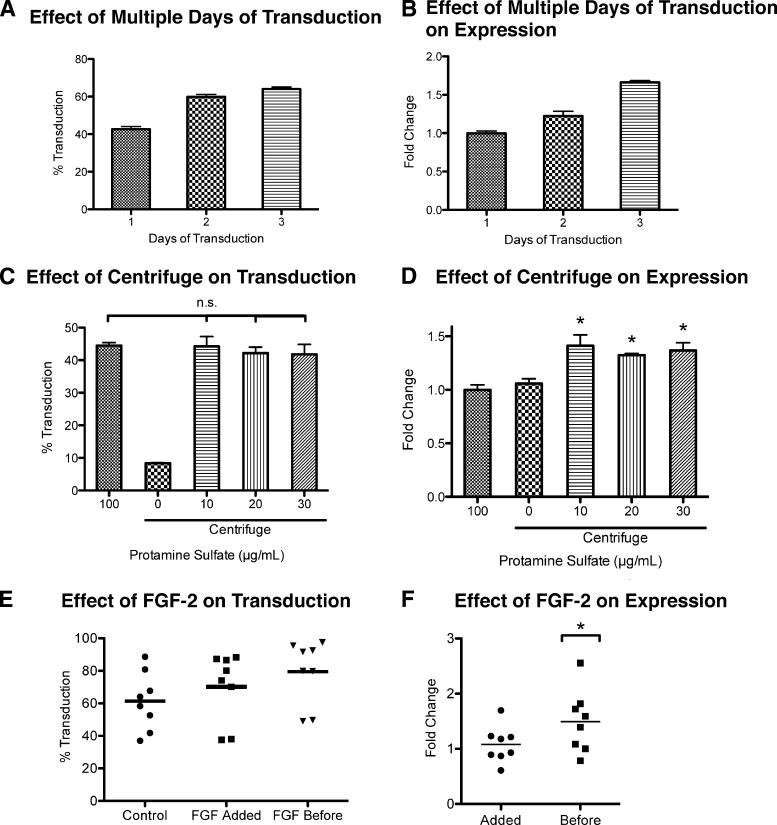
Figure 2.
Optimizing transduction efficiency and transgene expression. (A, B): The change in transduction efficiency (p < .05 between all groups) (A) and relative change in transgene expression (p < .01 between all groups) (B) of transducing over 2 to 3 days compared with a 1-day transduction. The total amount of virus was kept the same. For 1 day, MOI = 5; 2 days, MOI = 2.5; and 3 days, MOI = 1.67. Transduction volume (1 ml per well of a six-well plate) and protamine sulfate concentration (100 μg/ml) were held constant. Expression levels were compared with 1-day transduction. Values are mean ± SD. (C, D): The change in transduction efficiency (C) and relative change in transgene expression (D) of cells centrifuged with different concentrations of protamine sulfate compared with hMSCs transduced in static culture at 100 μg/ml protamine sulfate. At 10–30 μg/ml protamine sulfate, there was no statistical difference in transduction efficiency between static culture and centrifugation, whereas there was a statistical difference in transgene expression. *, p < .01 vs. static 100 μg/ml and centrifuge 0 μg/ml, Tukey's t test. Expression levels were compared with static culture. MOI = 5. Values are mean ± SD. (E, F): The change in transduction efficiency (p < .05, between all conditions, n = 8) (E) and transgene expression (*, p < .05, n = 8) (F) of hMSCs treated with FGF-2 prior to transduction (before) or at the time of transduction (added) compared with hMSCs cultured without FGF-2 (control). Expression levels were compared with control. All experiments involving FGF-2 were done with 100 μg/ml protamine sulfate and MOI = 5. Each data point is a mean of three replicates of one experiment. Abbreviation: FGF-2, fibroblast growth factor-2.
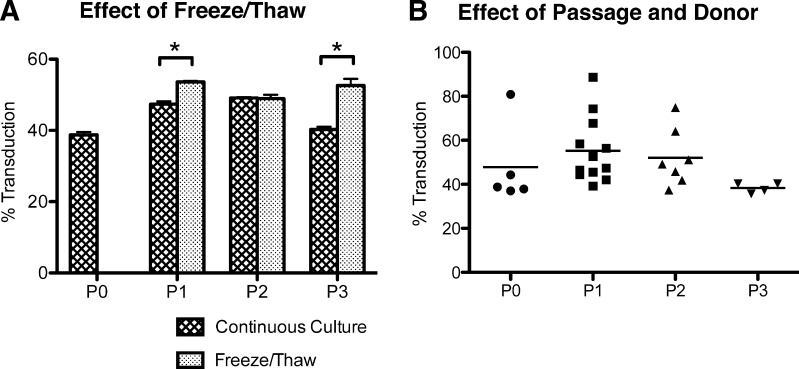
Figure 3.
Consistency of MSC transduction. (A): The transduction efficiency of hMSCs frozen and thawed at different passages compared with those that were maintained continuously in culture. MOI = 5; protamine sulfate = 100 μg/ml. Values are mean ± SD. *, p < .001. (B): A graph of all the transductions from the different donors at different passages. n = 5, 12, 7, and 4 respectively for P0, P1, P2, and P3. MOI = 5; protamine sulfate = 100 μg/ml. No statistical significance was found between passages or within donors. Each data point is a mean of three replicates of one experiment. Abbreviation: P, passage.
