Abstract
Wound healing requires a coordinated interplay among cells, growth factors, and extracellular matrix proteins. Central to this process is the endogenous mesenchymal stem cell (MSC), which coordinates the repair response by recruiting other host cells and secreting growth factors and matrix proteins. MSCs are self-renewing multipotent stem cells that can differentiate into various lineages of mesenchymal origin such as bone, cartilage, tendon, and fat. In addition to multilineage differentiation capacity, MSCs regulate immune response and inflammation and possess powerful tissue protective and reparative mechanisms, making these cells attractive for treatment of different diseases. The beneficial effect of exogenous MSCs on wound healing was observed in a variety of animal models and in reported clinical cases. Specifically, they have been successfully used to treat chronic wounds and stimulate stalled healing processes. Recent studies revealed that human placental membranes are a rich source of MSCs for tissue regeneration and repair. This review provides a concise summary of current knowledge of biological properties of MSCs and describes the use of MSCs for wound healing. In particular, the scope of this review focuses on the role MSCs have in each phase of the wound-healing process. In addition, characterization of MSCs containing skin substitutes is described, demonstrating the presence of key growth factors and cytokines uniquely suited to aid in wound repair.
Keywords: Mesenchymal stem cells, Cellular therapy, Placenta, Tissue regeneration
Introduction
Nonhealing chronic wounds are a large and growing problem with an incidence of 5–7 million cases per year in the United States [1], and ∼50% of those wounds do not respond to current treatments [2]. Accumulated data indicate that wound-care products should have a composition equivalent to that of the skin: a combination of particular growth factors and extra cellular matrix (ECM) proteins endogenous to the skin, together with viable epithelial cells, fibroblasts, and mesenchymal stem cells (MSCs). Recently, strategies consisting of bioengineered dressings and cell-based products have emerged for widespread clinical use; however, their performance is not optimal because chronic wounds persist as a serious unmet medical need. The presence of MSCs in normal skin [3] and their critical role in wound healing [4] suggest that the application of exogenous MSCs is a promising solution to treat nonhealing wounds.
MSCs have been well characterized to be multipotent cells that can differentiate into multiple tissue-forming cell lineages, such as osteoblasts, adipocytes, chondrocytes, tenocytes, and myocytes [5, 6]. In addition, they can be readily expanded ex vivo for several passages without losing their self-renewal capacity [7, 8]. In addition to the multilineage differentiation capacity that is useful for regeneration, MSCs regulate immune and inflammatory responses. These functions provide therapeutic potential for treating conditions characterized by the presence of an inflammatory component. MSCs can also have a reparative effect through paracrine signaling by releasing biologically active molecules that affect cell migration, proliferation, and survival of the surrounding cells.
The involvement of MSCs in the wound-healing process is critical, in particular for difficult nonhealing wounds resulting from trauma, diabetes, vascular insufficiency, and numerous other conditions. MSCs have a role in the inflammatory, proliferative, and remodeling phases of wound healing, and their presence supports healthy physiologic functioning towards successful healing. As such, therapeutic application of MSCs has been shown to enhance and improve wound healing in clinical settings.
Although bone marrow is one of the most frequently used and most readily available sources of MSCs, they are present throughout the body and have been isolated from adipose tissue, periosteum, tendon, muscle, synovial membrane, skin, and others [9]. In spite of the differences in gene and cytokine expression that can be observed in MSCs derived from different origins [10, 11], a set of core gene expressions are preserved [12] and MSCs from different tissues share properties, allowing identification of these cells as MSCs [13]. Moreover, at the present time there are no data points showing an advantage of a particular tissue origin of MSCs for wound healing or for other clinical applications. One source that has recently become a target for research and use is the human placenta. Comparison between bone marrow-derived MSCs and placental-derived MSCs showed minimal differences of cell phenotype, differentiation, and immunomodulative properties [14–17]. Like MSCs from other sources, placental MSCs are immune-privileged, allowing for allogeneic use [18, 19].
In this review, the role of MSCs in wound healing is examined, specifically for complex nonhealing wounds. In particular, the important role MSCs have in each phase of the wound-healing process is described. The use of allogeneic MSCs in placental-derived tissue for the treatment of wounds is also described.
Three Phases of Normal Wound Healing
Normal wound healing is a dynamic and complex process involving a series of coordinated events, including bleeding and coagulation, acute inflammation, cell migration, proliferation, differentiation, angiogenesis, re-epithelialization, and synthesis and remodeling of ECM. These complex events occur in three overlapping phases: (a) inflammatory, (b) proliferative, and (c) remodeling.
Inflammatory
Immediately after injury, coagulation and hemostasis serve to minimize blood loss from the wound site. The coagulation cascade is activated through extrinsic and intrinsic pathways, leading to platelet aggregation and clot formation [20]. While hemostasis is achieved, the inflammation phase begins. Neutrophils are the predominant cell type present 24–36 hours after injury. Guided by chemokines and other chemotactic agents (transforming growth factor-β [TGF-β], formylmethionyl peptides produced by bacteria, and others), neutrophils move from the circulating blood into the wound environment through the processes of margination and diapedesis [21]. The neutrophils remove foreign material, bacteria, dead cells, and damaged ECM by phagocytosis [22]. Mast cells are also active and release granules filled with enzymes, histamine, and other active amines. These mediators are responsible for the characteristic signs of inflammation around the wound site: rubor (redness), calor (heat), tumor (swelling), and dolor (pain) [23].
Monocytes, the precursors to macrophages, appear in the wound 48–72 hours after injury and continue the process of phagocytosis [24]. They are attracted to the wound site by a myriad of chemoattractive agents, such as clotting factors, platelet-derived growth factor (PDGF), TGF-β, and elastin and collagen breakdown products [25]. Macrophages also act as key regulatory cells and produce numerous potent tissue growth factors, including TGF-β, tumor necrosis factor-α (TNF-α), heparin binding epidermal growth factor, and fibroblast growth factor (FGF) [26]. These factors are integral in activating keratinocytes, fibroblasts, and endothelial cells [22].
