Abstract
Whereas cardiac-derived c-kit+ stem cells (CSCs) and bone marrow-derived mesenchymal stem cells (MSCs) are undergoing clinical trials testing safety and efficacy as a cell-based therapy, the relative therapeutic and biologic efficacy of these two cell types is unknown. We hypothesized that human CSCs have greater ability than MSCs to engraft, differentiate, and improve cardiac function. We compared intramyocardial injection of human fetal CSCs (36,000) with two doses of adult MSCs (36,000 and 1,000,000) or control (phosphate buffered saline) in nonobese diabetic/severe combined immune deficiency mice after coronary artery ligation. The myocardial infarction-induced enlargement in left ventricular chamber dimensions was ameliorated by CSCs (p < .05 for diastolic and systolic volumes), as was the decline in ejection fraction (EF; p < .05). Whereas 1 × 106 MSCs partially ameliorated ventricular remodeling and improved EF to a similar degree as CSCs, 36,000 MSCs did not influence chamber architecture or function. All cell therapies improved myocardial contractility, but CSCs preferentially reduced scar size and reduced vascular afterload. Engraftment and trilineage differentiation was substantially greater with CSCs than with MSCs. Adult-cultured c-kit+CSCs were less effective than fetal, but were still more potent than high-dose MSCs. These data demonstrate enhanced CSC engraftment, differentiation, and improved cardiac remodeling and function in ischemic heart failure. MSCs required a 30-fold greater dose than CSCs to improve cardiac function and anatomy. Together, these findings demonstrate a greater potency of CSCs than bone marrow MSCs in cardiac repair.
Keywords: Mesenchymal stem cells, Cardiac, c-kit, Myocardial infarction, Ventricular remodeling
Introduction
Currently, there is major enthusiasm for the notion that cells may form the basis of a new class of therapeutics for cardiac injury [1]. Major new insights gleaned from both experimental studies and clinical trials [2] support a growing body of evidence indicating that this approach is safe, practical, and provisionally effective in filling a major gap in the therapeutic armamentarium for chronic heart disease [3, 4]. Whereas clinical trials have generally used bone marrow-derived cells, resulting in some improvement in cardiac function [5, 6], research has focused on a variety of cell sources, ranging from embryonic stem cells [7] and induced pluripotent stem [8] (iPS) cells to adult bone marrow and cardiac stem cells. However, major gaps in knowledge still exist about the best source of cells for cardiac repair.
Substantial progress has been made with two potential adult stem cell sources—those from the heart itself [9] and those from bone marrow [3]. In the heart, a variety of resident stem cells have been described. These cells are characterized by either cell surface markers, such as c-kit+ (CD117+) [9], Sca-1 [10], and abcg2+ [11, 12], or transcription factors, such as WT1 [13] or Isl-1 [14]. Cardiac stem cells (CSCs) have been prepared by antigenic panning or culturing cardiac tissue to obtain “cardiospheres” [15]. Both bone marrow-derived [16] and cardiac-derived [17] stem cells express mesenchymal stromal/stem cell (MSC) markers, but Koninckx et al. showed that bone-marrow derived MSCs, but not c-kit+ CSCs, can differentiate into adipocytes and osteocytes, whereas heart-derived progenitor cells have greater cardiomyogenic potential than bone marrow-derived cells [18]. Current clinical trials are testing the safety of c-kit+ cells amplified after antigenic panning [19] or expanded in culture as a tissue mixture [20, 21]. Bone marrow-derived MSCs amplified in culture exhibit a constellation of effects contributing to tissue repair, including multilineage differentiation capacity, antifibrotic effects, paracrine signaling, and immunomodulatory effects [3, 4, 22–27].
We tested the prediction that CSCs would have greater efficacy than MSCs for cardiac repair following acute myocardial infarction (MI) by performing rigorous in vivo comparisons of the efficacy and biologic activity of these two stem cell sources. We studied primarily freshly isolated c-kit+ CSCs to avoid the loss of c-kit expression that normally occurs upon passage of these cells [28], so as to focus on the potential of these cells for engraftment and differentiation and to repair both cardiac structure and function. We also used adult-cultured cardiac CSCs for additional comparative studies.
Materials and Methods
An expanded Methods section is available in the supplemental online data.
Isolation of C-Kit+ CSCs From Human Heart Tissue
Using magnetic-activated cell sorting (MACS), CD117-positive cells were separated from human fetal heart tissues and were suspended in phosphate-buffered saline (PBS) prior to injection. Adult CSCs were cultured from explanted human left-ventricular assist device cores using the method of magnetic microbeads coupled with specific (anti-human CD117) antibodies [28].
Mesenchymal Stem Cells
Human bone marrow aspirates were obtained from AllCells LLC (Emeryville, CA, http://allcells.com). Low-density mononuclear cells were separated from human bone marrow aspirates and cultured as described (see supplemental online data). Adherent MSCs were frozen at passage 4 or 5 and thawed prior to injection.
Animal Studies
All experiments on live animals were performed in accordance with the protocol approved by the Institutional Animal Care and Use Committee at the University of Miami. Briefly, myocardial infarction was produced in nonobese diabetic/severe combined immune deficiency mice by permanent ligation of the left anterior descending coronary artery [29]. CSCs (36 × 103 cells, n = 17) or human adult bone marrow-derived mesenchymal stem cells (MSCs), either 36 × 103 (low-dose, n = 16) or 1 × 106 cells (high-dose, n = 17), were delivered through intramyocardial injection immediately after MI. Nineteen mice received PBS as control. In addition, a group of mice received adult human CSCs (36 × 103 cells, n = 10). Animals were followed for 8 weeks after injection.
