Human cardiosphere-derived cells (hCDCs) were exposed to trastuzumab (TZM) to evaluate whether TZM cardiotoxicity involves inhibition of human adult cardiac-derived stem cells, in addition to previously reported direct adverse effects on cardiomyocytes. The results indicated that TZM inhibits the cardiomyogenic and angiogenic capacities of hCDCs in vitro and abrogates the morphological and functional benefits of hCDC transplantation in vivo. Thus, TZM impairs the function of human resident cardiac stem cells, potentially contributing to TZM cardiotoxicity.
Keywords: Adult stem cells, Cardiac, Chemotherapy, Angiogenesis
Abstract
Trastuzumab (TZM), a monoclonal antibody against the ERBB2 protein, increases survival in ERBB2-positive breast cancer patients. Its clinical use, however, is limited by cardiotoxicity. We sought to evaluate whether TZM cardiotoxicity involves inhibition of human adult cardiac-derived stem cells, in addition to previously reported direct adverse effects on cardiomyocytes. To test this idea, we exposed human cardiosphere-derived cells (hCDCs), a natural mixture of cardiac stem cells and supporting cells that has been shown to exert potent regenerative effects, to TZM and tested the effects in vitro and in vivo. We found that ERBB2 mRNA and protein are expressed in hCDCs at levels comparable to those in human myocardium. Although clinically relevant concentrations of TZM had no effect on proliferation, apoptosis, or size of the c-kit-positive hCDC subpopulation, in vitro assays demonstrated diminished potential for cardiogenic differentiation and impaired ability to form microvascular networks in TZM-treated cells. The functional benefit of hCDCs injected into the border zone of acutely infarcted mouse hearts was abrogated by TZM: infarcted animals treated with TZM + hCDCs had a lower ejection fraction, thinner infarct scar, and reduced capillary density in the infarct border zone compared with animals that received hCDCs alone (n = 12 per group). Collectively, these results indicate that TZM inhibits the cardiomyogenic and angiogenic capacities of hCDCs in vitro and abrogates the morphological and functional benefits of hCDC transplantation in vivo. Thus, TZM impairs the function of human resident cardiac stem cells, potentially contributing to TZM cardiotoxicity.
Introduction
Breast cancer is the most prevalent form of cancer in women. In 2012, an estimated 226,870 women will be diagnosed with breast cancer in the U.S., and more than 39,510 will die from this disease [1]. Tyrosine kinase inhibitors have become an important treatment option for breast cancer patients. A series of large-scale studies have shown that trastuzumab (TZM) (Herceptin; Genentech, Inc., South San Francisco, CA, http://www.gene.com) a monoclonal antibody targeting the HER2/neu (ERBB2) tyrosine kinase, can substantially reduce the risk of recurrence and early death in women with ERBB2-positive breast cancer [2–4]. However, the use of TZM has been associated with adverse cardiovascular effects. The incidence of cardiac dysfunction ranged from 4% to 7% with TZM monotherapy but reached up to 27% when the regimen also included anthracyclines [4, 5]. Additionally, preexisting cardiac dysfunction, common in the older breast cancer population, calls for frequent monitoring to detect further functional deterioration, which usually requires temporary or permanent cessation of this important therapy. In this study, we sought to better understand the pathophysiologic basis of TZM-associated cardiotoxicity and hypothesized that cardiac dysfunction induced by TZM may be mediated, at least in part, by adverse effects on endogenous cardiac stem cells. The cardiac progenitor cell population used in the present study was isolated from explant cultures of adult human endomyocardial biopsies using an intermediate cardiosphere (CSp) step. CSps are self-assembling spherical clusters that constitute a niche-like environment with undifferentiated cells proliferating in the core and cardiac-committed cells on the periphery [6–8]. Human cardiosphere-derived cells (hCDCs) can be expanded many fold as monolayers, achieving cell numbers suitable for cell therapy (as in the ongoing CADUCEUS trial; NCT00893360, http://clinicaltrials.gov). Our previous work on hCDCs [7–9] and that of others [10, 11] support the notion that such cells can directly regenerate myocardium and blood vessels. The fact that cardiosphere-derived cells (CDCs) are also clonogenic qualifies them as cardiac-derived stem cells [12]. In the present study, we investigated whether functional impairment of hCDCs could contribute to TZM-induced cardiotoxicity in vitro and in vivo.
Materials and Methods
Biopsy Specimen Processing and Cell Culture
Percutaneous endomyocardial biopsy specimens (n = 12) were obtained from the right ventricular septal wall during clinically indicated procedures after informed consent was obtained, in an institutional review board-approved protocol. CDCs were isolated from these human myocardial specimens as described previously [7–9]. Human dermal fibroblasts and the breast cancer cell line MCF-7 served as controls and were cultured in the same medium as hCDCs.
Reverse Transcription SYBR Green Polymerase Chain Reaction (Quantitative Reverse Transcription-Polymerase Chain Reaction)
Total RNA was extracted from hCDCs using the RNeasy RNA extraction kit (Qiagen, Valencia, CA, http://www.qiagen.com). RNA samples were treated with RNase-free DNase Set (Qiagen) to eliminate genomic DNA contamination, and complementary DNA was synthesized from 1 μg of total RNA using AffinityScript multiple temperature reverse transcriptase (Stratagene, La Jolla, CA, http://www.stratagene.com) and oligo(dT)12–18 primer (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) following the manufacturer's instructions.
Primers for the genes of interest were designed using the National Center for Biotechnology Information primer design tool Primer-BLAST. Specificity of the primers was confirmed by a single band of the polymerase chain reaction (PCR) product on an agarose gel and a single peak of the dissociation curve (SYBR Green reverse transcription [RT]-PCR). Gene expression was normalized to ribosomal protein 18S. RT-PCR was performed in duplicate for each sample with 25 ng of cDNA and 300 nmol/l primer in the Applied Biosystems 7900HT RT-PCR system (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com) using the QuantiTect SYBR Green PCR Kit according to the recommendations of the manufacturer (Qiagen) as previously described [13]. Human control RNA was purchased from BioChain (BioChain Institute, Inc., Hayward, CA, http://www.biochain.com).
