Abstract
The neural crest (NC) is a transient, multipotent, migratory cell population unique to vertebrates that gives rise to diverse cell lineages. Much of our knowledge of NC development comes from studies of organisms such as chicken and zebrafish because human NC is difficult to obtain because of its transient nature and the limited availability of human fetal cells. Here we examined the process of NC induction from human pluripotent stem cells, including human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs). We showed that NC cells could be efficiently induced from hESCs by a combination of growth factors in medium conditioned on stromal cells and that NC stem cells (NCSCs) could be purified by p75 using fluorescence-activated cell sorting (FACS). FACS-isolated NCSCs could be propagated in vitro in five passages and cryopreserved while maintaining NCSC identity characterized by the expression of a panel of NC markers such as p75, Sox9, Sox10, CD44, and HNK1. In vitro-expanded NCSCs were able to differentiate into neurons and glia (Schwann cells) of the peripheral nervous system, as well as mesenchymal derivatives. hESC-derived NCSCs appeared to behave similarly to endogenous embryonic NC cells when injected in chicken embryos. Using a defined medium, we were able to generate and propagate a nearly pure population of Schwann cells that uniformly expressed glial fibrillary acidic protein, S100, and p75. Schwann cells generated by our protocol myelinated rat dorsal root ganglia neurons in vitro. To our knowledge, this is the first report on myelination by hESC- or iPSC-derived Schwann cells.
Keywords: Neural crest, Human embryonic stem cells, Differentiation, Schwann cells, Peripheral neuron
Introduction
The neural crest (NC) is a transient population of multipotent progenitors arising at the lateral edge of the neural plate in vertebrate embryos that then migrate throughout the body to generate a diverse array of tissues, such as the peripheral nervous system (PNS), endocrine cells in the adrenal and thyroid glands, skin melanocytes, craniofacial cartilage, bone, and teeth [1, 2]. Human NC has not been studied because of the difficulty in obtaining 3–5-week human embryos and the transient nature of this stem cell population.
Nevertheless, much has been learned about NC in Xenopus, zebrafish, avian, and rodent models using a variety of techniques ranging from transplants in chick quail chimeras to large-scale mutagenic screens in zebrafish and conditional knockouts in mouse models [2–7]. The emerging model of development suggests that NC arises early in development from the margins of the neural plate and epithelium as the neural tube undergoes closure. Cells undergo an epithelial to mesenchymal transition and migrate along medial and lateral pathways around the developing somites. As they migrate, NC stem cells (NCSCs) undergo progressive restriction of cell fate to give rise to the diverse differentiated phenotypes characteristic of this stem cell population.
Analysis of the developmental potentials of NC in clonal cultures suggests that the migratory NC is a collection of heterogeneous progenitors, including various types of intermediate precursors and highly multipotent cells, some of which are endowed with self-renewal capacity, including a common progenitor for mesenchymal derivatives and neural/melanocytic cells [8]. In addition, initial fate acquisition of premigratory NC is not dependent on the migratory environment. The fate of crest cells has been restricted prior to emigration [9]. These results are consistent with a hierarchical model of lineage segregation analogous to that proposed for the hematopoietic system wherein environmental cytokines control the fate of progenitors and stem cells.
Several key steps and signaling pathways involved in NC development (e.g., Wnt and transforming growth factor β) have been identified and shown to be conserved across various species [8, 10–13]. Evidence that these pathways are likely conserved in humans has come from analyzing hereditary disorders, including neurocristopathies and peripheral neuropathies, that affect different lineages in the PNS. It appears that the Sox family of genes is important for NC induction and self-renewal [14], whereas c-kit/stem cell factor and endothelin are important for melanocyte differentiation [15–17], neurogenins are important for sensory neuron differentiation [18, 19], MASH and HAND genes are important for autonomic differentiation [20], neuregulins are important for Schwann cells [21], and RET/GDNF is important for enteric neurons [22].
To develop a model system for a finer analysis of these key signaling pathways, several investigators have examined the process of NC induction from human embryonic stem cells (hESCs) [23–27] because hESCs offer a potentially unlimited source of this transient stem cell population. Although the methodologies differ, the basic process has been to induce hESCs to a neural stem cell (NSC) stage in either adherent or suspension culture and then induce NC as a heterogeneous population using coculture or defined medium and subsequently allowing the cells to mature into a variety of phenotypes [25, 28]. In general, the efficiencies have been low, and it has been difficult to sort for enrichment of purified cells and to perform clonal analysis and examine differentiation in detail.
In this article, we describe a system to analyze NC and Schwann cell development. We show that NCSCs can be obtained from cells migrating out of neural rosettes. The migrating population can be purified using cell surface markers and be propagated and cryopreserved without losing their differentiation ability. The purified and in vitro-expanded NCSCs can then be directed to differentiate into neural and non-neural derivatives in vitro and in ovo. In addition, NCSCs and their differentiated derivatives can be transfected using a novel high-efficiency transduction protocol, making it feasible to perturb specific pathways during NC development. Importantly, cells differentiated from NCSCs appeared to be functional, at least for Schwann cells, because they can myelinate rat dorsal root ganglia (DRG) neurons. We have also extended this process to induced pluripotent stem cells (iPSCs) and shown that a similar protocol can be used to obtain models of human disease.
Materials and Methods
Cell Culture
hESC and iPSC lines were either maintained on inactivated mouse embryonic fibroblast (MEF) feeder cells in medium composed of knockout Dulbecco's modified Eagle's medium/Ham's F-12 supplemented with 20% knockout serum replacement, 2 mM nonessential amino acids, 4 mM l-glutamine, 0.1 mM β-mercaptoethanol, 50 μg/ml penicillin/streptomycin (all from Invitrogen, Carlsbad, CA, http://www.invitrogen.com), and 4 ng/ml basic fibroblast growth factor (Sigma-Aldrich, St. Louis, http://www.sigmaaldrich.com) or on Geltrex (Invitrogen)-coated dishes in defined medium or medium conditioned with MEF for 24 hours as previously described [29–31].
Induction and Purification of Neural Crest Stem Cells from hESCs/iPSCs
hESC or iPSC colonies were cultured in suspension as EBs in Petri dishes on a rocking platform [32] for 10 days in NC induction medium containing 50% NSC medium (Neurobasal medium with MEM nonessential amino acids, GlutaMAX [Invitrogen], B27, and 20 ng/ml fibroblast growth factor 2 [FGF2]) and 50% medium conditioned by the stromal cell line PA6 [29] with Rock inhibitor (10 μM) and ascorbic acid (200 μM). The EBs were then plated on cell culture plates coated with Geltrex (Invitrogen). Four days after differentiation, NCSCs were sorted with fluorescence-activated cell sorting (FACS) by p75. Specifically, the cells were dissociated by Accutase (Invitrogen), passed through a 70-μm nylon filter, and resuspended in NC induction medium. After 20 minutes of incubation with anti-CD271-PE (1 μl per million cells; Miltenyi Biotec, Bergisch Gladbach, Germany, http://www.miltenyibiotec.com), the cells were washed once in staining medium and resuspended in staining medium at a density of 3 million per milliliter. The samples were sorted on a BD FACSAria special order system by the FACSDiva 6.1.1 software. Sorted cells were centrifuged and plated onto culture dishes coated with Geltrex in NC induction medium. NCSCs were expanded in the same medium for at least five passages and used for differentiation or other analysis. The cells were passaged by Accutase, and the medium was changed every other day.