Proliferative
The proliferative phase typically starts on the third day after the initial insult and lasts for about 2 weeks. It is characterized by fibroblast migration, deposition of newly synthesized extracellular matrix, and an abundant formation of granulation tissue [22]. The TGF-β released earlier by platelets and macrophages is a critical signal, as it increases the overall production of matrix components, including collagen, proteoglycans, and fibronectin [24]. At the same time TGF-β decreases the secretion of proteases responsible for the breakdown of the matrix and stimulates the production of tissue inhibitor of metalloproteinases (TIMP) [26]. Other cytokines considered to be important during this phase are interleukins, FGFs, and TNF-α. During the proliferation phase, the process of epithelialization is stimulated by the presence of epidermal growth factor (EGF) that is produced by platelets and keratinocytes and TGF-α that is produced by activated wound macrophages [27].
Local factors in the wound microenvironment (low pH, reduced oxygen tension, and increased lactate) initiate angiogenesis [28]. Angiogenesis is stimulated by vascular endothelial cell growth factor (VEGF), basic fibroblast growth factor (bFGF), and TGF-β produced by epidermal cells, fibroblasts, macrophages, and vascular endothelial cells [28].
In the proliferative phase, fibroblasts produce the new matrix needed to restore the structure of the injured tissue. Fibroblasts attach to the fibrin matrix and begin to produce collagen (predominantly type I) [29]. As the collagen matures, more and more intramolecular and intermolecular cross-links are created, serving to increase the strength of the weak tissue.
Remodeling
Remodeling is the final phase of wound healing. This phase may last 1–2 years or even longer [25]. The remodeling of an acute wound is designed to maintain a balance between degradation and synthesis, resulting in collagen bundles increasing in diameter and hyaluronic acid and fibronectin being degraded. The tensile strength of the wound increases progressively in parallel with collagen deposition [30]. Gradually, the activity of TIMPs increases, resulting in a drop in activity of metalloproteinase enzymes and an increase new matrix accumulation [31]. Although the initial deposition of collagen bundles is highly disorganized, the new collagen matrix becomes more oriented and cross-linked over time. Its subsequent organization is achieved during the remodeling phase at the same time as wound contraction that has already begun in the proliferative phase. The underlying connective tissue shrinks in size and brings the wound margins closer together. The process is regulated by a number of factors, with PDGF, TGF-βs, and FGFs being the most important [32]. Finally, as the wound heals, the density of fibroblasts and macrophages is reduced by apoptosis. With time, the growth of capillaries stops, blood flow to the area declines, and metabolic activity decreases, resulting in a fully healed wound [22].
Nonhealing, Chronic Wounds
Chronic wounds are those that fail to progress through the three normal stages of healing, resulting in a tissue injury that is not repaired within the typical time period. Chronic wounds result from various underlying disorders, including diabetes, pressure, vascular insufficiency, burns, and vasculitis [23]. The healing process can be disturbed by various factors, which prolong one or more phases of inflammation, proliferation, or remodeling. These factors include one or more of the following: infection, tissue hypoxia, necrosis, exudates, and excess levels of inflammatory cytokines [31]. A continuous state of inflammation in the wound creates a cascade that perpetuates a nonhealing state.
Excessive infiltration by neutrophils, which is responsible for chronic inflammation, is a biological marker for chronic wounds [26]. The neutrophils release significant amounts of enzymes such as collagenase that break down the ECM [33]. In addition, the neutrophils release the enzyme elastase, which is capable of destroying important healing factors such as PDGF and TGF-β [34]. These wounds typically will not respond even to advanced therapies unless there is a component that addresses chronic inflammation.
The Mechanisms of MSCs in Three Phases of Wound Healing
MSCs are involved in all three phases of wound healing to varying degrees (Fig. 1). They also influence the wound's ability to progress beyond the inflammatory phase and not regress to a chronic wound state. A significant component of the mechanism of action of MSCs is that they directly attenuate inflammatory response. Studies have shown that the addition of MSCs to an active immune response decreases secretion of the proinflammatory cytokines TNF-α and interferon-γ (IFN-γ) while simultaneously increasing the production of anti-inflammatory cytokines interleukin-10 (IL-10) and IL-4 [35]. It is these anti-inflammatory properties of MSCs that make them particularly beneficial to chronic wound treatment, as they can restart healing in stalled wounds by advancing the wound past a chronic inflammatory state into the next stage of healing. Accumulated data indicate the importance of MSC anti-inflammatory and immunomodulative activities in wound healing, detailed mechanisms of which are described in several reviews [36, 37].
Figure 1.
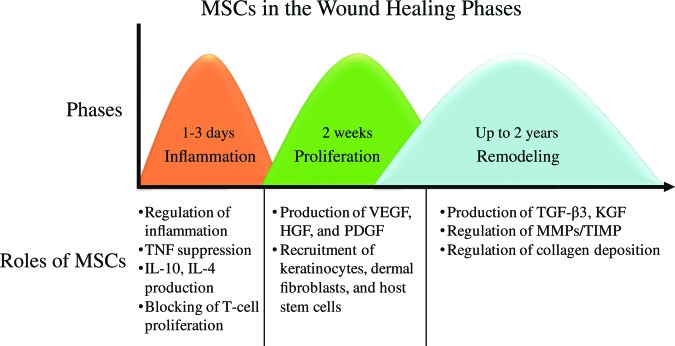
Mesenchymal stem cell roles in each phase of the wound-healing process. Abbreviations: HGF, hepatocyte growth factor; IL, interleukin; KGF, keratinocyte growth factor; MMP, matrix metalloproteinase; PDGF, platelet-derived growth factor; TGF, transforming growth factor; TIMP, tissue inhibitor of metalloproteinases; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
At the present time it is recognized that MSCs have antimicrobial activity, which is critical for wound clearance from infection. MSC antimicrobial activity is mediated by two mechanisms: direct, via secretion of antimicrobial factors such as LL-37 [38], and indirect, via secretion of immune-modulative factors, which will upregulate bacterial killing and phagocytosis by immune cells [39].
