This study identifies three dimensions with which to classify heuristically the routes to widespread adoption of cellular therapies: the relative involvement of clinicians and companies, cell type and scale of manufacture, and therapeutic intervention as a procedure or product. It is suggested that for those cellular therapies that require therapeutic procedures, close collaboration between companies and clinicians will reduce the time to widespread adoption. For selected cellular therapies, predictions are made of the likely time to widespread adoption.
Keywords: Cellular therapy, Clinical translation, Stem cell transplantation, Transplantation, Bone marrow, Embryonic stem cells, Adult stem cells
Abstract
We identify three dimensions with which to classify heuristically the routes to widespread adoption of cellular therapies. The first dimension is based on the relative involvement of clinicians and companies in a particular cellular therapy. The second dimension is based on cell type and consequent scale of manufacture. The third dimension classifies the therapeutic intervention as a procedure or product and has perhaps received less attention. We suggest that for those cellular therapies that require therapeutic procedures, close collaboration between companies and clinicians will reduce the time to widespread adoption. For selected cellular therapies we make predictions of the likely time to widespread adoption.
Introduction
Using cells to cure patients is an attractive and potentially revolutionary therapy [1]. The opportunity offered by potentially restorative treatments has been seized on the one hand by clinicians and on the other by companies, despite a challenging regulatory environment [2].
Clinician-led cellular therapies are increasingly common with a growing number demonstrating benefit to patients [1, 3]. Company-led programs for cellular therapies are also being vigorously pursued, and the first licensed cellular advanced therapeutic medicinal product (ATMP) is available in Europe [4]. The relative involvement of clinicians and companies in a particular cellular therapy, as evidenced by clinical trial authorizations, defines one dimension of analysis. The second dimension of analysis is the distinction between autologous and allogeneic therapies that lead to differences in the manufacturing technologies, the scale associated, and the disease indications to which they are suited [5].
The third dimension might be loosely thought of as the degree of integration of a particular cellular therapy program with clinical practice. On this dimension, the focus of the development of a cell therapy may be on a clinical procedure rather than a clinical product. Focus on product is likely to be commoner in those developing a therapy in which the prevalence of the indication is high.
We shall discuss how analysis along these three dimensions using heuristic classification [6] can lead to a better understanding of the route to market for cellular therapies by identifying factors that have a differential impact. Put another way, how will widespread adoption of cellular therapies best be achieved?
Materials and Methods
We have selected characteristics of cellular therapeutic interventions that we believe will have an impact on the time it will take for market approval and widespread adoption to be achieved. We have used heuristic classification as a method, as identified by Clancey [6]. In the first instance we have identified three dimensions that differentiate cell therapies from one another in terms of factors that we believe will play a key role in development and adoption in order to develop a heuristic classification of cell therapies existing or in development. These dimension are as follows.
The autologous-allogeneic dimension.
Here autologous is defined by donor cells that are derived from the patient who is the sole recipient of the cell product. Allogeneic is defined as donor cells that are derived from a source other than the patient to manufacture a cell bank from which a cell product is derived to treat many patients [7].
The clinician-company dimension.
This dimension has been defined according using publicly available clinical trial data (ClinicalTrials.gov, http://www.clinicaltrials.gov). Clinician-led cell therapies are those where the clinical trial is sponsored by an institution, whereas company-led cell therapies are defined as those where the clinical trials are sponsored by a company.
The procedure-product dimension.
We have defined this dimension to map the complexity of the intervention. If intervention is minimal (e.g., cells are applied to skin or injected either intramuscularly or intravenously), then the therapy is defined as a product. A cell therapy with a complex route of administration (e.g., surgery) is defined as a procedure.
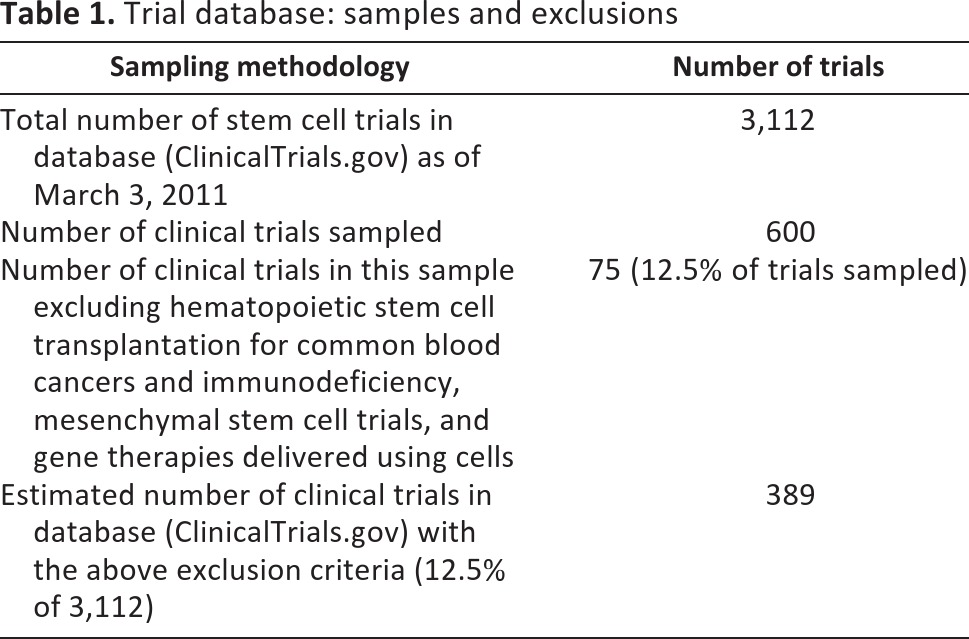
We used the clinical trials database (ClinicalTrials.gov) to develop an overview of cell therapies in clinical trials. In the first instance, we searched for “stem cells” and refined the search for interventional trials. From the 3,112 trials listed as of March 3, 2011, we then analyzed a randomly generated data set containing 600 trials. Hematopoietic stem cell transplantation for common blood cancers and immunodeficiency is now a well-developed clinical therapy [8] and although trials continue for these indications, we included only those that involve new therapeutic indications. We also excluded gene therapies delivered using cells (the occurrence of which was rare because of the search terms used), mesenchymal stem cell therapies (because mesenchymal stem cell therapies are based on immunomodulation and not cell replacement [9]; we analyzed these separately), and trials that did not have a therapeutic aim. This left a total of 75 trials. On the basis of this sampling paradigm, we predict that of the 3,112 trials registered, 389 (12.5%) are cell therapy trials that escape the exclusion criteria (Table 1).
Table 1.
Trial database: samples and exclusions
A separate analysis of mesenchymal stem cells was completed using the clinical trials database (ClinicalTrials.gov). Of the 150 trials listed as of March 3, 2011, 129 met our criteria. Those excluded were either hematological or nontherapeutic. On the basis of our sampling, we estimated that approximately 4% of all stem cell trials listed in the clinical trials database (ClinicalTrials.gov) are mesenchymal stem cell trials.
