Prospective donation of tissue specimens for induced pluripotent stem cell (iPSC) research requires an approach to informed consent that is constructed for this context. Approaches to informed consent have been variable in ways that threaten the simultaneous goals of protecting donors and safeguarding future research and translation, and investigators are seeking guidance. This analysis addresses this need by providing concrete recommendations for informed consent that balance the goals of iPSC and regenerative medicine researchers with the interests of individual research participants.
Keywords: Clinical translation, Ethics, iPS, Induced pluripotent stem cells
Abstract
Induced pluripotent stem cells (iPSCs) have elicited excitement in both the scientific and ethics communities for their potential to advance basic and translational research. They have been hailed as an alternative to derivation from embryos that provides a virtually unlimited source of pluripotent stem cells for research and therapeutic applications. However, research with iPSCs is ethically complex, uniquely encompassing the concerns associated with genomics, immortalized cell lines, transplantation, human reproduction, and biobanking. Prospective donation of tissue specimens for iPSC research thus requires an approach to informed consent that is constructed for this context. Even in the nascent stages of this field, approaches to informed consent have been variable in ways that threaten the simultaneous goals of protecting donors and safeguarding future research and translation, and investigators are seeking guidance. We address this need by providing concrete recommendations for informed consent that balance the perspectives of a variety of stakeholders. Our work combines analysis of consent form language collected from investigators worldwide with a conceptual balancing of normative ethical concerns, policy precedents, and scientific realities. Our framework asks people to consent prospectively to a broad umbrella of foreseeable research, including future therapeutic applications, with recontact possible in limited circumstances. We argue that the long-term goals of regenerative medicine, interest in sharing iPSC lines, and uncertain landscape of future research all would be served by a framework of ongoing communication with donors. Our approach balances the goals of iPSC and regenerative medicine researchers with the interests of individual research participants.
Introduction
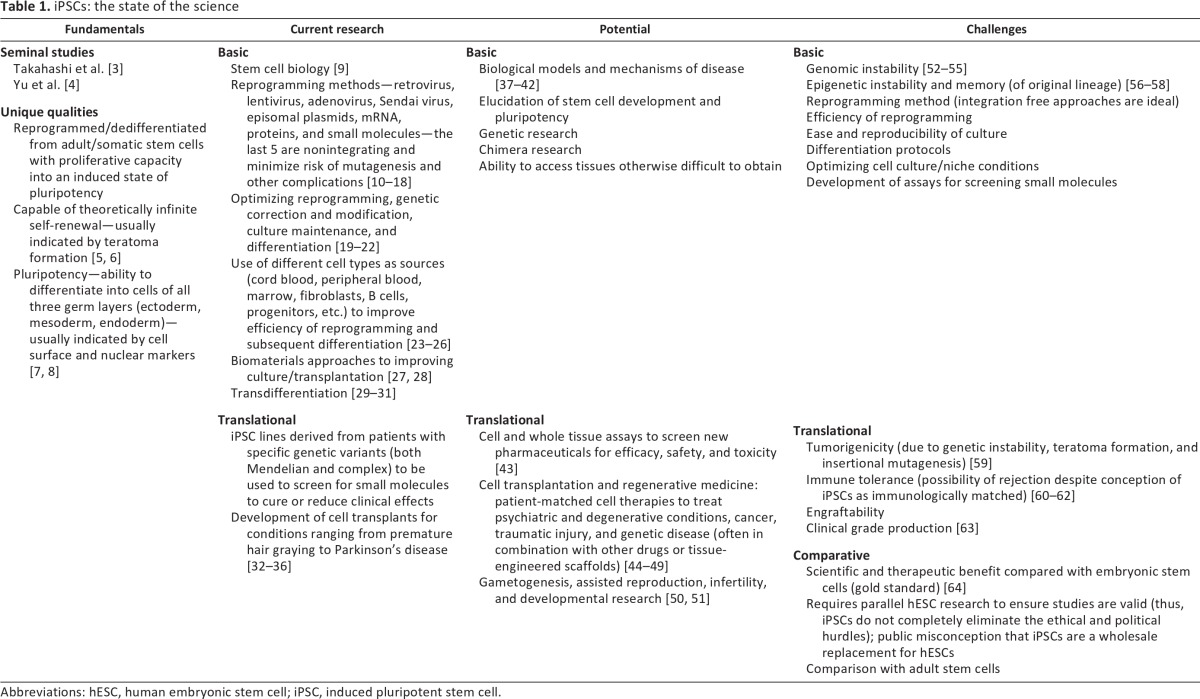
Human induced pluripotent stem cell (iPSC) research activity has seen a monumental expansion since the discovery of somatic cell reprogramming in 2006 [1, 2]. These cells have been hailed as a panacea for basic and translational medical research; the potential of this technology ranges from disease modeling and genetic analysis of otherwise inaccessible tissues to candidate drug screening to immunologically matched cell transplant therapies and novel infertility treatments (Table 1) [3–51]. Although recent evidence has tempered the hope that translating these technologies toward new therapies will be easy [52–64], there is great interest in using iPSC lines to advance translational goals [65]. A broad range of human tissue types are currently being procured to facilitate the generation of iPSC lines [13, 23–26]; ensuring that the prospective collection of specimens for this research proceeds with appropriate informed consent is thus a central objective [66, 67].
Table 1.
iPSCs: the state of the science
Abbreviations: hESC, human embryonic stem cell; iPSC, induced pluripotent stem cell.
Prospective donation of tissue specimens for iPSC research requires a nuanced approach to informed consent. Many of the salient features of iPSC research are no different from research involving other pluripotent stem cells, immortalized cell lines, human biospecimens, genetic and genomic analyses, reproductive research, and transplantation [67]. However, although the individual issues associated with these cells are not new, iPSC research uniquely encompasses them all. iPSCs are immortal, creating longitudinal challenges related to withdrawal, confidentiality, and sharing. They are pluripotent, and the range of possible uses of these cells is ever-growing. They are likely to undergo genomic analyses for intensive characterization and disease screening, marrying these cells to the ethical concerns of genomics. Furthermore, because these cells can be sourced from almost any kind of specimen and can be used for potentially limitless rounds of derivation and paths of differentiation, there is the potential for unprecedented flexibility in derivation, sharing, and banking. The scientific potential of iPSCs and the future therapies they make possible are extraordinary. In addition, research with iPSCs is unique because of the drive to transition rapidly toward therapy, making translational goals fundamental to this research from the outset.
Although a clear set of research and translational goals for iPSC research has been articulated [65], the precise nature of future research is largely unknown at this time, and providing appropriate information to prospective donors of specimens for iPSC research via the consent process is especially challenging. This is important, given data about public attitudes that suggests there may be hesitation to participate in the earliest stages of research and significant concerns about germline cell derivation and reproductive applications, although there is general support for iPSC research with appropriate ethical and regulatory oversight [68]. The process of contributing specimens to iPSC research can open doors to significant applications and associated concerns that are not yet foreseeable by scientists, the magnitude of which are incongruous with the relatively facile donation process and are difficult to convey in a consent form.
We argue here that the long-term goals of regenerative medicine, broad interest in sharing iPSC lines among scientists, the largely unknown landscape of future research, and sensitivities associated with some potential uses all point to a consent process that comprehensively addresses these goals and concerns. We describe an approach that asks people to consent prospectively to a broad umbrella of foreseeable research, anticipating downstream issues and goals to the extent possible by including future therapeutic applications of iPSCs. We believe this is the best approach among multiple ethically acceptable avenues. It balances the goals of iPSC and regenerative medicine researchers with the interests of individual research participants. By granting that participants have longitudinal interests in the use of their specimens, and by providing narrowly defined mechanisms by which to exercise those interests, our approach encourages a framework of ongoing communication between researchers and participants. This approach will allow scientific progress to continue forward while ensuring that donors of specimens are respected when providing consent to such a broad scope of future research.