Immunocytochemistry
Immunocytochemistry and staining procedures were as described previously [33]. Briefly, the cells were fixed with 4% paraformaldehyde for 20 minutes, blocked in blocking buffer (10% goat serum, 1% bovine serum albumin [BSA], 0.1% Triton X-100) for 1 hour followed by incubation with the primary antibody at 4°C overnight in 8% goat serum, 1% BSA, 0.1% Triton X-100. Appropriately coupled secondary antibodies, Alexa 350, Alexa 488, Alexa 546, Alexa 594, and Alexa 633 (Molecular Probes, Eugene, OR, http://probes.invitrogen.com; Jackson Immunoresearch Laboratories, West Grove, PA, http://www.jacksonimmuno.com), were used for single or double labeling. All of the secondary antibodies were tested for cross-reactivity and nonspecific immunoreactivity. The following primary antibodies were used: nestin (611658; BD Biosciences, San Diego, http://www.bdbiosciences.com; 1:500), β-III-tubulin (clone SDL.3D10, T8660; Sigma; 1:1,000), glial fibrillary acidic protein (GFAP) (Z0334; DakoCytomation, Glostrup, Denmark, http://www.dakocytomation.com; 1:2,000), p75 (AB52987; Abcam, Cambridge, U.K., http://www.abcam.com; 1:50), tyrosine hydroxylase (TH) (P40101; Pel-Freeze Biologicals, Rogers, AR, http://www.pelfreez-bio.com/; 1:500), peripherin (MAB5380; Chemicon, Temecula, CA, http://www.chemicon.com; 1:250), S100b (S2532; Sigma; 1:500), Sox10 (Ab25978-100; Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com; 1:250), Sox9 (SC20095; Abcam; 1:250), CD44 (Ab24504; Abcam; 1:500), dopamine β-hydroxylase (DBH) (Ab96615; Abcam; 1:200), human nuclei (MAB1281; Millipore, Billerica, MA, http://www.millipore.com; 1:250), HNK1 (C0678; Sigma; 1:500), Pax3 (mouse IgG2a; Developmental Studies Hybridoma Bank, Iowa City, IA, http://www.uiowa.edu/∼dshbwww; 1:300), Islet1 (394D5; Developmental Studies Hybridoma Bank; 1:200), neurofilament (3A10; Developmental Studies Hybridoma Bank; 1:200), and smooth muscle actin (Ab5694; Abcam; 1:300).
The quantification of immunoreactive cells in culture was performed by analyzing fluorescent images using Photoshop (Adobe Systems Inc., San Jose, CA, http://www.adobe.com) on a minimum of 5,000 cells of at least 10 randomly chosen fields derived from three or more independent experiments. The number of Hoechst-labeled nuclei on each image was referred as total cell number (100%).
Gene Expression and Reverse Transcription-Polymerase Chain Reaction Analysis
Total RNA was prepared using the RNeasy Mini kit according to the manufacturer's instructions (Qiagen, Hilden, Germany, http://www.qiagen.com). RNAs isolated from NCSC and Schwann cell populations were hybridized to Illumina Human HT-12 BeadChip (Illumina Inc., San Diego, http://www.illumina.com; performed by the microarray core facility at the Burnham Institute for Medical Research). All the data processing and analysis was performed using the algorithms included with the Illumina BeadStudio software. The background method was used for normalization. For the processed data, the dendrogram was conducted by global array clustering of genes across all the tested samples by using the complete linkage method. A differentially expressed gene was defined if the gene showed twofold expression change between any two samples. Unsupervised two-way hierarchical clustering of differentially expressed genes using log2 signal values for each gene across all samples was analyzed with The Institute for Genomic Research (Rockville, MD, http://www.jcvi.org) Multiexperiments Viewer v4.5.1, which used complete linkage and Euclidean distance metric to generate the heat map. All cell line correlations were a measure of Pearson's coefficient, implemented in R system (R Foundation for Statistical Computing, Vienna, Austria, http://www.r-project.org).
Approximately 2 μg of RNAs isolated from ESCs, NSCs, NCSCs, NCSC-derived Schwann cells, and an immortalized Schwann cell line were reverse-transcribed into single-strand cDNA using oligo(dT) 18-mer primers and the SuperScript III First-Strand Synthesis System (Invitrogen) according to the manufacturer's recommendations. Primer sequences and annealing temperatures are provided (supplemental online Table 1).
In Ovo NCSC Injection
Fertile White Leghorn chicken (Gallus gallus) eggs were obtained from Merrill's Hatchery (Merrill Poultry Farm Inc., Paul, ID) and incubated to the desired stage in a humidified incubator at 38°C. The embryos were injected between the 7th and 11th somite stages beneath the cranial ectoderm adjacent to the neural tube in the migratory path of endogenous neural crest. Human NCSCs (hNCSCs) were cultured as described and harvested by Accutase treatment. Pelleted hNCSCs were injected into the chicken embryo through a glass needle by picospritzer. The embryos were incubated and allowed to develop for 24, 48, 72, and 96 hours. The embryos were collected and prepared for cryosectioning and immunohistochemical staining as previously described [34, 35].
Schwann Cell Differentiation
Schwann cell differentiation was initiated by culturing NCSCs in MesenPRO medium (Invitrogen) supplemented with 20 ng/ml heregulin-β1 for 40 days. The medium was changed every other day.
Peripheral Neuronal Differentiation
For neuronal differentiation, NCSCs were plated on polyornithine-laminin-coated culture dishes in Neurobasal medium supplemented with B27 (1×), brain-derived neurotrophic factor (BDNF) (10 ng/ml), ascorbic acid (200 μM), nerve growth factor (NGF) (10 ng/ml), and dibutyryl cyclic AMP (dcAMP) (0.1 mM). After 5 days of differentiation, the dcAMP was withdrawn, and the cells were continuously differentiated for 3–4 weeks with a medium change every other day.
Non-Neural Differentiation
Osteogenic Differentiation
NCSCs were cultured on Geltrex-coated plates in MesenPRO medium for 2 weeks followed by 4 weeks in osteogenesis differentiation medium (Invitrogen). Differentiation was assessed by alizarin red S staining.
Chondrogenic Differentiation
NCSCs were plated into Geltrex-coated tissue culture plates and cultured in MesenPRO medium for 2 weeks. The desired number of cells (250,000 per pellet) was then cultured as pellets in 15-ml conical tubes in complete chondrogenic medium (Invitrogen) with a medium change every 3–4 days. The cultures were fixed with 4% formaldehyde for 30 minutes after 4 weeks, and chondrogenic differentiation was assessed by immunohistochemistry (Alcian Blue).
Adipogenic Differentiation
NCSCs were plated on Geltrex-coated tissue culture plates in MesenPRO medium (Invitrogen) for 2 weeks. The cells were then cultured in complete adipogenesis differentiation medium (Invitrogen) for 4 weeks with a medium change every 3–4 days. The cultures were fixed with 4% formaldehyde for 30 minutes after 4 weeks, and adipogenic differentiation was assessed by Oil Red O staining.
Myelinating Cultures
Myelinating cultures were prepared as described previously [36]. Embryonic DRGs were prepared from embryonic day 15 rat pups. After trypsinization, rat DRG neurons were plated on collagen-coated plastic dishes (Sigma-Aldrich). To eliminate endogenous Schwann cells and fibroblasts, the cultures were treated twice for 24 hours with cytosine arabinoside (10 μM) and were subsequently maintained for 5–7 days in neurobasal medium (Invitrogen) supplemented with 1% fetal bovine serum (FBS) (HyClone, Logan, UT, http://www.hyclone.com), 2 M l-glutamine, 2% B27 serum-free supplement (Invitrogen), and 10 ng/ml NGF (Sigma-Aldrich). In the case of significant contamination with endogenous non-neuronal cells, cycles of cytosine arabinoside treatment were repeated until all glial cells were eliminated. For coculture experiments, human Schwann cells were seeded onto rat DRG neurons at a density of 10,000 cells per well on 24-well plates or 50,000 cells per well in 35-mm plastic dishes. To initiate myelination, cell cultures were switched to myelination medium containing Eagle's medium with Earle's salts and 15% FBS, 10 ng/ml NGF, and 50 μg/ml ascorbic acid (Sigma-Aldrich). Medium was replenished every 2–3 days, and after 3 weeks of culturing, cells were fixed and stained with a combination of anti-human nuclei monoclonal antibody (MAB 1281; Millipore; 1:400), anti-myelin basic protein (MBP) (05-675; Millipore; 1:500), and anti-neurofilament antibodies (AB1989 [Millipore] or AB1982 [Chemicon]; 1:500).
Baculovirus Preparation and Transduction
Baculoviral vector carrying a green fluorescent protein (GFP) driven by the cytomegalovirus (CMV) promoter was obtained from Life Technologies (Rockville, MD, http://www.lifetech.com), and viral particles were prepared accordingly to the manufacturer's instructions. For transduction, hESC-derived NCSCs and NCSC-derived Schwann cells were infected with baculoviral particles at ratio of 500–1,000 multiplicity of infection for 30 minutes at room temperature on a rocker. Transduced cells were plated on culture dishes without virus removal and cultured overnight at 37°C. Viral particles were removed by a medium change the next day.
