The Committee for Advanced Therapies of the European Medicines Agency recently classified autologous bone marrow-derived mononuclear cells and CD133+ stem cells as tissue-engineered products when intended for regeneration through stem cell-induced angiogenesis in ischemic heart tissue. These scientific recommendations are reviewed in light of the scientific knowledge acquired over the last few years about the mechanisms of postnatal neovascularization. The negative impact that the consideration of these treatments as medicinal products, instead of cell transplants, will have on European public health services is also analyzed.
Keywords: Bone marrow transplant, Adult stem cells, Stem cell transplantation, Tissue-specific stem cells, Clinical trials, Bone marrow CD133+ cells, Advanced therapy medicinal product
Abstract
In November of 2011, the Committee for Advanced Therapies (CAT) of the European Medicines Agency (EMA) published two scientific recommendations regarding the classification of autologous bone marrow-derived mononuclear cells (BM-MNCs) and autologous bone marrow-derived CD133+ cells as advanced therapy medicinal products (ATMPs), specifically tissue-engineered products, when intended for regeneration in ischemic heart tissue on the basis that they are not used for the same essential function (hematological restoration) that they fulfill in the donor. In vitro and in vivo evidence demonstrates that bone marrow cells are physiologically involved in adult neovascularization and tissue repair, making their therapeutic use for these purposes a simple exploitation of their own essential functions. Therefore, from a scientific/legal point of view, nonsubstantially manipulated BM-MNCs and CD133+ cells are not an ATMP, because they have a physiological role in the processes of postnatal neovascularization and, when used therapeutically for vascular restoration in ischemic tissues, they are carrying out one of their essential physiological functions (the legal definition recognizes that cells can have several essential functions). The consequences of classifying BM-MNCs and CD133+ cells as medicinal products instead of cellular transplantation, like bone marrow transplantation, in terms of costs and time for these products to be introduced into clinical practice, make this an issue of crucial importance. Therefore, the recommendations of EMA/CAT could be reviewed in collaboration with scientific societies, in light of organizational and economic consequences as well as scientific knowledge recently acquired about the mechanisms of postnatal neovascularization and the function of bone marrow in the regeneration of remote tissues.
Introduction
Since the 1950s, when the Nobel Prize winner E. Donnall Thomas demonstrated the feasibility of bone marrow transplantation (BMT) in patients with leukemia [1], this therapeutic approach has saved the lives of thousands of patients suffering from a great variety of diseases, mainly affecting the hematopoietic or immunological system. Later on, the use of cellular fractions contained in bone marrow (BM), such as BM-derived mononuclear cells (BM-MNCs), or even more specific cells, such as CD34+ or CD133+ stem cells, started to become a common technique available in a large number of hospitals. More recently, in the 2000s, these selected types of cells began to be used for new clinical purposes different from those for which BMT was traditionally carried out, including the treatment of ischemic diseases, such as acute and chronic ischemic heart disease [2–6] or, even more recently, peripheral arterial disease [7, 8]. Since that time, an increasing number of clinical trials for these “nontraditional” purposes have been carried out all over the world, most of which are sponsored by hospitals, universities, or foundations.
One important issue regarding the development of these “new” treatments, which make use of these “old” cellular products, is whether they should be considered medicinal products or cellular transplantation. The consequences of classifying them as medicinal products, in terms of costs and time spent on the introduction of these approaches into clinical practice, make this an issue of crucial importance, not only for the clinical research groups involved in this field but also for the patients who may benefit from these treatments, whose safety has been largely proven for restoration of the hematological or immunological system.
Very recently, on November 23 and 24, 2011, the Committee for Advanced Therapies (CAT) of the European Medicines Agency (EMA) published two scientific recommendations regarding the classification of autologous BM-MNCs [9] and autologous bone marrow-derived CD133+ stem cells [10] as medicinal products, specifically tissue-engineered products (TEPs). In this article, we examine the definitions of some advanced therapy medicinal products (ATMPs) according to the European regulatory framework, as well as the scientific knowledge developed over several years to point out that the legal definition of TEPs may not apply in these cases, as, in fact, it does not apply in the case of BMT.
Definition of ATMPs
ATMPs include gene therapy and somatic cell therapy medicinal products (SCTMPs) as they are defined in Commission Directive 2009/120/EC. They also include TEPs and combined advanced therapy medicinal products as defined in Regulation (EC) No. 1394/2007.
According to Commission Directive 2009/120/EC, “Somatic cell therapy medicinal product means a biological medicinal product which has the following characteristics: (a) contains or consists of cells or tissues that have been subject to substantial manipulation so that biological characteristics, physiological functions or structural properties relevant for the intended clinical use have been altered, or of cells or tissues that are not intended to be used for the same essential function(s) in the recipient and the donor; (b) is presented as having properties for, or is used in or administered to human beings with a view to treating, preventing or diagnosing a disease through the pharmacological, immunological or metabolic action of its cells or tissues.” For the purposes of point (a), certain types of manipulations (as they are listed in Annex I to Regulation (EC) No. 1394/2007) shall not be considered substantial manipulations, such as grinding, shaping, centrifugation, soaking in antibiotic or antimicrobial solutions, sterilization, irradiation, cell separation, concentration or purification, filtering, lyophilization, freezing, cryopreservation, and vitrification.