Myocardial Infarction, Cell Injection, and Echocardiography
Myocardial infarction was created in adult male SCID-beige mice 10–20 weeks of age as described previously [8] under an approved animal protocol. CDCs were injected in a total volume of 10 μl of phosphate-buffered saline (PBS) at two sites bordering the infarct, as previously described [8]. PBS and human skin fibroblasts served as negative controls. All mice underwent echocardiography before and immediately after surgery (baseline) and 3 weeks after surgery. Left ventricular ejection fraction and fractional area were calculated with VisualSonics v1.3.8 software (VisualSonics Inc., Toronto, http://www.visualsonics.com) from two-dimensional long-axis views taken through the infarcted area [8]. To account for the shorter half-life of TZM in mice compared with patients, animals in the TZM group received biweekly intraperitoneal injections of 20 mg TZM/kg for 3 weeks [14].
Flow Cytometry
Flow cytometry was used to quantify the percentage of ERBB2 and c-kit-positive hCDCs after staining with a human ERBB2 antibody, followed by staining with an anti-rabbit Alexa 647 secondary antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com). Likewise, the percentage of dead and apoptotic hCDCs was determined by flow cytometry using 7-aminoactinomycin and Annexin V-APC (BD Biosciences, San Jose, CA, http://www.bdbiosciences.com), respectively.
Functional In Vitro Assays for Proliferation, Angiogenesis, and Cardiac Differentiation
Proliferation of control and TZM-treated hCDCs was assessed using the colorimetric WST-8 assay (Cell Counting Kit-8; Dojindo Molecular Technologies, Gaithersburg, MD, http://www.dojindo.com). Cells were incubated with 10 μl of WST-8 tetrazolium salt for 2 hours, and absorbance was measured at 450 nm (Spectramax M2; Molecular Devices, Sunnyvale, CA, http://www.moleculardevices.com). Proliferation experiments were repeated five times, with each condition tested in triplicate.
In vitro angiogenesis assays were performed as recommended by the manufacturer (Becton, Dickinson and Company, Franklin Lakes, NJ, http://www.bd.com) in triplicate in a 96-well plate with 104 hCDCs in endothelial growth medium (Cambrex, Walkersville, MD, http://www.cambrex.com). Vascular tube formation was documented within 5 hours after plating of hCDCs using an inverted microscope. Human umbilical vascular endothelial cells (HUVECs) served as positive control. A ×2 magnification lens covering the entire well and a Nikon digital camera (Nikon, Tokyo, http://www.nikon.com) were used to document vascular tube formation. Quantification was performed using NIH ImageJ software.
A third-generation lentiviral vector system was used to transduce hCDCs with the firefly luciferase reporter gene under the transcriptional control of the cardiac-specific promoter of the sodium-calcium exchanger, as described previously [13]. In brief, low passage number hCDCs (<6 passages) were plated at 2 × 104 cells per well in a 24-well plate and transduced with lentivirus (4.5 × 104 pg of p24, corresponding to a multiplicity of infection, i.e., the ratio of infectious agents [virus] to infection targets [cells], of 35) in a total volume of 2 ml in the presence of 8 μg/ml polybrene. Sixteen hours later, viral vector-containing medium was replaced with fresh medium, and cells were incubated with medium containing 1 μg/ml of the small molecules compounds JL-265-037 and JL-265-010 (kindly provided by Dr. Jay Schneider, University of Texas Southwestern Medical Center, Dallas, TX), shown to be potent inducers of cardiac differentiation [13, 15], in the presence and absence of TZM. Luciferase activity was measured 12 days after transduction, using an in vitro luciferase assay (Promega, Madison, WI, http://www.promega.com) as per the manufacturer's protocol with a Monolight 2010 luminometer (Analytical Luminescence Laboratory, San Diego). Twenty microliters of each sample (cell lysate) was mixed with 100 μl of luciferase assay reagent (Promega) in 75-mm glass tubes (VWR International, LLC, Radnor, PA, http://www.vwr.com) and placed in the instrument (2-second measurements). Results were reported as relative light units normalized to cell number.
Measurement of Infarct Size, Scar Thickness, and Capillary Density
In a subset of infarcted animals (hCDCs + PBS, n = 5; hCDCs + TZM, n = 5), hearts were excised under deep anesthesia, trimmed of the atria, washed with saline, embedded in O.C.T. compound (Sakura Finetek, Torrance, CA, http://www.sakura.com), and frozen in a bath of 2-methylbutane with dry ice. The hearts were stored at −80°C until they were sliced transversely with a cryostat at 6 μm thickness with 500 μm between sections. Sections were mounted on glass slides and stained with Masson trichrome [16]. Infarct thickness was measured in three or four consecutive sections.
For capillary staining, sections were incubated with fluorescein isothiocyanate-conjugated isolectin B4 antibody (Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com), which has been reported to be useful as a marker for endothelial cells from humans and nonprimates. Coincubation of isolectin B4 with 500 mM galactose was used as a negative control. The ImageJ software was used for binary threshold of fluorescent images for each channel and consequent particle count. Green pixels were normalized to the number of mouse nuclei (stained with 4′,6-diamidino-2-phenylindole) in the infarct border zone area [9].
Statistical Analysis
All results are presented as mean ± SEM. Significance of differences between any two groups was determined by Student's t test. A final value of p < .05 was considered significant for all analyses. All probability values reported are two-sided.