Results
Isolation and Culture of NCSCs from hESCs
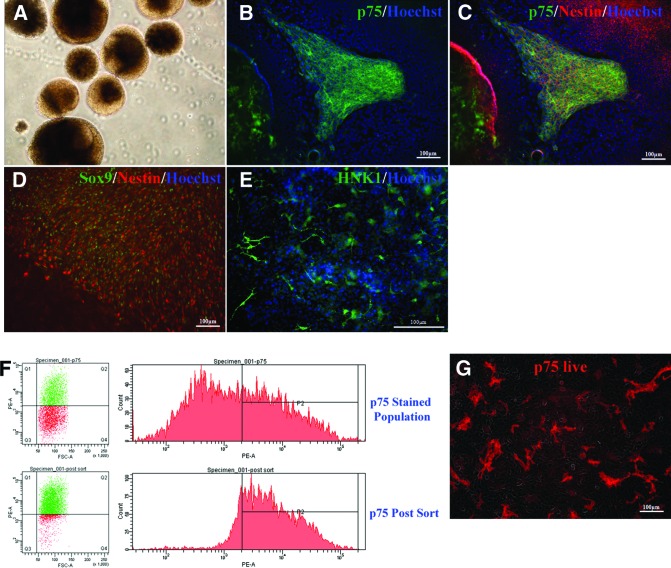
We have previously shown that hESCs cultured under conditions for NSC generation via EB formation form rosettes that give rise to NSCs and their derivatives that comprise the central nervous system (CNS) [29]. We observed a substantial number of cells migrating out of the rosettes and hypothesize that this population contains NCSCs based on the origin of NC from in vivo developmental studies and previous work with hESC model [25, 37–40]. To initiate NC differentiation, hESCs colonies were detached and cultured as EBs (Fig. 1A) in a novel medium (1:1 ratio of NSC medium and medium conditioned on stromal cells) that appeared to promote NC induction in the presence of rock inhibitor and ascorbic acid for 10 days followed by adherent culture in the same medium. Four days after EB attachment (14 days after initiation of hESC differentiation), the majority of the cells migrated out of the rosettes expressed p75, a marker of NCSC lineage, and the neural marker nestin (Fig. 1B, 1C). Furthermore, these p75- and nestin-positive cells were colabeled with Sox9, another marker of NCSCs (Fig. 1D). In addition, the migratory cells expressed HNK1, a gene that is expressed in NC (Fig. 1E). In general, the cells that migrated out of the rosettes expressed a high level of NC markers p75, Sox9, and HNK1, whereas the rosettes at the center of the EBs were negative for Sox9 and HNK1; both populations expressed neural marker nestin. This result indicated that NCSCs can be induced efficiently by extrinsic signals.
Figure 1.
Induction and purification of neural crest stem cells (NCSCs) from human embryonic stem cells (hESCs). When hESCs differentiated via EB formation, a subpopulation of cells appeared to have a neural crest phenotype. (A): hESC were detached and cultured as EBs for 10 days followed by adherent culture for 4 days. (B, C): The cells migrating out of the rosettes were immunostained for p75 (B) and nestin (C). (D): Sox9 staining was observed in those nestin-positive cells. (E): The migrating cells also expressed neural crest marker HNK1. (F): For fluorescence-activated cell sorting (FACS), dissociated cells from attached EBs were sorted for expression of p75. (G): The FACS-sorted cells were highly enriched in p75 population by live staining. The cell nuclei were visualized by 4′,6-diamidino-2-phenylindole (blue). Abbreviations: FSC-A, forward scatter area; PE-A, phycoerythrin area.
Since the majority of the cells migrated out can be live-stained with p75, we purified p75-positive cells by FACS. As seen in Figure 1F, approximately 46% of total cells were isolated by FACS with p75 after 14 days of differentiation (supplemental online Fig. 1). The FACS-isolated NCSCs were uniformly expressing p75 by live staining (Fig. 1G). Furthermore, these cells expressed Sox9 and Sox10, which are transcription factors expressed during NC specification, and CD44, which is a marker of premigratory and migratory cranial NC [41].
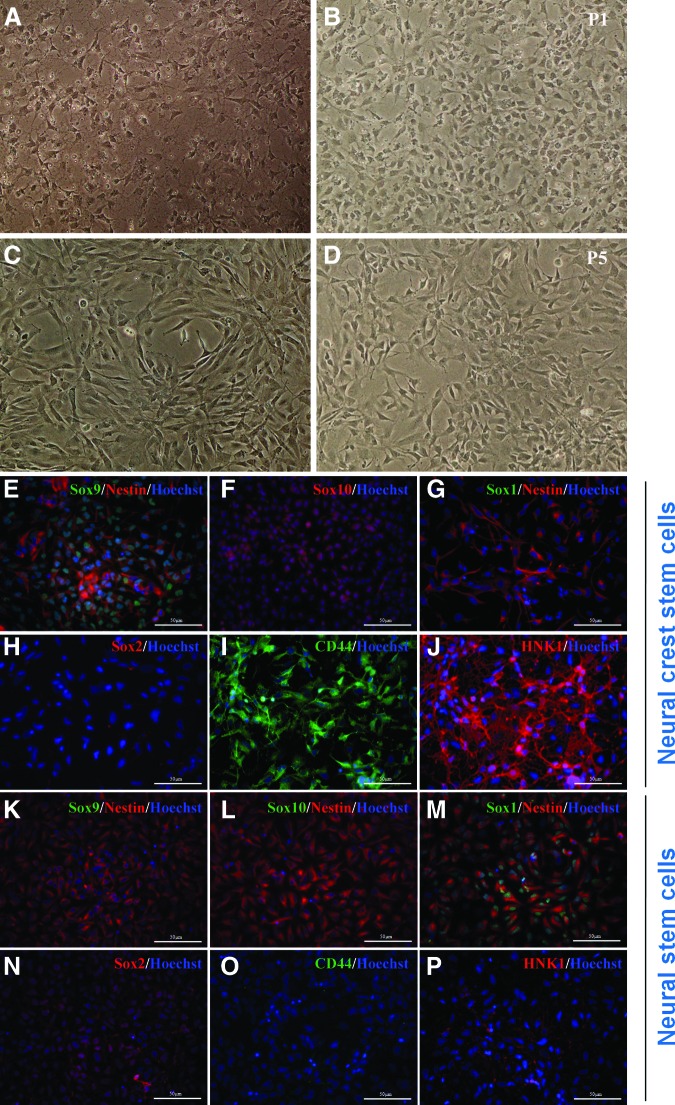
NCSCs Can Be Propagated In Vitro While Maintaining Their NCSC Identity
Although several research groups have generated NC lineage from hESCs [25, 27, 28, 38, 42], one major issue encountered with studying NC is the difficulty of expanding NCSCs in vitro without losing NCSC identity (the ability to differentiate into NC lineage) because of the transient nature of this stem cell population. We showed that stage-specific exposure of hESCs to growth factor withdrawal and to medium conditioned on stromal cells was sufficient to induce NCSCs and wished to test whether this medium and growth factor combination would allow us to propagate NCSCs for an extended period in vitro. As seen in Figure 2A–2D, morphologically, NCSCs at passage 5 (P5) (Fig. 2C, 2D) were similar to NCSCs initially isolated by FACS (Fig. 2A). Immunocytochemical analysis showed that P5 NCSCs were positive for Sox9 and Sox10 but negative for Sox1 and Sox2 (Fig. 2E–2H), which is in contrast to hESC/iPSC-derived NSCs of the CNS that are positive for Sox1 and Sox2 but negative for Sox9 and Sox10 (Fig. 2K–2N). Generation and characterization of NSCs from hESCs and iPSCs have previously been described in great detail [29, 43]. In brief, a homogeneous population of NSCs was isolated and expanded from the rosette structures induced by FGF2 during hESC/iPSC differentiation via EB formation. Sox2 is a widely accepted marker for early neuroepithelial cells such as NSCs but is downregulated in migratory NC progenitors [44–46]. Moreover, P5 NCSCs continued expressing NC markers CD44 and HNK1 (Fig. 2I, 2J), which are absent in NSCs (Fig. 2O, 2P).
Figure 2.
Fluorescence-activated cell sorting-isolated neural crest stem cells (NCSCs) can be propagated for more than five passages in NCSC medium. In vitro-propagated NCSCs expressed p75, Sox9, and Sox10 and retained the ability to differentiate into various NC lineages. (A–D): Morphology of NCSCs at passage 1 before freezing (A) and after thawing (B) and passage 5 before freezing (C) and after thawing (D). (E–P): NCSCs expressed the NCSC markers Sox9 (E), Sox10 (F), CD44 (I), and HNK1 (J) but negative for Sox1 (G) and Sox2 (H), in contrast to central nervous system NSCs derived from the same ESC line, which expressed Sox1 (M) and Sox2 (N) but were negative for Sox9 (K), Sox10 (L), CD44 (O), and HNK1 (P). The cell nuclei were visualized by 4′,6-diamidino-2-phenylindole (blue). Scale bars = 50 μm.