We also used a combination of publicly available data and predictions to map the time to widespread adoption of a selection of cell therapies that are either approved or in development. Mapping of historic activity is based on publicly available data such as that from clinical trials databases and publications (referenced in Table 2). Mapping of future activity is based on known data such as market size and production capacity and predicted data such as uptake rates and clinical trial approval (Table 2).
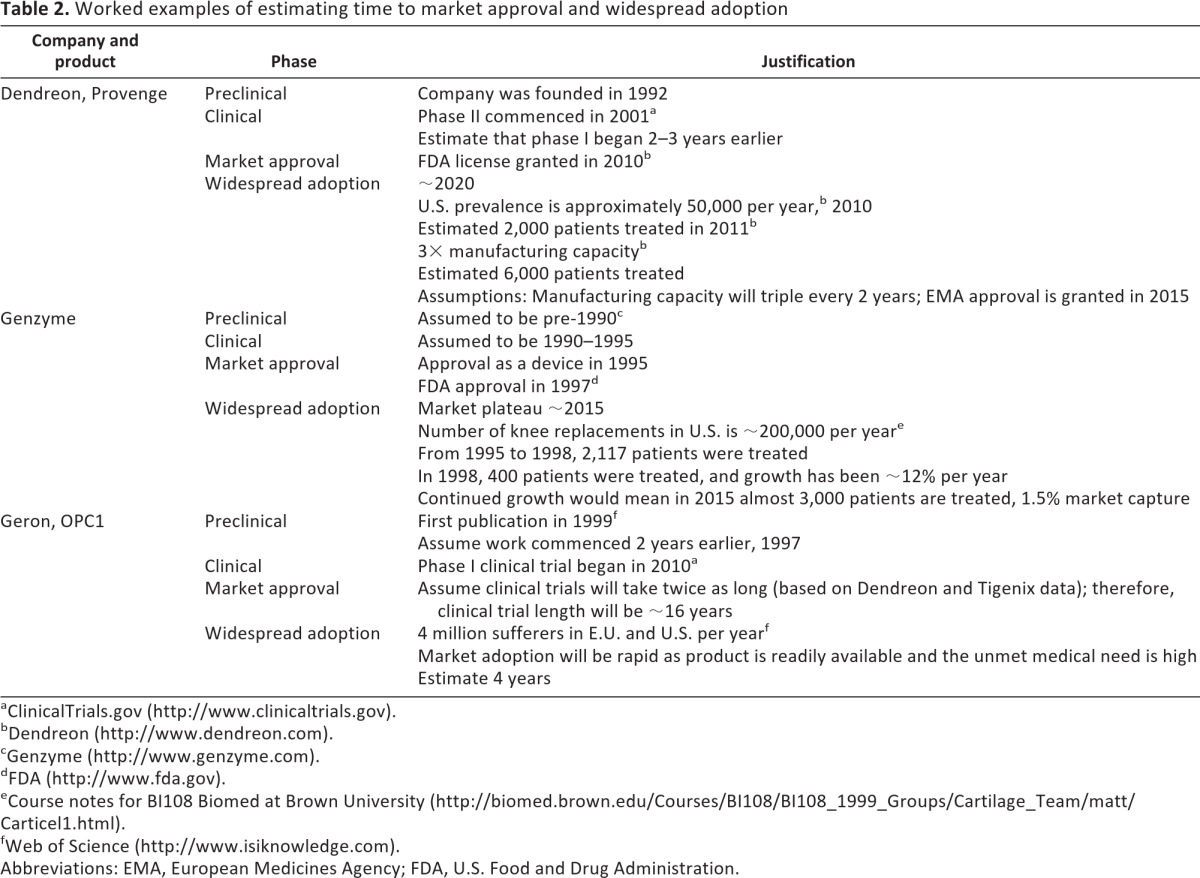
Table 2.
Worked examples of estimating time to market approval and widespread adoption
ClinicalTrials.gov (http://www.clinicaltrials.gov).
Dendreon (http://www.dendreon.com).
Genzyme (http://www.genzyme.com).
FDA (http://www.fda.gov).
Course notes for BI108 Biomed at Brown University (http://biomed.brown.edu/Courses/BI108/BI108_1999_Groups/Cartilage_Team/matt/Carticel1.html).
Web of Science (http://www.isiknowledge.com).
Abbreviations: EMA, European Medicines Agency; FDA, U.S. Food and Drug Administration.
The Clinician-Company Dimension
The clinician-company dimension is defined by the identity of the party that develops a cellular therapy and takes it to clinical trial (ClinicalTrials.gov).
The Company-Led Model
Most companies have adopted a route to market for their product based on the practices of the biopharmaceutical industry [7]. The biopharmaceutical industry in turn is heavily dependent on the production practices that pharmaceutical companies use to produce low molecular mass drugs. The successful production of biopharmaceuticals required considerable innovation of technologies of production, purification, and storage, innovation that is essential to bring the larger, less stable biopharmaceuticals to market. By analogy cellular therapy products have required substantial innovation associated, with the even more demanding challenges of delivering living cells to the patient reproducibly and safely [10]. In both cases innovation has been intense, but the overall shape of the production and distribution process is unchanged.
This unchanging shape is illustrated in Figure 1. The challenges facing the production chain of cellular therapies delivered in this way are the same as those of both pharmaceutical and biopharmaceutical production: cost of goods, scale-up, purity, shelf life, and route of administration [11]. In the company-led model clinicians are of course deeply involved in identifying clinical need and in the research that may lead to a product, but if a cellular product is developed using the biopharmaceutical model, then they have no major involvement in the technical aspects of the development and design of the production chain and their role in delivering a therapy is to collaborate in administering the product [10].
Figure 1.
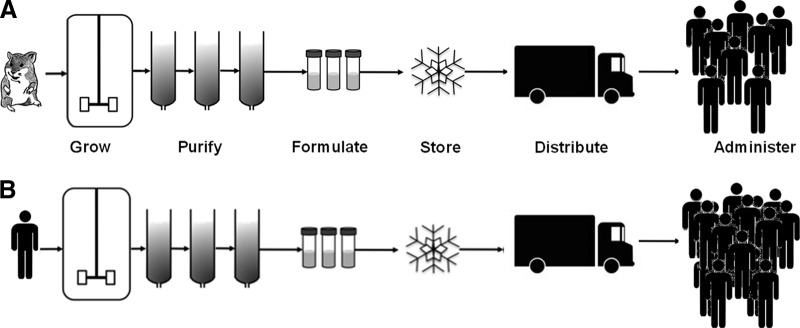
Schematic representing biopharmaceutical production (A) and allogeneic cell therapy production (B).