MSCs in vivo can migrate to sites of injury in response to chemotactic signals modulating inflammation, repairing damaged tissue, and facilitating tissue regeneration [36]. Differentiation and paracrine signaling have both been implicated as mechanisms by which MSCs improve tissue repair. MSC differentiation contributes by regenerating damaged tissue, whereas MSC paracrine signaling regulates the local cellular responses to injury. Current data suggest that the contribution of MSC differentiation is limited due to poor engraftment and survival of MSCs at the site of injury. MSC paracrine signaling is likely the primary mechanism for the beneficial effects of MSCs on wounds, that is, to reduce inflammation, promote angiogenesis, and induce cell migration and proliferation [40].
Analyses of MSC-conditioned medium indicate that MSCs secrete many known mediators of tissue repair including growth factors, cytokines, and chemokines, specifically VEGF, PDGF, bFGF, EGF, keratinocyte growth factor (KGF), and TGF-β [40, 41]. Studies indicate that many cell types, including epithelial cells, endothelial cells, keratinocytes, and fibroblasts, are responsive to MSC paracrine signaling, which regulates a number of different cellular responses including cell survival, proliferation, migration, and gene expression [42]. MSC-conditioned medium acts as a chemoattractant for macrophages, endothelial cells, epidermal keratinocytes, and dermal fibroblasts in vitro [41, 43]. The presence of either MSCs or MSC-conditioned medium has been shown to promote dermal fibroblasts to accelerate wound closure [44]. MSCs also secrete mitogens that stimulate proliferation of keratinocytes, dermal fibroblasts, and endothelial cells in vitro [44, 45]. Further investigation has shown that dermal fibroblasts secrete increased amounts of collagen type I and alter gene expression in response to either MSCs in coculture or MSC-conditioned medium [44]. Overall, these data suggest that MSCs release soluble factors that stimulate proliferation and migration of the predominant cell types in the wound. In addition, the paracrine signaling of MSCs provides antiscarring properties through the secretion of VEGF and hepatocyte growth factor (HGF) and maintaining the proper balance between TGF-β1 and TGF-β3 [46–48]. The molecular mechanisms of MSC involvement in wound healing are complex, and further details of these processes can be found in recent reviews [49, 50].
Evidence of MSC Importance in Healing
In vivo studies have also demonstrated the advantages of using exogenous MSCs for the treatment of wounds. Several studies have shown that the administration of MSCs to either acute or diabetic wounds in rodents improves wound closure by accelerating epithelialization, increasing granulation tissue formation, and increasing angiogenesis. Nakagawa et al. [51] suggested that MSCs, together with bFGF in a skin defect model, accelerate wound healing and showed that the human MSCs transdifferentiated into the epithelium in rats. Shumakov et al. [52] showed that the transplantation of MSCs on the surface of deep burn wounds in rats decreased inflammatory cell infiltration and accelerated the formation of new vessels and granulation tissue. The cells were also shown to produce bioactive substances that seemed to accelerate the regeneration process. Collectively, these data demonstrate that MSC treatment impacts all phases of wound repair, including inflammation, epithelialization, granulation tissue formation, and tissue remodeling.
Clinical results using MSCs to enhance the healing of wounds have also been promising, and selected key studies are outlined in Table 1. In a human study of chronic nonhealing wounds, Badiavas and Falanga [53] showed that direct application of bone marrow-derived cells can lead to wound closure and rebuilding of tissues. One study of chronic diabetic foot ulcers by Vojtassák et al. [54] combined autologous fibroblasts and MSCs on a biodegradable collagen membrane. In the same study, MSCs were also injected into the edges of the wound on days 7 and 17. The wound size decreased, and the vascularity of the dermis and dermal thickness of the wound increased. A unique delivery system using fibrin glue was investigated in both acute and chronic wounds by Falanga et al. [55]. Bone marrow-derived MSCs combined with a fibrin spray were applied topically up to 3 times. Surgical defects created from excision of nonmelanoma skin cancers healed within 8 weeks, suggesting that MSCs contributed to accelerated resurfacing. Chronic lower-extremity wounds present for longer than 1 year significantly decreased in size or healed completely by 20 weeks. The study also found a correlation between the surface density of MSCs and the reduction in ulcer size. One of the largest series by Yoshikawa et al. [56] consisted of 20 patients with various nonhealing wounds that were treated with autologous bone marrow-derived MSCs cultured on a collagen sponge. Ninety percent of the wounds healed completely when treated with the cell composite graft, and the addition of MSCs facilitated tissue regeneration.
Table 1.