Results
Protamine Sulfate
To investigate whether protamine sulfate is a viable alternative for polybrene, hMSCs were transduced at various concentrations of protamine sulfate with a modified dual reporter gene (LR) that codes for a fusion protein containing both luciferase (Luc) and mRFP functional domains [31]. The transduction efficiency was determined by analyzing the fluorescence with flow cytometry (Fig. 1A). An example of the flow cytometry analysis is shown in supplemental online Figure 1. Protamine sulfate concentrations below 8 μg/ml showed no effect on transduction efficiency. However, increasing the protamine sulfate concentration to 50 μg/ml resulted in a 2.4-fold increase in efficiency from 15.7% (at 0 μg/ml) to 37.6%. Further increase in protamine sulfate concentration resulted in a slight but not statistically significant increase in efficiency, although cell detachment became pronounced at concentrations above 100 μg/ml. Hence subsequent experiments were done at 100 μg/ml unless otherwise specified. Unlike polybrene, there was no statistical difference in cell proliferation between untreated cells and cells treated with protamine sulfate at any concentration tested for an extended period of time (7 days) (Fig. 1B). Similarly, trypan blue staining during cell passage showed an identical percentage of dead cells (between 0% and 1%) as controls. This was confirmed with a 4′,6-diamidino-2-phenylindole live/dead assay (supplemental online Fig. 2).
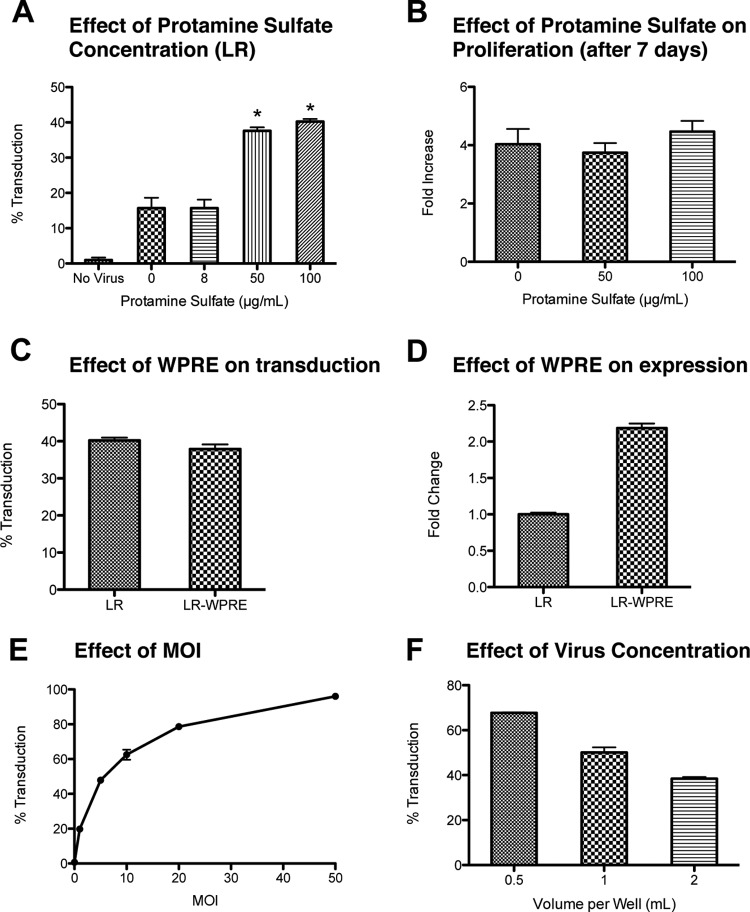
Figure 1.
Effect of protamine sulfate, WPRE, and viral concentration. (A): Human mesenchymal stem cells (hMSCs) were incubated with different concentrations of protamine sulfate at a constant concentration of LR coding for a luciferase/monomeric red fluorescent fusion protein. The transduction efficiency was measured by the red fluorescent intensity. *, p < .001 vs. 0 and 8 μg/ml, Tukey's t test. MOI = 5. (B): The increase in cell numbers was measured after 1 week in culture for hMSCs cultured in 0 μg/ml protamine sulfate (control) and at different concentrations of protamine sulfate. There was no statistical difference between the groups. (C): Transduction efficiency of the lentivirus with WPRE (LR-WPRE) or without WPRE (LR). No statistical difference (t test). (D): Difference in relative fluorescence values between the gene construct with or without WPRE. p < .001, t test. (E): Transduction efficiency of hMSCs incubated at different MOI but the same transduction volume (1 ml per well of a six-well plate) and protamine sulfate concentration (100 μg/ml). (F): Transduction efficiency at different transduction volumes in a six-well plate, but the same MOI (MOI = 5) and protamine sulfate concentration (100 μg/ml); p < .001 between all groups. All values are mean ± SD. Abbreviations: LR, lentivirus with luciferase and monomeric red fluorescent protein; MOI, multiplicity of infection; WPRE, woodchuck hepatitis post-transcriptional regulatory element.
Woodchuck Hepatitis Post-Transcriptional Regulatory Element
In addition to increasing transduction efficiency, labeling of hMSCs can be enhanced by increasing transgene expression. In an attempt to increase expression of the transgene, a WPRE was added at the end of the transgene in the vector construct. The addition of WPRE did not increase transduction efficiency (Fig. 1C), but it significantly increased the expression level of the transgene by 2.2-fold (Fig. 1D). This was confirmed by Western blot analysis, which showed a 2.4-fold increase in expression (supplemental online Fig. 3). Consequently, the remaining experiments were done with the LR-WPRE vector.
Viral Concentration
To investigate whether there is a concentration dependence with viral transduction, hMSCs were transduced with increasing MOI while maintaining a consistent protamine sulfate concentration (100 μg/ml). This resulted in a sharp initial rise in transduction efficiency (up to 20 MOI) followed by smaller increments in increased efficiency with further increasing amounts of lentivirus (Fig. 1E). The high viral titers also had no significant effect on cell proliferation (supplemental online Fig. 4). Therefore, the remaining experiments were performed with an MOI of 5, which showed a transduction efficiency of approximately 50%, allowing additional opportunities to investigate further improvements in efficiency.