Background
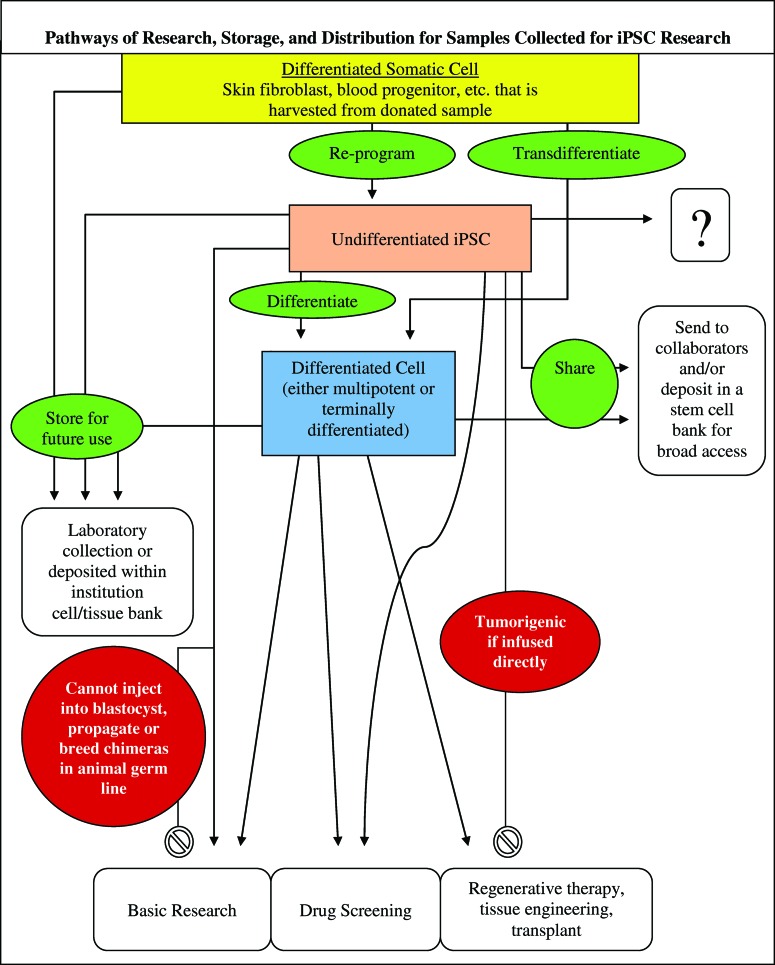
There is value in developing a consistent approach to informed consent across various research institutions in these still-early stages of regenerative medicine. Inadequate consent processes can both undermine the public's trust in research with cell lines, as the HeLa cell case teaches us [69], and hinder future research with cell lines. Problematic variability has been documented in consent forms for research involving the collection, storage, and future use of biological specimens [70, 71]. Such variation has complicated the ability of researchers to use embryonic stem cell lines [72–74]. Anticipating future applications and associated regulatory requirements now, to the extent possible, will help balance the goals of protecting participants with maximizing the utility of iPSC lines in research. Although it is not possible to predict which iPSC lines will be the “next HeLa” and lead to important therapeutic discoveries, following careful consent procedures for all generated iPSC lines will both ensure ethical provenance of all cell lines as they traverse many potential research and distribution pathways (Fig. 1) and protect the ability to utilize rare and valuable discoveries associated with any of the cell lines down the road.
Figure 1.
A flowchart depicting the ways in which a specimen obtained for iPSC research can traverse multiple pathways: reprogramming, differentiation, transdifferentiation, storage, banking, and final research purposes. Abbreviation: iPSC, induced pluripotent stem cell.
A variety of approaches to informed consent for research on specimens can be ethically justified [75, 76]. Some argue that research on specimens is well-suited to broad and open-ended consent approaches that involve a single interaction between the researcher and participant [75, 77, 78]. Policies that facilitate the systematic use of one-time consent for specimen research have been incorporated into proposed reforms to the U.S. human subjects regulations [79]. Such models assume that ongoing interaction with participants is neither necessary for the research nor desirable from the participant's perspective, and studies suggest that many participants are indeed comfortable with a one-time approach to consent for research with their specimens [77, 80].
However, there remain concerns about broad, one-time consent: specifically, questions about whether and how prospective participants can give adequate informed consent if they cannot be informed at the time of donation about some of the possible ways that their specimens will be used in the future. Allowing a participant to give carte blanche permission to unbounded future research may be inadequate in the case of iPSCs, as it is reasonable to assume that most participants are not truly aware of what such a broad permission could entail [81]. Broad, open-ended approaches may deter a small number of participants from enrolling in research. Although this may be a reasonable trade-off for some categories of research, it would be problematic if those with acute or rare diseases, who have the most at stake and whose specimens are also likely valuable, are disproportionately deterred from participation by being required to sign on so broadly.
At the other end of the spectrum are proposals to construct consent forms narrowly, limiting them to study specifics that are known at the present time [75, 76, 82]. Such approaches would likely assume that it is premature to prognosticate about future therapeutic applications of iPSCs, at least in most cases. Although broad up-front consent might be allowable in a limited number of cases—for example, if scientists had the ability to determine that a specific cell line would be particularly useful—researchers would generally be required to recontact participants to get consent for each new project and application that uses their iPSCs. However, there are several problems with this approach. First, a requirement for repeated reconsent in most cases is inefficient and would hinder the ability to share and use samples and cell lines broadly. In addition, it is highly unlikely that researchers can predict at this time which cell lines will be clinically useful in the future; this is not currently a useful metric for choosing between broad versus narrow consent. The current state of scientific knowledge requires us to assume that all iPSC lines have potential future clinical utility, even if only a few lines will actually go on to be used in therapeutic applications. Clinical trials with human embryonic stem cells (hESCs) show that it is possible to make clinically compliant samples from research grade materials [63], and samples obtained through blood and cord blood banks and bone marrow registries are already collected in a clinical-grade manner [23]. Finally, we are skeptical that narrowly tailored consent approaches that require frequent recontact of participants substantively enhance protections or respect for participants in iPSC research more so than approaches that include broader descriptions of iPSCs and their future applications [72].
On balance, these concerns about overly broad and overly narrow approaches to consent point to a process that provides accurate information about the broad aims of iPSC research and downstream goals, draws boundaries around the scope of the consent, and establishes an ongoing dialogue with participants that allows for reconsent in some cases. We believe such a middle ground exists.
Regulatory and Ethical Frameworks
Before delving into the content of consent forms, it is helpful to define the purpose of informed consent for iPSC research. The overarching goal is to provide sufficient baseline information that enables potential participants to decide whether to give permission for iPSC research to proceed with their specimens [83]. The consent process respects the autonomy of participants by giving them reasonable control over the use of their specimens. Informed consent also has the potential to establish a broader relationship between investigators and participants aimed at mutual benefit, trust, and education [84]. The degree of control that is granted to participants, the frequency of interactions between researchers and participants, and the level of detail about possible research applications are all dimensions of the consent process that need to be decided.
Tracing the development of the discourse surrounding iPSCs also helps frame the requirements for informed consent. Because iPSCs obviate the need for source blastocysts, they were initially hailed as ethically superior to hESCs [85–88]. Yet iPSCs are not wholly free from the encumbrances of prior ethical debates regarding hESCs [89–92]. Global objections to iPSC research related to its complicity with embryonic stem cell research (arising from the necessary symbiosis between the two research programs), its ability to alter our conceptions of human life, and its implications for human-animal chimeras have been raised [66, 93–102]. Although these questions continue to be debated, both publicly and privately funded research with iPSCs is proceeding with broad scientific, political, and public support and without the heavy regulation that has characterized research with hESCs [103, 104].
Only recently have iPSCs been recognized to have more complex ethical dimensions [67], presenting concrete ethical and logistical issues analogous to those with which biobanks, stem cell research oversight (SCRO) committees, institutional review boards (IRBs), and other bodies have previously struggled including terms of use, confidentiality, tracking, governance, and withdrawal [105–109]. There is also uncertainty about the role that the U.S. Food and Drug Administration (FDA) will play in regulating this research and translation [110–115]. (Of particular concern for researchers involved in regenerative medicine is anticipating how FDA oversight and regulation will affect the technology transfer and translation of iPSC-related materials and therapies, what up-front and ongoing requirements will need to be satisfied [infectious disease testing, at a minimum], and what role, if any, informed consent will play.) The informed consent process for research with iPSCs needs to take this complex ethical backdrop into account [75, 116, 117]. An emerging literature highlights the value of “tiered” approaches that allow participants to make choices [67], and describes traceability and withdrawal concerns as they apply to consent for stem cell banking [84]. Guidelines at all levels of oversight—international bodies and working groups [118–121], federal agencies [122, 123], state boards and foundations [124, 125], even institution-level IRBs and SCROs—are beginning to speak to the complexities of regulation and informed consent in these contexts by recommending new approaches, including specific provisions for human transplantation, recontact, stem cell banking, opt-out for return of results, tiered consent, and partial withdrawal. Our analysis builds upon this foundation.