Results
ERBB2 mRNA and Protein Are Expressed in Human CDCs
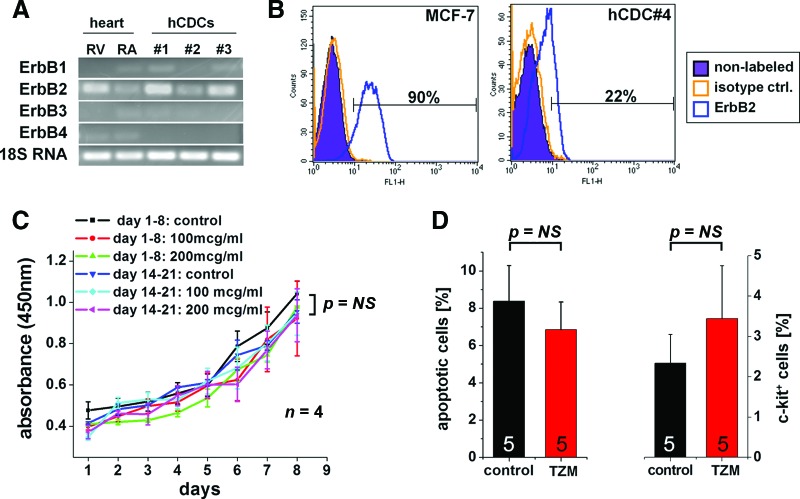
Among the different ERBB isoforms, ERBB2 mRNA was found to be most abundant in hCDCs, followed by ERBB1 and ERBB3, whereas ERBB4 mRNA was not detected. At the transcript level, comparable levels of ERBB2 were identified in hCDCs and in human right ventricular and right atrial myocardium from nonfailing control hearts (Fig. 1A). In addition, a significant proportion of hCDCs also expressed ERBB2 protein, as assessed by flow cytometry (20 ± 3%, n = 4; Fig. 1B).
Figure 1.
ERBB2 mRNA and protein expression in human CDCs. (A): Reverse transcription-polymerase chain reaction shows expression of ERBB isoforms in human myocardium (RV and RA both obtained from a nonfailing human control heart) and hCDCs isolated from three different patients. 18S RNA served as a control. (B): Representative analysis of ERBB2 protein quantified by flow cytometry (20 ± 3% of hCDCs expressed ERBB2 protein, n = 4). The breast cancer cell line MCF-7, known to express ERBB2, served as positive control. (C): In vitro proliferation of human cardiosphere-derived cells (hCDCs) was not affected by TZM. No statistically significant differences were seen for proliferation of freshly isolated hCDCs (days 1–8) versus hCDCs that were first incubated for up to 13 days with TZM, and proliferation on days 14–21 was then measured using the WST-8 assay (at least n = 4 different hCDCs isolates for each condition). (D): TZM did not change the percentage of apoptotic or c-kit-positive hCDCs (n = 5 different human CDCs). Abbreviations: hCDC, human cardiosphere-derived cell; NS, not significant; RA, right atrium; RV, right ventricle; TZM, trastuzumab.
In Vitro Effects of Trastuzumab on Human CDCs
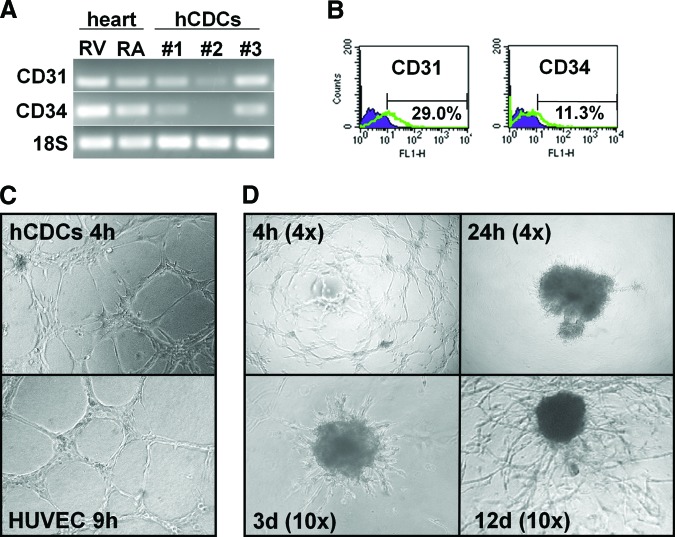
At clinically relevant concentrations (100 μg/ml), TZM did not slow proliferation of hCDCs, nor did it increase the percentage of apoptotic cells or alter the c-kit-positive cell population (Fig. 1C, 1D). Of note, no effect on proliferation was seen when cells were incubated with TZM for up to 21 days. To study longer exposures with TZM, cells were first incubated for up to 13 days with 100 μg/ml TZM, and then proliferation was measured with the WST-8 assay (Fig. 1C). Nevertheless, assessment of cell number and viability may not entirely reflect the functional capacity of cells in vivo [17]; thus, additional testing is required to verify proper cell function. As hCDCs express vascular markers, including CD31 and CD34, at the mRNA and protein levels (Fig. 2A and 2B, respectively), we tested the cells' ability to form vascular tubes in an in vitro angiogenesis assay. As compared with HUVECs, which are the gold standard for this angiogenesis assay, vascular tubes formed more quickly with hCDCs (4 hours for hCDCs vs. 9–16 hours for HUVECs; Fig. 2C). However, there was no difference in the complexity of vascular networks formed by HUVECs and hCDCs (assessed by the number of branching points; data not shown). At later time points (>8–12 hours), hCDCs then involuted to form a central cell cluster from which secondary vascular structures started to migrate out within the following days. The initially linear sprouts of endothelial cells progressively branched, anastomosed, and formed a microvascular network that covered the entire well after 12–20 days (Fig. 2D). Thus, this late migratory response seen on Matrigel resembles the outgrowth seen with the aortic ring assay. Importantly, the size and complexity of the microvascular network were significantly greater in the control compared with the TZM group: continued incubation with TZM-containing medium slowed the growth and reduced the complexity of the microvascular network by >50% (Fig. 3A, 3B).
Figure 2.