In addition to changing the ratio of virus to cell (i.e., MOI) in solution, viral concentration can also be affected by changing the transduction volume. To investigate whether viral concentration or the absolute amount of viral particles is more important in determining transduction efficiencies, the same amount of virus (MOI = 5) was added to different volumes of transduction medium (Fig. 1F). Halving the transduction volume to 0.5 ml per well increased transduction efficiency from 50% to 68% (Fig. 1F), similar to the transduction efficiency of 61% when the MOI is doubled to 10 (Fig. 1E). In contrast, changing the seeding density and adjusting the viral particles to maintain a consistent MOI had no effect on efficiency (supplemental online Fig. 5).
Transducing over Multiple Days
To investigate the role that the cells play in the transduction process, we transduced hMSCs over the course of 2 days (MOI = 2.5 × 2 days) or 3 days (MOI = 1.67 × 3 days) while keeping the volume and overall total amount of virus particles constant (MOI = 5). A 2-day consecutive transduction increased transduction efficiency by 40%. Three days of transduction resulted in an additional smaller, although statistically significant, increase of 7% from the 2-day transduction (Fig. 2A). The increased transduction efficiency also correlated with higher expression levels (Fig. 2B), since a 2-day transduction increased expression by 22%, and a 3-day transduction by another 44%. There was no difference in cell proliferation as compared with untreated cells (supplemental online Fig. 6A), and there was no difference in cell proliferation of transduced cells cultured in 60- and 100-mm dishes, and T175 and T500 flasks at up to seven passages (supplemental online Fig. 6B, 6C).
Centrifugation
Another method commonly used to increase transduction efficiency is centrifugation after the addition of virus to the medium [33]. Unlike the case with static cultures, centrifugation caused the cells to detach at much lower concentrations of protamine sulfate even without the addition of virus. Major cell detachment was observed at concentrations greater than 30 μg/ml. Importantly, centrifugation alone (without the addition of protamine sulfate) did not improve transduction efficiency. However, 10–30 μg/ml protamine sulfate combined with centrifugation was sufficient to increase efficiency to the same degree as 100 μg/ml under static conditions (Fig. 2C). Whereas centrifugation did not have an effect on transduction efficiency above what can be achieved under static conditions, it did improve the expression level by approximately 36% (Fig. 2D). Centrifuging at lower than 1,500 rpm (411g) had no effect (data not shown).
FGF-2
Although a key characteristic of the lentivirus is its ability to transduce nondividing cells, it transduces dividing cells more efficiently [34, 35]. This led us to hypothesize that treatment of hMSCs with FGF-2, a known mitogen [36], could have a positive impact on transduction efficiency. To determine whether the timing of the addition of FGF-2 had an effect, hMSCs were either cultured in FGF-2 at least 1 week prior to transduction or FGF-2 was added at the time of transduction. In both cases, FGF-2 significantly increased transduction efficiency (Fig. 2E). On average, the efficiency increased from 61% to 70% when FGF-2 was added at the time of transduction, and it further increased to 79% with cells cultured in FGF-2 prior to transduction. Cells treated with FGF-2 before transduction also showed a significant increase in transgene expression, ranging from a 9% to a 155% increase (mean, 49%) (Fig. 2F).
Consistency of Transduction Efficiency
Storage of hMSCs in liquid N2 is commonly practiced to preserve the cells for later use. Freeze-thaw of the cells was shown to not negatively impact the transduction efficiency of the cells at any passage (Fig. 3A). We did notice a slight but significant increase in transduction efficiency with cells that were previously frozen in P1 and P3. We have also observed that thawed cells can exhibit increased proliferation (unpublished data), which could explain this increase in transduction efficiency.
Viral transduction of hMSCs was also slightly better at P1 and P2 compared with P0 and P3 (55% and 52% vs. 48% and 38%, respectively), although it was not significant due to the high variability at P1 and P2, for which the transduction efficiencies ranged from 39% to 89% (Fig. 3B). For cells transduced at P0, one donor had an efficiency of 80%, but the remaining four donors clustered tightly around 40%. There can also be high intradonor variability as one donor had a transduction efficiency of 39% and 75% at P1 and P2, respectively, whereas another had an efficiency of 89% and 42% at the same passages. Such donor-to-donor variations are normal observations with hMSCs [37]. Donor age (27–61 years old) and gender had no significant effect.