The Clinician-Led Model
Some clinicians have been very quick to see the benefit to patients offered by cellular therapies (for example, autologous chondrocyte implantation [ACI] [12], autologous limbal stem cell transplantation [3, 13], and allogeneic islet transplantation [14]). This has led them to develop their own therapeutic approaches, often in collaboration with basic scientists [15]. These approaches are usually highly innovative, but they are conservative in their approach to risk and in requiring minimal changes to the clinical context of existing treatment. There is often a pattern of serial innovation in a single institution that remains an island of potentially transformative therapy unconnected to the mainland of routine clinical practice. An example of this is the application of cultured epidermal stem cells by Pellegrini and others (University of Modena and Reggio Emilia) to treat a range of indications [16–18].
Bone marrow transplantation is a historical example of a clinician-led cellular therapy pioneered by Thomas in a single institution [19]. After 15 years of work with patients with advanced disease, Thomas, in the late 1970s, first used transplants for leukemia in first remission. Eight of these first 19 patients were still alive 20 years later [19]. In those intervening 20 years transplantation became a widely adopted therapy for bone marrow cancers. Bone marrow transplantation was developed and adopted by clinicians entirely within the context of clinical care. It generated and stimulated the provision of new technologies into hospitals, for example, fluorescence-activated cell sorting and clean room facilities for cellular processing. Cellular processing expertise developed in hospitals for bone marrow transplantation has facilitated the development of other novel cellular therapies in a clinical context (for example, mesenchymal stem cell therapies [20] and limbal stem cell transplantation [3]).
Bone marrow transplantation also illustrates the importance of clinical champions and clinical networks. The adoption by and promotion of a novel therapy through professional networks by clinicians leading their fields has always been a very important element of therapeutic innovation.
The Clinician-Company Dimension and the Regulatory Environment
Until recently one advantage for clinicians in conceiving and implementing cellular therapies has been the relative ease with which motivated clinicians could fulfill the ethical and regulatory requirements surrounding these novel therapies. In contrast companies have necessarily had to traverse a more complex regulatory route to justify the use of cellular products in patients. Now the hand of the regulators has reached into the clinic, and the current regulatory requirements have placed a high and wide barrier in front of clinician-led innovation [21]. These regulatory changes have caused a good deal of consternation but in fact have stimulated some hospitals to develop the expertise and facilities necessary for compliance with the regulations surrounding cellular therapies. The development of regulatory knowhow now means that these hospitals are likely to be much better placed to develop in-house cellular therapies with demonstrable safety and efficacy on a par with company products.
The Autologous-Allogeneic Dimension
Autologous therapies involve taking cells from the patient and offering them as a therapy to the same patient. Allogeneic therapies take cells from a donor and offer them as a therapy to many different patients.
Allogeneic Therapies
The majority of newly proposed allogeneic cellular therapies are based on the premise of one donor and many recipients. They fit well with the biopharmaceutical model, as they require manufacturing at a large scale, some inevitable downstream processing, and close attention to storage and administration.
Large-scale manufacturing is dictated by the economies of scale required to deliver an acceptable cost of goods [11]. It is estimated that production costs for an allogeneic cell therapy are as high as $20,000 per dose (based on data for insulin-dependent diabetes [22] and a cost of $20 per million cells [23]) before any product development is completed; extensive process development and scale-up, although costly, may ultimately drive costs down to $250 per dose [24].
The attendant need for substantial amplification of cell mass in its turn dictates a highly proliferate stem cell origin for the product. The extent of downstream processing to differentiate and purify the therapeutic product can be substantial.
Two major challenges for allogeneic therapies are high cost of goods and safety. Even with substantial scale-up, the complexities of cellular manufacturing set a limit to reductions in cost per unit volume, not least because cellular manufacture remains very labor intensive [25]. The labor intensity of cellular manufacture also increases the chance of human error leading to product variability and potential safety concerns. Improvements in both cost and product variability may eventually be achieved through automation [26] or the use of large-scale suspension culture [27]. In addition to the usual safety concerns that apply to all cellular therapies, for the subclass of cellular therapies derived from pluripotent cells, a nagging safety concern will always remain the presence of small numbers of undifferentiated pluripotent cells in the final product. These concerns lead to substantial delays in the approval of a clinical trial for spinal cord injury [28] and markedly increase the contribution of risk to the risk-benefit ratio. It is for these reasons that current trials of embryonic stem cell-based cellular therapies have fierce exclusion criteria for recruitment into trials to minimize risk and in the hope of demonstrating maximal benefit [29, 30].
The grand challenge for allogeneic therapies is tissue incompatibility. Cells derived from a single individual and administered to others will likely cause a tissue rejection syndrome [31, 32] except in relatively immunoprivileged regions such as the central nervous system (which includes the retina) [33]. Overcoming rejection using long-term immunosuppression carries a substantial risk of morbidity and mortality from infection and oncogenesis.
Autologous Therapies
Although autologous cellular therapies are more newly arrived, there is a close analogy with bone marrow transplantation. The analogy lies in the matching of one donor with one patient, the therapy consisting in the removal of cells, their manipulation, and their return to the patient.
For the vast majority of indications [22], amplification of the donor cells may take place in autologous cellular therapies, but scale-up is modest as only small amounts of product are needed for the single recipient (Fig. 2). This minimizes the number of cell divisions required to generate the therapeutic product, an approach inherently safer from the point of view of teratogenesis [34]. Processed tissue is genetically identical to the recipient; no immunosuppression is necessary [5].
Figure 2.
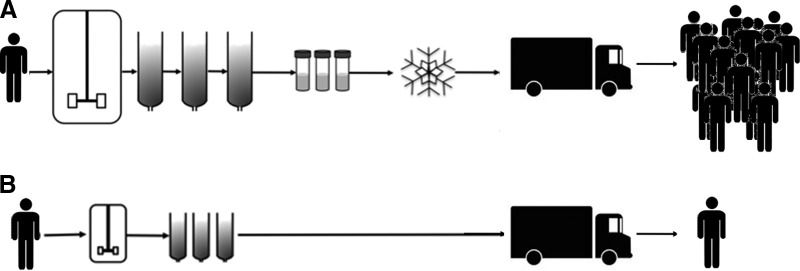
Schematic representing the differing scales and processing complexities of allogeneic cell therapies (A) and autologous cell therapies (B).
The smaller scale of production needed for autologous therapies offers alternative manufacturing and distribution approaches to the biopharmaceutical model [10]. Cost of goods remains an essential element in designing an autologous cellular therapy, but these cannot be reduced through large-scale manufacture. One way to reduce costs in an autologous therapy program is to have a central processing hub that deals with many individual samples that are shipped from and to the patient. The savings are likely to be largely in capital costs and regulatory compliance rather than in the recurrent consumable and labor costs associated with each individual procedure. Unit costs can be reduced in other ways perhaps, for example, by developing small, closed system—automated cellular processing machines—close to the patient [35]. Or again, existing hospital biomanufacturing facilities and staff, present in most tertiary care centers, can be made available.