We then tested whether NCSCs can be cryopreserved and found that these cells could be thawed with greater than 90% viability. Cytogenetic analysis of P7 cells confirmed that in vitro-expanded NCSCs retained a normal karyotype (data not shown). We also validated that late passaged NCSCs retained the ability to differentiate into PNS neurons and glia as well as non-neuronal lineages (see below).
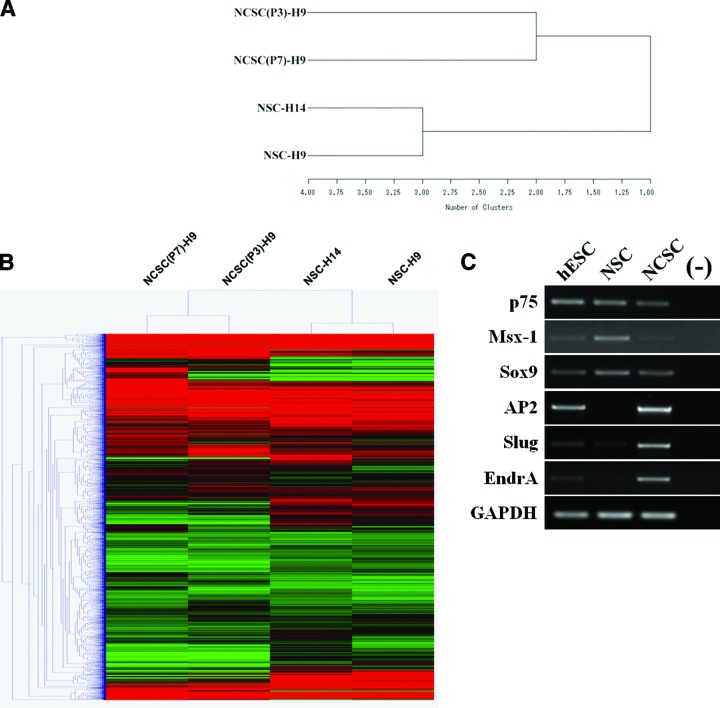
To further characterize in vitro-cultured NCSCs, we performed a large-scale microarray analysis of gene expression profiling of NCSC populations at P2 and P7 and compared the expression profiles of NSCs derived from the same hESC line H9 (Fig. 3A, 3B). Table 1 provides a list of genes that are highly expressed in the NCSC populations versus the NSC population. Transcripts that are upregulated in both P2 and P7 NCSCs included key markers of neural plate border induction (Msx1 and BMP2), neural plate border specifiers and NC specifier (Snai1, Sox9, ZIC1, and AP2), and epithelial to mesenchymal transition (Slug and cadherin). Expression of a subset of these genes was validated by reverse transcription (RT)-polymerase chain reaction (PCR) and/or real-time PCR analysis. Previously validated genes include p75, Sox9, AP2, and Slug (Fig. 3C and supplemental online Fig. 2A, 2B).
Figure 3.
Gene expression profiling of NCSCs by Illumina microarray. Gene expression profiling of four samples (NSCs derived from undifferentiated hESC lines H9 and H14 and NCSCs derived from H9 at passages 2 and 7) revealed similarities and differences between NSCs and NCSCs. (A): A dendrogram of unsupervised one-way hierarchical clustering analysis of global gene expression data in NSCs derived from H9 and H14 and H9-derived NCSCs at passages 2 and 7. (B): Unsupervised two-way hierarchical cluster analysis of differentially expressed genes illustrated in a heat map. NSCs derived from H9 and H14 hESC lines and H9-derived NCSCs at passages 2 and 7 are clustered into two groups. Expression values are presented as the log2 signal value of the given gene. (C): Reverse transcription-polymerase chain reaction analysis of marker genes for NCSCs derived from H9 compared with NSCs as well as naive hESCs. Expression of the GAPDH housekeeping gene was used as an internal control. Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; hESC, human embryonic stem cell; NCSC, neural crest stem cell..
Table 1.
A list of genes that are highly expressed in the NCSC population versus the NSC population
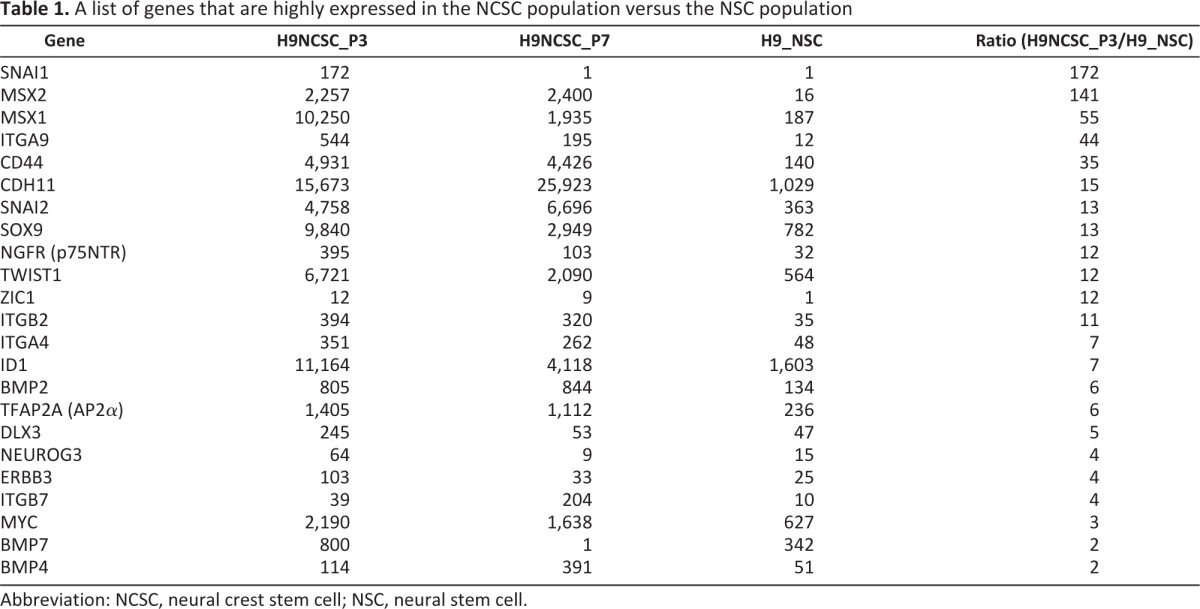
Abbreviation: NCSC, neural crest stem cell; NSC, neural stem cell.
In Ovo NCSC Injection
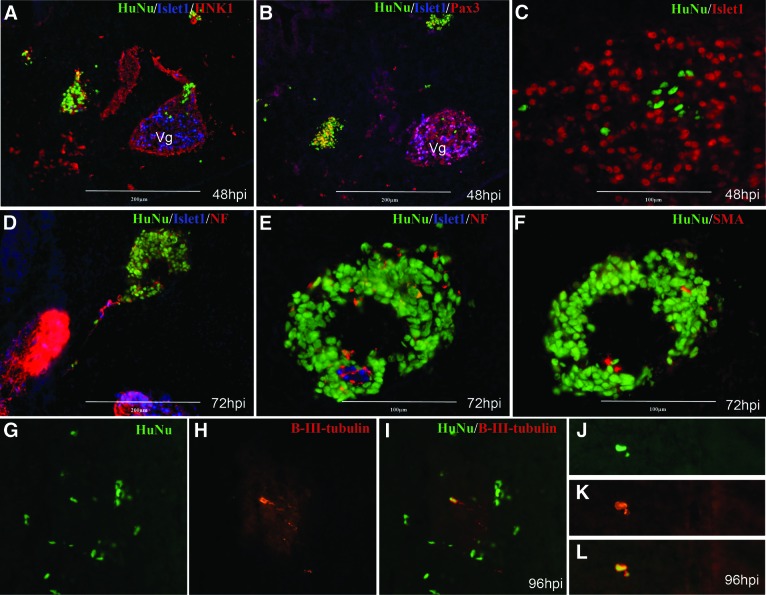
To evaluate the behavior of hESC-derived NCSCs in an environment where NC cells are known to differentiate, hESC-derived NCSCs were transplanted by picospritzer injection into the heads of developing chick embryos at somite stages 7–11 when endogenous chick NC cells begin to migrate from the dorsal neural folds. At 24 and 48 hours postinjection (hpi), human cells could be identified by immunostaining for a human nuclear stain (HuNu) and were located primarily as conglomerates adjacent to cranial ganglia or within the forming cranial ganglia. Some individual cells were also observed throughout the head mesenchyme (Fig. 4A–4C). HuNu+ cells were HNK1 immunoreactive and were typically found intermingled with HNK1+ chicken NC cells. Some human NCSCs were also weakly Pax3+, as is typical of migratory chick neural crest cells. There was no indication of neuronal differentiation, which at this stage is primarily restricted to differentiating placode-derived neurons, which are also Islet1+. At 72 hours, when neuronal differentiation of chick neural crest cells is more apparent, the cells were similarly located, and careful examination of human cell aggregates revealed that they did not express Islet1; a few cells appeared to be immunoreactive for neurofilament (NF), and many cells were found near NF+ cell processes within and near the human cell aggregates (Fig. 4D–4F).