Differentiation Potential
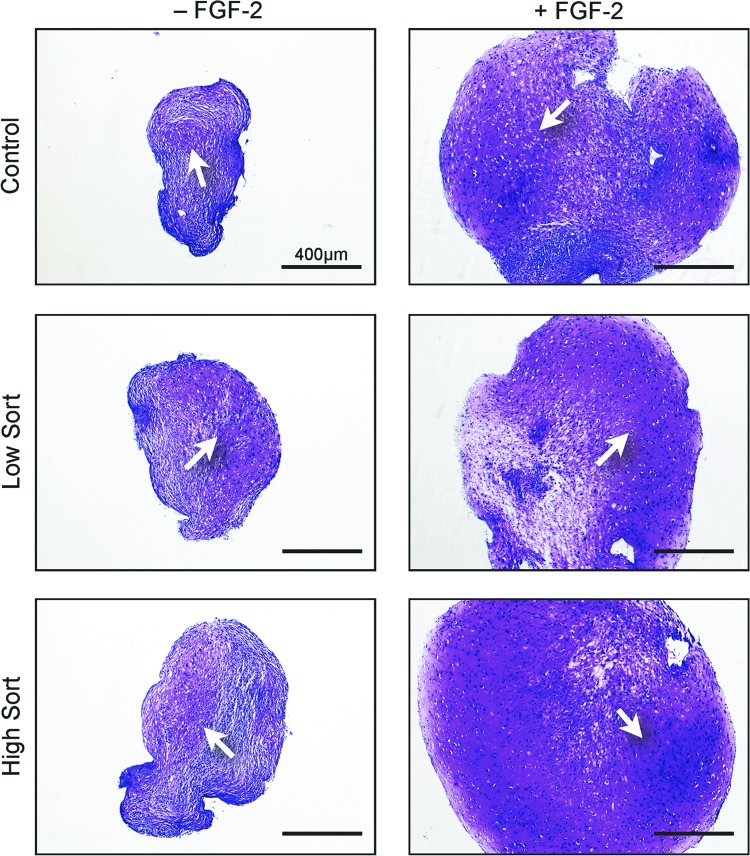
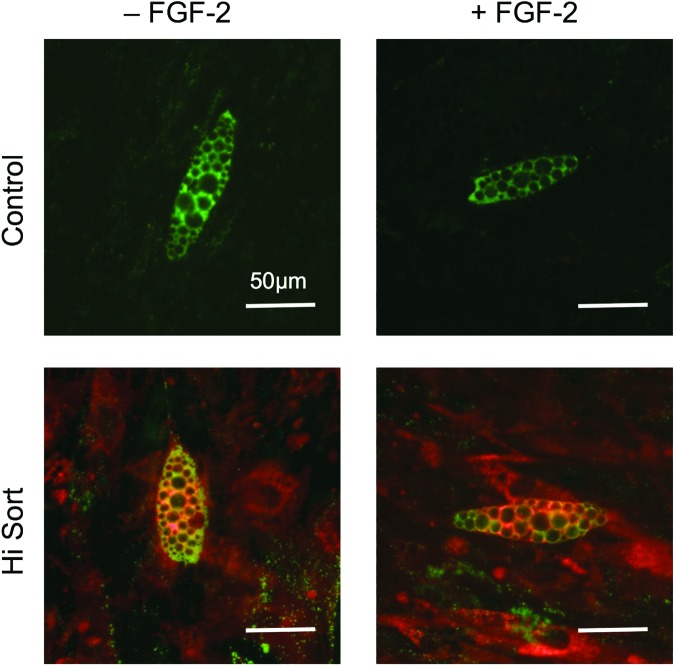
In order to test whether transduction and the insertion of the transgene affects the differentiation potential of hMSCs, the brightest and dimmest 30% of the cells based on their fluorescent intensity were sorted, and chondrogenic, adipogenic, and osteogenic assays were performed. In the chondrogenic pellet assay, both low and high expressing cells showed cartilage formation similar to that of untransduced cells. Furthermore, cells expanded in the presence of FGF-2 formed larger pellets and had more matrix (Fig. 4).
Figure 4.
Chondrogenic pellet assay. Human mesenchymal stem cells were transduced with or without prior FGF-2 treatment and then sorted into the top 30% highest expressing (high sort) and bottom 30% lowest expressing (low sort) cells and tested for their ability to form cartilage in pellet cultures compared with nontransduced cells. The white arrows point to the cartilage matrix. Scale bars = 400 μm. Abbreviation: FGF-2, fibroblast growth factor-2.
In the adipogenic assay, lipid formation was observed in all conditions. To confirm that the lipid formation was not isolated to untransduced cells, the MSCs were stained for adipophilin. Colocalization of red fluorescence with adipophilin staining indicated lipid formation in lentiviral transduced hMSCs (Fig. 5).
Figure 5.
Adipogenic assay. Human mesenchymal stem cells were cultured in adipogenic medium for 21 days. The cultures were imaged for red fluorescence and merged with the immunofluorescence stain for adipophilin (green). Transduced cells show both red fluorescence and adipophilin staining. Scale bars = 50 μm. Abbreviation: FGF-2, fibroblast growth factor-2.
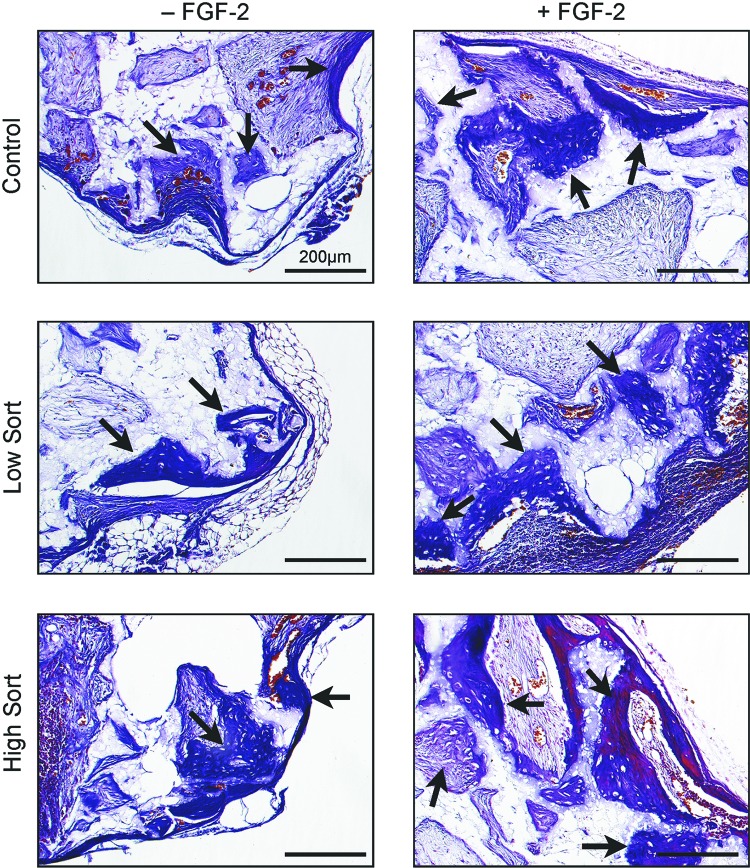
To test osteogenic potential, transduced MSCs were loaded into porous calcium phosphate ceramic scaffolds that were implanted subcutaneously on the dorsal surface of immunocompromised mice. After 6 weeks in vivo, bioluminescent imaging showed strong transgene expression (Fig. 6A). Concurrently, the same cells cultured in vitro confirmed that fluorescent expression remained consistent for at least seven passages (about 7 weeks) (Fig. 6B). Histologic analysis of the implanted ceramic cubes showed that bone formation was similar in high and low sort and control cells. There was no statistical difference in the ceramic cube scores between the transduced and the control cells in both FGF-2 and non-FGF-2-treated cells. Interestingly, cubes loaded with FGF-2-treated cells had more bone formation (Fig. 7).