Echocardiography
Cardiac function and anatomy were evaluated by serial echocardiography at baseline and at 48 hours and 1, 2, 4, and 8 weeks following injection. End diastolic volume (EDV), end systolic volume (ESV), and ejection fractions were calculated and compared.
Hemodynamic Study
Eight weeks after injections, pressure volume loops were recorded using a Millar conductance manometry catheter (Millar Instruments, Inc., Houston, TX, http://millar.com) at baseline and during vena cava occlusion.
Immunohistochemistry
Hearts were harvested after hemodynamic recording and were fixed in formalin. Scar size was defined by measuring the perimeter of the Trichrome-Masson (TM)-stained scar. Alu staining BioGenex (San Ramon, CA, http://www.biogenex.com) was used to detect injected human cells in murine tissue. Costaining for cardiac proteins was performed to assess vascular or myocyte differentiation.
Quantification of Cellular Engraftment
We used two midventricular sections of heart, 1.5 and 3 mm below the left atrial edge. After Alu staining, the slides were scanned with a Zeiss Mirax150 fluorescent scanner (Carl Zeiss, Jena, Germany, http://www.zeiss.com). Images were analyzed with a panoramic viewer, and positive cells were counted in Photoshop CS3 (Adobe systems Inc., San Jose, CA, http://www.adobe.com).
Results
Impact of Cell Therapy on Post-MI Remodeling
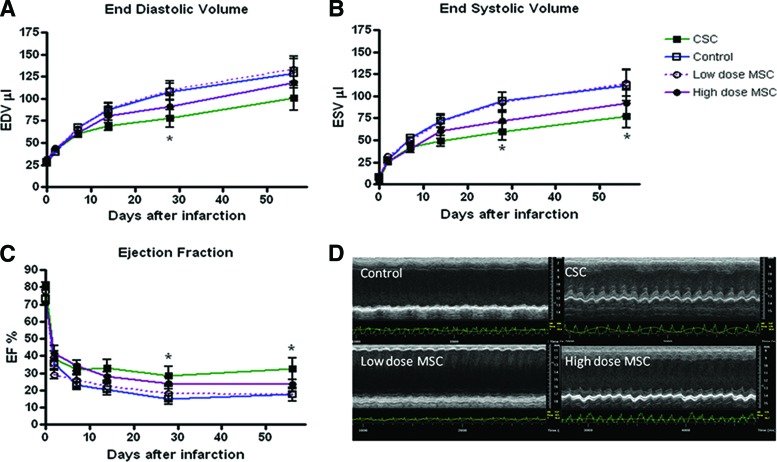
Myocardial infarction injury produced a time-dependent ventricular dilatation and dysfunction, the degree of which was similar in all groups 48 hours after injection (Fig. 1 and supplemental online Table 1). CSCs had the greatest impact on ameliorating left ventricular (LV) chamber enlargement. At 8 weeks following MI, LV EDV was 100.7 ± 14.2 μl in the CSC group versus 128.1 ± 15.7 μl in control (p = .002). EDV was also significantly better in the CSC group compared with low-dose MSC at 4 and 8 weeks (133.5 ± 14.5 μl) (p < .0001, Fig. 1A). These data show that there was no significant change in the CSC-treated group after week 1 in EDV and EF. In contrast, control and low-dose MSC hearts exhibited an increase in their diastolic dimensions and a progressive decrease in ejection fraction after MI (p < .05). Hearts treated with high-dose MSC showed a drop in these functions between day 2 and week 2 and then remained stable (p < .05). ESV was also significantly reduced at 4 and 8 weeks in the CSC-injected hearts compared with control, whereas EF was augmented (p < .05) (Fig. 1B). In contrast, an equivalent number of MSCs was ineffective at offsetting chamber remodeling, whereas an approximately 30-fold greater dose of MSCs (1 × 106 cells) ameliorated the change in chamber size and attenuated the decline in EF similar to CSC treatment (Fig. 1).
Figure 1.
C-kit+ CSCs improve cardiovascular function as assessed by echocardiography. (A): EDV. (B): ESV. (C): EF, p < .05. (D): Representative echocardiographic M-mode short axis images showing preserved anatomy and function of LV in CSC-treated mice and severe dyskinesia and ventricular dilatation in control and low-dose MSC heart. *, p < .05. Abbreviations: CSC, cardiac stem cell; EDV, end diastolic volume; EF, ejection fraction; ESV, end systolic volume; MSC, mesenchymal stem cell.
Impact of Adult CSCs
To address the relative efficacy of adult CSCs, reported to have less potency than fetal [23, 30], we performed additional experiments using 36,000 adult CSCs (n = 10). The reduction in EF was ameliorated to a lesser degree than the fetal CSCs but was not different from the level achieved with the high-dose MSC group. EFs at 8 weeks were 24.05 ± 6.9% versus 22.9 ± 2.8% in adult CSCs versus high-dose MSCs, respectively.