Methods
We developed a model consent template for the prospective creation of iPSC lines for research. This template was informed by (a) a conceptual analysis and normative balancing of issues associated with the various informed consent content domains, and (b) a content analysis of 25 iPSC-specific consent forms and previously approved language that were shared with us by investigators and administrators from a variety of U.S. and international institutions (Table 2). Our analysis incorporates a review of relevant literature and careful tailoring of various consent domains to the iPSC context. The example consent forms were reviewed for clarity as well as content and compared with each other to uncover qualitative patterns. In addition, our proposed template was informed by conversations with a variety of stakeholders, including investigators, bioethicists, IRB chairs, lawyers, research administrators, and those involved in federal and state-level stem cell research policy and regulation.
Table 2.
Sources of consent forms: list of affiliations of collaborating investigators

As part of this process, we reviewed consent forms from NIH intramural investigators at multiple stages of protocol and institutional review board review and through multiple iterations of editing.
This project was a joint effort between the newly formed National Institutes of Health (NIH) Center for Regenerative Medicine and the NIH Clinical Center Department of Bioethics, with the goal of producing a consent template for prospective collection of fresh specimens to create iPSCs for research that would be useful both for our intramural investigators and the broader research community. The guidance provided here is meant to take advantage of a window of opportunity to harmonize the guidance provided to investigators and institutions, but of course is not a substitute for IRB approval of individual studies.
A summary of the provisions that were incorporated into our model consent template (supplemental online data) is provided in Tables 3 and 4 and discussed below.
Table 3.
Consent recommendations for scope and limits of iPSC research

Some unspecified or unknown future research uses might be straightforward and will not be sensitive or controversial in nature. These could conceivably be covered under the original consent (i.e., as Foundational research and applications in the table), as long as the distinction is explained.
This provision requires a mechanism for tracking subjects' willingness to be recontacted (e.g., check box on consent form) and ability to follow up in the future when novel applications in this category are being pursued.
If a participant cannot be recontacted for affirmative reconsent in the case of sensitive future research projects, it should be assumed that the participant has not consented to these projects, and other samples must be obtained and used.
Abbreviation: iPSC, induced pluripotent stem cell.
Table 4.
Other consent recommendations
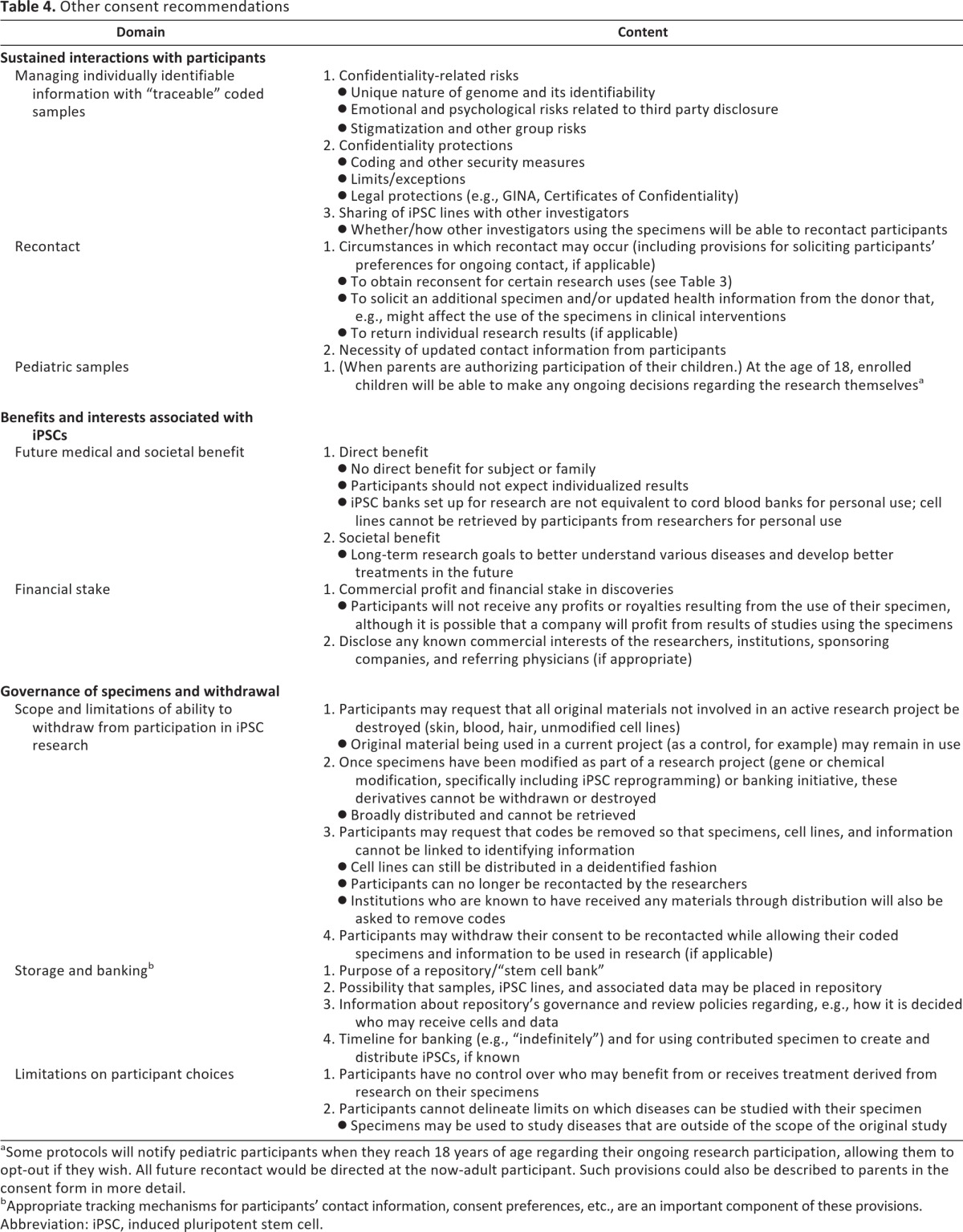
Some protocols will notify pediatric participants when they reach 18 years of age regarding their ongoing research participation, allowing them to opt-out if they wish. All future recontact would be directed at the now-adult participant. Such provisions could also be described to parents in the consent form in more detail.
Appropriate tracking mechanisms for participants' contact information, consent preferences, etc., are an important component of these provisions.
Abbreviation: iPSC, induced pluripotent stem cell.
Analysis of Consent Domains
We evaluated the content domains of informed consent that are most relevant to iPSC research and concluded that the most salient ethical concerns arise from the scope of possible future research applications, both foreseeable and unknown, of iPSCs. Many potential uses—reproductive research and gamete generation, mixing of human and animal biological materials, pharmaceutical screening (separation between “research purposes” and commercial development for profit), transplantation into humans for regenerative medicine, and genetic sequencing and manipulation—are essential as both methods and paths for future exploration yet are potentially sensitive in nature and may reasonably elicit objection from prospective participants. These uses sort into three general categories: core foundational methodologies, regenerative medicine, and reproductive research. Furthermore, it can be assumed that there are potential uses of these cells that cannot yet be predicted, some of which may be sensitive. The consent process should provide sufficient information to allow participants to be aware of all of these possibilities.
We acknowledge that participants have an inherent stake in how their donated specimens are used, and thus have longitudinal interests in the research performed on the generated iPSC lines generated. This gives rise to several additional concerns that are appropriate to address in the informed consent process. These concerns can be sorted by the general principles and categories of informed consent. Although these categories are not necessarily unique to iPSC research, the discussion proceeds with an eye toward how they should be applied to that context.
Confidentiality, Traceability, and Sustained Interaction
A description of risks to confidentiality and measures that will be taken to minimize these risks are required elements of informed consent (as per 45 CFR 46.116) [126]. This information is important in part because it allows participants to make informed decisions about the acceptable amount of privacy risk.
Traceability (i.e., maintaining accessible coded identifiers rather than de-linking samples and associated data) may be beneficial in the case of pluripotent stem cell banking. A system of traceable specimens can facilitate ongoing communication, participation, and information exchange. A “sustained interaction,” so defined, would benefit the research enterprise by fostering public trust and streamlining the translation of research [84]. Traceability can also facilitate longitudinal data collection, create an avenue for return of research results, and enable participants to be informed of research outcomes [84].