Selected key clinical studies using mesenchymal stem cells for the treatment of wounds
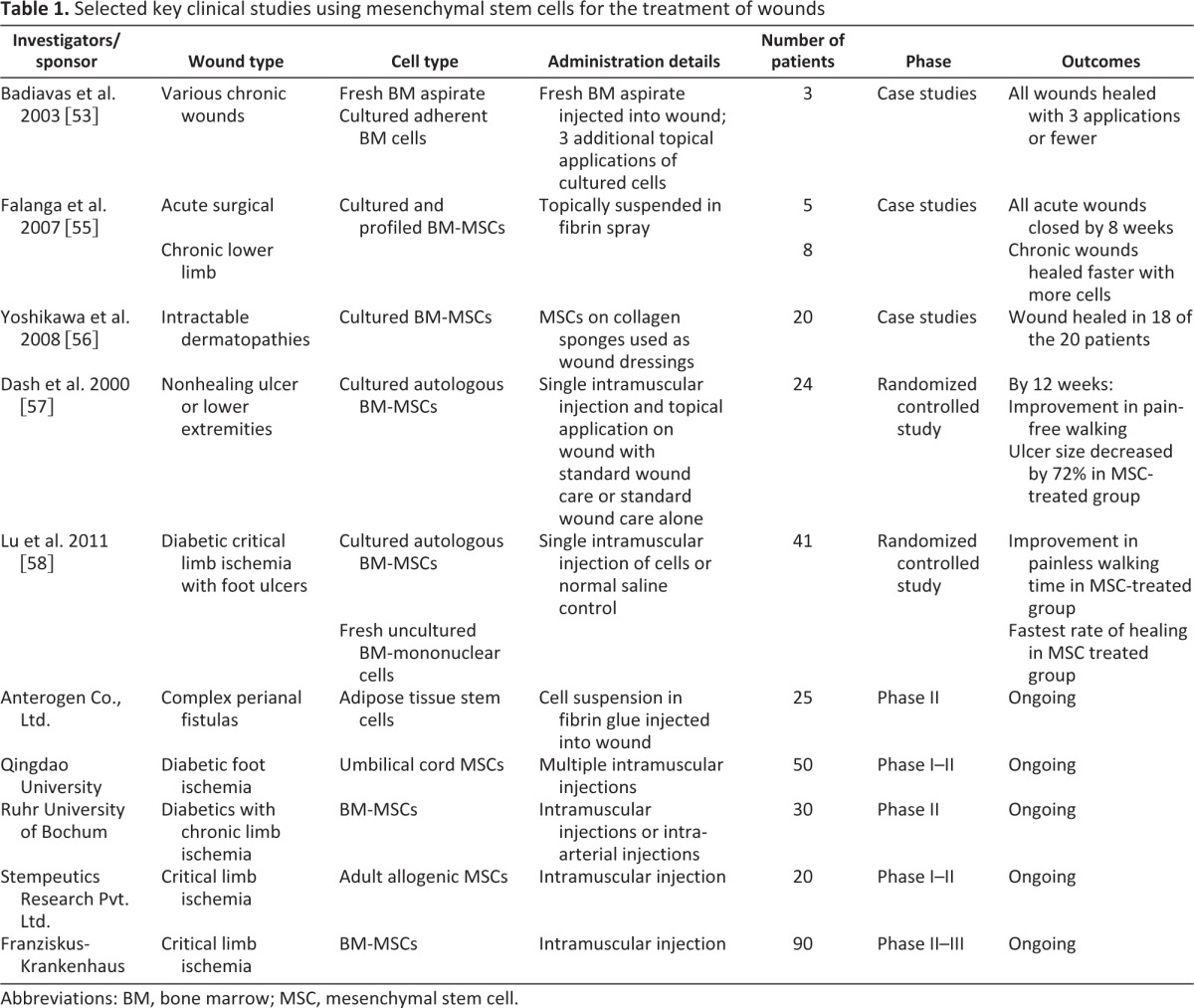
Abbreviations: BM, bone marrow; MSC, mesenchymal stem cell.
Systemic administration of MSCs has also been observed to promote healing in chronic wounds, particularly when there is an underlying condition such as diabetes and other systemic disorders. In a randomized controlled study of 24 patients with nonhealing ulcers of lower extremities by Dash et al. [57], the authors simultaneously administered cultured autologous bone marrow-derived MSCs intramuscularly into the affected limb and topically directly onto the ulcer. Within 12 weeks, significant improvement in pain and a greater decrease in wound size (72% versus 25%) were observed in the MSC-treated group compared to the control group. Clinical benefit of systemic administration of MSCs was also observed in a randomized controlled study conducted by Lu et al. [58]. Briefly, one limb of the patient was injected intramuscularly with cultured autologous bone marrow-derived MSCs or fresh nonculture bone marrow-derived mononuclear cells. The contralateral leg was injected with normal saline as a control for each patient. Compared to control groups, both MSCs and mononuclear cell injections resulted in marked improvement in pain-free walking at 24 weeks and significant increase in ulcer healing rate. Furthermore, the MSC-treated group demonstrated significantly greater increase in ulcer healing rate compared to the mononuclear-injected group.
Altogether, these clinical results suggest that MSCs provide clinical benefits when treating chronic wounds either topically or systemically. Although these studies have shown promising results, there are still numerous areas of future study including the effect of the source of the MSCs, the benefits of MSCs alone or within a matrix, the timing and frequency of MSC administration, and the number of cells administered.
MSCs from Placental Tissue in Wound Healing
The use of placental tissue for wound treatment started more than 100 years ago [59, 60]. The first reported case series used amnion membrane (AM) and chorionic membrane (CM) as skin substitutes for burned and ulcerated surfaces [61–63]. More recently, placental tissue has been studied as an alternative source of MSCs, providing multipotent differentiation and beneficial immunosuppressive capabilities similar to MSCs derived from other tissues. Miao et al. [15] compared placental-derived MSCs with bone marrow-derived MSCs in terms of morphology, growth, membrane markers, and differentiation potential. MSCs from placenta presented the same morphology and growth characteristics, as well as markers such as CD105, CD29, and CD44. There was no expression detected of the hematopoietic markers CD34, CD45, and HLA-DR. The authors also demonstrated differentiation potential of placental MSCs into endothelial and neuronal cells.
Other studies have confirmed the presence of cell markers commonly found in MSC populations in cells derived from placental membranes. Specifically, CD44, CD73, CD90, and CD105 membrane markers were identified [64]. Additional studies have demonstrated trilineage differentiation capabilities of placental-derived MSCs [65–67], as well as their lack of immunogenicity and positive immunomodulatory effects in vitro [18].
The beneficial activity of MSCs in wound healing is complemented by the effects of growth factors and ECM produced by the native placenta tissue cells. Analysis of cryopreserved AM growth factor and growth factor receptor content by reverse transcriptase-polymerase chain reaction and enzyme-linked immunosorbent assay have identified EGF, KGF, HGF, bFGF, and the family of TGFs [68]. As previously described, these factors are critical in the wound-healing process.
Research on the properties of AM and CM tissue and improved understanding of the MSC constituents have led to renewed interest in their clinical use. A recent report on the treatment of venous leg ulcers with allogeneic AM transplantation highlights the antiadhesive, wound protection, and proper re-epithelialization (antiscarring) effects as extremely beneficial in serious wounds [69]. Allogeneic transplants for wound healing are a significant improvement over autologous skin grafts that require inconvenient harvesting with frequent morbidity.