In vitro angiogenesis assay. Endothelial markers CD31 and CD34 were expressed at the mRNA level (A) and protein level (B). In summary, 15 ± 7% and 7 ± 2% of hCDCs expressed CD31 and CD34 protein, respectively; n = 6. (C): Tube formation on Matrigel angiogenesis assay was similar in hCDCs (top) and HUVECs (bottom). Magnification, ×10. (D): Representative images of a time-course experiment. Early angiogenic response after 4 hours was highlighted by formation of vascular tubes giving rise to a complex mesh-like structure. After 24 hours, cells formed a central cluster in the middle of the well (top right). Several days later, cells started migrating out from the central cell cluster, gradually covering the entire well over the next 2–3 weeks (bottom). Magnification, ×4 (top) and ×10 (bottom). Abbreviations: d, days; h, hours; hCDCs, human cardiosphere-derived cells; HUVEC, human umbilical vein endothelial cells; RA, right atrium; RV, right ventricle.
Figure 3.
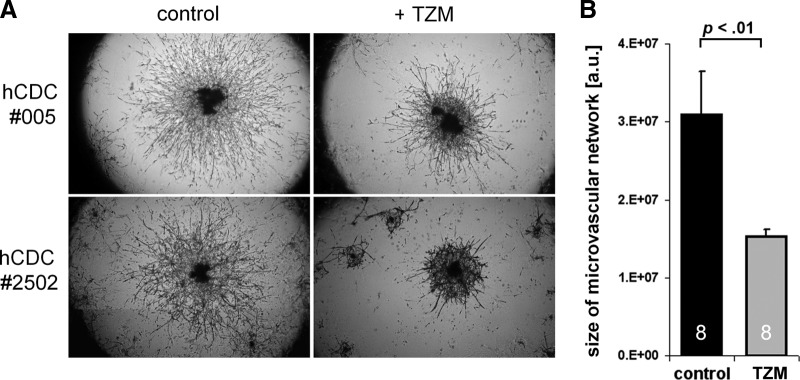
Effect of TZM on in vitro angiogenesis. (A): The size and complexity of the microvascular network on Matrigel matrix after 17 days in culture differed significantly between control and TZM-treated hCDCs. (B): Continued incubation with TZM-containing medium slowed the growth and reduced the complexity of the microvascular network by >50% (n = 8 different human CDCs, each plated and analyzed in triplicate for control and TZM groups). Abbreviations: a.u., arbitrary units; hCDC, human cardiosphere-derived cells; TZM, trastuzumab.
In addition to the adverse effects on angiogenesis, TZM led to an impairment of cardiac differentiation of hCDCs in vitro, which became apparent when the cells were incubated with cardiogenic sulfonyl hydrazone compounds [13, 15]. Using a previously validated system for testing cardiac differentiation in vitro (cardiac-specific promoter of the sodium-calcium exchanger [NCX1] driving the expression of firefly luciferase [13]), we found luciferase activity under control conditions to be significantly higher than after incubation with 100 μg/ml TZM, when hCDCs were exposed to two known cardiogenic sulfonyl hydrazone compounds (JL-265-010 and -037; Fig. 4A). In agreement with these results, the expression of transcripts indicative of early cardiac differentiation, including pro-brain natriuretic peptide, cardiac troponin I, and SERCA2, was more than twofold higher in the control versus the TZM group (Fig. 4B). Previously, we demonstrated that NCX1-green fluorescent protein-positive CDC subpopulations showed prolonged expression of a variety of additional cardiac markers, including l-type calcium channel and the cardiac-specific transcription factors GATA4 and TBX5 [13].
Figure 4.
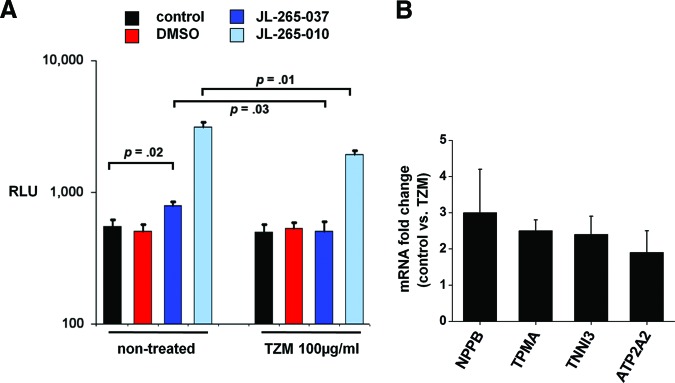
In vitro assay for early cardiogenic differentiation. CDCs were transduced with lentiviral vectors expressing firefly luciferase under the transcriptional control of the cardiac-specific promoter of the sodium-calcium exchanger. Subsequently, hCDCs were incubated for 11 days with control medium, medium + DMSO, or medium with either of the two cardiogenic sulfonyl hydrazone compounds, JL-265-010 and JL-265-037. Then, luciferase activity was determined with in vitro luciferase assays, and results were normalized to cell number. (A): Cardiogenic differentiation was significantly inhibited by TZM compared with control (n = 5). (B): Quantitative real-time reverse transcription-polymerase chain reaction showed expression of cardiac-specific mRNAs, including NPPB, TPMA, TNNI3, and ATP2A2, to be twofold higher in the control versus the TZM group (n = 5, p < .05 for all comparisons). Abbreviations: ATP2A2, SERCA2; DMSO, dimethyl sulfoxide; NPPB, pro-brain natriuretic peptide; RLU, relative light units; TNNI3, cardiac troponin I; TPMA, tropomyosin; TZM, trastuzumab.