Traceability does entail greater risks to confidentiality, requiring explicit language in the consent form about the protections being afforded. Although de-linking or “anonymization” significantly reduces risks to confidentiality, these measures may limit the scientific utility of the lines, as well as any associated benefits to participants. Furthermore, these approaches do not address the ethical problems associated with the inherent stake that participants have in the ongoing and future uses of their specimens [72], and limited empirical evidence shows that participants do not necessarily favor this approach [127]. A balance must be struck that takes into account scientific considerations, privacy concerns, obligations to maintain ongoing contact with participants, and practical administrative realities.
Return of Benefit and Information
Closely related is the idea of return of benefit through the provision of research results. Prospective participants are unlikely to benefit from iPSC research directly—either through a direct medical intervention or through future access to “personal” stem cells. However, there remain open questions about obligations to return either aggregate or individual research results, validity of individual results when obtained downstream from mutated or genetically altered cell lines, and methods to capture participants' preferences regarding receipt of such results. Any plans for recontact and return of results, whether robust or limited, should be described up front in the consent process.
Commercial Product Development
Because iPSCs will potentially be used as drug screening models or direct transplant interventions, commercial profits may be possible. The potential for profits connected to iPSC research should be clearly expressed to participants, as this may affect a participant's conception of his or her “donation.” Such disclosures are prompted largely by legal precedents [128] but are motivated by ethical considerations as well. Some argue that sponsorship, commercialization, and financial interests are relevant to a participant's assessments of risks and benefits, and call for exhaustive disclosure of commercial aspects prior to enrollment. However, this must be weighed against the need for concise consent forms to better promote understanding, which is already quite challenging [129, 130]. The potential for commercialization may be relevant to participants' decisions to allow their cells to be used therapeutically.
Withdrawal
A right to withdraw from participation in biomedical research is widely acknowledged in the human subjects research ethics literature [131]. How this right extends to donated specimens and pluripotent cell lines is unclear, however, particularly for those samples that will be stored and distributed widely. The extent of a right to withdraw is further complicated by questions about whether cell line derivatives still constitute human subjects research. There are many possible avenues for withdrawal that vary greatly in both practicality and adequacy: ceasing recontact, anonymization (destruction of identifiers), destruction of original specimens, ceasing distribution of any materials (original or derivative) and information, complete destruction of iPSC lines, and efforts to ask partner institutions to anonymize or cease use of the shared iPSC lines. The choice of an appropriate approach should take into account religious beliefs and cultural traditions that require specimens to be returned to participants [132].
Storage, Banking, and Exchange
Provisions for sharing and storage of iPSC lines may be of particular interest to participants, including whether these cells will stay with the primary investigator, will be shared nationally or internationally, and/or will be banked in a widely available cell repository. Institutions may vary in their governance of the use of these cells and in confidentiality protections, and restrictions promised by the initial investigator may not transfer when shared. Participants should be informed about oversight assurances and provisions for future uses, regardless of goal or location of distribution.
Recontact and Reconsent
Recontact permits evolving issues related to the research to be addressed with participants on an ongoing basis. Some have recommended recontact of participants in pursuit of reconsent if iPSCs derived from their specimens are to be used either for potentially sensitive applications, such as therapy or gamete generation, or for uses that were unknown at the time of consent. Data on participant attitudes in the field of genomics supports a reconsent approach when samples and data are being used for substantively different purposes than originally proposed [133]. There is also debate about whether pediatric participants should be recontacted to seek consent once they reach the age of majority [134]. All of this must be weighed against the administrative burdens that reconsent provisions may have, including the potential of such provisions to slow down or otherwise impede needed research (especially if particular therapeutic benefit is found in a cell line but the initial participant cannot be found to get the needed permission).
With pluripotent stem cell research, the need for recontact does not end with future research permission. It may also be appropriate to recontact a participant for a variety of different reasons: disclosing incidental or individualized research findings, soliciting updated health information from the participant (e.g., to verify a specimen's safety for human transplantation) [110–112], recruitment for future studies, describing changes in the scope of the current research study, and ensuring continued consent for use of a specimen obtained from a pediatric participant.
Reassurance
A consent form can be used as a tool to assure participants of the ethical use and governance of their specimen. However, providing detailed information about what will not be done with a participant's specimen can be problematic. It may be sensible to reassure a participant that certain uses are prohibited, specifically reproductive cloning and germ-line introduction. Going beyond this, however, may facilitate misconceptions and may hinder research in unintended ways.
Our Approach to Consent for iPSC Research
The consent form template that we developed is included as supplemental online data, and the general structure is described below in Tables 3 and 4. The following sections provide the rationale for the content and structure decisions that we made, including provisions for future research uses, sustained interactions with participants, prospects for benefits and payments, and withdrawal from research, as well as logistical and governance issues. This analysis, at the very least, represents the necessary categories that must be addressed in formulating an iPSC consent process. We recognize that institutions and states will have specific regulations and requirements that may conflict with our recommendations; the consent form will have to be tailored appropriately in each case.
Our recommendations rely on several assumptions about the direction of the fields of iPSC research and regenerative medicine. First, as noted earlier, there is an intense focus on prospective planning to rapidly translate these cell lines to clinical use, both for drug development platforms and for cell transplantation and tissue engineering (Table 1). Second, we believe that the iPSC research enterprise will benefit from longitudinal relationships between specimen donors and researchers, supported by the fact that banking is most useful and ethical when ongoing commitments predicated on a thorough up-front consent can be honored.
Future Research Uses
The approach we take in addressing the scope of iPSC research (Table 3) is geared toward an “omnibus” consent model in which the research purposes are open-ended and include broad future uses and applications, within limits. Our framework draws a line past which prospective consent cannot go at this time. This line is structured such that cases of future problematic research will be relatively rare but would require recontact for reconsent. Our template assumes that some future research uses will require affirmative reconsent, including (a) reproductive research and (b) particularly sensitive areas of research that have not yet been anticipated. An inability to get reconsent from participants would proscribe using their iPSC lines for these research uses. We also followed the lead of some existing consent models and encourage participants to provide updated contact information so that ongoing contact will be possible.
We assume that including therapeutic goals—specifically, regenerative medicine and cell transplantation—is ethically permissible within the broad scope of a prospective consent, given that translation is one of the fundamental goals of iPSC research. This is a potentially controversial stance, and it is not the only approach currently being taken. Others have suggested that, like reproductive research, introducing derivatives of iPSCs made from a participant's samples directly into other patients has special status and thus should require specific reconsent in the future [67], and this approach was reflected in some of the existing consent forms that we reviewed. However, there is precedent for consent approaches that clearly describe the prospects for therapeutic applications of iPSCs so that subjects can decide whether they are comfortable accepting those possibilities.
Sustained Interaction
Our consent template reflects an intention for sustained interactions [84] with participants in select circumstances about the ongoing uses of their coded specimens. This will be important for both the original and secondary researchers, as the need for recontact increases with time and scientific advancement. Provisions that support a sustained interaction include a description of plans for recontact in multiple but limited circumstances, the coding and linkability of specimens, and plans for engaging pediatric participants once they reach the age of majority (Table 4).
Our review of existing consent forms revealed significant variation in plans for recontact, including plans to de-link, which may be problematic as previously discussed. Our approach is consistent with the scope of permissible future research discussed above, permitting cell lines to be used for both research and translational applications and allowing researchers to meet obligations to provide participants with feedback over time.
Sustained interactions are also relevant in the case of pediatric subjects, who eventually will become adults over the course of the research and will have autonomous interests in the use of their specimens at that time. Accordingly, we have incorporated plans for pediatric reconsent that allow children to be informed upon reaching adulthood of the ability to review the previous consent. We endorse pediatric repository models that notify children when they reach the age of majority that their specimen is being used in research and that any decision making regarding the future use of those specimens, withdrawal, etc. now falls to them; we realize, however, that there might be difficulties in locating and tracking these participants [134, 135].
We acknowledge that our recommendations for sustained interactions with participants lead to additional logistical steps, such as developing web interfaces and newsletters that inform participants of the types of research being done, the results, the implications, and the goals. This increased communication will inform participants of the research to which they are integrally contributing, provide opportunities for feedback, and create openness within the research enterprise.