It is not yet clear which of these approaches will be most suitable; indeed, different approaches may suit different disease indications, depending on the prevalence of the disease and the complexity of processing and subsequent administration. The production, facilities, logistics, personnel, storage, and administration requirements may differ substantially for different indications.
Mapping Indications to This Dimension
Autologous therapies have not been attractive to companies, especially large companies, perhaps because the lack of clear economies of scale makes them an unattractive investment [36]. This is illustrated in comments to the U.K. Parliamentary Science and Technology Committee by Prof. Christopher Mason: “The other thing, I think, as Martin Evans, again, correctly identified, is that autologous cell therapies may not be that attractive to big pharma; in fact, Ruth McKernan at Pfizer has said that they would only look at allogeneics, for example universal cell therapies, for scalability issues” [37].
Autologous therapies have, however, been taken up by clinicians, perhaps partly because, in the absence of company interest, there is an unmet need. The need for initial patient biopsy and a second subsequent restorative procedure must also be a factor. A consequence of the sole participation of clinicians in autologous cellular therapies is, in general, the smaller scale of studies. This is undoubtedly because of the not insubstantial but nonetheless limited financial resources available to individual clinicians. For the moment, the development of autologous therapies is for rarer indications or for small trials on patients with more prevalent indications.
The inherently lower risks of autologous cellular therapies much improve the risk-benefit ratio to the extent that it is trials of autologous therapies that predominate. There are also substantial numbers of allogeneic therapy-based trials [38], but these involve mesenchymal stem cells, cells that themselves have immunomodulatory properties that tend to reduce or eliminate allotype-related inflammation and that persist in the recipient for only short periods of time. By comparison, there are so far only two approved trials based on pluripotent stem cells [39]. The risk-benefit equation for allogeneic therapies points to their use, at least initially, only for indications that carry a high cost of care and are life-threatening or severely debilitating. A major health care challenge is of course the much more prevalent but less severe indications that make up the bulk of degenerative disease.
The Procedure-Product Dimension
We postulate that a therapy is primarily a product when it is produced in a form that can be administered in a straightforward way to the patient, perhaps by a nurse. A therapy is primarily a procedure when, although involving a cellular product, it requires a complex clinical intervention performed by a clinician in an operating theater.
Therapeutic Products
Classical pharmaceutical products are designed to be easy to administer [40]. The oral route overwhelmingly predominates. This route no longer meets all clinical need: the biopharmaceutical industry's biological products cannot be administered by the same route, as they are susceptible to parenteral degradation. Biopharmaceutical therapeutics must be administered by subcutaneous, intramuscular, or intravenous injection (erythropoietin is administered subcutaneously or intravenously [41] and interferons are administered either by intravenous or intramuscular injection [42]). Cellular therapeutic products can involve even more complex routes of administration [43], for example, stereotactic introduction of cells into the brain (Clinical Trial NCT01151124, ClinicalTrials.gov), intravenous catheterization of the heart (Clinical Trial NCT00747708, ClinicalTrials.gov), and portal vein administration of islets [44]. The more complex the procedure, the greater the clinical procedural risk.
The complexity of the administrative route for therapeutic product determines the amount of time and effort needed from the clinician to administer the product. The cost of the procedure must be added to the cost of the product to determine the overall therapeutic cost to the health care provider even if, as with many medical devices, the procedure and the product are coded separately by health care procurers [45].
Therapeutic Procedures
The more invasive the therapeutic procedure, the costlier and riskier it becomes. Riskier because the more extensively the body's barriers against infection and insult are breached, the likelier it is that harm will be done; costlier because of the complexity of the clinical environment in terms of skills and sterility required to mitigate these risks increases. This cost-benefit equation applies to biopharmaceutical products; for example, a biopharmaceutical that is suited to intramuscular injection can be self-administered, whereas a biopharmaceutical suited only to intravenous injection always requires a degree of clinical supervision. The cost-benefit equation for cellular therapies offers even greater challenges given the requirement for more invasive and often multiple interventions.
Therapeutic procedures are themselves tightly regulated. Altered or entirely novel procedures carry substantial costs of testing and training to ensure safety under good clinical practice (GCP) [46]. So, to the costs of the procedures themselves must be added any development costs for new procedures if they are required to administer new cellular therapies.
Therapeutic Adoption
Adoption costs for any new therapy are large. Achieving the adoption of orally administered drugs can cost pharmaceutical companies many millions from their sales and marketing budgets [47]. There are also adoption costs for health care providers, but these are readily estimated as the difference in price between the new drug and the one it is supplanting. However, therapeutic procedures are unchanged: the clinician substitutes one drug for another on a prescription.
Adoption costs for cellular therapeutics may be similarly large, but they are borne directly by the health care provider rather than, in the case of the orally administered drug, through the cost of the product, which of course includes the sales and marketing costs incurred by the supplier. Although the adoption costs to the health care provider can be readily monetized, they will involve changes to health care practice far greater than simply prescribing a different drug. As is well known, changing health care practices involves a great deal more than mere money [48]. Adoption costs for cellular therapies can be minimized only by minimizing changes to clinical practice.
Having developed a cost-effective cellular therapy that can be adopted with minimal change to clinical practice, a final hurdle to widespread adoption is procurement and reimbursement by health care providers. Price relative to existing treatments is a key issue, but perhaps more significant is the fact that procurement codes for cellular therapies do not exist. Obtaining an appropriate procurement code requires detailed discussion with health care providers, in many cases at the regional level rather than the national level and sometimes with individual hospitals. These discussions are multiple, complex, and time-consuming.
Clinicians Have Procedures and Companies Have Products That May Disrupt the Clinical Care Pathway
In general, when clinicians lead or are very closely involved in developing a novel cellular therapy, the therapy is developed paying close attention to existing clinical procedures. This is the case largely because the individual clinician does not have the means to alter markedly either the procedure or the clinical pathway. When companies develop novel cellular therapies they pay close attention to manufacture but pay less attention to any changes in clinical procedures or clinical pathways, ignoring the fact that the customer is the health care provider and the end user is the clinician. Conversely, clinicians rarely consider the manufacturing challenges that arise from their potentially successful cellular therapies. Both companies and clinicians are very sensitive to clinical need, but each ignores a key component of the procedure-product dimension. Ideally, if cellular therapies are to be readily adopted, clinical procedures and cellular products should be brought much closer together. There is evidence that some companies are considering means to deliver their cellular product using a clinical procedure, as half of the cellular products in company trials involve a clinical procedure. Nonetheless it remains a challenge to adapt these new procedures to the clinical pathway of treatment. Time to market for cellular therapies should be reduced markedly if clinicians think more about manufacturing and distribution and companies think more about minimizing disruptions to existing clinical pathways. This is most easily achieved if clinicians, health economists, and companies are speaking to one another from a very early point in the development of a cellular therapy [49].