Figure 4.
Neural crest stem cell (NCSC) behavior in an embryonic environment. Human embryonic stem cell (hESC)-derived NCSCs were grafted into the endogenous neural crest cell migratory streams in early chicken embryos. (A–C): Immunostaining 48 hours after NCSC cell injection revealed human cells colocalized with chick neural crest cells, including cells localized to the Vg, as evidenced by HuNu and HNK1 immunoreactivity. Some NCSCs also expressed Pax3 weakly, similar to adjacent chick neural crest cells. High levels of Pax3 expression, along with expression of an early neuronal differentiation marker, Islet1, indicate nearby placode-derived neurons. (D–F): Immunostaining 72 hours after NCSC cell injection showed human cells coalescing near cranial ganglia and neurofilament-reactive tissues. Most human cells did not appear to be neurons themselves, because only a few cells human cells showed immunoreactivity for neurofilament protein, and none were Islet1-positive. The cells were also negative for smooth muscle actin. (G–L): Immunostaining 96 hours after NCSC cell injection showed human cells robustly expressing the neuronal marker β-III-tubulin. The particular antibody used is strongly immunoreactive for mammalian β-III-tubulin and only weakly immunoreactive for chicken β-III-tubulin, thereby making it possible to clearly identify the human cells and neuronal processes in contrast to regions of chick neuronal tissue. Abbreviations: HuNu, human nuclear stain; hpi, hours postinjection; NF, neurofilament; SMA, smooth muscle actin; Vg, trigeminal ganglion.
Additional experiments were then carried out to examine the potential of NCSCs to generate neurons. The cells were again injected as described, and the embryos were allowed to develop to 96 hpi and assayed for the neuronal-specific β-III-tubulin marker. In embryo sections, several human cells expressed β-III-tubulin (Fig. 4G–4I), indicating that human NCSCs can differentiate to neurons as in an embryonic environment.
Differentiation of NCSCs to Schwann Cells
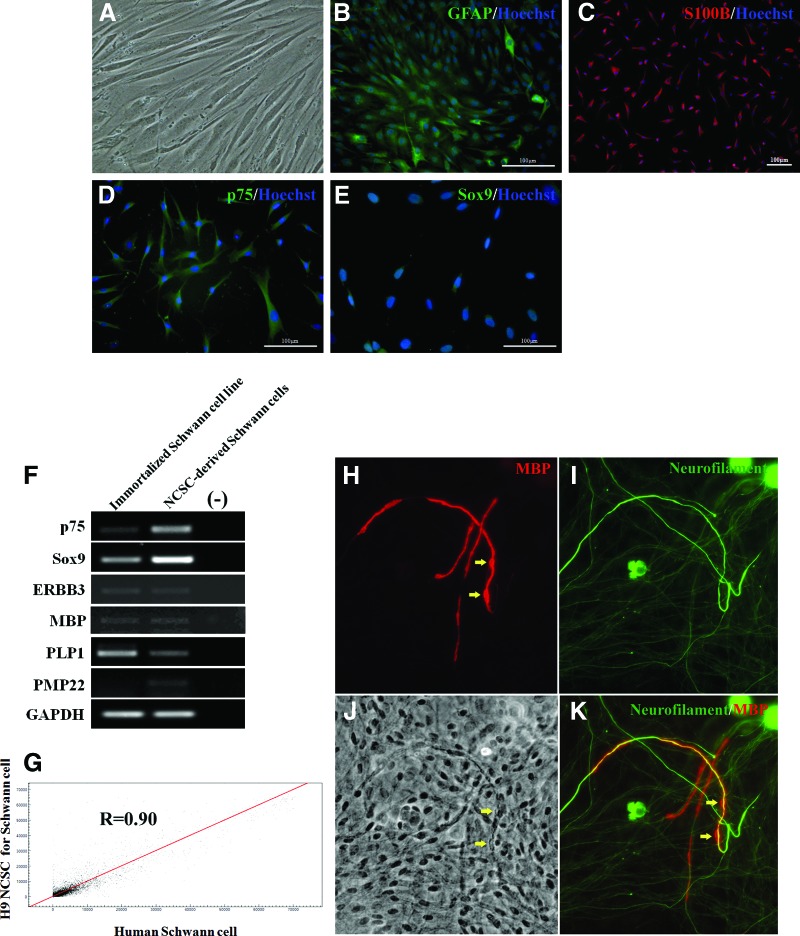
Schwann cells are the principal glia of the PNS that keep peripheral nerve fibers (both myelinated and unmyelinated) alive. To test whether hESC-derived NCSCs (both early and late passaged cells, as well as cells that were cryopreserved) can differentiate into Schwann cells, we tested several protocols for generation of Schwann cells from hESC-derived NCSCs. We found that the combination of several growth factors in a defined basal medium (mesenchymal stem cell [MSC] medium) promoted efficient Schwann cell differentiation. Morphologically, most of the NCSCs were changed from a monolayer of polygon shape to a bipolar, spindle-like shape 3 weeks postdifferentiation (Fig. 5A). Immunocytochemical analysis showed that approximately 78% and 85% of the differentiated cells were positive for GFAP and S100β, respectively (Fig. 5B, 5C), after 6 weeks of differentiation. To distinguish these cells from GFAP+ astrocytes of the CNS, we examined the expression of additional markers by immunocytochemistry and found that more than 90% of the differentiated cells were positive for p75 and Sox9 (Fig. 5D, 5E). RT-PCR and real-time PCR analyses showed that several glial-specific markers (ErbB3, MBP, PLP1, and PMP22) were expressed in the differentiated Schwann cell population, although the expression level of MBP was low (Fig. 5F and supplemental online Fig. 2C). These results indicated that the cells generated by our differentiation protocol are similar to genuine Schwann cells in terms of marker expression.
Figure 5.
Propagated NCSCs can differentiate into Schwann cells that myelinate rat dorsal root ganglia (DRG) neurons in vitro. (A–E): The figure shows the immunostaining of Schwann cells, which are efficiently generated from human embryonic stem cell (hESC)-derived NCSCs. (A): hESC-derived NCSCs grown for 3 weeks in defined based medium combining with growth factors exhibited Schwann cell precursor-like bipolar morphology. (B–E): Immunocytochemical analysis. (B, C): Immunostaining for Schwann cell markers GFAP (B) and S100β (C) after 6 weeks of differentiation. (D, E): Expression of Schwann cell markers p75 (D) and Sox9 (E). The cell nuclei were visualized by 4′,6-diamidino-2-phenylindole. (F): Reverse transcription-polymerase chain reaction analysis for Schwann cell markers for hESC-derived Schwann cells and immortalized Schwann cell line. The figure shows expression of transcripts for Schwann cell markers p75, Sox9, and ERBB3 and myelin markers PMP-22, MBP, and PLP. Expression of the GAPDH housekeeping gene was used as an internal control. (G): The overall correlation coefficient (R) between hESC-derived Schwann cells and immortalized Schwann cells was measured by Pearson's coefficient using gene expression data. After 3 weeks of coculture, hESC-derived Schwann cells myelinated segments of rat DRG neurons. (H): Image of myelinated segments as stained with anti-MBP and anti-human nuclear antigen (arrows point to hESC-derived Schwann cell nuclei). (I): Anti-neurofilament-stained rat DRG neurons. (J): Phase-contrast image shows the nuclei that were stained with anti-human nuclear antigen in (A). (K): Composite image pointing to hESC-derived Schwann cells myelinating rat DRG axons. Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GFAP, glial fibrillary acidic protein; MBP, myelin basic protein; NCSC, neural crest stem cell.