Impact of Cell Therapy on LV Hemodynamics
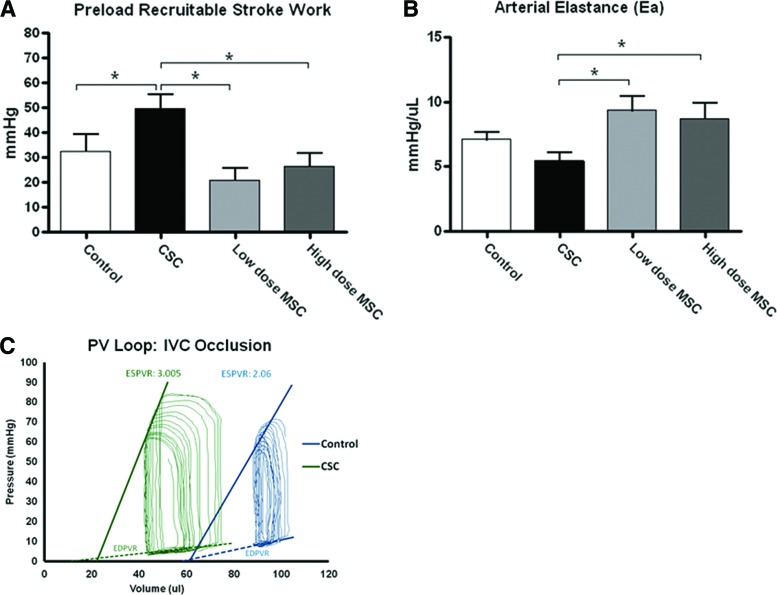
We used a micromanometer-conductance catheter to measure integrated cardiovascular performance 8 weeks after MI (Fig. 2). Whereas CSCs had the greatest impact on augmenting LV contractility measured as preload recruitable stroke work (PRSW) (49.5 ± 5.7 vs. 32.5 ± 6.6 mmHg), neither dose of MSCs increased this parameter (26.3 ± 5.3 mmHg: high-dose and 20.9 ± 4.9 mmHg: low-dose) (p < .05, Fig. 2A). When evaluated by the slope of the end systolic pressure volume relationship (ESPVR), contractility was increased in all cell-treated hearts: fetal CSCs (3.08 ± 0.57 mmHg/μl) and low-dose (3.03 ± 0.40 mmHg/μl) or high-dose (3.05 ± 0.49 mmHg/μl) MSCs versus control hearts (1.25 ± 0.36 mmHg/μl). Whereas CSC treatment did not influence preload (LV end-diastolic pressure was 19.6 ± 2.4 mmHg in CSCs and 22.5 ± 1.7 mmHg in controls), it did favorably affect arterial elastance (Ea), which was lower in the CSC-injected hearts (5.4 ± 0.6 mmHg/μl) than in either the low- or high-dose MSC-injected hearts (low: 9.3 ± 1.0 mmHg/μl, p < .05; high: 8.7 ± 1.2 mmHg/μl, p < .05), indicating that the CSC group exhibited less arterial load (Fig. 2B). Figure 2C shows a representative pressure volume loop and the corresponding ESPVR for one control and one CSC-treated animal.
Figure 2.
CSCs improve hemodynamic parameters more than MSCs. (A): Preload recruitable stroke work recorded during IVC occlusion. (B): Arterial elastance. (C): Representative PV loops showing preserved loop morphometry in the CSC-treated heart and increased ventricular volumes and decreased systolic function in control heart. *, p < .05. Abbreviations: CSC, cardiac stem cell; IVC, inferior vena cava; MSC, mesenchymal stem cell; PV, pressure volume.
Impact of Cell Therapy on MI Size
Scar size analysis using the ratio of scar to left ventricle on Trichrome Masson-stained sections showed that scar size was reduced in CSC-treated mice: 32.7 ± 5.9% versus control: 53.3 ± 6.9% (p < .05). Mice injected with either the low (47.7 ± 3.6%) or high (38.7 ± 4.5%) dose of MSC had scar sizes that were not significantly different from control (Fig. 3).
Figure 3.
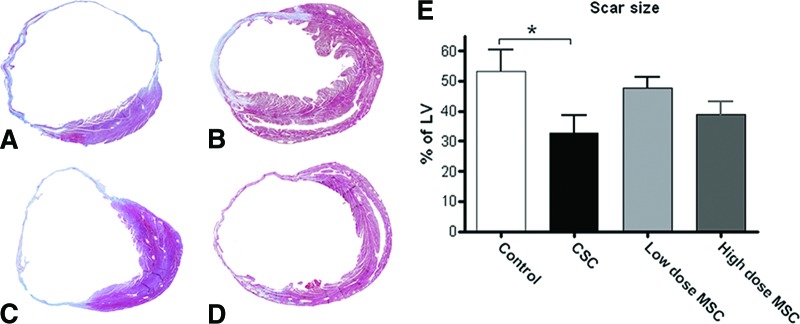
CSC therapy reduces scar size. Trichrome Masson (TM) staining of (A): control, (B): CSC, (C): low-dose MSC, and (D): high-dose MSC-treated hearts. (E): Quantitation of scar size following TM staining. *, p < .05. Abbreviations: CSC, cardiac stem cell; LV, left ventricle; MSC, mesenchymal stem cell.