Mapping Cell Therapies to the Three Dimensions
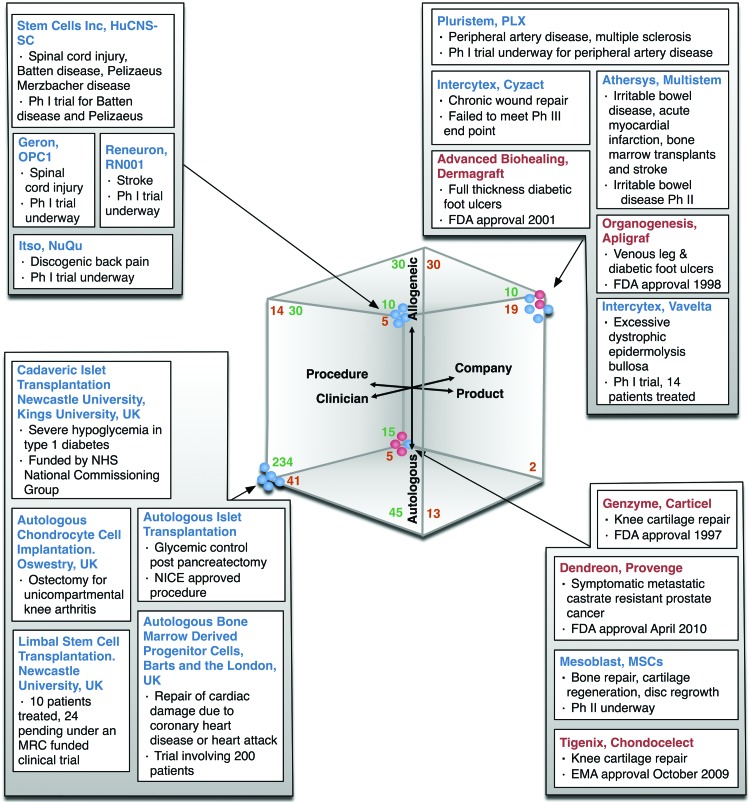
We have mapped a number of existing and potential cellular therapies to our three dimensions. We have excluded cellular therapies that involve genetic modification, as we believe these should be considered separately. We have chosen these examples as a representative cross-section of therapies that are close to the clinic. We have analyzed mesenchymal stem cell therapies separately. We have used the criteria set out in the Materials and Methods section to determine their placement on each of the three dimensions. This approach is illustrated in Figure 3. It is interesting to note that the three cellular products that have had European Medicines Agency (EMA) or U.S. Food and Drug Administration (FDA) approval as cellular therapies are autologous therapies.
Figure 3.
Heuristic classification of a selection of cell therapies to the three dimensions. Therapies shown in red have received a market authorization. Numbers in green are our estimate of all stem cell trials in the clinical trials database that met our criteria, mapped in proportion to our sample of 75. Numbers in orange are mesenchymal stem cell clinical trials. Abbreviations: EMA, European Medicines Agency; FDA, U.S. Food and Drug Administration; MRC, Medical Research Council; MSC, mesenchymal stem cell; NHS, National Health Service; NICE, National Institute for Health and Clinical Excellence; Ph, phase.
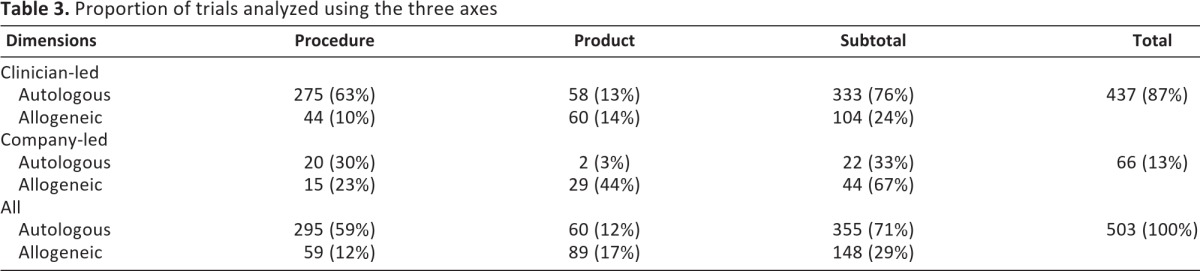
Perhaps not surprisingly, of the cellular therapies in development identified from the clinical trials database there are two prominent groups: clinician-led cellular therapies that focus on procedures and are autologous (275) represent 63% of all clinician-led trials, whereas company-led cellular therapies that are product-focused and allogeneic [29] represent 44% of all company-led trials. The analysis also shows that only 22 of 66 company-led therapies are autologous (33%), whereas 333 of 437 clinician-led trials (involving both procedures and products) are autologous (76%). Overall, of the 503 trials sampled and estimated, 437 are led by clinicians (87%) and 66 by companies (13%), and 149 (30%) are products and 354 (70%) are procedures (Table 3).
Table 3.
Proportion of trials analyzed using the three axes
As well as sampling the clinical trials database, we have selected additional trials that we analyze in more detail. Of the clinician-led therapies, limbal stem cell therapies show very promising initial trial results [3], cardiac mesenchymal stem cell therapies show no signs of substantial clinical benefit [50, 51], and therapies for cartilage and bone are well developed and show modest clinical benefit [52, 53]. Of the company-led therapies, Intercytex (Manchester, U.K., http://www.intercytex.com) has already brought an allogeneic fibroblast-based therapy to market (this product was originally not recognized by the Medicines and Healthcare Products Regulatory Agency as a medicinal product or device and was delivered under GCP; under the new regulations it is now grandfathered until December 2012, when it will have to be licensed as an ATMP or under hospital exemption) and is now in trials to improve healing in patients with epidermolysis bullosa [54]. Reneuron (Surrey, U.K., http://www.reneuron.com) and Geron (Menlo Park, CA, http://www.geron.com/) central nervous system and spinal injury products are fetal and embryonic stem cell-based, respectively, and are aimed at indications where existing treatment cost [55, 56] is higher. Perhaps more interesting than the identification of two prominent categories (clinician-led autologous procedures and company-led allogeneic products) is the observation from our analysis above that some cellular therapy approaches fall outside these two main groups.
Dendreon's (Seattle, WA, http://www.dendreon.com) autologous dendritic cell therapy for prostate cancer is already on the market in the U.S., pointing to the advantages offered by an autologous cell therapy in the context of prostate cancer [57, 58]. Allogeneic islet transplantation is procedure-based and clinician-led, reflecting the severity of its indication (severe hypoglycemic episodes in type 1 diabetes) and a consequent risk-benefit analysis that justifies immunosuppression [58]. These two are clear examples of the administration of cells that themselves have been shown not to be injurious in order to alleviate a severe indication.