To further characterize the properties of NCSC-derived Schwann cells, we performed a whole-genome expression analysis using the Illumina microarray and compared the gene expression profile of hESC-derived Schwann cells with that of an immortalized Schwann cell line derived from human fetal tissue [36]. In general, the expression profiles of these two populations are similar, as the correlation of Schwann cells differentiated from hESC-derived NCSCs and immortalized human Schwann cell line is 0.90 (p < .01) (Fig. 5G). Expression of selected genes categorized by transcription factors, growth factors, and receptors is shown in supplemental online Table 2. As expected, Schwann cell-specific markers, such as transcription factors Slug (Snail2), S100A, ERBB3, and integrin A4, as well as NC markers expressed by Schwann cells (p75, Sox9, and TWIST), were detected in NCSC-derived Schwann cell samples. Several genes were highly expressed in both populations, suggesting that these markers might be useful for human Schwann cell-specific markers.
NCSC-Derived Schwann Cell Myelinated Rat Sensory Axons
The model of Schwann cells cocultured with DRG neurons, followed by the induction of myelination with progesterone and ascorbic acid, provides an excellent experimental setting to examine myelination by Schwann cells. To determine whether Schwann cells differentiated from hESC-derived NCSCs have the ability to myelinate peripheral axons in vitro, Schwann cells were added to established cultures of rat embryonic DRG sensory neurons. After 3 weeks in culture, hESC-derived Schwann cells had ensheathed bundles of sensory axons, and myelin segments could be visualized with anti-MBP staining (Fig. 5H–5K). An analysis of single myelin segments confirmed that human Schwann cells and not residual contaminating rat glial cells were the myelin-forming cells as revealed by Schwann cell nuclei labeled with anti-human nuclear antibody (Fig. 5J). The number of myelinated internodes in cell cultures of hESC-derived Schwann cells was low, similar to our previous report with immortalized human Schwann cells, approximately 100-fold less in comparison with rat Schwann cells [36].
Differentiation of NCSCs to Peripheral Neurons and Mesenchymal Lineages
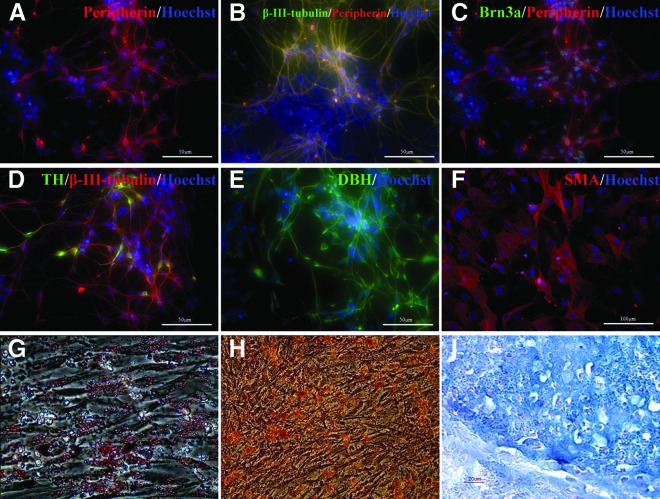
In vivo, the NC gives rise to a wide range of derivatives in the vertebrate embryo, including neurons in the PNS. We examined whether NCSCs derived and expanded from hESCs under our culture conditions can differentiate into peripheral neurons such as sensory neurons and sympathetic neurons. Neuronal differentiation was induced by treatment of NCSCs with NGF, BDNF, and dibutyryladenosine 3′,5′-cyclic monophosphate (dbcAMP) for 5 days followed by withdrawal of dbcAMP but in the presence of NGF and BDNF for 4–6 weeks. After 15 days of differentiation, the majority of the differentiated cells resembled neurons and coexpressed β-III-tubulin/peripherin (Fig. 6A, 6B). A subset of these neurons also expressed markers of peripheral sensory neurons Brn3a (Fig. 6C) or of sympathetic neurons, TH, and β-III-tubulin (Fig. 6D). Quantitative analysis showed that an average of 25% of cells expressed Brn3a, whereas 2% of cells expressed TH. Most TH-positive cells also expressed the noradrenergic marker DBH (Fig. 6E).
Figure 6.
Neural crest stem cells (NCSCs) derived from human embryonic stem cells (hESCs) differentiate into neuronal and non-neuronal cells. (A–E): The figure shows that hESC-derived NCSCs can be specified to go toward peripheral neurons, as shown by immunocytochemistry. (A, B): The majority of the cells coexpressed β-III-tubulin and peripherin after 2 weeks of differentiation. (C): Peripherin and Brn3a staining indicates differentiation into peripheral sensory neurons. (D): TH and β-III-tubulin staining indicates differentiation into sympathetic neurons. (E): Expression of noradrenergic marker DBH in TH+ neurons differentiated into mesenchymal lineage. (F): The mesenchymal precursor cells positive for SMA by immunostaining emerged 2 weeks after differentiation. (G–I): Adipogenic, chondrogenic, and osteogenic differentiation of mesenchymal precursors derived from NCSCs. (G): Oil Red O staining was used to detect adipocytes. (H, I): Alizarin red showed osteogenic cells (H), and Alcian Blue staining of cross-section of day 21 chondrogenic pellet showed the chondrogenic differentiation (I). Abbreviations: DBH, dopamine β-hydroxylase; SMA, smooth muscle actin; TH, tyrosine hydroxylase.
To determine whether hESC-derived NCSCs can differentiate into mesenchymal lineages, we cultured NCSCs under conditions that favored for MSC culture. After 2 weeks of differentiation in MSC medium, cells with mesenchymal morphology and marker expression smooth muscle actin emerged (Fig. 6F). After 4 weeks of further culture, these mesenchymal precursors differentiated into several cell types, including adipocytes stained by Oil Red O, osteogenic cells stained by alizarin red, and chondrocytes stained by Alcian Blue (Fig. 6G–6I). These results suggested that in vitro-expanded NCSCs retained the ability to differentiate to cells of the mesenchymal lineage.
Baculoviral Vector-Mediated Transgene Expression in NCSCs and Schwann Cells
For the study of PNS disorders, it is crucial to be able to introduce and express exogenous constructs in NC lineages in order to assess gene function. We have previously developed a platform technology that uses an insect virus backbone (baculovirus) to deliver large payloads efficiently in hESC-derived NSCs and postmitotic neurons [43]. We tested this delivery system with a ubiquitous promoter (CMV) driving GFP in hESC-derived NCSCs and Schwann cells derived from them. As seen in supplemental online Figure 4A and 4B, approximately 60% of NCSCs were expressing GFP 24 hours after infection as assessed by FACS analysis (supplemental online Fig. 3). Likewise, transduction is efficient in NCSC-derived Schwann cells as 80% of cells expressed GFP 2 days after infection (supplemental online Fig. 4C, 4D).
The Same NCSC Protocol Can Be Used for iPSC and Engineered hESC Lines
The ability to reprogram human somatic cells to iPSCs makes it possible to generate iPSC lines from patients with PNS disorders for disease mechanism studies and potential personalized medicine. Likewise, novel technologies of more efficient gene targeting in hESCs (e.g., zinc finger nuclease technology) allow for functional gene analysis using knockin/knockout hESC lines targeting genes involved in NC development. We therefore wished to address whether NCSCs can be generated from iPSC lines and genetically engineered hESC lines and be differentiated into the relevant cell types using the identical protocols.
We first tested whether iPSCs can differentiate into NCSCs using an iPSC line generated by integration-free technology [47]. Similar to hESC differentiation, cells migrating out of the rosette structures in the EBs expressed p75 and could be isolated by FACS. FACS-isolated NCSCs can be propagated in vitro for five passages without losing the ability to differentiate into Schwann cells and PNS neurons (supplemental online Fig. 5A–5F). We did not observe significant differences between iPSC and hESCs lines regarding the efficiency of NC induction and timeline.
We then tested a genetically modified hESC line H9 (iC23) generated by site-specific recombination in which a GFP is tagged to a known locus on chromosome 13 [48]. We have previously shown that this line differentiates into many lineages, including midbrain dopaminergic neurons, without any gene silencing issues. Here we showed that iC23 could differentiate into NCSCs and Schwann cells similar to its parent line H9 and NCSCs derived from iC23 uniformly expressed GFP (supplemental online Fig. 5G). Importantly, GFP expression was persistent when NCSCs differentiated into Schwann cells (supplemental online Fig. 5H, 5I).