Prospects for Benefits and Payments
Given that the promise of iPSC research carries with it heightened expectations about the translational benefits and a risk of therapeutic misconception because of the emphasis on “personalized treatments” in public discourse, as well as questions about future commercial potential and patenting, our consent form template attempts to temper participants' expectations [105, 136–139]. (Therapeutic misconception is the mistaken belief that research is both designed for and likely to help research subjects personally. It is common among research participants. The misconception is concerning because it might lead participants to believe that research on their samples will be prioritized for their particular conditions and that treatments will be developed for them [or their families] personally as part of the research study in which they are participating.) The template explicitly informs participants that direct benefits to them are unlikely and that they will not benefit financially from any commercial product developments based on their specimens (Table 4). We also included language underscoring that participants will not be able to retrieve cell lines developed from their specimens for personal use, nor can they choose who can receive future treatments that may be developed from their iPSC lines (Table 4). This language parallels the NIH requirements for hESC and consent [140].
We generally found that existing consent forms frame these discussions about benefits and profits in similar ways, although we found some problematic language that hints at a possibility of direct medical benefit. We also identified variation in the description of plans for the management and disclosure of incidental research findings. Because the ethical discourse on these questions is far from settled and because individualized findings may not be valid after cell lines have mutated or been reprogrammed, we have not taken a position on what (if any) obligations exist to return findings in this context, only facilitating the diversity of approaches to disclosure.
Withdrawal from Research Participation
Our approach permits various forms of withdrawal at different stages of the research process (Table 4). Although the logistics of a staged approach are more complicated and require tracking mechanisms, it yields a net benefit for both research participants and the research process. For example, allowing withdrawal of just the identifying information associated with a specimen can help preserve some of the scientific utility of that specimen. Some existing consent forms describe more extensive opportunities for the withdrawal of original or derivative materials, which can be justified as long as these promises can reasonably be tracked and honored.
Logistics and Governance
Our group struggled with how many separate decisions to grant participants within the consent form. Should participants be asked to agree to ongoing research as proposed, given the opportunity to agree to ongoing contact in certain cases, and/or given explicit choices (via check boxes) about the various aspects of the research? We attempted to carve out the simplest and broadest system we could justify, keeping in mind that check boxes, although attractive, could be logistically difficult to track and also may register participants' reflexive reactions rather than their well-considered values. Any provisions allowing for explicit participant decisions that include check boxes necessitate a tracking mechanism. Although burdensome, it may be beneficial for iPSC banks to develop a standardized system to track and interpret these consent provisions in order to most efficiently respect the initial participant requests, especially when the specimens are banked and shared (Table 4). A standardized consent form and consistent approaches to tracking participants and their preferences go hand-in-hand. A broader conversation needs to happen within the research community about infrastructure across institutions to track iPSC lines and the associated consent provisions and terms of use. Finally, to facilitate understanding of the complexities of iPSC research, we recommend that supplementary educational materials describing iPSCs be distributed to participants along with the consent form. The manual developed by the International Society for Stem Cell Research [141] is a useful model.
Conclusion
Approaches to informed consent for iPSC research thus far have been variable, often in ways that could create confusion for participants and hinder research collaborations and future uses. This variation goes beyond what we might expect based on differences in study design and institutional policies; it seems to reflect fundamental disagreements about the appropriate scope of the consent process at this time, the manner in which future plans should be described, and the necessity of ongoing contact with participants. This lack of consensus is problematic in a field with accelerating momentum that will rely on the sharing of cell lines.
Our proposal reflects the broadest possible approach to consent that we believe can be ethically justified, incorporating all of the currently foreseeable ways in which iPSC research will play out. Although our approach does risk excluding some participants who are not comfortable with the stated long-term goals, we accept this as the trade-off for making a model that is practically useful to researchers as well as to a majority of research participants.
On its surface, our reappraisal of informed consent for iPSC research may seem to have limited consequences. It is important, however, as a first step in the larger process of improving dialogue between the research enterprise and the public. Although empirical data are limited, there is some evidence that the public has visceral discomfort, both legitimate and unfounded, with certain forms of research. As Aalto-Setälä et al. warn, “if the perception that iPSC research poses no ethical concerns is not corrected, there could be a backlash against iPSCs later” [67].
The importance of informed consent extends beyond the physical, economic, and psychosocial risks associated with iPSC research [72]. Research participants have autonomy-based interests in the longitudinal uses of their samples, especially when they might object to some uses on moral grounds. Thoughtful informed consent, accordingly, plays a role that is critical to the success of research with iPSCs: assuring the public that researchers will “honor both the letter and spirit of the agreements between researchers and subjects” [72]. We believe that the proposal described herein represents a significant step toward these goals.
Acknowledgments
We gratefully acknowledge the investigators and administrators who generously shared their consent forms, templates, and guidance documents with us. We thank Samantha Finstad, Hans Hermans, and Mine Kimura for providing us with thorough English translations of the Dutch and Japanese consent forms. We are also grateful to Miriam Rosenbaum for her assistance at the inception of this project, and to the many people who provided helpful feedback on earlier drafts of the manuscript and consent template, including Benjamin Berkman, Barbara Karp, and members of the NIH Office of General Counsel, the NIH Human Subjects Research Advisory Committee, the NIH Clinical Center's Department of Bioethics, and the NIH hESC Administrative Review Committee. This research was supported by the Intramural Research Program of the NIH.
Author Contributions
J.L.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript; S.L.: conception and design, final approval of manuscript, liaison to investigators during consent form drafting process; M.R.: conception and design, administrative support, final approval of manuscript; S.C.H.: conception and design, financial support, administrative support, provision of study material, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, De Los Angeles A, Zhang J. The art of human induced pluripotent stem cells: The past, the present and the future. Open Stem Cell J. 2010;2:2–7. [Google Scholar]
- 3.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez-Aranda I, Ramos-Mejia V, Bueno C, et al. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells. 2010;28:1568–1570. doi: 10.1002/stem.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 7.Ma J, Guo L, Fiene SJ, et al. High purity human-induced pluripotent stem cell-derived cardiomyocytes: Electrophysiological properties of action potentials and ionic currents. Am J Physiol Heart Circ Physiol. 2011;301:H2006–H2017. doi: 10.1152/ajpheart.00694.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bharti K, Miller SS, Arnheiter H. The new paradigm: Retinal pigment epithelium cells generated from embryonic or induced pluripotent stem cells. Pigment Cell Melanoma Res. 2011;24:21–34. doi: 10.1111/j.1755-148X.2010.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phanstiel DH, Brumbaugh J, Wenger CD, et al. Proteomic and phosphoproteomic comparison of human ES and iPS cells. Nat Methods. 2011;8:821–827. doi: 10.1038/nmeth.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stadtfeld M, Nagaya M, Utikal J, et al. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu J, Hu K, Smuga-Otto K, et al. Human induced pluripotent stem cells free of vector and transgene. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warren L, Manos PD, Ahfeldt T, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giorgetti A, Montserrat N, Rodriguez-Piza I, et al. Generation of induced pluripotent stem cells from human cord blood cells with only two factors: Oct4 and Sox2. Nat Protoc. 2010;5:811–820. doi: 10.1038/nprot.2010.16. [DOI] [PubMed] [Google Scholar]
- 14.Anokye-Danso F, Trivedi CM, Juhr D, et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8:376–388. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng B, Ng JH, Heng JC, et al. Molecules that promote or enhance reprogramming of somatic cells to induced pluripotent stem cells. Cell Stem Cell. 2009;4:301–312. doi: 10.1016/j.stem.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Ho R, Chronis C, Plath K. Mechanistic insights into reprogramming to induced pluripotency. J Cell Physiol. 2011;226:868–878. doi: 10.1002/jcp.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W, Ding S. Small molecules that modulate embryonic stem cell fate and somatic cell reprogramming. Trends Pharmacol Sci. 2010;31:36–45. doi: 10.1016/j.tips.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Li W, Jiang K, Ding S. A chemical approach to controlling cell fate and function. Stem Cells. 2012;30:61–68. doi: 10.1002/stem.768. [DOI] [PubMed] [Google Scholar]
- 19.Burridge PW, Thompson S, Millrod MA, et al. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS One. 2011;6:e18293. doi: 10.1371/journal.pone.0018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanna J, Saha K, Pando B, et al. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature. 2009;462:595–601. doi: 10.1038/nature08592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zwi-Dantsis L, Mizrahi I, Arbel G, et al. Scalable production of cardiomyocytes derived from c-Myc free induced pluripotent stem cells. Tissue Eng Part A. 2011;17:1027–1037. doi: 10.1089/ten.TEA.2010.0235. [DOI] [PubMed] [Google Scholar]