Where companies have developed cellular therapies that require complex procedures, our examples (Fig. 3) show that they have worked closely with clinicians to develop appropriate protocols for the delivery of the cellular therapy to the site of action. Tigenix's (Leuven, Belgium, http://www.tigenix.com) autologous chondrocyte therapy is the first licensed ATMP in Europe, its safety in the context of tumorigenicity largely guaranteed by the use of low passage number autologous cells. Geron and Reneuron have also worked very closely with clinicians to develop routes of administration of cellular products into the central nervous system. These close collaborations between companies and clinicians should do much to mitigate the risks associated with the use of stem cell-derived products.
Mapping Market Approval and Widespread Adoption of Cellular Therapies
We can use the three dimensions that we have defined to help analyze projected times to market approval, defined as acquiring regulatory approval from the EMA or the FDA, and widespread adoption of cellular therapies. Figure 4 illustrates our estimates of the time to market approval and widespread adoption for the therapies shown in Figure 3. We have made a number of assumptions that we set out in the legend to Figure 4. The three major assumptions are that (a) the therapy will prove effective, (b) adoption will be more rapid for less prevalent orphan indications for which there is by definition and no effective existing treatment and for which there are regulatory and intellectual property concessions, and (c) clinical trials authorized under ATMP regulations are likely to take twice as long to complete as conventional clinical trials. This last assumption is based on the historical experience of the Tigenix and Dendreon phase III trials, which were 6–7 years in duration with a further 2 years for market approval, and on projected trial duration given on ClinicalTrials.gov from a sample of six current trials. Two other factors that contribute to the length of cellular therapy trials are time to clear clinical end point (Tigenix) and slow rates of enrolment of patients in to trials due to strict exclusion criteria.
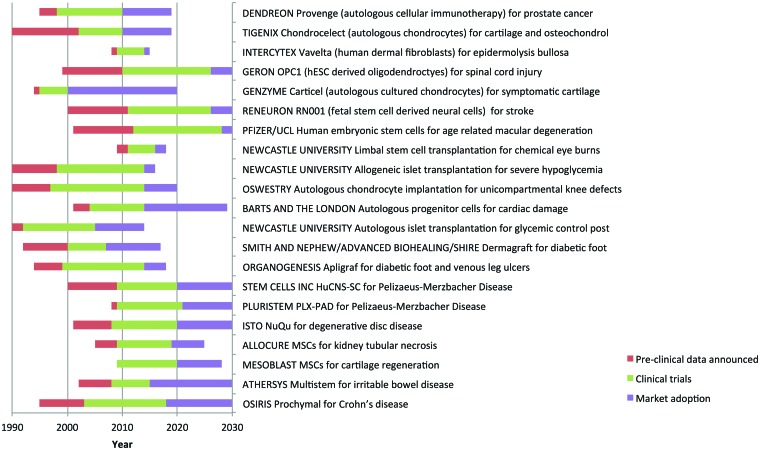
Figure 4.
Time lines of the development of the cellular therapies illustrated in Figure 3, with predictions beyond 2010. Three phases are shown: preclinical development (red), the clinical trials phase (green), and the time to market adoption (purple). Events before the end of 2010 are taken from information in the public domain. We have made the following assumptions in arriving at our estimates beyond 2010: (a) the cell therapy is safe and effective in clinical trials; (b) adoption will be more rapid and for longer periods of time for orphan indications as there is no existing treatment, they are fast tracked by the regulators, and they have extended intellectual property protection; (c) where not complete, clinical trials are estimated to take twice as long as those documented by the pharmaceutical industry [59]; (d) cellular therapies will first be taken through either the U.S. Food and Drug Administration or the European Medicines Agency, not both simultaneously, and the granting of a license (advanced therapeutic medicinal product or human cells, tissues, and cellular and tissue-based products) by one regulator will be followed 3 years later by the other; and (e) widespread adoption does not, in all cases, represent full market coverage; it is perhaps best defined as the market plateau. In estimating time to widespread adoption, we have considered the following factors: (a) market size; (b) clinical complexity; (c) substantial benefit relative to existing treatments; (d) competing cellular therapies; (e) supply; and (f) cost of goods. As an illustration, three worked examples are shown in Table 2. Abbreviations: hESC, human embryonic stem cell; MSC, mesenchymal stem cell.
We predict that widespread adoption of company-led autologous therapies will be constrained by the need to convince individual clinicians and procurement agencies of the value of the therapy particularly in the diverse European health care market. The major factor in time to market approval and widespread adoption of company-led allogeneic embryonic stem cell therapies will be the length of clinical trials. Because we estimate that market approval for these therapies will not be gained until 2025 at the earliest, it is difficult to predict how long it will take to achieve widespread adoption for these therapies; it is possible that if attention is paid during the trials to clinicians and procurers, then adoption may be relatively rapid.
Clinician-led therapies fall into two classes: those where a degree of clinical adoption already exists and those where regulator-approved trials will be carried out under more recently arrived regulatory constraints. Take the example of ACI, a therapy developed in a commercial context by Genzyme (Cambridge, MA, http://www.genzyme.com) with approximately 20,000 patients treated across the U.S. since approval in 1997. Since 1997 and quite independently, more than 400 patients have received this treatment in a routine setting in a single U.K. hospital. A multicenter trial is now under way (ACTIVE Trial, http://www.active-trial.org.uk) to compare ACI with existing best practice to provide evidence that may convince health care providers to adopt it more widely as a therapy. Again, clinician-led allogeneic islet transplantation is approved as a therapy by the U.K.'s National Commissioning group (National Institute for Health and Clinical Excellence, http://www.nice.org.uk/IPG274); this program aims to provide national coverage by 2015. These therapies follow the historical pattern of steady adoption into health care; however, there is a question mark: it seems likely that one or both of these therapies may be classified as using an advanced therapeutic medicinal product. If this is the case it is unclear whether the existing demonstrations of safety and efficacy will satisfy the regulatory authorities, as the data were collected without a clinical trial authorization.
Clinician-led therapies now entering clinical trials with clinical trials authorizations will not follow this traditional route. A survey of 150 clinical trials on the ClinicalTrials.gov Web site (search criterion: “stem cell”) showed that 53% of clinician-led registered trials were multiphase (phase I/II or phase II/III), whereas only 12% of company-led cell therapies fell into this criterion. We predict that this is the case because safety and efficacy have already been demonstrated in a clinical nontrial setting and that the overall time in trial will be shorter than for company-led cellular therapies that will individual phase I, phase II, and phase III trials. The time to adoption after market authorization for this class of cellular therapies will vary depending upon the prevalence of the indication. For example, limbal stem cell treatment for limbal stem cell deficiency should show relatively rapid adoption, as only two or three treatment centers will be required for the 200 or so cases annually in the U.K. In contrast, mesenchymal stem cell autologous therapy for orthopedic applications will take much longer in large part because of the prevalence of the indication, but also perhaps because it may take longer to reach an end point that demonstrates its efficacy relative to existing treatment.