Discussion
The NC gives rise to neurons and glia in the PNS, which is an important component of the nervous system. Disorders of different components in the PNS result in a wide variety of functional deficits. These disorders range from peripheral demyelination disorders to aganglionic megacolon (Hirschsprung disease) and sensory neuropathies. Such diseases have been difficult to examine because human sources of these populations are not readily available, and the existing mouse models, although useful, do not fully replicate these disorders. The recent advances in obtaining pluripotent stem cells from normal and diseased individuals and our ability to direct differentiation of pluripotent stem cells along specific lineages (e.g., NC lineage) allowed us for the first time to develop in vitro models for these diseases. In the present study, we report the induction and purification of NCSCs from hESCs and iPSCs that can be propagated in vitro for an extended period and be cryopreserved without losing the ability to differentiate into PNS neurons and glia, as well as cells of the mesenchymal lineage. We also describe a novel method of generating a nearly pure population of functional Schwann cells from hESC-derived NCSCs, which can be cryopreserved and were capable of myelinating primary neurons in coculture.
One critical aspect of the process of NC differentiation is whether NCSCs can be purified, expanded in vitro, and stored while maintaining the NCSC identity. To address this issue, we developed a stage-specific differentiation process that can be broken into the following steps: (a) induction of migrating NCSCs that express NC markers p75, Sox9, and Sox10; (b) purification of NCSCs by FACS with p75; (c) in vitro culture/expansion of NCSCs; and (d) differentiation of NCSCs to various cell types. Each of these steps can use a medium with a combination of growth factors and a defined substrate. Although several research groups have reported NC differentiation from hESCs, most of the previously described methods used a continuous differentiation protocol to generate NC lineages (e.g., sensory neurons and Schwann cells) directly from pluripotent cells [25, 27, 28, 38, 42]. Such methods are difficult to scale up and in general take many weeks if not months to obtain a certain cell type and with limited purity. We have focused our effort on developing a method/medium that can arrest the cells at the NCSC stage in an attempt to improve differentiation efficiency and to enable scaling up. We found that a combination of a medium conditioned on stromal cells with a medium we previously used for NSC expansion promoted NCSC induction and proliferation. NCSCs cultured under this condition for seven passages and/or cryopreserved were similar to initial FACS-isolated NCSCs in terms of gene expression profiles by microarray and immunocytochemistry and the ability to differentiate into various NC lineages in vitro and in developing chicken embryos.
Schwann and sensory lineages are very important, and many diseases (e.g., Charcot-Marie-Tooth, Schwannomatosis, and phantom limb syndrome) affect them. For an average-size myelinated peripheral nerve, motor or sensory, the ratio of the volume of axoplasm to neuronal cytoplasm is staggeringly large. To date most of the “neuroprotective” strategies have focused on rescuing the neuronal cell body or preventing cell death, but in many diseases affecting the PNS, there is often no cell death. Even a minimal distal degeneration of axons, mediated by local events, can lead to a functional loss of the neuron. There is a growing body of literature suggesting that mechanisms underlying cell death in neurons and axonal degeneration are different and that we need to pay attention to local events in the axon. Another issue that brings up the relevance of Schwann cells in neurodegenerative disorders is the realization that disorders such as amyotrophic lateral sclerosis are likely to be non-cell-autonomous, that is, non-neuronal cells play an important role in disease pathogenesis. Most of the recent research has, however, focused attention on CNS glia or the target tissues and ignored the Schwann cells, which has a much higher cell-to-neuron ratio. We need to consider dysfunction in Schwann cells as a potential target for investigating pathogenesis in neurodegenerative disorders affecting the PNS. In addition, transplant and repair in PNS are much easier than in CNS. To facilitate such studies, it is therefore critical that a large number of functional Schwann cells can be generated. Our method not only provides a protocol of generating pure Schwann cells but also allows us to passage and store Schwann cells in vitro, making scale-up feasible. Most importantly, Schwann cells generated by this method can myelinate rat DRG neurons. We noted, however, that the efficiency of myelination was low compared with that of rat Schwann cells (similar to immortalized human Schwann cell line) and are currently testing myelination in a human neuron culture system.
Recent advances in iPSC technology suggest that it is possible to perform normal and disease comparisons, run screens, and generate sufficient cells for therapy [49–54]. Here we demonstrate that NC lineages can be derived from iPSCs in a similar manner as from hESC lines. Our results also show that the same NC differentiation process can be applied to genetically modified hESCs, which will be useful for in vitro and in vivo analysis of NC development if hESC lines targeting genes regulating NC fate can be generated. In addition, we showed that NCSCs and Schwann cells could be efficiently transduced by a baculoviral vector delivering episomal DNA, demonstrating that gene function studies and disease modeling can be readily performed in this population of cells.
Conclusion
We have developed a scalable process of deriving and expanding NCSCs from human pluripotent stem cells (hPSCs) that can give rise to many cell types of the NC lineage, including Schwann cells, that are able to myelinate rat DRG neurons. Our work provides a path to generate large numbers of cells of the NC lineage from hESC and patient-specific iPSCs for potential cell therapy and drug screening. We acknowledge that our protocols currently are not xeno-free, but as with our previous reports on hPSC-derived dopaminergic neurons, we believe we can easily extend these observations to make clinical grade PNS derivatives as well.
Acknowledgments
This work was supported in part by the California Institute for Regenerative Medicine Grants CL1-00501-1 (to X.Z.) and TG2-01155 (to X.Z.) and Maryland Stem Cell Research Fund Grant 302385 (to A.H.). We thank Dr. L. Cheng at the Johns Hopkins University for providing the iPSC line and Drs. J. Peng and A. Swistowski (a previous member of our laboratory) for technical assistance. We also acknowledge the Developmental Studies Hybridoma Bank maintained by the University of Iowa (Iowa City, IA) for supply of monoclonal antibodies.
Author Contributions
Q.L. and M.R.S.: collection and/or assembly of data, data analysis and interpretation, manuscript writing; S.C.S., R.M., and R.N.T.L.: collection and/or assembly of data; A.H.: data analysis and interpretation; M.S.R: conception and design, manuscript writing; X.Z.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Le Douarin N. New York: Cambridge University Press; 1982. The neural crest. [Google Scholar]
- 2.Le Douarin N, Kalcheim C. New York: Cambridge University Press; 1999. The neural crest. [Google Scholar]
- 3.Couly GF, Coltey PM, Le Douarin NM. The triple origin of skull in higher vertebrates: A study in quail-chick chimeras. Development. 1993;117:409–429. doi: 10.1242/dev.117.2.409. [DOI] [PubMed] [Google Scholar]
- 4.Gross JB, Hanken J. Use of fluorescent dextran conjugates as a long-term marker of osteogenic neural crest in frogs. Dev Dyn. 2004;230:100–106. doi: 10.1002/dvdy.20036. [DOI] [PubMed] [Google Scholar]
- 5.Gross JB, Hanken J. Cranial neural crest contributes to the bony skull vault in adult Xenopus laevis: Insights from cell labeling studies. J Exp Zool B Mol Dev Evol. 2005;304:169–176. doi: 10.1002/jez.b.21028. [DOI] [PubMed] [Google Scholar]
- 6.Jiang X, Iseki S, Maxson RE, et al. Tissue origins and interactions in the mammalian skull vault. Dev Biol. 2002;241:106–116. doi: 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- 7.Kelsh RN, Brand M, Jiang YJ, et al. Zebrafish pigmentation mutations and the processes of neural crest development. Development. 1996;123:369–389. doi: 10.1242/dev.123.1.369. [DOI] [PubMed] [Google Scholar]
- 8.Le Douarin NM, Dupin E. Multipotentiality of the neural crest. Curr Opin Genet Dev. 2003;13:529–536. doi: 10.1016/j.gde.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Krispin S, Nitzan E, Kassem Y, et al. Evidence for a dynamic spatiotemporal fate map and early fate restrictions of premigratory avian neural crest. Development. 2010;137:585–595. doi: 10.1242/dev.041509. [DOI] [PubMed] [Google Scholar]
- 10.Hari L, Brault V, Kleber M, et al. Lineage-specific requirements of beta-catenin in neural crest development. J Cell Biol. 2002;159:867–880. doi: 10.1083/jcb.200209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kléber M, Sommer L. Wnt signaling and the regulation of stem cell function. Curr Opin Cell Biol. 2004;16:681–687. doi: 10.1016/j.ceb.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Lee HY, Kleber M, Hari L, et al. Instructive role of Wnt/beta-catenin in sensory fate specification in neural crest stem cells. Science. 2004;303:1020–1023. doi: 10.1126/science.1091611. [DOI] [PubMed] [Google Scholar]