In Vivo Effects of Trastuzumab on hCDCs in the Infarcted Heart
In various studies, we [8, 9, 18–20] and others [10, 21, 22] have demonstrated a consistent benefit of transplanted CDCs on ventricular function in small and large animal models of acute myocardial infarction. Given that TZM inhibits the cardiomyogenic and angiogenic capacities of hCDCs in vitro, we tested the hypothesis that TZM abrogates the functional benefits of hCDC transplantation in vivo. Biweekly injections of TZM over a period of 3 weeks did not lead to left ventricular dysfunction in healthy mice (Fig. 5A). In contrast, when hCDCs were injected into the border zone of acutely infarcted murine left ventricular myocardium, biweekly intraperitoneal administration of TZM abrogated the functional benefit of hCDCs, as evidenced by a lower ejection fraction in animals that received hCDCs and TZM compared with hCDCs alone. Of note, there was no difference in the baseline ejection fraction after acute myocardial infarction between the study groups (data not shown). Although hCDCs alone (hCDC + PBS) improved function, consistent with previous reports [8, 9, 20, 23], the hCDCs + TZM group and the two control groups (injection of human dermal fibroblasts or injection of PBS) were indistinguishable in terms of ejection fraction (Fig. 5B). Additionally, animals injected with hCDCs + PBS exhibited less thinning of infarcted walls and a tendency to lower infarct area than animals in the TZM group, signifying that TZM prevents the beneficial effects of hCDCs on postischemic remodeling (Fig. 5C). Left-ventricular end-systolic and end-diastolic dimensions were not statistically different between groups at 3 weeks.
Figure 5.
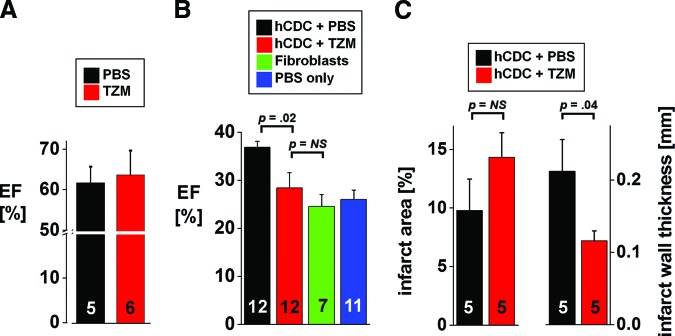
In vivo effect of trastuzumab. (A): Biweekly intraperitoneal injection of TZM for 3 weeks did not lead to left ventricular dysfunction in SCID-beige mice. (B): Biweekly intraperitoneal administration of TZM abrogated the functional benefit of hCDCs injected into the border zone of acutely infarcted murine left ventricular myocardium. Injection of hCDCs alone led to a 10% increase in ejection fraction compared with animals that received hCDCs and TZM. Of note, there was no difference between the treatment group (hCDCs + TZM) and the two negative control groups included in the study, that is, injection of human dermal fibroblasts and injection of PBS. (C): Differences in postinfarct remodeling were evident by a thinner infarct scar in animals receiving TZM, whereas the difference in infarct area between the two groups was not statistically significant (the numbers of animals are indicated on the bars). Abbreviations: EF, ejection fraction; hCDC, human cardiosphere-derived cells; NS, not significant; PBS, phosphate-buffered saline; TZM, trastuzumab.
The profound antiangiogenic effects observed in vitro prompted us to test the hypothesis that TZM adversely affects the density of capillaries in vivo. As a result, we found that the capillary density (detected by isolectin B4 staining and normalized to the number of nuclei) was significantly reduced in the infarct border zone of animals that received hCDCs and TZM compared with animals that received hCDCs alone (Fig. 6A, 6B).
Figure 6.
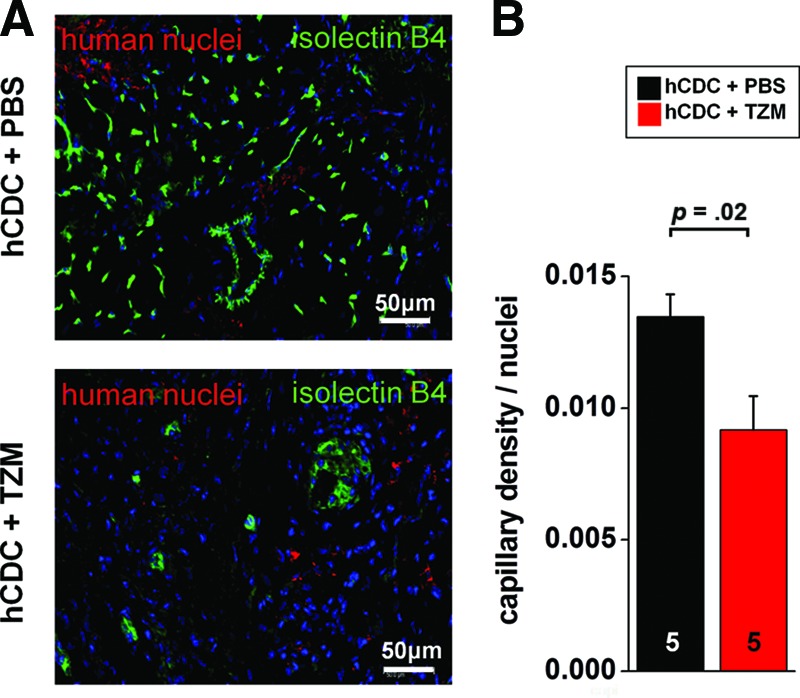
In vivo effect on angiogenesis. (A): Representative images of the infarct border zone myocardium of hCDCs + PBS-treated (top) and hCDCs + TZM-treated (bottom) animals, demonstrating a reduced number of capillaries in TZM-treated animals. Capillaries were stained with isolectin B4-fluorescein (green). Nuclei were stained with 4′,6-diamidino-2-phenylindole (blue) and with an antibody targeting human nuclear antigen (red). (B): After capillaries were stained with isolectin B4-fluorescein, green pixels were normalized to the number of total nuclei in the imaged infarct border zone area. Similar results were obtained when green pixels were divided by the number of human nuclei, stained with an antibody targeting the human nuclear antigen (Chemicon; data not shown). Images were taken at ×20 magnification. Scale bars = 50 μm. Abbreviations: hCDC, human cardiosphere-derived cells; PBS, phosphate-buffered saline; TZM, trastuzumab.