Human MSC-Containing Skin Substitutes
Human MSC-containing skin substitutes derived from placental tissues are an attractive source of MSCs to lead to improved wound-healing therapies, in particular for the treatment of chronic wounds and burns. Skin substitutes based on cryopreserved placental membranes must be processed to selectively remove antigenic components and preserve the tissue's native ECM architecture, growth factors, and cytokines as well as high-potential cells, including MSCs, which promote the complex sequence of events required for physiologic wound healing.
Human MSC-containing skin substitutes have been characterized to identify key components necessary for proper wound healing [70]. A unique feature of these skin substitutes is the presence of viable cells, including MSCs, fibroblasts, and epithelial cells. Fluorescence-activated cell sorting analysis of cells within the skin substitutes reveals the expression of MSC markers, CD105 and CD166, and the absence of CD45, confirming their stem cell identity. Because CD45-positive cells are potentially immunogenic, the absence of this antigen indicates a lack of cell-mediated immunogenicity. The exact number of MSCs present within the skin substitute is unpublished. However, for a reference point, the published cell concentration within placental membranes ranges from 1 to 4 × 104 cells/cm2 [16, 71]. The viability of cells is also confirmed, ensuring that functioning cells are delivered at the time of use. Post-thaw, the product's cell viability must be determined to be greater than 70% before it can be released for clinical use. Figure 2A illustrates in situ staining showing the high density of viable cells in the layers of the human MSC-containing skin substitutes. Although the presence of viable MSCs within the skin substitute is beneficial for wound repair in that the cells actively produce tissue reparative paracrine factors [72, 73], it is the combination of viable MSCs, native ECM, and growth factors within the skin substitute that is integral in promoting wound repair.
Figure 2.
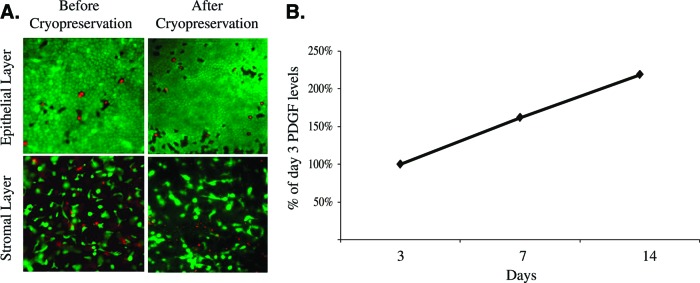
Characterization of human mesenchymal stem cell-containing skin substitute. (A): Cell viability: live cells were stained with fluorescent green cytoplasmic dye, and dead cells were stained with red nuclear fluorescent dye. Magnification, ×4 (Live/Dead Viability/Cytotoxicity Staining Kit, Molecular Probes). (B): Sustained growth factor release. PDGF was measured by enzyme-linked immunosorbent assay in conditioned supernatants collected through 2 weeks of culture. Amounts are presented normalized to the initial measurement. Abbreviation: PDGF, platelet-derived growth factor.
A protein profile of the skin substitute reveals the presence of an extensive array of beneficial proteins, which include physiological growth factors needed to carry out the phases of normal healing—inflammatory, proliferative, and remodeling (Table 2). Several anti-inflammatory and antimicrobial factors are present in the placental-derived MSC-containing skin substitute including defensins, N-Gal, IL-1RA, and several others [70]. These factors help to transit from the inflammatory phase to the proliferative phase of wound healing as well as to clear infected wounds. Other key proteins present within the skin substitute are the angiogenic proteins VEGF, bFGF, and PDGF; the epithelial cell stimulatory proteins KGF and EGF; and the antiscarring proteins TGF-β3, IFN-α2, and HGF. As described previously, physiological levels of growth factors and cytokines are critical to ensure healing of chronic wounds. Recombinant growth factors used to treat chronic wounds undergo rapid degradation and require repeated administration of nonphysiologically high concentrations of growth factors to support healing of chronic wounds, which may lead to adverse side effects [74, 75]. However, the unique population of viable cells allows for the sustained release of a cocktail of growth factors, persisting at physiological levels over extended periods of time (Fig. 2B) and eliminating the need for frequent reapplication. Functionally, the skin substitutes have been shown to promote cell migration and wound closure in in vitro wound-healing assays [70].
Table 2.
Functional classes of wound healing proteins in human mesenchymal stem cell-containing skin substitutes
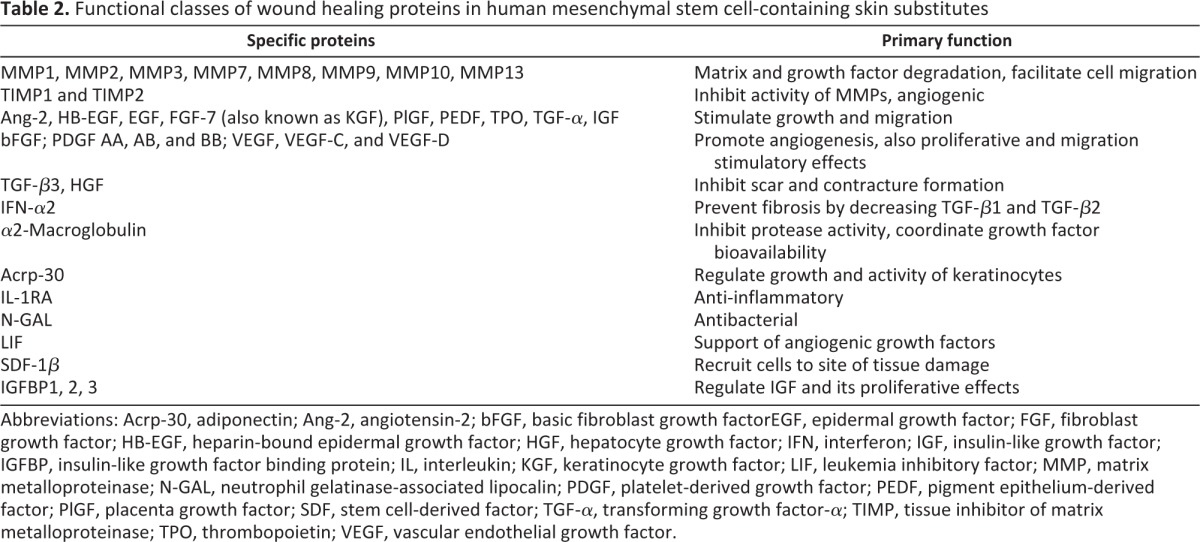
Abbreviations: Acrp-30, adiponectin; Ang-2, angiotensin-2; bFGF, basic fibroblast growth factorEGF, epidermal growth factor; FGF, fibroblast growth factor; HB-EGF, heparin-bound epidermal growth factor; HGF, hepatocyte growth factor; IFN, interferon; IGF, insulin-like growth factor; IGFBP, insulin-like growth factor binding protein; IL, interleukin; KGF, keratinocyte growth factor; LIF, leukemia inhibitory factor; MMP, matrix metalloproteinase; N-GAL, neutrophil gelatinase-associated lipocalin; PDGF, platelet-derived growth factor; PEDF, pigment epithelium-derived factor; PlGF, placenta growth factor; SDF, stem cell-derived factor; TGF-α, transforming growth factor-α; TIMP, tissue inhibitor of matrix metalloproteinase; TPO, thrombopoietin; VEGF, vascular endothelial growth factor.