Cell Engraftment and Differentiation
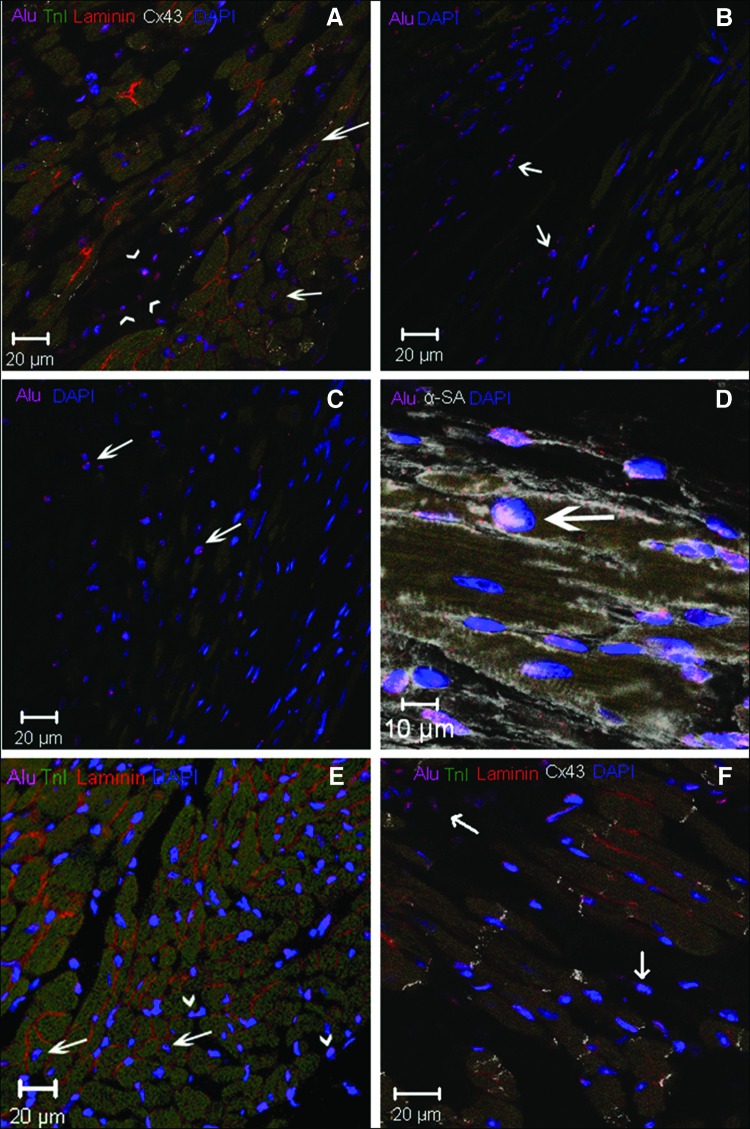
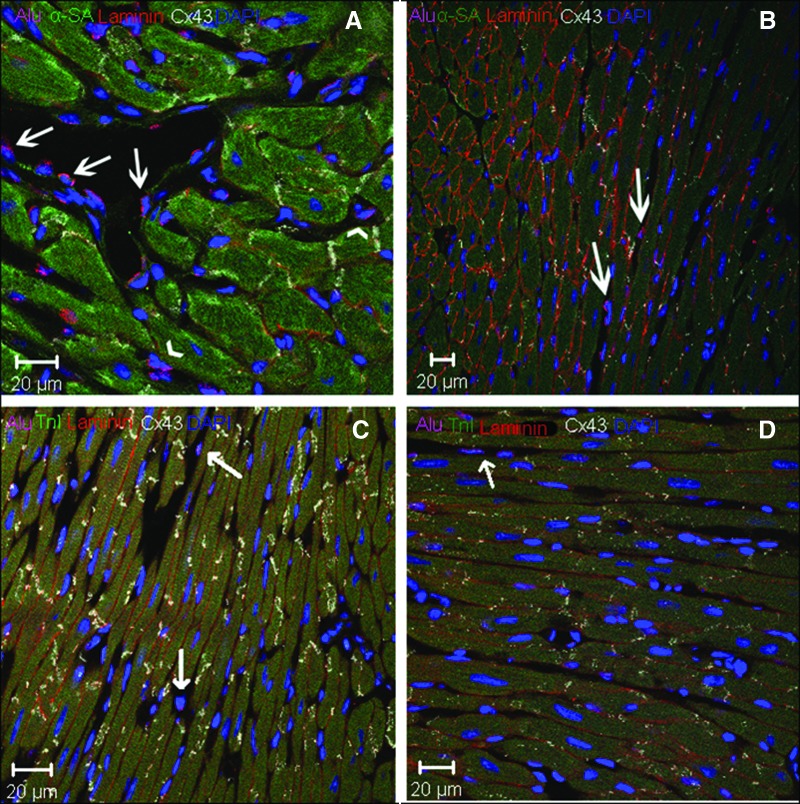
We used human-specific DNA probes (Alu) to detect injected CSCs or MSCs in mouse cardiac tissue (Fig. 4). In stained samples, Alu+ cells were identified in infarcted areas (Fig. 4C), in border zones between infarcted and healthy tissue (Fig. 4B, 4D, 4F), and in remote areas away from the infarction (Fig. 4A, 4E). Alu+ cells were also found in proximity to blood vessel walls (Fig. 4A).
Figure 4.
Cardiac stem cell-injected hearts (×40). (A): Remote area: Alu (pink), Cx43 (white), Laminin (green), TnI (long arrow). (B), (C): Alu+ cells in the (B) peri-infarct area and (C) infarcted area. (D): Border zone: Alu (pink), αSA, (white), coexpression (arrow). (E): Remote area: Alu and TnI costaining (long arrow), laminin (orange). Intercellular space: Alu+ (short arrows). (F): Border zone. Abbreviations: Cx43, connexin 43; DAPI, 4′,6-diamidino-2-phenylindole; TnI, troponin I.
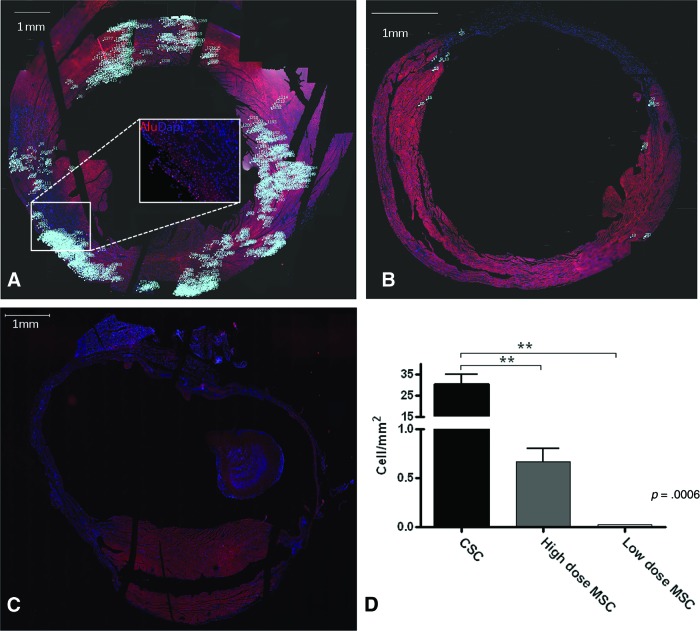
The midventricular sections of the heart 3 mm below the left atrium, a level corresponding to the location of cell injections, were used to quantify Alu+ cells. CSC-treated hearts (Fig. 5A) showed significantly higher incorporation of stem cells (30.3 ± 4.5 cell/mm2) compared with hearts that received the high-dose MSC treatment (0.7 ± 0.1 cell/mm2, p = .0006) (Fig. 5B). CSCs were found primarily in three clusters that corresponded to the three injection sites. Additional clusters suggested migration of the CSCs from the injection sites (Fig. 5A). Adult CSCs exhibited lower degrees of engraftment than the fetal CSCs (data not shown).