Discussion
Trastuzumab, a recombinant humanized monoclonal antibody against the tyrosine kinase receptor ERBB2, dramatically improves survival in patients with advanced breast cancer. However, initial studies in metastatic disease revealed a surprisingly high incidence of congestive heart failure, especially in patients who were concurrently treated with anthracyclines. More recent adjuvant studies in early-stage breast cancer incorporating well-designed prospective cardiac monitoring suggest a lower incidence of symptomatic heart failure (up to 4% when anthracycline and TZM are given in a sequential fashion) [24, 25]. TZM cardiotoxicity appears to be dose-independent and largely reversible, suggesting a mechanism different from that of anthracyclines [26, 27]. In recent years, several studies have shed light on the role on ERBB receptors and its main ligand neuregulin 1 (NRG-1) in the developing and mature heart. Neuregulin-ERBB signaling is essential for cardiac development [28–30] and for growth and survival in cultured neonatal and adult rat myocytes [31]. Importantly, postnatal disruption of NRG-1/ERBB signaling by gene targeting in mice leads to dilated cardiomyopathy [32], mimicking TZM-associated cardiotoxicity.
Here, we have tested the hypothesis that TZM-associated cardiotoxicity may be mediated by direct adverse effects of TZM on adult cardiac stem cells, in addition to adverse effects on cardiomyocytes. Even though the mammalian heart has been traditionally viewed as a terminally differentiated postmitotic organ, the concept of endogenous cardiac regeneration is now firmly established; through use of radiocarbon dating of human post-mortem cardiac tissue, it has been documented that cardiomyocyte turnover in the adult human heart occurs at an age-dependent rate of 0.4%–1% per year, with ∼40% of the mature heart composed of postnatally generated myocytes [33] (whereas a recent study using immunohistochemistry reported strikingly higher turnover rates [34]). In addition, multiple antigenically distinctive populations of putative endogenous or heart-derived stem cells (defined by an ability to differentiate into multiple cardiac lineages in vitro and in vivo), have now been identified in rodents and humans, including c-Kit+ (CD117, the receptor for stem cell factor) [35] cells, Sca-1+ (stem cell antigen-1) [36] cells, side population cells (characterized by the ability to efflux metabolic markers such as Hoechst because of high expression of membrane pumps) [37], cardiospheres (three-dimensional microtissues of heart stem cells and supporting cells) [7], and hCDCs [8]. With a view to therapeutic application, we have developed a technique to yield clinically relevant numbers of cardiac stem cells and heart-derived stromal cells from small amounts of starting tissue material (such as percutaneous endomyocardial biopsies) in a timely manner [8]. Cardiac biopsies are minced and placed in primary culture, spontaneously shedding outgrowth cells, which can be harvested by gentle enzymatic digestion. When placed in suspension culture, these cells self-organize into multicellular spherical clusters, termed CSps [7]. CSps are replated and further expanded in monolayer culture to yield CDCs. CDCs (in contrast to antigenically purified cells) are a naturally heterogeneous cardiac-derived cell population of nonhematological origin [38], rich in cardiac stem and mesenchymal cells. When grown according to established methods, CDCs exhibit clonogenicity, self-renewal capacity, and multipotentiality [12], thus fulfilling key criteria for stem cells. In vivo, we have demonstrated that CDCs can safely engraft and improve cardiac function post-myocardial infarction (MI) (through both direct and indirect mechanisms) in mice [6, 8, 9], rats [19, 23, 39], and pigs [19, 20]. Importantly, CDCs can be safely delivered via the intracoronary route within a defined dosage range [20]. These cells differentiate primarily into cardiomyocytes and, to a lesser extent, also into endothelial and smooth muscle cells [8, 12, 40]. Here, we demonstrated that hCDCs express ERBB2 receptors at levels comparable to those of adult myocardium. Even though TZM had no effect on proliferation, apoptosis, or size of the c-kit-positive hCDC subpopulation in vitro, the functional benefit of hCDCs injected into the border zone of acutely infarcted immunocompromised SCID-beige mice was abrogated by TZM in vivo. Infarcted animals treated with TZM + hCDCs had a lower ejection fraction, a thinner infarct scar, and a reduced capillary density compared with animals that received hCDCs alone. Functional in vitro assays demonstrated that hCDCs exposed to clinically relevant concentrations of TZM were functionally inhibited, as demonstrated by diminished potential for early cardiogenic differentiation and impaired ability to form microvascular networks in angiogenesis assays. The neuregulin-1/ErbB-signaling axis has been shown to enhance differentiation of cardiomyocytes from embryonic stem cells [41–43] and to upregulate the expression of the cardiac-restricted transcription factors Nkx2.5 and GATA-4 [42]. In line with these findings, TZM led to a reduced expression of markers of early cardiogenic differentiation in hCDCs in the present study (Fig. 4).
Although our work identifies TZM-mediated inhibition of cardiomyogenesis and angiogenesis as potential culprits, the relative roles of these parallel effects, in addition to known adverse effects on cardiomyocytes, are as yet unclear. Given that the median length of TZM therapy is limited to 12 months in the adjuvant setting [2] but could exceed that in the metastatic setting [26], prolonged inhibition of the physiologic turnover of cardiomyocytes could impede the myocardium's ability to recover from myocardial injury. In addition to inhibitory effects on cardiogenesis, trastuzumab's antiangiogenic effects have been well-documented, evident by downregulation of angiogenic factors such as vascular endothelial growth factor, angiopoetin-1, plasminogen activator inhibitor-1, and thrombospondin 1 [44, 45]. In agreement with previous studies [8, 12], we found hCDCs to express several endothelial surface markers, including CD31 and CD34, and to form microvascular tubes on a Matrigel angiogenesis assay. However, there are important differences between hCDCs and endothelial cells (e.g., HUVECs) with respect to angiogenesis. For instance, tube formation occurs much earlier in hCDCs than in HUVECs (3–4 vs. 9–18 hours, respectively), possibly as a result of the mixed cell composition of hCDCs. The recognition that angiogenesis in vivo involves not only endothelial cells but also their surrounding cells has more recently led to the use of organ culture methods to assess angiogenesis. Of these, the rat aortic ring assay has become the most widely used, where the isolated rat aorta is cut into segments and placed in culture, generally in a matrix-containing environment such as Matrigel. Over the next 7–14 days, the explants are monitored for the outgrowth of endothelial (and other) cells, as this is affected by the addition of test substances [46]. In analogy to the aortic ring assay, a microvascular network started to be seen as early as 2–3 days after hCDCs were plated on Matrigel, and cells continued to grow in medium containing 2.5% fetal bovine serum for more than 4 weeks. Consistent with TZM's antiangiogenic properties, the size and complexity of the microvascular outgrowth was dramatically inhibited in hCDCs cultured in TZM-containing medium compared with control. Moreover, TZM-treated animals had a reduced capillary density in the infarct border zone compared with animals that received hCDCs alone.