Conclusion
Wound healing is a complex process that requires the coordinated interplay of ECM, growth factors, and cells. MSCs, in particular, play an important role in mediating each phase of the wound-healing process—inflammatory, proliferative, and remodeling. During the inflammatory phase, MSCs coordinate the effects of inflammatory cells and inhibit the deleterious effects of inflammatory cytokines such as TNF and IFN-γ. In addition, MSCs support wound clearance from infection via direct secretion of antimicrobial factors and by stimulating phagocytosis by immune cells. The ability of MSCs to promote the transition from the inflammatory to the proliferative phase is particularly critical for treating chronic wounds where high levels of inflammation prevent healing. MSCs also contribute to the proliferative phase by expressing growth factors such as VEGF, bFGF, and KGF to promote granulation and epithelialization. Lastly, MSCs regulate remodeling of the healed wound by promoting organized ECM deposition. As such, the benefits of MSCs in wound healing have been demonstrated in several preclinical and clinical studies. Thus, multiple mechanisms are involved in MSC-mediated wound healing, including antiinflammatory and antimicrobial, immunomodulative, and tissue reparative activities.
Although numerous products are currently available to treat wounds, very few therapies exist that incorporate the beneficial effects of MSCs, which are especially critical for difficult-to-heal wounds. Many efforts are under way to develop novel bioengineered wound-healing products, and considering the role of MSCs in the wound-healing process, it is important to consider their inclusion.
Acknowledgments
The authors thank Chris Alder, Erica Elchin, and Dayna Healy for their insightful review and comments.
Author Contributions
S.M., E.A.L., D.Y., A.D.-M.: conception and design, manuscript writing; M.A.L.: conception and design, final approval of manuscript.
References
- 1.Hanson SE, Bentz ML, Hematti P. Mesenchymal stem cell therapy for nonhealing cutaneous wounds. Plast Reconstr Surg. 2010;125:510–516. doi: 10.1097/PRS.0b013e3181c722bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mustoe TA, O'Shaughnessy K, Kloeters O. Chronic wound pathogenesis and current treatment strategies: A unifying hypothesis. Plast Reconstr Surg. 2006;117(7 Suppl):35S–41S. doi: 10.1097/01.prs.0000225431.63010.1b. [DOI] [PubMed] [Google Scholar]
- 3.Sellheyer K, Krahl D. Cutaneous mesenchymal stem cells. Current status of research and potential clinical applications. Hautarzt. 2010;61:429–434. doi: 10.1007/s00105-010-1919-6. [DOI] [PubMed] [Google Scholar]
- 4.Paquet-Fifield S, Schlueter H, Li A, et al. A role for pericytes as microenvironmental regulators of human skin tissue regeneration. J Clin Invest. 2009;119:2795–2806. doi: 10.1172/JCI38535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krampera M, Pizzolo G, Aprili G, et al. Mesenchymal stem cells for bone, cartilage, tendon and skeletal muscle repair. Bone. 2006;39:678–683. doi: 10.1016/j.bone.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Rahaman MN, Mao JJ. Stem cell-based composite tissue constructs for regenerative medicine. Biotechnol Bioeng. 2005;91:261–284. doi: 10.1002/bit.20292. [DOI] [PubMed] [Google Scholar]
- 7.Polak JM, Bishop AE. Stem cells and tissue engineering: Past, present, and future. Ann N Y Acad Sci. 2006;1068:352–366. doi: 10.1196/annals.1346.001. [DOI] [PubMed] [Google Scholar]
- 8.Raghunath J, Salacinski HJ, Sales KM, et al. Advancing cartilage tissue engineering: The application of stem cell technology. Curr Opin Biotechnol. 2005;16:503–509. doi: 10.1016/j.copbio.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 9.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi Y, Sekiya I, Yagishita K, et al. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 11.Hwang JH, Shim SS, Seok OS, et al. Comparison of cytokine expression in mesenchymal stem cells from human placenta, cord blood, and bone marrow. J Korean Med Sci. 2009;24:547–554. doi: 10.3346/jkms.2009.24.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai MS, Hwang SM, Chen KD, et al. Functional network analysis of the transcriptomes of mesenchymal stem cells derived from amniotic fluid, amniotic membrane, cord blood, and bone marrow. Stem Cells. 2007;25:2511–2523. doi: 10.1634/stemcells.2007-0023. [DOI] [PubMed] [Google Scholar]
- 13.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 14.Barlow S, Brooke G, Chatterjee K, et al. Comparison of human placenta- and bone marrow-derived multipotent mesenchymal stem cells. Stem Cells Dev. 2008;17:1095–1107. doi: 10.1089/scd.2007.0154. [DOI] [PubMed] [Google Scholar]
- 15.Miao Z, Jin J, Chen L, et al. Isolation of mesenchymal stem cells from human placenta: Comparison with human bone marrow mesenchymal stem cells. Cell Biol Int. 2006;30:681–687. doi: 10.1016/j.cellbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Parolini O, Alviano F, Bagnara GP, et al. Concise review: Isolation and characterization of cells from human term placenta: Outcome of the first International Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26:300–311. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- 17.Poloni A, Rosini V, Mondini E, et al. Characterization and expansion of mesenchymal progenitor cells from first-trimester chorionic villi of human placenta. Cytotherapy. 2008;10:690–697. doi: 10.1080/14653240802419310. [DOI] [PubMed] [Google Scholar]