Figure 6.
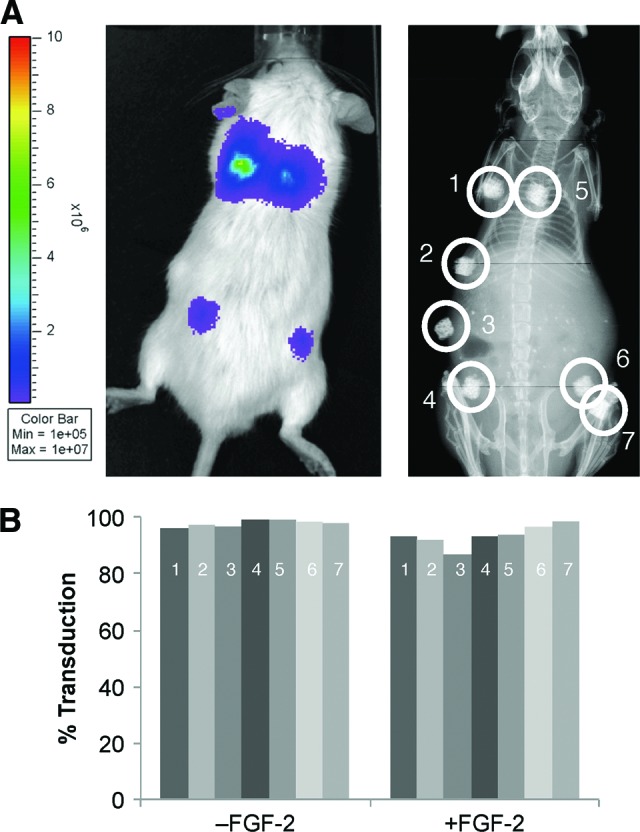
Ceramic cube assay. (A): Sorted human mesenchymal stem cells (hMSCs) were seeded into ceramic cubes and implanted subcutaneously. The transduced hMSCs continued to show strong bioluminescence after 6 weeks in vivo. An x-ray image shows the location of the ceramic cubes. Numbers indicate: hMSCs cultured without FGF-2, high sort (1) and low sort (4); hMSCs cultured with FGF-2, high sort (5) and low sort (7); nontransduced hMSCs (2, 3, 6). (B): The high sort cells were cultured for another seven passages, equivalent to 7 weeks, and their transduction efficiency was measured at each passage. The numbers in the bars indicate the passage numbers. Abbreviation: FGF-2, fibroblast growth factor-2.
Figure 7.
Ceramic cube assay. After 6 weeks, the ceramic cubes were harvested and stained for histologic analysis. The black arrows point to the bone matrix found in all ceramics. FGF-2-treated cells showed more bone matrix overall. Scale bars = 200 μm. Abbreviation: FGF-2, fibroblast growth factor-2.
Discussion
Currently, the addition of polybrene during transduction is required to obtain efficient lentiviral transduction with hMSCs. Unfortunately, we have found that polybrene dramatically impacts hMSC proliferation and possibly their differentiation capabilities as well [27]. In our attempts to devise an alternative method to preserve hMSC proliferation and differentiation, improve hMSC transduction, and enhance transgene expression, we first established a baseline for comparison with 100 μg/ml protamine sulfate (as a polybrene alternative), the inclusion of WPRE at the end of the transgene, and an MOI of 5. Next we investigated variables affecting the virus, the cells, and the protocol. Those variables included viral concentration, number of rounds of transduction, centrifugation, the effect of FGF-2, freeze/thaw cycle, passage number, donor variability, and seeding density. All these methods had an impact on both transduction efficiency and transgene expression.