- 22.Narsinh KH, Wu JC. Gene correction in human embryonic and induced pluripotent stem cells: Promises and challenges ahead. Mol Ther. 2010;18:1061–1063. doi: 10.1038/mt.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao M, Ahrlund-Richter L, Kaufman DS. Concise review: Cord blood banking, transplantation and induced pluripotent stem cell: Success and opportunities. Stem Cells. 2012;30:55–60. doi: 10.1002/stem.770. [DOI] [PubMed] [Google Scholar]
- 24.Rajesh D, Dickerson SJ, Yu J, et al. Human lymphoblastoid B-cell lines reprogrammed to EBV-free induced pluripotent stem cells. Blood. 2011;118:1797–1800. doi: 10.1182/blood-2011-01-332064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haase A, Olmer R, Schwanke K, et al. Generation of induced pluripotent stem cells from human cord blood. Cell Stem Cell. 2009;5:434–441. doi: 10.1016/j.stem.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 26.Tan KY, Eminli S, Hettmer S, et al. Efficient generation of iPS cells from skeletal muscle stem cells. PLoS One. 2011;6:e26406. doi: 10.1371/journal.pone.0026406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mei Y, Saha K, Bogatyrev SR, et al. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat Mater. 2010;9:768–778. doi: 10.1038/nmat2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saha K, Mei Y, Reisterer CM, et al. Surface-engineered substrates for improved human pluripotent stem cell culture under fully defined conditions. Proc Natl Acad Sci USA. 2011;108:18714–18719. doi: 10.1073/pnas.1114854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Efe JA, Yuan X, Jiang K, et al. Development unchained: How cellular reprogramming is redefining our view of cell fate and identity. Sci Prog. 2011;94:298–322. doi: 10.3184/003685011X13131588500975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ieda M, Fu JD, Delgado-Olguin P, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J, Efe JA, Zhu S, et al. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci USA. 2011;108:7838–7843. doi: 10.1073/pnas.1103113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dyson SC, Barker RA. Cell-based therapies for Parkinson's disease. Expert Rev Neurother. 2011;11:831–844. doi: 10.1586/ern.11.33. [DOI] [PubMed] [Google Scholar]
- 33.Galach M, Utikal J. From skin to the treatment of diseases—the possibilities of iPS cell research in dermatology. Exp Dermatol. 2011;20:523–528. doi: 10.1111/j.1600-0625.2011.01282.x. [DOI] [PubMed] [Google Scholar]
- 34.Rhee YH, Ko JY, Chang MY, et al. Protein-based human iPS cells efficiently generate functional dopamine neurons and can treat a rat model of Parkinson disease. J Clin Invest. 2011;121:2326–2335. doi: 10.1172/JCI45794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaccarino FM, Stevens HE, Kocabas A, et al. Induced pluripotent stem cells: A new tool to confront the challenge of neuropsychiatric disorders. Neuropharmacology. 2011;60:1355–1363. doi: 10.1016/j.neuropharm.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wetsel RA, Wang D, Calame DG. Therapeutic potential of lung epithelial progenitor cells derived from embryonic and induced pluripotent stem cells. Annu Rev Med. 2011;62:95–105. doi: 10.1146/annurev-med-052009-172110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai W, Zhang Y, Kamp TJ. Imaging of induced pluripotent stem cells: From cellular reprogramming to transplantation. Am J Nucl Med Mol Imaging. 2011;1:18–28. [PMC free article] [PubMed] [Google Scholar]
- 38.Itzhaki I, Maizels L, Huber I, et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature. 2011;471:225–229. doi: 10.1038/nature09747. [DOI] [PubMed] [Google Scholar]
- 39.Park IH, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134:877–886. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saha K, Jaenisch R. Technical challenges in using human induced pluripotent stem cells to model disease. Cell Stem Cell. 2009;5:584–595. doi: 10.1016/j.stem.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saha K, Hurlbut JB. Disease modeling using pluripotent stem cells: Making sense of disease from bench to bedside. Swiss Med Wkly. 2011;141:w13144. doi: 10.4414/smw.2011.13144. [DOI] [PubMed] [Google Scholar]
- 42.Urbach A, Bar-Nur O, Daley GQ, et al. Differential modeling of fragile X syndrome by human embryonic stem cells and induced pluripotent stem cells. Cell Stem Cell. 2010;6:407–411. doi: 10.1016/j.stem.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inoue H, Yamanaka S. The use of induced pluripotent stem cells in drug development. Clin Pharmacol Ther. 2011;89:655–661. doi: 10.1038/clpt.2011.38. [DOI] [PubMed] [Google Scholar]
- 44.Sun N, Longaker MT, Wu JC. Human iPS cell-based therapy: Considerations before clinical applications. Cell Cycle. 2010;9:880–885. doi: 10.4161/cc.9.5.10827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Power C, Rasko JE. Promises and challenges of stem cell research for regenerative medicine. Ann Intern Med. 2011;155:706–713. doi: 10.7326/0003-4819-155-10-201111150-00010. W217. [DOI] [PubMed] [Google Scholar]
- 46.Liras A. Induced human pluripotent stem cells and advanced therapies: Future perspectives for the treatment of haemophilia? Thromb Res. 2011;128:8–13. doi: 10.1016/j.thromres.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 47.Parker KK, Ingber DE. Extracellular matrix, mechanotransduction and structural hierarchies in heart tissue engineering. Philos Trans R Soc Lond B Biol Sci. 2007;362:1267–1279. doi: 10.1098/rstb.2007.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tucker BA, Park IH, Qi SD, et al. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS One. 2011;6:e18992. doi: 10.1371/journal.pone.0018992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yusa K, Rashid ST, Strick-Marchand H, et al. Targeted gene correction of alpha1-antitrypsin deficiency in induced pluripotent stem cells. Nature. 2011;478:391–394. doi: 10.1038/nature10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boland MJ, Hazen JL, Nazor KL, et al. Adult mice generated from induced pluripotent stem cells. Nature. 2009;461:91–94. doi: 10.1038/nature08310. [DOI] [PubMed] [Google Scholar]
- 51.Yao L, Yu X, Hui N, et al. Application of iPS in assisted reproductive technology: Sperm from somatic cells? Stem Cell Rev. 2011;7:714–721. doi: 10.1007/s12015-011-9236-8. [DOI] [PubMed] [Google Scholar]
- 52.Gore A, Li Z, Fung HL, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471:63–67. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hussein SM, Batada NN, Vuoristo S, et al. Copy number variation and selection during reprogramming to pluripotency. Nature. 2011;471:58–62. doi: 10.1038/nature09871. [DOI] [PubMed] [Google Scholar]
- 54.Laurent LC, Ulitsky I, Slavin I, et al. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell. 2011;8:106–118. doi: 10.1016/j.stem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mayshar Y, Ben-David U, Lavon N, et al. Identification and classification of chromosomal aberrations in human induced pluripotent stem cells. Cell Stem Cell. 2010;7:521–531. doi: 10.1016/j.stem.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 56.Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lister R, Pelizzola M, Kida YS, et al. Hotspots of aberrant epigenomic reprogramming in human induced pluripotent stem cells. Nature. 2011;471:68–73. doi: 10.1038/nature09798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohm JE, Mali P, Van Neste L, et al. Cancer-related epigenome changes associated with reprogramming to induced pluripotent stem cells. Cancer Res. 2010;70:7662–7673. doi: 10.1158/0008-5472.CAN-10-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ben-David U, Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11:268–277. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 60.Apostolou E, Hochedlinger K. Stem cells: iPS cells under attack. Nature. 2011;474:165–166. doi: 10.1038/474165a. [DOI] [PubMed] [Google Scholar]
- 61.Pera MF. Stem cells: The dark side of induced pluripotency. Nature. 2011;471:46–47. doi: 10.1038/471046a. [DOI] [PubMed] [Google Scholar]
- 62.Zhao T, Zhang ZN, Rong Z, et al. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 63.Crook JM, Peura TT, Kravets L, et al. The generation of six clinical-grade human embryonic stem cell lines. Cell Stem Cell. 2007;1:490–494. doi: 10.1016/j.stem.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 64.Ng VY, Choo ABH. iPS and ES cells: Do both roads lead to Rome? Open Stem Cell J. 2010;2:8–17. [Google Scholar]
- 65.Rao MS, Collins FS. Steering a new course for stem cell research: NIH's intramural center for regenerative medicine. Stem Cells Translational Medicine. 2012;1:15–17. doi: 10.5966/sctm.2011-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lehrman S. Undifferentiated ethics. Sci Am. 2010;303:18. doi: 10.1038/scientificamerican0910-18. 20. [DOI] [PubMed] [Google Scholar]
- 67.Aalto-Setälä K, Conklin BR, Lo B. Obtaining consent for future research with induced pluripotent cells: Opportunities and challenges. PLoS Biol. 2009;7:e42. doi: 10.1371/journal.pbio.1000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shineha R, Kawakami M, Kawakami K, et al. Familiarity and prudence of the Japanese public with research into induced pluripotent stem cells, and their desire for its proper regulation. Stem Cell Rev. 2010;6:1–7. doi: 10.1007/s12015-009-9111-z. [DOI] [PubMed] [Google Scholar]
- 69.Skloot R. 1st pbk. ed. New York: Broadway Paperbacks; 2011. The Immortal Life of Henrietta Lacks. [Google Scholar]
- 70.Hull SC, Gooding H, Klein AP, et al. Genetic research involving human biological materials: A need to tailor current consent forms. IRB. 2004;26:1–7. [PubMed] [Google Scholar]
- 71.White MT, Gamm J. Informed consent for research on stored blood and tissue samples: A survey of institutional review board practices. Account Res. 2002;9:1–16. doi: 10.1080/08989620210354. [DOI] [PubMed] [Google Scholar]
- 72.Streiffer R. Informed consent and federal funding for stem cell research. Hastings Cent Rep. 2008;38:40–47. doi: 10.1353/hcr.0.0013. [DOI] [PubMed] [Google Scholar]
- 73.Baker M. Consent issues restrict stem-cell use. Nature. 2008;454:556. doi: 10.1038/454556a. [DOI] [PubMed] [Google Scholar]
- 74.Dolgin E. NIH accused of being overly literal on stem cell approvals. Nat Med. 2012;18:325. doi: 10.1038/nm0312-325. [DOI] [PubMed] [Google Scholar]
- 75.Scott CT, Caulfield T, Borgelt E, et al. Personal medicine—the new banking crisis. Nat Biotechnol. 2012;30:141–147. doi: 10.1038/nbt.2116. [DOI] [PubMed] [Google Scholar]
- 76.National Bioethics Advisory Commission. Research Involving Human Biological Materials: Ethical Issues and Policy Guidance. 1999. Aug, [Accessed January 22, 2012]. Available at http://bioethics.georgetown.edu/nbac/hbm_exec.pdf.