- 18.Banas RA, Trumpower C, Bentlejewski C, et al. Immunogenicity and immunomodulatory effects of amnion-derived multipotent progenitor cells. Hum Immunol. 2008;69:321–328. doi: 10.1016/j.humimm.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Magatti M, De Munari S, Vertua E, et al. Human amnion mesenchyme harbors cells with allogeneic T-cell suppression and stimulation capabilities. Stem Cells. 2008;26:182–192. doi: 10.1634/stemcells.2007-0491. [DOI] [PubMed] [Google Scholar]
- 20.Pool JG. Normal Hemostatic Mechanisms: A review. Am J Med Technol. 1977;43:776–780. [PubMed] [Google Scholar]
- 21.Robson MC, Steed DL, Franz MG. Wound healing: Biologic features and approaches to maximize healing trajectories. Curr Probl Surg. 2001;38:72–140. doi: 10.1067/msg.2001.111167. [DOI] [PubMed] [Google Scholar]
- 22.Velnar T, Bailey T, Smrkoli V. The wound healing process: An overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528–1542. doi: 10.1177/147323000903700531. [DOI] [PubMed] [Google Scholar]
- 23.Degreef HJ. How to heal a wound fast. Dermatol Clin. 1998;16:365–375. doi: 10.1016/s0733-8635(05)70019-x. [DOI] [PubMed] [Google Scholar]
- 24.Hunt TK. The physiology of wound healing. Ann Emergency Med. 1988;17:1265–1273. doi: 10.1016/s0196-0644(88)80351-2. [DOI] [PubMed] [Google Scholar]
- 25.Ramasastry SS. Acute wounds. Clin Plast Surg. 2005;32:195–208. doi: 10.1016/j.cps.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Diegelmann RF, Evans MC. Wound healing: An overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 27.Schultz G, Rotatori DS, Clark W. EGF and TGF-alpha in wound healing and repair. J Cell Biochem. 1991;45:346–352. doi: 10.1002/jcb.240450407. [DOI] [PubMed] [Google Scholar]
- 28.Tonnesen MG, Feng XD, Clark RAF. Angiogenesis in wound healing. J Invest Dermatol Symp Proc. 2000;5:40–46. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 29.Clark RAF. Fibrin and wound healing. Ann N Y Acad Sci. 2001;936:355–367. doi: 10.1111/j.1749-6632.2001.tb03522.x. [DOI] [PubMed] [Google Scholar]
- 30.Baum CL, Arpey CJ. Normal cutaneous wound healing: Clinical correlation with cellular and molecular events. Dermatol Surg. 2005;31:674–686. doi: 10.1111/j.1524-4725.2005.31612. [DOI] [PubMed] [Google Scholar]
- 31.Mulder GD, Berg JSV. Cellular senescence and matrix metalloproteinase activity in chronic wounds. Relevance to debridement and new technologies. J Am Podiatr Med Assoc. 2002;92:34–37. doi: 10.7547/87507315-92-1-34. [DOI] [PubMed] [Google Scholar]
- 32.Clark RAF. Regulation of Fibroplasia in Cutaneous Wound Repair. Am J Med Sci. 1993;306:42–48. doi: 10.1097/00000441-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Nwomeh BC, Liang HX, Cohen IK, et al. MMP-8 is the predominant collagenase in healing wounds and nonhealing ulcers. J Surg Res. 1999;81:189–195. doi: 10.1006/jsre.1998.5495. [DOI] [PubMed] [Google Scholar]
- 34.Yager DR, Zhang LY, Liang HX, et al. Wound fluids from human pressure ulcers contain elevated matrix metalloproteinase levels and activity compared to surgical wound fluids. J Invest Dermatol. 1996;107:743–748. doi: 10.1111/1523-1747.ep12365637. [DOI] [PubMed] [Google Scholar]
- 35.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 36.Newman R, Yoo D, LeRoux MA, et al. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets. 2009;8:110–123. doi: 10.2174/187152809788462635. [DOI] [PubMed] [Google Scholar]
- 37.Singer NG, Caplan AI. Mesenchymal stem cells: Mechanisms of inflammation. Annu Rev Pathol. 2011;6:457–478. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- 38.Krasnodembskaya A, Song Y, Fang X, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28:2229–2238. doi: 10.1002/stem.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mei SH, Haitsma JJ, Dos Santos CC, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182:1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 40.Gnecchi M, Zhang Z, Ni A, et al. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L, Tredget EE, Wu PY, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hocking AM, Gibran NS. Mesenchymal stem cells: Paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res. 2010;316:2213–2219. doi: 10.1016/j.yexcr.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 44.Smith AN, Willis E, Chan VT, et al. Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp Cell Res. 2010;316:48–54. doi: 10.1016/j.yexcr.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee EY, Xia Y, Kim W-S, et al. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: Increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009;17:540–547. doi: 10.1111/j.1524-475X.2009.00499.x. [DOI] [PubMed] [Google Scholar]
- 46.Ono I, Yamashita T, Hida T, et al. Combined administration of basic fibroblast growth factor protein and the hepatocyte growth factor gene enhances the regeneration of dermis in acute incisional wounds. Wound Repair Regen. 2004;12:67–79. doi: 10.1111/j.1067-1927.2004.012113.x. [DOI] [PubMed] [Google Scholar]
- 47.Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108:985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- 48.Colwell AS, Beanes SR, Soo C, et al. Increased angiogenesis and expression of vascular endothelial growth factor during scarless repair. Plast Reconstr Surg. 2005;115:204–212. [PubMed] [Google Scholar]