Figure 5.
Human CSCs engraft better and are more widely distributed than human MSCs. Reconstructed images after tiling fluorescent scans at the midventricular level of Alu-stained hearts 8 weeks after cell delivery. Numbers = count of Alu+ cells. (A): CSCs, box outlines region of inset. Inset: an example of Alu+ cells (pink-stained nuclei) that were counted. (B): High-dose and (C): low-dose MSCs. DAPI (blue-stained nuclei). (D): Quantitation of Alu+ cells (p = .0006). **, p < .01. Abbreviations: CSC, cardiac stem cell; DAPI, 4′,6-diamidino-2-phenylindole; MSC, mesenchymal stem cell.
Next, we assessed the degree of cell differentiation by co-staining with Alu sequence probes and antisera to cardiac proteins, α sarcomeric actin (αSA), and troponin I (TnI). Using this approach, we documented that Alu+ cells expressed both TnI and αSA (Figs. 4A, 4D), signifying cardiomyogenic differentiation. To confirm the localization of these cardiac markers within Alu+ cells, we examined laminin staining and used confocal z-stacking to identify the cell borders (Fig. 4A, 4E, 4F).
We also detected connexin 43 (Cx43) in cells that contained Alu sequence staining (Fig. 4A, 4F). The observation that these Alu+ cells not only express contractile proteins but also have the potential to form gap junctions suggests that the injected CSC cells underwent myocardial differentiation. In addition, groups of Alu+ cells that had not differentiated into cardiomyocytes were observed in the intercellular space (Fig. 4E, 4F), suggestive of reservoirs of immature cells.
In MSC-injected hearts, Alu+ cells were located primarily in intercellular spaces between native cardiomyocytes in the border (Fig. 6A) and remote zone (Fig. 6B). High-dose MSC hearts contained Alu+ cells in proximity to the vascular walls of the border zone (Fig. 6A) and the remote zone (Fig. 6B). There was no clear evidence of myocyte differentiation based upon an absence of costaining for Alu and either αSA (Fig. 6A) or TnI (Fig. 6B). Cell engraftment was much more apparent in the high-dose MSC group compared with the low-dose group, which had only a few cells within the intercellular spaces. None of these cells co-stained for either TnI or αSA, and there were no detectable cells in the vascular wall (Fig. 6C, 6D, 5B, 5C).
Figure 6.
Immunohistochemistry of mesenchymal stem cell (MSC)-injected hearts, (A), (B): High-dose MSC. A: Border/peri infarct zone: Alu+ cells (pink) in proximity to the vascular wall (white arrows) and between myocardial cells (arrow head). (B): Remote zone: α-SA (green), Laminin (orange). (C), (D): 36 × 103 MSCs: Few Alu- and no TnI-positive cells (arrows).
Discussion
The major new findings of this study are that human fetal heart-derived c-kit+ cardiac stem cells attenuate cardiac remodeling, decrease infarct size, improve left ventricular function, and differentiate into blood vessels and myocardium with greater potency than bone marrow-derived MSCs. MSCs also exert cardio-reparative effects, but a 30-fold greater cell number had less capacity for engraftment and differentiation than CSCs [31]. Together these findings offer key new insights into the cellular characteristics underlying successful cell-based cardiac repair.
Our study was conducted in the context of a growing body of evidence that stem cells may be used as a therapy to repair the detrimental effects that result from MI. Currently, there is enormous interest in cell-based cardiac repair, and a major effort is being expended to identify the optimal cell sources. In this regard, experimental studies and clinical trials have used the gamut of cells, ranging from autologous whole bone marrow [32] to pluripotent stem cells [33, 34]. Two very promising avenues of work are currently testing the hypothesis that bone marrow-derived MSCs or cardiac-derived stem cells have the capacity for engraftment within the heart and the ability to differentiate into the cellular elements that have been lost to injury. As yet, no stem cell therapy has succeeded in fully replacing scarred or injured myocardium with new contractile and perfused contractile myocardium. As we continue to strive toward that goal, one of the major gaps in our knowledge derives from an absence of a direct comparison of various cell preparations to stimulate cardiac repair and for their capacity to engraft and differentiate into cardiac myocytes and vascular elements.
Beltrami et al. first reported the existence of c-kit-positive cardiac stem cells in 2003 [35]. CD 117 (c-kit) is a tyrosine kinase receptor for stem cell factor. Although c-kit is normally present on hematopoietic stem cells and neonatal cardiac cells [34], it can also be found, albeit in lower numbers, in adult cardiac tissue, where these cells retain their properties of stemness [35, 36, 37]. Cardiac c-kit+ cells have been identified in the mammalian heart, including that of humans, and are self-renewing; are capable of differentiation into myocytes, vascular smooth muscle, and endothelium; and are clonogenic [36, 37].