- 13.Dorsky RI, Moon RT, Raible DW. Control of neural crest cell fate by the Wnt signalling pathway. Nature. 1998;396:370–373. doi: 10.1038/24620. [DOI] [PubMed] [Google Scholar]
- 14.Wong CE, Paratore C, Dours-Zimmermann MT, et al. Neural crest-derived cells with stem cell features can be traced back to multiple lineages in the adult skin. J Cell Biol. 2006;175:1005–1015. doi: 10.1083/jcb.200606062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spritz RA, Giebel LB, Holmes SA. Dominant negative and loss of function mutations of the c-kit (mast/stem cell growth factor receptor) proto-oncogene in human piebaldism. Am J Hum Genet. 1992;50:261–269. [PMC free article] [PubMed] [Google Scholar]
- 16.Baynash AG, Hosoda K, Giaid A, et al. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994;79:1277–1285. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 17.Kunisada T, Yoshida H, Yamazaki H, et al. Transgene expression of steel factor in the basal layer of epidermis promotes survival, proliferation, differentiation and migration of melanocyte precursors. Development. 1998;125:2915–2923. doi: 10.1242/dev.125.15.2915. [DOI] [PubMed] [Google Scholar]
- 18.Anderson DJ. Stem cells and pattern formation in the nervous system: The possible versus the actual. Neuron. 2001;30:19–35. doi: 10.1016/s0896-6273(01)00260-4. [DOI] [PubMed] [Google Scholar]
- 19.Zirlinger M, Lo L, McMahon J, et al. Transient expression of the bHLH factor neurogenin-2 marks a subpopulation of neural crest cells biased for a sensory but not a neuronal fate. Proc Natl Acad Sci USA. 2002;99:8084–8089. doi: 10.1073/pnas.122231199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guillemot F, Lo LC, Johnson JE, et al. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–476. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- 21.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 22.Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung's disease: Advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8:466–479. doi: 10.1038/nrn2137. [DOI] [PubMed] [Google Scholar]
- 23.Kawaguchi J, Mee PJ, Smith AG. Osteogenic and chondrogenic differentiation of embryonic stem cells in response to specific growth factors. Bone. 2005;36:758–769. doi: 10.1016/j.bone.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 24.Lee G, Chambers SM, Tomishima MJ, et al. Derivation of neural crest cells from human pluripotent stem cells. Nat Protoc. 2010;5:688–701. doi: 10.1038/nprot.2010.35. [DOI] [PubMed] [Google Scholar]
- 25.Lee G, Kim H, Elkabetz Y, et al. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nat Biotechnol. 2007;25:1468–1475. doi: 10.1038/nbt1365. [DOI] [PubMed] [Google Scholar]
- 26.Mizuseki K, Sakamoto T, Watanabe K, et al. Generation of neural crest-derived peripheral neurons and floor plate cells from mouse and primate embryonic stem cells. Proc Natl Acad Sci USA. 2003;100:5828–5833. doi: 10.1073/pnas.1037282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y, Snead ML. Derivation of cranial neural crest-like cells from human embryonic stem cells. Biochem Biophys Res Commun. 2008;376:542–547. doi: 10.1016/j.bbrc.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curchoe CL, Maurer J, McKeown SJ, et al. Early acquisition of neural crest competence during hESCs neuralization. PLoS One. 2010;5:e13890. doi: 10.1371/journal.pone.0013890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swistowska AM, da Cruz AB, Han Y, et al. Stage-specific role for shh in dopaminergic differentiation of human embryonic stem cells induced by stromal cells. Stem Cells Dev. 2010;19:71–82. doi: 10.1089/scd.2009.0107. [DOI] [PubMed] [Google Scholar]
- 30.Zeng X, Miura T, Luo Y, et al. Properties of pluripotent human embryonic stem cells BG01 and BG02. Stem Cells. 2004;22:292–312. doi: 10.1634/stemcells.22-3-292. [DOI] [PubMed] [Google Scholar]
- 31.Swistowski A, Peng J, Han Y, et al. Xeno-free defined conditions for culture of human embryonic stem cells, neural stem cells and dopaminergic neurons derived from them. PLoS One. 2009;4:e6233. doi: 10.1371/journal.pone.0006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rungarunlert S, Techakumphu M, Pirity MK, et al. Embryoid body formation from embryonic and induced pluripotent stem cells: Benefits of bioreactors. World J Stem Cells. 2009;1:11–21. doi: 10.4252/wjsc.v1.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng X, Chen J, Sanchez JF, et al. Stable expression of hrGFP by mouse embryonic stem cells: Promoter activity in the undifferentiated state and during dopaminergic neural differentiation. Stem Cells. 2003;21:647–653. doi: 10.1634/stemcells.21-6-647. [DOI] [PubMed] [Google Scholar]
- 34.Lassiter RN, Dude CM, Reynolds SB, et al. Canonical Wnt signaling is required for ophthalmic trigeminal placode cell fate determination and maintenance. Dev Biol. 2007;308:392–406. doi: 10.1016/j.ydbio.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lassiter RN, Reynolds SB, Marin KD, et al. FGF signaling is essential for ophthalmic trigeminal placode cell delamination and differentiation. Dev Dyn. 2009;238:1073–1082. doi: 10.1002/dvdy.21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehmann HC, Chen W, Mi R, et al. Human Schwann cells retain essential phenotype characteristics after immortalization. Stem Cells Dev. 2012;21:423–431. doi: 10.1089/scd.2010.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lazzari G, Colleoni S, Giannelli SG, et al. Direct derivation of neural rosettes from cloned bovine blastocysts: A model of early neurulation events and neural crest specification in vitro. Stem Cells. 2006;24:2514–2521. doi: 10.1634/stemcells.2006-0149. [DOI] [PubMed] [Google Scholar]
- 38.Pomp O, Brokhman I, Ben-Dor I, et al. Generation of peripheral sensory and sympathetic neurons and neural crest cells from human embryonic stem cells. Stem Cells. 2005;23:923–930. doi: 10.1634/stemcells.2005-0038. [DOI] [PubMed] [Google Scholar]
- 39.Creuzet S, Couly G, Le Douarin NM. Patterning the neural crest derivatives during development of the vertebrate head: Insights from avian studies. J Anat. 2005;207:447–459. doi: 10.1111/j.1469-7580.2005.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Douarin NM, Calloni GW, Dupin E. The stem cells of the neural crest. Cell Cycle. 2008;7:1013–1019. doi: 10.4161/cc.7.8.5641. [DOI] [PubMed] [Google Scholar]
- 41.Perissinotto D, Iacopetti P, Bellina I, et al. Avian neural crest cell migration is diversely regulated by the two major hyaluronan-binding proteoglycans PG-M/versican and aggrecan. Development. 2000;127:2823–2842. doi: 10.1242/dev.127.13.2823. [DOI] [PubMed] [Google Scholar]
- 42.Pomp O, Brokhman I, Ziegler L, et al. PA6-induced human embryonic stem cell-derived neurospheres: A new source of human peripheral sensory neurons and neural crest cells. Brain Res. 2008;1230:50–60. doi: 10.1016/j.brainres.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 43.Swistowski A, Peng J, Liu Q, et al. Efficient generation of functional dopaminergic neurons from human induced pluripotent stem cells under defined conditions. Stem Cells. 2010;28:1893–1904. doi: 10.1002/stem.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biernaskie J, Paris M, Morozova O, et al. SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell. 2009;5:610–623. doi: 10.1016/j.stem.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cimadamore F, Fishwick K, Giusto E, et al. Human ESC-derived neural crest model reveals a key role for SOX2 in sensory neurogenesis. Cell Stem Cell. 2011;8:538–551. doi: 10.1016/j.stem.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heanue TA, Pachnis V. Prospective identification and isolation of enteric nervous system progenitors using Sox2. Stem Cells. 2011;29:128–140. doi: 10.1002/stem.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chou BK, Mali P, Huang X, et al. Efficient human iPS cell derivation by a non-integrating plasmid from blood cells with unique epigenetic and gene expression signatures. Cell Res. 2011;21:518–529. doi: 10.1038/cr.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Macarthur CC, Xue H, Van Hoof D, et al. Chromatin insulator elements block transgene silencing in engineered human embryonic stem cell lines at a defined chromosome 13 locus. Stem Cells Dev. 2011;21:191–205. doi: 10.1089/scd.2011.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park IH, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ebert AD, Yu J, Rose FF, Jr., et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dimos JT, Rodolfa KT, Niakan KK, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 52.Nguyen HN, Byers B, Cord B, et al. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell. 2011;8:267–280. doi: 10.1016/j.stem.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee G, Papapetrou EP, Kim H, et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature. 2009;461:402–406. doi: 10.1038/nature08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soldner F, Hockemeyer D, Beard C, et al. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]