Protamine sulfate is a Food and Drug Administration-approved, relatively inexpensive polycation most commonly used to neutralize heparin overdose. It is believed to improve transduction by neutralizing the negative charge between the cell surface and the viral particles [38]. It was first suggested as an alternative to polybrene by Cornetta and Anderson in 1989 [39]. In that report, 5–10 μg/ml protamine sulfate resulted in the highest transduction efficiencies with the retrovirus, with either increasing toxicity or decreased transduction efficiency at higher concentrations. However, in addition to using a different viral vector, the cells used were rapidly dividing NIH 3T3 cells and another cell line, which typically experience greater transduction efficiencies than primary cells [39]. When these authors investigated transduction of primary cultures at 10 μg/ml, they were unable to obtain efficiencies above 1% without coculture with the retroviral producer cells that provided continuous high viral titers. Investigators have since used protamine sulfate for labeling of MSCs with superparamagnetic iron oxide for cell tracking by magnetic resonance imaging [40] and attempted to use for transduction with lentivirus [41, 42]. As it has always been used at concentrations below 8 μg/ml, which we found to have no effect on hMSC lentiviral transduction, their efforts have had limited success. As a consequence, protamine sulfate is rarely used for hMSC transduction. In contrast, when the concentration was raised to 50 μg/ml, transduction efficiencies increased 2.4-fold, although concentrations above 100 μg/ml affect the ability of hMSCs to bind to the surface of the plate during seeding or cause the cells to detach from the dish. The improvement was great enough that in some experiments at an MOI of 5, protamine sulfate alone was sufficient to increase transduction efficiency to as high as 88%. Thus ultracentrifugation is not required to concentrate the viral supernatant in our studies. Furthermore, unlike polybrene, protamine sulfate did not affect the cells' ability to divide, thus allowing lower amounts of virus to be used for clinical or experimental purposes, potentially decreasing the initial number of MSCs required for therapy, and thereby enhancing the utility for therapeutic applications in which MSCs serve as regenerative cells. Although not as drastic, we also found that polybrene affected the proliferation of human umbilical vein endothelial cells, whereas protamine sulfate does not (data not shown). Thus, these results are potentially applicable to other cell types.
In addition to improving transduction efficiencies, we sought to improve expression levels with the addition of WPRE at the end of the gene construct, which had been shown to improve transgene expression of the viral vector [43, 44]. Although constructs with WPRE have been used previously with hMSCs [45–47], the degree of enhancement on lentiviral transduction of hMSCs was unknown because such vectors have not previously been directly compared with constructs not containing this element.
The addition of the WPRE resulted in two benefits to our system. First, whereas the transduction efficiency did not increase with WPRE, the expression level of the transgene increased 2.2-fold. This confirmed previous results with 293T cells, for which the addition of the WPRE did not increase the percentage of transduced cells, affect the copy number of the transgenes, or affect the frequency of transcription, but instead increased the expression of the vector, possibly through stabilization of the transcript in the nucleus [43]. Second, we observed that the addition of WPRE benefited viral production as viral titers approximately doubled.
It is clear that viral concentration is key to obtaining high transduction efficiency. Increasing viral concentration can occur from either increasing the amount of virus in the transduction solution (increasing MOI) or by decreasing the transduction solution volume while keeping the MOI constant. Both resulted in higher transduction efficiencies. Unfortunately, since hMSCs are not grown in suspension, the volume is limited to the lowest volume possible that does not desiccate the substrate-attached cells.
An alternative method to increase transduction efficiency is the centrifugation of the cells in transduction medium, which is also termed spinoculation. Despite the small size of the virus and the relatively low g force, centrifugation is believed to function by effectively increasing the concentration of viral particles near the cells [33]. However, unlike the case with T cells, centrifugation alone had no effect on improving hMSC transduction and protamine sulfate continues to be required. Interestingly, centrifugation causes extensive cell detachment at protamine sulfate concentrations above 30 μg/ml, even without the addition of virus; this is not observed in static cultures. One possible explanation is that centrifugation can also concentrate the protamine sulfate, despite its small size (∼5.1 kDa). Alternatively, it is possible that the stress from centrifugation causes the cells to be more susceptible to deleterious effects of protamine sulfate.
Ultimately spinoculation of hMSCs presents a number of different challenges. A larger volume is needed to provide sufficient medium coverage, which decreases the viral concentration and counteracts any increase in concentration from the spinoculation itself. In the end, this resulted in the observation that transduction efficiencies were not different from those of static cultures, although a lower concentration of protamine sulfate was needed and transgene expression increased by 36%. There are also issues with scalability. It is difficult to expand beyond the six-well plates used in these experiments. Our attempts at spinning T75 flasks have led to regions of cell death on the flask due to desiccation.
Although important, viral concentration is not the only viral factor that affects transduction efficiency. When the total amount of virus was held constant but spread out over multiple days, efficiencies improved despite the lower viral concentration at each day. This suggests that at any given time, there are a limited number of cells that are susceptible to transduction.
In addition to manipulating the virus, it is possible to affect the cell as well. One common treatment used to modify hMSCs is the addition of FGF-2 in culture. Human MSCs are slowly proliferating cells that can lose their differentiation potential with increasing passage [36], thus limiting the number of cells available from each donor. The addition of FGF-2 to cultures has the advantage of increasing the proliferation rate while delaying the loss of chondrogenic potential (senescence) and maintaining immune suppressive capabilities with increasing passage [36, 48]. In addition, FGF-2-treated hMSCs form larger pellets with more extracellular matrix in the chondrogenic pellet assay [4, 36]. Our results show for the first time that FGF-2-treated hMSCs also form more bone matrix in the in vivo ceramic cube assay, thus possibly extending FGF-2 treatment of hMSCs for osteogenic repair as well.
The increased proliferation of hMSCs provides another opportunity to increase transduction efficiency. Although it is true that lentiviruses can transduce nondividing cells [49], lentiviral transduction is superior with rapidly dividing cells [34, 35]. Our results with FGF-2-treated hMSCs are consistent with this. When FGF-2 was added during the time of transduction, the improvement in efficiency and increase in transgene expression was donor-dependent. It is possible that this relates to the length of time it takes for the cells of a particular donor to respond to the mitogen. In contrast, if the cells were cultured in FGF-2 for at least a week before transduction, we saw both increased efficiency and transgene expression.
Human MSCs are known to have donor-to-donor variability [37], and there have been reports of donor-to-donor variability with hMSC transduction [45]. Interestingly we found this variability to be dependent on passage number. hMSCs in primary culture and at passage 3 were fairly consistent, whereas high variability was found at passages 1 and 2. Furthermore, there can also be high intradonor variability. It is possible that this relates to the fact that proliferation rates vary between donors at passages 1 and 2, and that by passage 3 the proliferation rates stabilize.