According to Regulation (EC) No. 1394/2007, “Tissue engineered product means a product that contains or consists of engineered cells or tissues, and is presented as having properties for, or is used in or administered to human beings with a view to regenerating, repairing or replacing a human tissue.” When considering the concept of engineered cells or tissues, the Regulation states that “Cells or tissues shall be considered ‘engineered' if they fulfill at least one of the following conditions:
“The cells or tissues have been subject to substantial manipulation, so that biological characteristics, physiological functions or structural properties relevant for the intended regeneration, repair or replacement are achieved.
“The cells or tissues are not intended to be used for the same essential function or functions in the recipient as in the donor.”
Therefore, the composition of both SCTMPs and TEPs may be identical, but their mode of action is different.
The key points regarding the limits of the definition of SCTMPs or TEPs, to determine whether a cell therapy product is or is not a medicinal product, are based on the type of manipulation performed on the cells and their intended use—for the same or different essential function(s)—in the recipient and the donor, irrespective of whether the donor and the recipient are the same person.
The procedure to obtain BM-MNCs and CD133+ cells includes only manipulations considered nonsubstantial (cell separation, concentration, or purification). Therefore, their consideration or not as medicinal products will depend exclusively on the essential functions of the cells and their intended use.
European Medicines Agency Position
As has been mentioned earlier, in November 2011, EMA/CAT published two scientific recommendations—that are not binding—regarding the classification of autologous BM-MNCs [9] and autologous BM-derived CD133+ stem cells [10] as medicinal products (considered as TEPs). The recommendations assessed these two products “intended for improvement of heart function and quality of life in patients with ischemic heart disease, postacute myocardial infarction and in chronic ischemic heart disease.”
The EMA/CAT conclusions were made on the bases that “the products are not intended to be used for the same essential function (hematological restoration)” and that “the products are intended for regeneration through stem cell-induced angiogenesis in ischemic heart tissue by nonhematological differentiation of the bone marrow cells into vascular cells or by paracrine effects of the stem cells.” Finally, the EMA/CAT considers that the products fall within the definition of TEP as provided in Article 2.1 of Regulation (EC) No. 1394/2007.
Functions of Bone Marrow-Derived Mononuclear Cells and CD133+ Cells
The concept of BM as an exclusively hematopoietic organ changed more than a decade ago with the discovery of the existence of diverse nonhematopoietic stem cell populations, which share the same origin as hematopoietic cells and coexist in normal adult human BM [11, 12]. Simultaneously, the concept of adult tissue renovation also changed, because until then it had been restricted to some specific organs and tissues known for their high regenerative capacity, but the ability to homeostatically renovate tissues such as myocardium, neural tissue, or blood vessels, among others, was then unknown.
As a result of these discoveries, the view that, under physiological conditions, BM fulfills an exclusively hematopoietic function has become obsolete. Over the last 10 years, it has been scientifically demonstrated that BM carries out a further regenerative function of remote tissues under homeostatic conditions. Besides the evidence obtained from animal models, the principal evidence from humans can be grouped as follows.
Presence of Different Adult Stem Cell Lineages in Normal Human Adult BM
The term BM-MNC is simply used to collectively denominate all cells present in BM whose nuclei are unilobulated or rounded and lack granules in the cytoplasm. These characteristics give the BM-MNCs a similar density and size, which is different from that of myeloid cells and red-cell progenitors, making them easy to separate by physical means. Among adult human BM-MNCs are hematopoietic progenitor cells at different stages of maturation as well as lymphoid cells (lymphocytes, plasmatic cells), monocytes, and macrophages. Furthermore, several cells of nonhematopoietic lineage, or which can differentiate into nonhematopoietic cells, have been identified in the mononuclear fraction of normal human adult BM. Among these are the side population cells, which present a phenotype and functionality characteristic of primitive stem cells having multipotent capacity [13]; mesenchymal stromal cells [14]; very small embryonic-like stem cells, which have characteristics similar to embryonic stem cells [15]; multipotent adult progenitor cells [16]; hemangioblasts (progenitor cells that are common for hematopoietic and vasculogenic lineages) [17]; endothelial progenitor cells (EPCs) [18]; and tissue-committed stem cells [19].
Microchimerism in BMT Recipients
Transplanted BM-derived cells have been found to engraft in almost all tissues of the organism and contribute to tissue repair after injury (e.g., women undergoing sex-mismatched BMT showing Y chromosome-positive cardiomyocytes) [20, 21]. Interestingly, animal experiments showed no detectable engraftment of marrow-derived cells in the absence of myocardial injury. These findings suggest that adult human BM cells have the capacity to participate in the homeostatic regeneration of multiple tissues under physiological or pathological conditions, proving the concept that remote tissue regeneration constitutes an essential function of adult human BM.