In line with the current results, previous studies have shown that the assessment of cell number and viability may not entirely reflect the functional capacity of cells in vivo [17]. Differences in cellular bioactivity due to distinct cell isolation protocols were shown to be responsible for discrepant results in clinical trials using bone marrow-derived progenitor cells for cardiac repair: cells that retained their functional activity, determined by conventional migration assays in Boyden chambers, showed a functional benefit in vivo as manifested by a statistically significant increase in ejection fraction, whereas clinical trials using cells with impaired migratory response did not [17]. Likewise, the importance of the cell isolation protocol was also highlighted in a study by Assmus et al., demonstrating that contaminating red blood cells affected the functionality of isolated bone marrow-derived progenitor cells and suggesting a bioactivity response relationship very much like a dose-response relationship in drug trials [47]. The present study underscores the importance of functional in vitro assays to test cellular bioactivity and potential therapeutic efficacy of stem cells. Prospective studies are needed to determine the relationship between differences in the results of angiogenesis and cardiogenic differentiation assays for CDCs isolated from different patients and the cells' functional ability to promote cardiogenic repair.
Conclusion
Collectively, these results indicate that TZM inhibits the cardiomyogenic and angiogenic capacities of hCDCs in vitro and abrogates the morphological and functional benefits of hCDC transplantation in vivo. Thus, functional impairment of human resident cardiac stem cells may represent a novel mechanism of TZM cardiotoxicity.
Study Limitations
The results of the present study indicate that TZM inhibits the angiogenic potential of hCDCs in vitro. Although we were able to demonstrate that TZM slowed the growth and reduced the complexity of the microvascular network, future studies need to address whether the antiangiogenic effect of TZM is mediated by inhibition of the migration or the angiogenic differentiation of hCDCs, or both, in vitro and in vivo.
Although we demonstrated that the cardiomyogenic and angiogenic capacities of hCDCs are inhibited in vitro by TZM, the relative contributions of these mechanisms, in addition to direct toxic effects on cardiomyocytes, to the overall adverse effect on cardiac function deserve further exploration. In the present study, we showed that TZM did not affect myocardial function of healthy mice. One potential limitation of the study is the absence of a trastuzumab-only group post-MI. It is theoretically possible that TZM alone might have effects on the mouse myocardium, such as inhibiting the cardiac healing potential post-MI. However, the marked species specificity of TZM for the human ERBB2 receptor makes such effects unlikely.
We demonstrated that ERBB2 is abundantly expressed in hCDCs, which are a natural mixture of cardiac stem cells and supporting cells with potent regenerative effects in vivo. Future studies need to determine whether particular subpopulations of hCDCs (e.g., c-kit-positive CDCs) express ERBB2 and whether the ERBB2-expressing cells are different from other subpopulations of hCDCs.
Acknowledgments
This study was supported by a grant from the German Research Foundation (Deutsche Forschungsgemeinschaft Grant BA 3341/1-1; to A.S.B.); by a Heart and Stroke Foundation of Canada Research Fellowship (to Y.Z.); by the Canadian Institutes of Health Research (Clinician Scientist Award; to D.R.D.); by the Michel Mirowski, M.D., Fellowship of the Heart Rhythm Society (to E.K.); and by the National Institutes of Health (Grant R01-HL083109). The authors gratefully acknowledge Michelle Leppo's technical assistance with the animal surgery.
Author Contributions
A.S.B.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; Y.Z., T.L., R.R.S., I.C., I.T., D.R.D., E.K., A.S.H., and B.O.: collection and/or assembly of data, data analysis and interpretation; A.C.W. and G.G.: conception and design, manuscript writing; E.M.: conception and design, data analysis and interpretation, manuscript writing, financial support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
E.M. holds equity in a private company (Capricor Inc.) that licenses techniques used to manufacture cardiac stem cells. R.R.S. is partially employed by Capricor Inc. Capricor provided no funding for the present work. The other authors indicate no potential conflicts of interest.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 3.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 4.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 5.Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 6.Li TS, Cheng K, Lee ST, et al. Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells. 2010;28:2088–2098. doi: 10.1002/stem.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messina E, De Angelis L, Frati G, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 8.Smith RR, Barile L, Cho HC, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 9.Chimenti I, Smith RR, Li TS, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–980. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takehara N, Tsutsumi Y, Tateishi K, et al. Controlled delivery of basic fibroblast growth factor promotes human cardiosphere-derived cell engraftment to enhance cardiac repair for chronic myocardial infarction. J Am Coll Cardiol. 2008;52:1858–1865. doi: 10.1016/j.jacc.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 11.Tang YL, Zhu W, Cheng M, et al. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:1209–1216. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis DR, Zhang Y, Smith RR, et al. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS One. 2009;4:e7195. doi: 10.1371/journal.pone.0007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barth AS, Kizana E, Smith RR, et al. Lentiviral vectors bearing the cardiac promoter of the Na+-Ca2+ exchanger report cardiogenic differentiation in stem cells. Mol Ther. 2008;16:957–964. doi: 10.1038/mt.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.du Manoir JM, Francia G, Man S, et al. Strategies for delaying or treating in vivo acquired resistance to trastuzumab in human breast cancer xenografts. Clin Cancer Res. 2006;12:904–916. doi: 10.1158/1078-0432.CCR-05-1109. [DOI] [PubMed] [Google Scholar]