Conclusion
Our studies indicate that hMSCs can be efficiently transduced using a protamine sulfate concentration of 100 μg/ml at low volumes over 3 days. Furthermore, inclusion of WPRE at the end of the transgene leads to higher viral titers and increased transgene expression. Transduction efficiency and transgene expression can be improved by transducing at passage 1 and by treatment with FGF-2 for 1 week prior to transduction. With the capacity to easily, reliably, and inexpensively transduce hMSCs, it will be possible to efficiently use genetic engineering to add or delete a function, improve or replace an existing one, and gain insight into the biology of these cells through the use of reporter genes. This will significantly impact not only scientific progress with these cells but also their clinical and therapeutic translational potential.
Acknowledgments
The authors thank Margie Harris for assistance in isolating the hMSCs, R. Michael Sramkoski and Jonida Toska for their assistance with the cell sorting, Yunhui Kim for her assistance with creating the viral construct, Amad Awadallah for his assistance with the histology, Vance Holt III and Dr. Danielle MacKay for their help with the immunohistochemical stain, and Christine Lee for her assistance with the Western blot. We also thank David Carrino for his help reviewing the manuscript and with the Western blot. This research was supported by the L. David and E. Virginia Baldwin Foundation. P.L. was supported in part by National Institutes of Health T32 GM007250.
Author Contributions
P.L.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; Y.L.: conception and design, collection and/or assembly of data, data analysis and interpretation; D.P.L.: collection and/or assembly of data, data analysis and interpretation; D.C. and M.S.: data analysis and interpretation; A.I.C.: conception and design, data analysis and interpretation, financial support.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 2.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Kadiyala S, Jaiswal N, Bruder SP. Culture-expanded, bone marrow-derived mesenchymal stem cells can regenerate a critical-sized segmental bone defect. Tissue Eng. 1997;3:173–185. [Google Scholar]
- 4.Solchaga LA, Penick KJ, Welter JF. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells: Tips and tricks. Methods Mol Biol. 2011;698:253–278. doi: 10.1007/978-1-60761-999-4_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai L, Lennon DP, Eaton V, et al. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57:1192–1203. doi: 10.1002/glia.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.González MA, Gonzalez-Rey E, Rico L, et al. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60:1006–1019. doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- 7.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 9.Bonfield TL, Koloze M, Lennon DP, et al. Human mesenchymal stem cells suppress chronic airway inflammation in the murine ovalbumin asthma model. Am J Physiol Lung Cell Mol Physiol. 2010;299:L760–L770. doi: 10.1152/ajplung.00182.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang M, Mal N, Kiedrowski M, et al. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21:3197–3207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 12.Mangi AA, Noiseux N, Kong D, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 13.Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 14.Studeny M, Marini FC, Champlin RE, et al. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 15.Kumar S, Ponnazhagan S. Bone homing of mesenchymal stem cells by ectopic alpha 4 integrin expression. FASEB J. 2007;21:3917–3927. doi: 10.1096/fj.07-8275com. [DOI] [PubMed] [Google Scholar]
- 16.Reiser J, Zhang XY, Hemenway CS, et al. Potential of mesenchymal stem cells in gene therapy approaches for inherited and acquired diseases. Expert Opin Biol Ther. 2005;5:1571–1584. doi: 10.1517/14712598.5.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004;363:1377–1385. doi: 10.1016/S0140-6736(04)16051-0. [DOI] [PubMed] [Google Scholar]
- 18.Caplan AI. Osteogenesis imperfecta, rehabilitation medicine, fundamental research and mesenchymal stem cells. Connect Tissue Res. 1995;31:S9–14. doi: 10.3109/03008209509116826. [DOI] [PubMed] [Google Scholar]
- 19.Allay JA, Dennis JE, Haynesworth SE, et al. LacZ and interleukin-3 expression in vivo after retroviral transduction of marrow-derived human osteogenic mesenchymal progenitors. Hum Gene Ther. 1997;8:1417–1427. doi: 10.1089/hum.1997.8.12-1417. [DOI] [PubMed] [Google Scholar]
- 20.Zielske SP, Livant DL, Lawrence TS. Radiation increases invasion of gene-modified mesenchymal stem cells into tumors. Int J Radiat Oncol Biol Phys. 2009;75:843–853. doi: 10.1016/j.ijrobp.2008.06.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kidd S, Spaeth E, Dembinski JL, et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 2009;27:2614–2623. doi: 10.1002/stem.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santos JL, Pandita D, Rodrigues J, et al. Nonviral gene delivery to mesenchymal stem cells: Methods, strategies and application in bone tissue engineering and regeneration. Curr Gene Ther. 2011;11:46–57. doi: 10.2174/156652311794520102. [DOI] [PubMed] [Google Scholar]
- 23.Park J, Ries J, Gelse K, et al. Bone regeneration in critical size defects by cell-mediated BMP-2 gene transfer: A comparison of adenoviral vectors and liposomes. Gene Ther. 2003;10:1089–1098. doi: 10.1038/sj.gt.3301960. [DOI] [PubMed] [Google Scholar]