- 49.Cha J, Falanga V. Stem cells in cutaneous wound healing. Clin Dermatol. 2007;25:73–78. doi: 10.1016/j.clindermatol.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Lau K, Paus R, Tiede S, et al. Exploring the role of stem cells in cutaneous wound healing. Exp Dermatol. 2009;18:921–933. doi: 10.1111/j.1600-0625.2009.00942.x. [DOI] [PubMed] [Google Scholar]
- 51.Nakagawa H, Akita S, Fukui M, et al. Human mesenchymal stem cells successfully improve skin-substitute wound healing. Br J Dermatol. 2005;153:29–36. doi: 10.1111/j.1365-2133.2005.06554.x. [DOI] [PubMed] [Google Scholar]
- 52.Shumakov VI, Onishchenko NA, Rasulov MF, et al. Mesenchymal bone marrow stem cells more effectively stimulate regeneration of deep burn wounds than embryonic fibroblasts. Bull Exp Biol Med. 2003;136:192–195. doi: 10.1023/a:1026387411627. [DOI] [PubMed] [Google Scholar]
- 53.Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol. 2003;139:510–516. doi: 10.1001/archderm.139.4.510. [DOI] [PubMed] [Google Scholar]
- 54.Vojtassák J, Danisovic Lu, Kubes M, et al. Autologous biograft and mesenchymal stem cells in treatment of the diabetic foot. Neuro Endocrinol Lett. 2006;27(suppl 2):134–137. [PubMed] [Google Scholar]
- 55.Falanga V, Iwamoto S, Chartier M, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007;13:1299–1312. doi: 10.1089/ten.2006.0278. [DOI] [PubMed] [Google Scholar]
- 56.Yoshikawa T, Mitsuno H, Nonaka I, et al. Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg. 2008;121:860–877. doi: 10.1097/01.prs.0000299922.96006.24. [DOI] [PubMed] [Google Scholar]
- 57.Dash NR, Dash SN, Routray P, et al. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res. 2009;12:359–366. doi: 10.1089/rej.2009.0872. [DOI] [PubMed] [Google Scholar]
- 58.Lu D, Chen B, Liang Z, et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: A double-blind, randomized, controlled trial. Diabetes Res Clin Pract. 2011;92:26–36. doi: 10.1016/j.diabres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 59.Kesting MR, Loeffelbein DJ, Steinstraesser L, et al. Cryopreserved human amniotic membrane for soft tissue repair in rats. Ann Plast Surg. 2008;60:684–691. doi: 10.1097/SAP.0b013e31814fb9d2. [DOI] [PubMed] [Google Scholar]
- 60.Kesting MR, Wolff KD, Hohlweg-Majert B, et al. The role of allogenic amniotic membrane in burn treatment. J Burn Care Res. 2008;29:907–916. doi: 10.1097/BCR.0b013e31818b9e40. [DOI] [PubMed] [Google Scholar]
- 61.Davis J. Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. Johns Hopkins Med J. 1910;15:307–396. [Google Scholar]
- 62.Sabella N. Use of fetal membranes in skin grafting. Med Rec, NY. 1913;83:478–480. [Google Scholar]
- 63.Stern M. The grafting of preserved amniotic membrane to burned and ulcerated skin surfaces substituting skin grafts. JAMA. 1913;60:973–974. [Google Scholar]
- 64.Toda A, Okabe M, Yoshida T, et al. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci. 2007;105:215–228. doi: 10.1254/jphs.cr0070034. [DOI] [PubMed] [Google Scholar]
- 65.Miki T, Strom SC. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. 2006;2:133–142. doi: 10.1007/s12015-006-0020-0. [DOI] [PubMed] [Google Scholar]
- 66.Miki T, Mitamura K, Ross MA, et al. Identification of stem cell marker-positive cells by immunofluorescence in term human amnion. J Reprod Immunol. 2007;75:91–96. doi: 10.1016/j.jri.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 67.Miki T, Marongiu F, Ellis E, et al. Isolation of amniotic epithelial stem cells. Curr Protoc Stem Cell Biol. 2007 doi: 10.1002/9780470151808.sc01e03s3. Chapter 1:Unit 1E.3. [DOI] [PubMed] [Google Scholar]
- 68.Koizumi NJ, Inatomi TJ, Sotozono CJ, et al. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20:173–177. [PubMed] [Google Scholar]
- 69.Mermet I, Pottier N, Sainthillier JM, et al. Use of amniotic membrane transplantation in the treatment of venous leg ulcers. Wound Repair Regen. 2007;15:459–464. doi: 10.1111/j.1524-475X.2007.00252.x. [DOI] [PubMed] [Google Scholar]
- 70.Yoo D, Jansen T, Kuang J, et al. Characterization of novel human mesenchymal stem cell-containing skin substitutes for the treatment of wounds. Ostomy Wound Manage. 2011;57:71. [Google Scholar]
- 71.Bieback K, Brinkmann I. Mesenchymal stromal cells from human perinatal tissues: From biology to cell therapy. World J Stem Cells. 2010;2:81–92. doi: 10.4252/wjsc.v2.i4.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yew TL, Hung YT, Li HY, et al. Enhancement of wound healing by human multipotent stromal cell conditioned medium: The paracrine factors and p38MAPK activation. Cell Transplant. 2011;20:693–706. doi: 10.3727/096368910X550198. [DOI] [PubMed] [Google Scholar]
- 73.Wolbank S, Hildner F, Redl H, et al. Impact of human amniotic membrane preparation on release of angiogenic factors. J Tissue Eng Regen Med. 2009;3:651–654. doi: 10.1002/term.207. [DOI] [PubMed] [Google Scholar]
- 74.FDA. Communication about an Ongoing Safety Review of Regranex. 2008. [Accessed Aug. 25, 2011]. Available at http://www.fda.gov/Drugs/DrugSafety/postmarketDrugSafetyInformationforpatientsandProviders/DrugSafetyInformationforHealthcareProfessionals/ucm072148.htm.
- 75.Andree C, Swain WF, Page CP, et al. In vivo transfer and expression of a human epidermal growth factor gene accelerates wound repair. Proc Natl Acad Sci U S A. 1994;91:12188–12192. doi: 10.1073/pnas.91.25.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]