- 77.Wendler D. One-time general consent for research on biological samples. BMJ. 2006;332:544–547. doi: 10.1136/bmj.332.7540.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hansson MG, Dillner J, Bartram CR, et al. Should donors be allowed to give broad consent to future biobank research? Lancet Oncol. 2006;7:266–269. doi: 10.1016/S1470-2045(06)70618-0. [DOI] [PubMed] [Google Scholar]
- 79.Emanuel EJ, Menikoff J. Reforming the regulations governing research with human subjects. N Engl J Med. 2011;365:1145–1150. doi: 10.1056/NEJMsb1106942. [DOI] [PubMed] [Google Scholar]
- 80.Chen DT, Rosenstein DL, Muthappan P, et al. Research with stored biological samples: What do research participants want? Arch Intern Med. 2005;165:652–655. doi: 10.1001/archinte.165.6.652. [DOI] [PubMed] [Google Scholar]
- 81.Miller FG, Wertheimer A. Facing up to paternalism in research ethics. Hastings Cent Rep. 2007;37:24–34. doi: 10.1353/hcr.2007.0044. [DOI] [PubMed] [Google Scholar]
- 82.Taube SE, Barr P, LiVolsi V, et al. Ensuring the availability of specimens for research. Breast J. 1998;4:391–395. [Google Scholar]
- 83.Emanuel EJ, Wendler D, Grady C. What makes clinical research ethical? JAMA. 2000;283:2701–2711. doi: 10.1001/jama.283.20.2701. [DOI] [PubMed] [Google Scholar]
- 84.Isasi R, Knoppers BM, Lomax G. Sustained interaction: The new normal for stem cell repositories? Regen Med. 2011;6:783–792. doi: 10.2217/rme.11.93. [DOI] [PubMed] [Google Scholar]
- 85.Rao M, Condic ML. Alternative sources of pluripotent stem cells: Scientific solutions to an ethical dilemma. Stem Cells Dev. 2008;17:1–10. doi: 10.1089/scd.2008.0013. [DOI] [PubMed] [Google Scholar]
- 86.President's Council on Bioethics. White Paper: Alternative Source of Pluripotent Stem Cells. 2005. May, [Accessed January 22, 2012]. Available at http://bioethics.georgetown.edu/pcbe/reports/white_paper/index.html.
- 87.Krauthammer C. Stem cell vindication. Washington Post. 2007. Nov 30, [Accessed February 15, 2012]. Available at http://www.washingtonpost.com/wp-dyn/content/article/2007/11/29/AR2007112901878.html.
- 88.Condic ML, Rao M. Alternative sources of pluripotent stem cells: Ethical and scientific issues revisited. Stem Cells Dev. 2010;19:1121–1129. doi: 10.1089/scd.2009.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Suaudeau J. From embryonic stem cells to iPS-an ethical perspective. Cell Prolif. 2011;44(suppl 1):70–84. doi: 10.1111/j.1365-2184.2010.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Power C, Rasko JE. Will cell reprogramming resolve the embryonic stem cell controversy? A narrative review. Ann Intern Med. 2011;155:114–121. doi: 10.7326/0003-4819-155-2-201107190-00007. [DOI] [PubMed] [Google Scholar]
- 91.Crook JM. Human iPS cells: Science and ethics. Open Stem Cell J. 2010;2:1. [Google Scholar]
- 92.Watt JC, Kobayashi NR. The bioethics of human pluripotent stem cells: Will induced pluripotent stem cells end the debate? Open Stem Cell J. 2010;2:18–24. [Google Scholar]
- 93.Brown MT. Moral complicity in induced pluripotent stem cell research. Kennedy Inst Ethic J. 2009;19:1–22. doi: 10.1353/ken.0.0270. [DOI] [PubMed] [Google Scholar]
- 94.Denker HW. Potentiality of embryonic stem cells: An ethical problem even with alternative stem cell sources. J Med Ethics. 2006;32:665–671. doi: 10.1136/jme.2005.014738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Devolder K. Complicity in stem cell research: The case of induced pluripotent stem cells. Hum Reprod. 2010;25:2175–2180. doi: 10.1093/humrep/deq176. [DOI] [PubMed] [Google Scholar]
- 96.Hyun I. Stem cells from skin cells: The ethical questions. Hastings Cent Rep. 2008;38:20–22. doi: 10.1353/hcr.2008.0004. [DOI] [PubMed] [Google Scholar]
- 97.Hyun I, Hochedlinger K, Jaenisch R, et al. New advances in iPS cell research do not obviate the need for human embryonic stem cells. Cell Stem Cell. 2007;1:367–368. doi: 10.1016/j.stem.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 98.Testa G, Borghese L, Steinbeck JA, et al. Breakdown of the potentiality principle and its impact on global stem cell research. Cell Stem Cell. 2007;1:153–156. doi: 10.1016/j.stem.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 99.Neri D. The race toward ‘ethically universally acceptable’ human pluripotent (embryonic-like) stem cells: Only a problem of sources? Bioethics. 2011;25:260–266. doi: 10.1111/j.1467-8519.2009.01784.x. [DOI] [PubMed] [Google Scholar]
- 100.Mathews DJ, Donovan PJ, Harris J, et al. Pluripotent stem cell-derived gametes: Truth and (potential) consequences. Cell Stem Cell. 2009;5:11–14. doi: 10.1016/j.stem.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ramalho-Santos J. Human procreation in unchartered territory: New twists in ethical discussions. Hum Reprod. 2011;26:1284–1287. doi: 10.1093/humrep/der093. [DOI] [PubMed] [Google Scholar]
- 102.Lo B, Parham L, Alvarez-Buylla A, et al. Cloning mice and men: Prohibiting the use of iPS cells for human reproductive cloning. Cell Stem Cell. 2010;6:16–20. doi: 10.1016/j.stem.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.International Society for Stem Cell Research. Guidelines for the Conduct of Human Embryonic Stem Cell Research. 2006. [Accessed February 24, 2012]. Available at http://www.isscr.org/guidelines/ISSCRhESCguidelines2006.pdf.