- 24.Haleem-Smith H, Derfoul A, Okafor C, et al. Optimization of high-efficiency transfection of adult human mesenchymal stem cells in vitro. Mol Biotechnol. 2005;30:9–20. doi: 10.1385/MB:30:1:009. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Cao F, De A, et al. Trafficking mesenchymal stem cell engraftment and differentiation in tumor-bearing mice by bioluminescence imaging. Stem Cells. 2009;27:1548–1558. doi: 10.1002/stem.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peister A, Mellad JA, Larson BL, et al. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 27.Lin P, Correa D, Lin Y, et al. Polybrene inhibits human mesenchymal stem cell proliferation during lentiviral transduction. PLoS One. 2011;6:e23891. doi: 10.1371/journal.pone.0023891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haynesworth SE, Goshima J, Goldberg VM, et al. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13:81–88. doi: 10.1016/8756-3282(92)90364-3. [DOI] [PubMed] [Google Scholar]
- 29.Lennon DP, Caplan AI. Mesenchymal stem cells for tissue engineering. In: Vunjak-Novakovic G, Freshney RI, editors. Culture of Cells for Tissue Engineering. Hoboken, NJ: John Wiley & Sons, Inc.; 2006. pp. 23–59. [Google Scholar]
- 30.Lennon D, Haynesworth S, Bruder S, et al. Human and animal mesenchymal progenitor cells from bone marrow: Identification of serum for optimal selection and proliferation. In Vitro Cell Dev Biol Anim. 1996;32:602–611. [Google Scholar]
- 31.Ray P, De A, minutes JJ, et al. Imaging tri-fusion multimodality reporter gene expression in living subjects. Cancer Res. 2004;64:1323–1330. doi: 10.1158/0008-5472.can-03-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dennis JE, Konstantakos EK, Arm D, et al. In vivo osteogenesis assay: A rapid method for quantitative analysis. Biomaterials. 1998;19:1323–1328. doi: 10.1016/s0142-9612(97)00170-1. [DOI] [PubMed] [Google Scholar]
- 33.O'Doherty U, Swiggard WJ, Malim MH. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J Virol. 2000;74:10074–10080. doi: 10.1128/jvi.74.21.10074-10080.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis P, Hensel M, Emerman M. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO J. 1992;11:3053–3058. doi: 10.1002/j.1460-2075.1992.tb05376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Millington M, Arndt A, Boyd M, et al. Towards a clinically relevant lentiviral transduction protocol for primary human CD34 hematopoietic stem/progenitor cells. PLoS One. 2009;4:e6461. doi: 10.1371/journal.pone.0006461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solchaga LA, Penick K, Goldberg VM, et al. Fibroblast growth factor-2 enhances proliferation and delays loss of chondrogenic potential in human adult bone-marrow-derived mesenchymal stem cells. Tissue Eng Part A. 2010;16:1009–1019. doi: 10.1089/ten.tea.2009.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: Effects of dexamethasone and IL-1α. J Cell Physiol. 1996;166:585–592. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 38.Yang YW, Hsieh YC. Protamine sulfate enhances the transduction efficiency of recombinant adeno-associated virus-mediated gene delivery. Pharm Res. 2001;18:922–927. doi: 10.1023/a:1010923924844. [DOI] [PubMed] [Google Scholar]
- 39.Cornetta K, Anderson WF. Protamine sulfate as an effective alternative to polybrene in retroviral-mediated gene-transfer: Implications for human gene therapy. J Virol Methods. 1989;23:187–194. doi: 10.1016/0166-0934(89)90132-8. [DOI] [PubMed] [Google Scholar]
- 40.Arbab AS, Yocum GT, Rad AM, et al. Labeling of cells with ferumoxides-protamine sulfate complexes does not inhibit function or differentiation capacity of hematopoietic or mesenchymal stem cells. NMR Biomed. 2005;18:553–559. doi: 10.1002/nbm.991. [DOI] [PubMed] [Google Scholar]
- 41.Chuah MK, Van Damme A, Zwinnen H, et al. Long-term persistence of human bone marrow stromal cells transduced with factor VIII-retroviral vectors and transient production of therapeutic levels of human factor VIII in nonmyeloablated immunodeficient mice. Hum Gene Ther. 2000;11:729–738. doi: 10.1089/10430340050015626. [DOI] [PubMed] [Google Scholar]
- 42.Sasportas LS, Kasmieh R, Wakimoto H, et al. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci USA. 2009;106:4822–4827. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zufferey R, Donello JE, Trono D, et al. Woodchuck hepatitis virus posttranscriptional regulatory element enhances expression of transgenes delivered by retroviral vectors. J Virol. 1999;73:2886–2892. doi: 10.1128/jvi.73.4.2886-2892.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Higashimoto T, Urbinati F, Perumbeti A, et al. The woodchuck hepatitis virus post-transcriptional regulatory element reduces readthrough transcription from retroviral vectors. Gene Ther. 2007;14:1298–1304. doi: 10.1038/sj.gt.3302979. [DOI] [PubMed] [Google Scholar]
- 45.Kallifatidis G, Beckermann BM, Groth A, et al. Improved lentiviral transduction of human mesenchymal stem cells for therapeutic intervention in pancreatic cancer. Cancer Gene Ther. 2008;15:231–240. doi: 10.1038/sj.cgt.7701097. [DOI] [PubMed] [Google Scholar]
- 46.Van Damme A, Thorrez L, Ma L, et al. Efficient lentiviral transduction and improved engraftment of human bone marrow mesenchymal cells. Stem Cells. 2006;24:896–907. doi: 10.1634/stemcells.2003-0106. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki M, McHugh J, Tork C, et al. Direct muscle delivery of GDNF with human mesenchymal stem cells improves motor neuron survival and function in a rat model of familial ALS. Mol Ther. 2008;16:2002–2010. doi: 10.1038/mt.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Auletta JJ, Zale EA, Welter JF, et al. Fibroblast growth factor-2 enhances expansion of human bone marrow-derived mesenchymal stromal cells without diminishing their immunosuppressive potential. Stem Cells Int. 2011;2011:235176. doi: 10.4061/2011/235176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naldini L, Blömer U, Gallay P, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]