- 15.Sadek H, Hannack B, Choe E, et al. Cardiogenic small molecules that enhance myocardial repair by stem cells. Proc Natl Acad Sci USA. 2008;105:6063–6068. doi: 10.1073/pnas.0711507105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takagawa J, Zhang Y, Wong ML, et al. Myocardial infarct size measurement in the mouse chronic infarction model: Comparison of area- and length-based approaches. J Appl Physiol. 2007;102:2104–2111. doi: 10.1152/japplphysiol.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seeger FH, Tonn T, Krzossok N, et al. Cell isolation procedures matter: A comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 2007;28:766–772. doi: 10.1093/eurheartj/ehl509. [DOI] [PubMed] [Google Scholar]
- 18.Davis DR, Kizana E, Terrovitis J, et al. Isolation and expansion of functionally-competent cardiac progenitor cells directly from heart biopsies. J Mol Cell Cardiol. 2010;49:312–321. doi: 10.1016/j.yjmcc.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis DR, Ruckdeschel Smith R, Marban E. Human cardiospheres are a source of stem cells with cardiomyogenic potential. Stem Cells. 2010;28:903–904. doi: 10.1002/stem.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston PV, Sasano T, Mills K, et al. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–1083. doi: 10.1161/CIRCULATIONAHA.108.816058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang XL, Rokosh G, Sanganalmath SK, et al. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 2010;121:293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zakharova L, Mastroeni D, Mutlu N, et al. Transplantation of cardiac progenitor cell sheet onto infarcted heart promotes cardiogenesis and improves function. Cardiovasc Res. 2010;87:40–49. doi: 10.1093/cvr/cvq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terrovitis J, Lautamaki R, Bonios M, et al. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. J Am Coll Cardiol. 2009;54:1619–1626. doi: 10.1016/j.jacc.2009.04.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Procter M, Suter TM, de Azambuja E, et al. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol. 2010;28:3422–3428. doi: 10.1200/JCO.2009.26.0463. [DOI] [PubMed] [Google Scholar]
- 25.Russell SD, Blackwell KL, Lawrence J, et al. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: A combined review of cardiac data from the National Surgical Adjuvant breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol. 2010;28:3416–3421. doi: 10.1200/JCO.2009.23.6950. [DOI] [PubMed] [Google Scholar]
- 26.Guarneri V, Lenihan DJ, Valero V, et al. Long-term cardiac tolerability of trastuzumab in metastatic breast cancer: The M.D. Anderson Cancer Center experience. J Clin Oncol. 2006;24:4107–4115. doi: 10.1200/JCO.2005.04.9551. [DOI] [PubMed] [Google Scholar]
- 27.Telli ML, Hunt SA, Carlson RW, Guardino AE. Trastuzumab-related cardiotoxicity: Calling into question the concept of reversibility. J Clin Oncol. 2007;25:3525–3533. doi: 10.1200/JCO.2007.11.0106. [DOI] [PubMed] [Google Scholar]
- 28.Gassmann M, Casagranda F, Orioli D, et al. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- 29.Lee KF, Simon H, Chen H, et al. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- 30.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 31.Zhao YY, Sawyer DR, Baliga RR, et al. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273:10261–10269. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
- 32.Ozcelik C, Erdmann B, Pilz B, et al. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc Natl Acad Sci USA. 2002;99:8880–8885. doi: 10.1073/pnas.122249299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kajstura J, Urbanek K, Perl S, et al. Cardiomyogenesis in the adult human heart. Circ Res. 2010;107:305–315. doi: 10.1161/CIRCRESAHA.110.223024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 36.Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: Homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin CM, Meeson AP, Robertson SM, et al. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Develop Biol. 2004;265:262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 38.White AJ, Smith RR, Matsushita S, et al. Intrinsic cardiac origin of human cardiosphere-derived cells. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr172. in press. [DOI] [PubMed] [Google Scholar]
- 39.Cheng K, Li TS, Malliaras K, et al. Magnetic targeting enhances engraftment and functional benefit of iron-labeled cardiosphere-derived cells in myocardial infarction. Circ Res. 2010;106:1570–1581. doi: 10.1161/CIRCRESAHA.109.212589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci USA. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun M, Yan X, Bian Y, et al. Improving murine embryonic stem cell differentiation into cardiomyocytes with neuregulin-1: Differential expression of microRNA. Am J Physiol Cell Physiol. 2011;301:C21–C30. doi: 10.1152/ajpcell.00141.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z, Xu G, Wu Y, et al. Neuregulin-1 enhances differentiation of cardiomyocytes from embryonic stem cells. Med Biol Eng Comput. 2009;47:41–48. doi: 10.1007/s11517-008-0383-2. [DOI] [PubMed] [Google Scholar]
- 43.Zhu WZ, Xie Y, Moyes KW, et al. Neuregulin/ErbB signaling regulates cardiac subtype specification in differentiating human embryonic stem cells. Circ Res. 2010;107:776–786. doi: 10.1161/CIRCRESAHA.110.223917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Güler M, Yilmaz T, Ozercan I, et al. The inhibitory effects of trastuzumab on corneal neovascularization. Am J Ophthalmol. 2009;147:703–708. doi: 10.1016/j.ajo.2008.09.022. e2. [DOI] [PubMed] [Google Scholar]
- 45.Izumi Y, Xu L, di Tomaso E, et al. Tumour biology: Herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416:279–280. doi: 10.1038/416279b. [DOI] [PubMed] [Google Scholar]
- 46.Auerbach R, Lewis R, Shinners B, et al. Angiogenesis assays: A critical overview. Clin Chem. 2003;49:32–40. doi: 10.1373/49.1.32. [DOI] [PubMed] [Google Scholar]
- 47.Assmus B, Tonn T, Seeger FH, et al. Red blood cell contamination of the final cell product impairs the efficacy of autologous bone marrow mononuclear cell therapy. J Am Coll Cardiol. 2010;55:1385–1394. doi: 10.1016/j.jacc.2009.10.059. [DOI] [PubMed] [Google Scholar]