Conclusion
Our analysis shows that clinicians work predominantly with autologous therapies that involve procedures and that companies work predominantly with allogeneic therapies, the majority of these involving products not procedures. Our broad analysis suggests that collaboration between companies and clinicians can reduce overall costs. This allows close attention to the patient pathway, reduces time to market by ensuring straightforward adoption into clinical practice, and improves the benefit to risk ratio either by identifying severe indications for which higher risks are acceptable or working to develop strategies to reduce postadministration risk, for example, by ablation of the transplanted cells.
Company push in developing cost-effective cellular therapies that attract reimbursement is only one of the forces favoring widespread adoption. It may prove that the most important consequence of close collaboration between companies and clinicians is a network effect. Well-regarded practitioners can act as national and international clinical champions for novel therapies, pulling new treatments into the clinic.
Acknowledgments
We thank Andrew Lyddiatt, Margaret Parton, James Shaw, Sajjad Ahmad, James Richardson, and Paul Kemp.
Author Contributions
L.F.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; M.W.: conception and design, data analysis and interpretation, manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Mimeault M, Hauke R, Batra S. Stem cells: A revolution in therapeutics-recent advances in stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. Clin Pharmacol Ther. 2007;82:252–264. doi: 10.1038/sj.clpt.6100301. [DOI] [PubMed] [Google Scholar]
- 2.Schneider C, Salmikangas P, Jilma B, et al. Challenges with advanced therapy medicinal products and how to meet them. Nat Rev Drug Discov. 2010;9:195–201. doi: 10.1038/nrd3052. [DOI] [PubMed] [Google Scholar]
- 3.Kolli S, Ahmad S, Lako M, et al. Successful clinical implementation of corneal epithelial stem cell therapy for treatment of unilateral limbal stem cell deficiency. Stem Cells. 2010;28:597–610. doi: 10.1002/stem.276. [DOI] [PubMed] [Google Scholar]
- 4.Sam T. Chondrocelect: The first advanced therapy medicinal product in Europe. Industrial Pharmacy. 2010;5:15–17. [Google Scholar]
- 5.Mason C, Dunnill P. Assessing the value of autologous and allogeneic cells for regenerative medicine. Regen Med. 2009;4:835–853. doi: 10.2217/rme.09.64. [DOI] [PubMed] [Google Scholar]
- 6.Clancey W. Classification Problem Solving. Austin, TX: Proceedings of the National Conference on Artificial Intelligence; 1984. pp. 49–55. [Google Scholar]
- 7.Behme S. Manufacturing of Pharmaceutical Proteins: From Technology to Economy. Weinheim, Germany: Wiley-VCH Verlag GmBH & Co. KGaA; 2009. [Google Scholar]
- 8.Daley GQ. Stem cells: Roadmap to the clinic. J Clin Invest. 2010;120:8–10. doi: 10.1172/JCI41801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Blanc K, Ringdén O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–525. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- 10.Mason C, Hoare M. Regenerative medicine bioprocessing: Building a conceptual framework based on early studies. Tissue Eng. 2007;13:301–311. doi: 10.1089/ten.2006.0177. [DOI] [PubMed] [Google Scholar]
- 11.Mason C, Hoare M. Regenerative medicine bioprocessing: the need to learn from the experience of other fields. Regen Med. 2006;1:615–623. doi: 10.2217/17460751.1.5.615. [DOI] [PubMed] [Google Scholar]
- 12.Roberts S, Mccall I, Darby A, et al. Autologous chondrocyte implantation for cartilage repair: Monitoring its success by magnetic resonance imaging and histology. Arthritis Res Ther. 2003;5:R60–R73. doi: 10.1186/ar613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pellegrini G, Luca MD, Arsenijevic Y. Towards therapeutic application of ocular stem cells. Semin Cell Dev Biol. 2007;18:805–818. doi: 10.1016/j.semcdb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro A, Ricordi C, Hering B, et al. International trial of the Edmonton protocol for islet transplantation. New Engl J Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 15.Strauer B, Kornowski R. Stem cell therapy in perspective. Circulation. 2003;107:929–934. doi: 10.1161/01.cir.0000057525.13182.24. [DOI] [PubMed] [Google Scholar]
- 16.Mavilio F, Pellegrini G, Ferrari S, et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat Med. 2006;12:1397–1402. doi: 10.1038/nm1504. [DOI] [PubMed] [Google Scholar]
- 17.Pellegrini G, Ranno R, Stracuzzi G, et al. The control of epidermal stem cells (holoclones) in the treatment of massive full-thickness burns with autologous keratinocytes cultured on fibrin. Transplantation. 1999;68:868–879. doi: 10.1097/00007890-199909270-00021. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrini G, Traverso CE, Franzi AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 19.Thomas ED. Bone marrow transplantation: A review. Semin Hematol. 1999;36:95–103. [PubMed] [Google Scholar]
- 20.Sotiropoulou P, Perez S, Salagianni M, et al. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006;24:462–471. doi: 10.1634/stemcells.2004-0331. [DOI] [PubMed] [Google Scholar]
- 21.Deal G. Stem cell therapy regulations: The US vs the EU. Regulatory Rapporteur. 2009;6:4–7. [Google Scholar]
- 22.Mason C, Dunnill P. Quantities of cells used for regenerative medicine and some implications for clinicians and bioprocessors. Regen Med. 2009;4:153–157. doi: 10.2217/17460751.4.2.153. [DOI] [PubMed] [Google Scholar]
- 23.Deans R, Zandstra P. Webinar on Cell Therapy Bioprocessing. [Accessed October 2011]. Available at http://www.aiche.org/SBE/RestrictedDocuments/DeansZandstraWebinar.aspx.
- 24.Glaser V. Status of cell and gene therapy keeps vacillating. Genetic Eng News. 2011:31. [Google Scholar]
- 25.Rowley JA. Developing cell therapy biomanufacturing processes. Chem Eng Progr (SBE Stem Cell Eng Suppl) 2010:50–55. [Google Scholar]
- 26.Liu Y, Hourd P, Chandra A, et al. Human cell culture process capability: A comparison of manual and automated production. J Tissue Eng Regen Med. 2010;4:45–54. doi: 10.1002/term.217. [DOI] [PubMed] [Google Scholar]
- 27.Zweigerdt R. Large scale production of stem cells and their derivatives. Adv Biochem Eng Biotechnol. 2009;114:201–235. doi: 10.1007/10_2008_27. [DOI] [PubMed] [Google Scholar]
- 28.Strauss S. Geron trial resumes, but standards for stem cell trials remain elusive. Nat Biotechnol. 2010;28:988–990. doi: 10.1038/nbt1010-989. [DOI] [PubMed] [Google Scholar]
- 29.Palmieri B, Tremblay JP, Daniele L. Past, present and future of myoblast transplantation in the treatment of Duchenne muscular dystrophy. Pediatr Transplant. 2010;14:813–819. doi: 10.1111/j.1399-3046.2010.01377.x. [DOI] [PubMed] [Google Scholar]
- 30.Pilot Investigation of Stem Cells in Stroke (PISCES) 2010. [Accessed May 5, 2012]. Available at http://www.clinicaltrials.gov/ct2/show/NCT01151124.