Both c-kit+ CSC and bone marrow-derived MSCs have entered clinical trials, supported by preclinical studies showing their promise for cardiac regeneration therapy [19, 20, 22]. Although there are numerous independent studies for these cells, they have never been compared directly for efficacy and cardiac regeneration in humans. MSCs are amplified from bone marrow based upon simple adherence to culture dishes and the capacity to grow in culture [38]. These cells have the capacity to differentiate into mesodermal lineages but exert other effects that contribute to cardiac repair, including trilineage cell differentiation, the production of cytokines and growth factors that promote repair, antifibrotic effects, immunomodulation, and, perhaps most importantly, the induction of endogenous stem cell activity [23, 39, 40]. Several preclinical studies with MSCs have shown improved cardiac function after MI [23, 30, 39], with the totality of evidence supporting a favorable impact and ability to both prevent and reverse ventricular remodeling. MSCs have been shown to be safe and provisionally effective in offsetting cardiac remodeling in a phase I clinical trial [27]. Some studies (conducted mainly in rodents with chronic arterial occlusions) have shown either no functional improvement [29] or functional improvement with limited or no cellular differentiation [39], leading to the conclusion that paracrine and vasculogenic effect represent predominant mechanisms.
Based on previous experiments with c-kit+ CSCs [35, 36, 37, 41], we hypothesized that, in a dose-matched experiment, c-kit+ cells from heart would be more potent than MSCs in repairing cardiac damage after ischemic injury. Previous studies have suggested that the cardiomyogenic potential of animal- and human-derived c-kit+ cells is greater both in vitro [18, 36, 42–44] and in vivo [17, 18]. Most studies have focused on injecting cultured cells into animals and have described the potential of these cells to differentiate into various elements of cardiac tissue [23, 25, 29, 39]. However, passaging c-kit+ cells potentially alters c-kit on the cell surface [28]. To surmount this problem, our study has, for the first time, carried out a direct comparison of the cardioprotective effects of fresh c-kit+ CSCs and cultured MSCs in an in vivo, ischemic, murine model. Furthermore, we compared these CSCs against both an equivalent (low-dose) and an approximately 30-fold excess (high-dose) number of MSCs. To address the issue of adult CSCs, we performed experiments using cultured adult c-kit+ CSCs; these cells, although not as potent as the fetal CSCs, still retained an efficacy achieving a similar amelioration of EF decline as did the high-dose MSCs. Importantly, this improvement was achieved using 30-fold lower cell concentration, illustrating the greater potency CSCs compared with MSCs.
After a relatively long-term follow-up (8 weeks), we identified human CSCs and MSCs in the heart by their staining for Alu-sequences. Surviving CSCs and MSCs from the high-dose group were embedded in the myocardium and appeared to be associated with vascular structures. However, only the CSCs differentiated into cardiomyocytes (Figs. 4, 5). In the low-dose MSC group, few cells were found embedded in the myocardium after 8 weeks, and no cells were seen in vascular walls. Quantification of Alu+ cells showed that CSCs have high survival and engraftment capacity in the toxic ischemic environment. Furthermore, they have a greater ability than MSCs to differentiate into cardiac elements, despite the relatively low number of CSCs that were injected. In addition, our results suggest that the ability of MSCs to elicit repair may depend on the dose of cells injected and may help explain the minimal improvements and inconsistencies in clinical trials using MSCs [4]. We found that 1 × 106 MSCs was an effective dose when injected into a mouse heart that normally has ∼8 × 106 cells [45]. An equivalent dose for humans would be ∼325 × 106 MSCs, since the human heart has ∼2.6 × 109 cells [45], which is somewhat higher, but of the order of magnitude currently used in ongoing clinical trials [46].
Importantly, we have previously described myocyte differentiation of MSCs in large animals using a reperfusion model [30, 47]. These animals have different hemodynamic characteristics than do rodents, and these in vivo experiments suggest that mechanical loading and tissue perfusion are crucial for myocyte differentiation of MSCs to occur. Thus, the lack of myocyte differentiation by MSCs in our mouse model suggests important differences in lineage differentiation between large and small animal models. Despite this limitation, it is clear that CSCs are superior in this regard and point to their potential advantages over MSCs to promote repair following ischemic heart damage. Furthermore, they are effective at a surprisingly low dose/efficacy ratio. Another significant aspect of this study is that we have used human cells, the candidate cells for clinical application. We avoided using fluorescent labeling, considering that green fluorescent protein labeling impairs actin myosin interaction [48].
Cultured MSCs are the typical cell source in most animal studies and clinical trials and represent a model against which all other cell types can be compared. They represent a (relatively) pure population of cells and are readily available for clinical use when needed. However, a high number of passages or time in culture might affect their protective effect [49] and might explain, in part, the inferiority of MSCs in this study compared with fresh CSCs. It is also important to note that there are various benefits of using MSCs. MSCs clearly have efficacy [30, 47], although more cells are required than CSCs, and MSCs can also be used as an allogeneic graft. Perhaps most importantly, we have previously shown that MSCs stimulate endogenous c-kit+ CSC proliferation and differentiation [30], and this stimulation may represent a crucial mechanism underlying MSC-mediated repair. This mechanism raises the intriguing hypothesis that the combination of MSCs and c-kit+ CSCs may yield a highly potent therapeutic regimen [20].
Limitation
The potential limitation to this study is that we used fetal hearts in order to have a source of cells containing more tissue than an adult heart biopsy sample. Fetal hearts are not clinically applicable as a stem cell source, and they potentially differ from adult CSCs in their differentiation capacity [50]. However, we also used cultured adult cardiac stem cells in additional experiments and revealed similar efficacy to the high concentration of MSCs.