- 104.National Research Council and Institute of Medicine. National Academy Press; [Accessed February 24, 2012]. 2008 Amendments to the National Academies' Guidelines for Human Embryonic Stem Cell Research. Available at http://www.nap.edu/catalog.php?record_id=12260. [Google Scholar]
- 105.Mathews DJ, Graff GD, Saha K, et al. Access to stem cells and data: Persons, property rights, and scientific progress. Science. 2011;331:725–727. doi: 10.1126/science.1201382. [DOI] [PubMed] [Google Scholar]
- 106.Lo B, Parham L. Ethical issues in stem cell research. Endocr Rev. 2009;30:204–213. doi: 10.1210/er.2008-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Caulfield T, Scott C, Hyun I, et al. Stem cell research policy and iPS cells. Nat Methods. 2010;7:28–33. doi: 10.1038/nmeth.f.282. [DOI] [PubMed] [Google Scholar]
- 108.Zarzeczny A, Scott C, Hyun I, et al. iPS cells: Mapping the policy issues. Cell. 2009;139:1032–1037. doi: 10.1016/j.cell.2009.11.039. [DOI] [PubMed] [Google Scholar]
- 109.Luong MX, Auerbach J, Crook JM, et al. A call for standardized naming and reporting of human ESC and iPSC lines. Cell Stem Cell. 2011;8:357–359. doi: 10.1016/j.stem.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 110.U.S. Food and Drug Administration. A Guide to Informed Consent—Information Sheet. 21.CFR. 50. [Accessed February 24, 2012]. Available at http://www.fda.gov/RegulatoryInformation/Guidances/ucm126431.htm.
- 111.U.S. Food and Drug Administration. 21.CFR. 1270. [Accessed February 24, 2012]. Available at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=1270.
- 112.U.S. Food and Drug Administration. 21.CFR. 1271. [Accessed February 24, 2012]. Available at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=1271.
- 113.Koleva G. Stem cells and the lawsuit that may shape our medical future. Forbes. 2012. Feb 10, [Accessed February 15, 2012]. Available at http://www.forbes.com/sites/gerganakoleva/2012/02/10/stem-cells-and-the-lawsuit-that-may-shape-our-medical-future.
- 114.Hyun I. Allowing innovative stem cell-based therapies outside of clinical trials: Ethical and policy challenges. J Law Med Ethics. 2010;38:277–285. doi: 10.1111/j.1748-720X.2010.00488.x. [DOI] [PubMed] [Google Scholar]
- 115.Regulators must step up stem cell oversight. Nat Med. 2010;16:492. doi: 10.1038/nm0510-492. [DOI] [PubMed] [Google Scholar]
- 116.Hyun I. The bioethics of stem cell research and therapy. J Clin Invest. 2010;120:71–75. doi: 10.1172/JCI40435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hyun I. The bioethics of iPS cell-based drug discovery. Clin Pharmacol Ther. 2011;89:646–647. doi: 10.1038/clpt.2010.308. [DOI] [PubMed] [Google Scholar]
- 118.Daley GQ, Ahrlund Richter L, Auerbach JM, et al. Ethics. The ISSCR guidelines for human embryonic stem cell research. Science. 2007;315:603–604. doi: 10.1126/science.1139337. [DOI] [PubMed] [Google Scholar]
- 119.Hyun I, Lindvall O, Ahrlund-Richter L, et al. New ISSCR guidelines underscore major principles for responsible translational stem cell research. Cell Stem Cell. 2008;3:607–609. doi: 10.1016/j.stem.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 120.International Society for Stem Cell Research. ISSCR Patient Guidelines for the Clinical Translation of Stem Cells. 2008. Dec 23, [Accessed January 12, 2012]. Available http://www.isscr.org/GuidelinesforClinicalTranslation/2480.htm.
- 121.The International Stem Cell Banking Initiative. Consensus guidance for banking and supply of human embryonic stem cell lines for research purposes. Stem Cell Rev Rep. 2009;5:301–314. doi: 10.1007/s12015-009-9085-x. [DOI] [PubMed] [Google Scholar]
- 122.National Research Council and Institute of Medicine. National Academy Press; [Accessed February 24, 2012]. Final Report of the National Academies' Human Embryonic Stem Cell Research Advisory Committee and 2010 Amendments to The National Academies' Guidelines for Human Embryonic Stem Cell Research. Available at http://www.nap.edu/catalog.php?record_id=12923. [PubMed] [Google Scholar]
- 123.U.S. National Institutes of Health. National Cancer Institute Office of Biorepositories and Biospecimen Research. 2011 Revised NCI Best Practices for Biospecimen Resources. [Accessed March 5, 2012]. Available at http://biospecimens.cancer.gov/practices/2011bp.asp.
- 124.California Institute for Regenerative Medicine. CIRM MES Regulations Title 17 California Code of Regulations Section 100010–100110. 2006. [Accessed February 12, 2012]. Available at http://www.cirm.ca.gov/files/PDFs/Standards/Reformatted_MES_Regs.pdf.
- 125.California Institute for Regenerative Medicine. Summary and Recommendations of the CIRM Human iPS Cell Banking Workshop. 2010. Nov 17–18, [Accessed February 12, 2012]. Available at http://www.cirm.ca.gov/MeetingReports.
- 126.U.S. Department of Health and Human Services. Protection of Human Subjects. 45.CFR. 46. [Accessed February 24, 2012]. Available at http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.htm.
- 127.Hull SC, Sharp RR, Botkin JR, et al. Patients' views on identifiability of samples and informed consent for genetic research. Am J Bioeth. 2008;8:62–70. doi: 10.1080/15265160802478404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Casebriefs. Moore v. Regents of the University of California. [Accessed February 15, 2012]. Available at http://www.casebriefs.com/blog/law/property/property-law-keyed-to-cribbet/non-traditional-objects-and-classifications-of-property/moore-v-regents-of-the-university-of-california-2.
- 129.Beskow LM, Friedman JY, Hardy NC, et al. Simplifying informed consent for biorepositories: Stakeholder perspectives. Genet Med. 2010;12:567–572. doi: 10.1097/GIM.0b013e3181ead64d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cressey D. Informed consent on trial. Nature. 2012;482:16. doi: 10.1038/482016a. [DOI] [PubMed] [Google Scholar]
- 131.Schaefer GO, Wertheimer A. The right to withdraw from research. Kennedy Inst Ethics J. 2010;20:329–352. [PubMed] [Google Scholar]
- 132.Mello MM, Wolf LE. The Havasupai Indian tribe case—lessons for research involving stored biologic samples. N Engl J Med. 2010;363:204–207. doi: 10.1056/NEJMp1005203. [DOI] [PubMed] [Google Scholar]
- 133.Ludman EJ, Fullerton SM, Spangler L, et al. Glad you asked: Participants' opinions of re-consent for dbGap data submission. J Empir Res Hum Res Ethics. 2010;5:9–16. doi: 10.1525/jer.2010.5.3.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Goldenberg AJ, Hull SC, Botkin JR, et al. Pediatric biobanks: Approaching informed consent for continuing research after children grow up. J Pediatr. 2009;155:578–583. doi: 10.1016/j.jpeds.2009.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hens K, Levesque E, Dierickx K. Children and biobanks: A review of the ethical and legal discussion. Hum Genet. 2011;130:403–413. doi: 10.1007/s00439-011-1031-8. [DOI] [PubMed] [Google Scholar]
- 136.Bahadur G, Morrison M. Patenting human pluripotent cells: Balancing commercial, academic and ethical interests. Hum Reprod. 2010;25:14–21. doi: 10.1093/humrep/dep369. [DOI] [PubMed] [Google Scholar]
- 137.Caulfield T, Rachul C. Science spin: iPS cell research in the news. Clin Pharmacol Ther. 2011;89:644–646. doi: 10.1038/clpt.2010.309. [DOI] [PubMed] [Google Scholar]
- 138.King NM, Henderson GE, Churchill LR, et al. Consent forms and the therapeutic misconception: The example of gene transfer research. IRB. 2005;27:1–8. [PubMed] [Google Scholar]
- 139.Scott CT, DeRouen MC, Crawley LM. The language of hope: Therapeutic intent in stem-cell clinical trials. AJOB Primary Res. 2010;1:4–11. [Google Scholar]
- 140.U.S. National Institutes of Health. National Institutes of Health Guidelines on Human Stem Cell Research. [Accessed January 22, 2012]. Available at http://stemcells.nih.gov/policy/2009guidelines.htm.
- 141.International Society for Stem Cell Research. ISSCR Patient Handbook on Stem Cell Therapies. 2008. Dec 23, [Accessed January 12, 2012]. Available at http://www.isscr.org/The_Patient_Handbook.htm.