- 31.Ryan J, Barry F, Murphy J, et al. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ward WF. The Rejection Syndrome and Induced Tolerance in Tissue Transplantation: A Report of an Independent Study. Maryville, TN: Maryville College; 1962. [Google Scholar]
- 33.Kijlstra A, Heij EL, Hendrikse F. Immunological factors in the pathogenesis and treatment of age-related macular degeneration. Ocul Immunol Inflamm. 2005;13:3–11. doi: 10.1080/09273940590909185. [DOI] [PubMed] [Google Scholar]
- 34.Sherley JL. Asymmetric cell kinetics genes: The key to expansion of adult stem cells in culture. ScientificWorldJournal. 2002;2:1906–1921. doi: 10.1100/tsw.2002.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirouac D, Zandstra P. The systematic production of cells for cell therapies. Cell Stem Cell. 2008;3:369–381. doi: 10.1016/j.stem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Giebel LB. Stem cells: A hard sell to investors. Nat Biotechnol. 2005;23:798–800. doi: 10.1038/nbt0705-798. [DOI] [PubMed] [Google Scholar]
- 37.Mason C, Elliot R. Oral evidence taken before the Science and Technology Committee (Evidence 30–39). Science and Technology Committee. [Accessed January, 2012]. U.K. Parliament, Available at http://www.publications.parliament.uk/pa/cm200910/cmselect/cmsctech/220/220.pdf.
- 38.Caplan A. Mesenchymal stem cells: The past, the present, the future. Cartilage. 2010;1:6–9. doi: 10.1177/1947603509354992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Escalera J. Stem cell-based drug therapies: A discussion on emerging technologies and future developments. Yale J Biol Med. 2009;82:111–112. [PMC free article] [PubMed] [Google Scholar]
- 40.Polak DJ. Regenerative medicine: Opportunities and challenges: A brief overview. J R Soc Interface. 2010;7(suppl 6):S777–S781. doi: 10.1098/rsif.2010.0362.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salmonson T, Danielson B, Wikstrom B. The pharmacokinetics of recombinant human erythropoietin after intravenous and subcutaneous administration to healthy subjects. Br J Clin Pharmacol. 1990;29:709–713. doi: 10.1111/j.1365-2125.1990.tb03692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quesada J, Talpaz M, Rios A, et al. Clinical toxicity of interferons in cancer patients: A review. J Clin Oncol. 1986;4:234–243. doi: 10.1200/JCO.1986.4.2.234. [DOI] [PubMed] [Google Scholar]
- 43.Mason C, Dunnill P. The crucial linkage required between regenerative medicine bioprocessors and clinicians. Regen Med. 2008;3:435–442. doi: 10.2217/17460751.3.4.435. [DOI] [PubMed] [Google Scholar]
- 44.Ryan EA, Lakey JRT, Rajotte RV, et al. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton Protocol. Diabetes. 2001;50:710–719. doi: 10.2337/diabetes.50.4.710. [DOI] [PubMed] [Google Scholar]
- 45.Fronk DM, Ferros JD. Human tissues for cardiovascular surgery: Regulatory requirements. Aortic Root Surg. 2010:581–587. [Google Scholar]
- 46.European Medicines Agency. CPMP/ICH/135/95: Guideline for Good Clinical Practice. 1997 [Google Scholar]
- 47.Gagnon M, Lexchin J. The cost of pushing pills: A new estimate of pharmaceutical promotion expenditures in the United States. PLoS Med. 2008;5:e1. doi: 10.1371/journal.pmed.0050001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams D, Wells O, Hourd P. Feeling the pain: Disruptive innovation in healthcare markets. Innovation in Manufacturing Networks. 2008;266:26–34. [Google Scholar]
- 49.Trounson A, Baum E, Gibbons D, et al. Developing a case study model for successful translation of stem cell therapies. Cell Stem Cell. 2010;6:513–516. doi: 10.1016/j.stem.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 50.Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: A systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 51.Giordano A, Galderisi U, Marino IR. From the laboratory bench to the patient's bedside: An update on clinical trials with mesenchymal stem cells. J Cell Physiol. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- 52.Smith G, Knutsen G, Richardson J. A clinical review of cartilage repair techniques. J Bone Joint Surg Br. 2005;87:445–449. doi: 10.1302/0301-620X.87B4.15971. [DOI] [PubMed] [Google Scholar]
- 53.Koga H, Engebretsen L, Brinchmann J, et al. Mesenchymal stem cell-based therapy for cartilage repair: A review. Knee Surg Sports Traumatol Arthrosc. 2009;17:1289–1297. doi: 10.1007/s00167-009-0782-4. [DOI] [PubMed] [Google Scholar]
- 54.Featherstone C. Epidermolysis bullosa: From fundamental molecular biology to clinical therapies. J Invest Dermatol. 2007;127:256–259. doi: 10.1038/sj.jid.5700731. [DOI] [PubMed] [Google Scholar]
- 55.Tator C, Duncan EG, Edmonds VE, et al. Complications and costs of management of acute spinal cord injury. Paraplegia. 1993;31:700–714. doi: 10.1038/sc.1993.112. [DOI] [PubMed] [Google Scholar]
- 56.Taylor T, Davis PH, Torner JC, et al. Lifetime cost of stroke in the United States. Stroke. 1996;27:1459–1466. doi: 10.1161/01.str.27.9.1459. [DOI] [PubMed] [Google Scholar]
- 57.Tarassoff CP, Arlen PM, Gulley JL. Therapeutic vaccines for prostate cancer. The Oncologist. 2006;11:451–462. doi: 10.1634/theoncologist.11-5-451. [DOI] [PubMed] [Google Scholar]
- 58.Hirshberg B, Rother KI, Digon BJ, et al. Benefits and risks of solitary islet transplantation for type 1 diabetes using steroid-sparing immunosuppression. Diabetes Care. 2003;26:3288–3295. doi: 10.2337/diacare.26.12.3288. [DOI] [PubMed] [Google Scholar]
- 59.DiMasi J, Grabowski HG. The cost of biopharmaceutical R&D: Is biotech different? Manage Decis Econ. 2007;28:469–479. [Google Scholar]