Conclusion
In summary, in a direct comparison, we have shown that human CSCs expressing c-kit have an enhanced cardiopoietic potential relative to human bone marrow-derived MSCs. Our findings help define the parameters around the usage of CSCs and MSCs for cardiac indications with regard to dosing and mechanism of action, and the present data support the ongoing rigorous testing of both CSCs and MSCs for their role in the armamentarium of cells that could be used in cell-based therapy for a variety of cardiac conditions, including ischemic heart disease and heart failure.
Acknowledgments
We thank Dr. Philip Ruiz and Dr. Tadafumi Asaoka (Department of Transplant Surgery, University of Miami) for fluorescent scanning service. We also thank Sophia Cedola for help in cell counting and Dr. Ellena Paulino for assistance in procedures. This work was supported by National Heart, Lung, and Blood Institute Grants U54-HL081028 (Specialized Center for Cell-Based Therapy), R01-HL084275, and P20-HL101443. J.M.H. is also supported by NIH Grants R01-AG025017, HL065455, and HL094849.
Author Contributions
B.N.O.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; G.L., C.J., A.V.T., S.L., J.D.S., K.H., and M.D.: collection and/or assembly of data; W.B.: data analysis and interpretation, manuscript writing; I.M.: conception and design, provision of study material; J.M.H.: conception and design, data analysis and interpretation, manuscript writing, financial support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28(2):208–216. doi: 10.1161/ATVBAHA.107.155317. [DOI] [PubMed] [Google Scholar]
- 2.Suncion VY, Schulman IH, Hare JM. Concise review: The role of clinical trials in deciphering mechanisms of action of cardiac cell-based therapy. Stem Cells Translational Medicine. 2012;1:29–35. doi: 10.5966/sctm.2011-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams AR, Trachtenberg B, Velazquez DL, et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: Functional recovery and reverse remodeling. Circ Res. 2011;108:792–796. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendrikx M, Hensen K, Clijsters C, et al. Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation: results from a randomized controlled clinical trial. Circulation. 2006;114(suppl 1):I101–I107. doi: 10.1161/CIRCULATIONAHA.105.000505. [DOI] [PubMed] [Google Scholar]
- 6.Schächinger V, Erbs S, Elsässer A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 7.Christoforou N, Oskouei BN, Esteso P, et al. Implantation of mouse embryonic stem cell-derived cardiac progenitor cells preserves function of infarcted murine hearts. PLoS One. 2010;5:e11536. doi: 10.1371/journal.pone.0011536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshida Y, Yamanaka S. iPS cells: A source of cardiac regeneration. J Mol Cell Cardiol. 2011;50:327–332. doi: 10.1016/j.yjmcc.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Leri A, Kajstura J, Anversa P. Role of cardiac stem cells in cardiac pathophysiology: A paradigm shift in human myocardial biology. Circ Res. 2011;109:941–961. doi: 10.1161/CIRCRESAHA.111.243154. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Forte G, Pietronave S, Nardone G, et al. Human cardiac progenitor cell grafts as unrestricted source of supernumerary cardiac cells in healthy murine hearts. Stem Cells. 2011;29:2051–2061. doi: 10.1002/stem.763. [DOI] [PubMed] [Google Scholar]
- 11.Alfakir M, Dawe N, Eyre R, et al. The temporal and spatial expression patterns of ABCG2 in the developing human heart. Int J Cardiol. 2010 doi: 10.1016/j.ijcard.2010.10.025. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higashikuni Y, Sainz J, Nakamura K, et al. The ATP-binding cassette transporter BCRP1/ABCG2 plays a pivotal role in cardiac repair after myocardial infarction via modulation of microvascular endothelial cell survival and function. Arterioscler Thromb Vasc Biol. 2010;30:2128–2135. doi: 10.1161/ATVBAHA.110.211755. [DOI] [PubMed] [Google Scholar]
- 13.Smart N, Bollini S, Dubé KN, et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature. 2011;474:640–644. doi: 10.1038/nature10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bu L, Jiang X, Martin-Puig S, et al. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature. 2009;460:113–117. doi: 10.1038/nature08191. [DOI] [PubMed] [Google Scholar]
- 15.Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 16.Gambini E, Pompilio G, Biondi A, et al. C-kit+ cardiac progenitors exhibit mesenchymal markers and preferential cardiovascular commitment. Cardiovasc Res. 2011;89:362–373. doi: 10.1093/cvr/cvq292. [DOI] [PubMed] [Google Scholar]
- 17.Tateishi K, Ashihara E, Honsho S, et al. Human cardiac stem cells exhibit mesenchymal features and are maintained through Akt/GSK-3beta signaling. Biochem Biophys Res Commun. 2007;352:635–641. doi: 10.1016/j.bbrc.2006.11.096. [DOI] [PubMed] [Google Scholar]
- 18.Koninckx R, Daniels A, Windmolders S, et al. Mesenchymal stem cells or cardiac progenitors for cardiac repair? A comparative study. Cell Mol Life Sci. 2011;68:2141–2156. doi: 10.1007/s00018-010-0560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolli R, Chugh AR, D'Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): Initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Williams AR, Hatzistergos KE, Carvalho D, et al. Synergistic effects of human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and restore cardiac function. Circulation. 2011;124(suppl):A13079. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng K, Malliaras K, Smith RR, et al. Human cardiosphere-derived cells from advanced heart failure patients exhibit augmented functional potency in a mouse model of myocardial infarction. Circulation. 2011;124(suppl):A17146. doi: 10.1016/j.jchf.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heldman AW, Zambrano JP, Hare JM. Cell therapy for heart disease: where are we in 2011? J Am Coll Cardiol. 2011;57:466–468. doi: 10.1016/j.jacc.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quevedo HC, Hatzistergos KE, Oskouei BN, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuleri KH, Amado LC, Boyle AJ, et al. Early improvement in cardiac tissue perfusion due to mesenchymal stem cells. Am J Physiol Heart Circ Physiol. 2008;294:H2002–H2011. doi: 10.1152/ajpheart.00762.2007. [DOI] [PubMed] [Google Scholar]
- 25.Amado LC, Schuleri KH, Saliaris AP, et al. Multimodality noninvasive imaging demonstrates in vivo cardiac regeneration after mesenchymal stem cell therapy. J Am Coll Cardiol. 2006;48:2116–2124. doi: 10.1016/j.jacc.2006.06.073. [DOI] [PubMed] [Google Scholar]
- 26.Davis DR, Zhang Y, Smith RR, et al. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS One. 2009;4:e7195. doi: 10.1371/journal.pone.0007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jankowski RJ, Haluszczak C, Trucco M, et al. Flow cytometric characterization of myogenic cell populations obtained via the preplate technique: Potential for rapid isolation of muscle-derived stem cells. Hum Gene Ther. 2001;12:619–628. doi: 10.1089/104303401300057306. [DOI] [PubMed] [Google Scholar]
- 29.van der Bogt KE, Schrepfer S, Yu J, et al. Comparison of transplantation of adipose tissue- and bone marrow-derived mesenchymal stem cells in the infarcted heart. Transplantation. 2009;87:642–652. doi: 10.1097/TP.0b013e31819609d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatzistergos KE, Quevedo H, Oskouei BN, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramos GA, Hare JM. Cardiac cell-based therapy: Cell types and mechanisms of actions. Cell Transplant. 2007;16:951–961. doi: 10.3727/096368907783338208. [DOI] [PubMed] [Google Scholar]
- 32.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow stem cells regenerate infarcted myocardium. Pediatr Transplant. 2003;7(suppl 3):86–88. doi: 10.1034/j.1399-3046.7.s3.13.x. [DOI] [PubMed] [Google Scholar]
- 33.Shiba Y, Hauch KD, Laflamme MA. Cardiac applications for human pluripotent stem cells. Curr Pharm Des. 2009;15:2791–2806. doi: 10.2174/138161209788923804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tallini YN, Greene KS, Craven M, et al. c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc Natl Acad Sci U S A. 2009;106:1808–1813. doi: 10.1073/pnas.0808920106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 36.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torella D, Ellison GM, Méndez-Ferrer S, et al. Resident human cardiac stem cells: Role in cardiac cellular homeostasis and potential for myocardial regeneration. Nat Clin Pract Cardiovasc Med. 2006;3(suppl 1):S8–S13. doi: 10.1038/ncpcardio0409. [DOI] [PubMed] [Google Scholar]
- 38.Boyle AJ, McNiece IK, Hare JM. Mesenchymal stem cell therapy for cardiac repair. Methods Mol Biol. 2010;660:65–84. doi: 10.1007/978-1-60761-705-1_5. [DOI] [PubMed] [Google Scholar]
- 39.Tang YL, Zhao Q, Qin X, et al. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg. 2005;80:229–236. doi: 10.1016/j.athoracsur.2005.02.072. [DOI] [PubMed] [Google Scholar]
- 40.Tang YL, Zhao Q, Zhang YC, et al. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept. 2004;117:3–10. doi: 10.1016/j.regpep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Zaruba MM, Soonpaa M, Reuter S, et al. Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts. Circulation. 2010;121:1992–2000. doi: 10.1161/CIRCULATIONAHA.109.909093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubo H, Jaleel N, Kumarapeli A, et al. Increased cardiac myocyte progenitors in failing human hearts. Circulation. 2008;118:649–657. doi: 10.1161/CIRCULATIONAHA.107.761031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linke A, Müller P, Nurzynska D, et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandstedt J, Jonsson M, Lindahl A, et al. C-kit+ CD45- cells found in the adult human heart represent a population of endothelial progenitor cells. Basic Res Cardiol. 2010;105:545–556. doi: 10.1007/s00395-010-0088-1. [DOI] [PubMed] [Google Scholar]
- 45.Adler CP, Neuburger M, Herget GW, et al. Regeneration processes in human myocardium after acute ischaemia—quantitative determination of DNA, cell number and collagen content. Virchows Arch. 1997;430:149–153. doi: 10.1007/BF01008036. [DOI] [PubMed] [Google Scholar]
- 46.Trachtenberg B, Velazquez DL, Williams AR, et al. Rationale and design of the Transendocardial Injection of Autologous Human Cells (bone marrow or mesenchymal) in Chronic Ischemic Left Ventricular Dysfunction and Heart Failure Secondary to Myocardial Infarction (TAC-HFT) trial: A randomized, double-blind, placebo-controlled study of safety and efficacy. Am Heart J. 2011;161:487–493. doi: 10.1016/j.ahj.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 47.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohnishi S, Ohgushi H, Kitamura S, et al. Mesenchymal stem cells for the treatment of heart failure. Int J Hematol. 2007;86:17–21. doi: 10.1532/IJH97.07041. [DOI] [PubMed] [Google Scholar]
- 49.Crisostomo PR, Wang M, Wairiuko GM, et al. High passage number of stem cells adversely affects stem cell activation and myocardial protection. Shock. 2006;26:575–580. doi: 10.1097/01.shk.0000235087.45798.93. [DOI] [PubMed] [Google Scholar]
- 50.van Vliet P, Smits AM, de Boer TP, et al. Foetal and adult cardiomyocyte progenitor cells have different developmental potential. J Cell Mol Med. 2010;14:861–870. doi: 10.1111/j.1582-4934.2010.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]