BM Cell Mobilization in Response to Tissue Damage
It has been shown that there is a mobilization of BM cells into peripheral blood in response to ischemia-induced cytokines secreted from remote tissues and that these cells reach damaged tissues, contributing to their regeneration under physiological circumstances of homeostasis. This has been observed particularly after acute myocardial infarction (AMI), giving rise to the concept of BM-myocardium axis [22], as in the cases of liver [23] and kidney [24] tissue damage, among others. Moreover, the level of mobilized marrow-derived EPCs in circulating blood after an AMI not only correlates with the cumulative cardiovascular risk [25] and vascular function [26] but also has a predictive value for the probability of new cardiovascular events and for the progression of arteriosclerosis in patients with coronary disease [27, 28]. At least four BM cell types have been shown to mobilize in response to tissue damage: one of these is EPCs, as we document below in greater detail.
Adult BM Cell Secretion of Cytokines with Cytoprotective Function for Diverse Cellular Lineages
There is evidence that one of the beneficial effects (probably the most important) of BM cells recruited by injured tissues results from a paracrine mechanism. This includes the production at the tissue level of significant amounts of cytokines and growth factors with different actions, including prevention of apoptosis, cytoprotection of native viable cells, anti-inflammatory effects, reduction of fibrosis, and recruitment of specific stem cells, leading to a robust stimulation of angiogenesis and tissue regenerative mechanisms directly mediated by resident progenitor cells [29, 30].
In addition to this general evidence for BM-MNCs, there are some other specific types of evidence for the population of CD133+ cells. Cells positive for CD133, which represent the best known human homolog of the murine protein prominin-1, were initially identified in populations of hematopoietic stem cells and progenitor cells of BM and peripheral blood [31], but later they have also been identified in a multitude of nonhematopoietic tissues, such as the endothelium, skin, liver, pancreas, muscle, kidney, prostate, and brain [32–35]. Their capacity to differentiate into tissues of mesodermal, ectodermal, and endodermal origin has been consistently shown in vivo and in vitro. When transplanted into nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice, they are able to generate multilineage hematopoiesis as well as repopulate and regenerate tissues, such as liver, lung, brain, heart, intestine, and muscle [36] and also to differentiate into mature endothelial cells. In fact, it has been found that in vitro CD133+ cells differentiate mainly into mature endothelial cells [37], and there is a general consensus that BM-derived EPCs express CD133 [38].
In the field of postnatal neovascularization, the discoveries of EPCs by Asahara et al. in 1997 [39] and their origin in BM 2 years later by the same group [40] constitute a genuine breakthrough. There exists growing evidence that postnatal neovascularization depends, to some extent, on the necessary contribution of mobilized marrow-derived EPCs [37, 41, 42]. Besides the direct incorporation of EPCs to neovascularization foci and their subsequent differentiation into mature endothelial cells [43], both EPCs [44] and other types of mobilized BM-MNCs constitute a source of proangiogenic cytokines and growth factors [45]. Therefore, the role of BM-derived cells in postnatal neovascularization can be summarized in two main functions: first, the direct mechanism by which EPCs differentiate into mature endothelial cells that incorporate into new vessels (vasculogenesis) [43] and, second, the indirect mechanism that involves, in turn, a paracrine-mediated stimulation of the local angiogenesis as well as a less known contribution of monocytes and/or macrophages to neoarteriogenesis [46, 47].
From the scientific point of view, all the above-mentioned functions of the bone marrow-derived cells that are mobilized by ischemia-induced stimuli (EPCs and probably other, less well known cell types) occur in a physiological way. Therefore, neovascularization should be considered an essential function of these types of cells.
Discussion
The legal definition of SCTMP and TEP refers to “the cells or tissues not intended to be used for the same essential function or functions in the recipient as in the donor.” Therefore, in line with the legal definition, BM-MNCs and CD133+ cells should not be classified as ATMPs when intended for the promotion of new blood vessel growth and ischemic tissue repair because of the evidence available today regarding their physiological role in the processes of postnatal neovascularization [42–46]. Ischemic or damaged tissues physiologically recruit CD133+ cells, which stimulate the mechanisms of neovascularization and tissue repair. From the physiological perspective, this process is cumulative, with continuous recruitment of cells over a varying period of time [42], and from the therapeutic perspective, the therapy simply facilitates the physiological means by which CD133+ cells reach the targeted tissue, that is, collecting them from BM and administering them intra-arterially.
It can be argued that neovascularization is not the exclusive essential function of BM-MNCs, because they are a heterogeneous combination of different types of progenitors, or even that we have not yet completely characterized all the cellular subtypes included in BM-MNCs. However, in the case of BMT, the infusion into the central venous system of BM-MNCs for hematopoiesis restoration also includes a heterogeneous group of cells whose exact functions are equally unknown, and it is not considered a medicinal product. In any case, the legal definition itself recognizes that cells or tissues can have several essential functions. Moreover, the definition mentions neither that the intended use has to be for the main essential function nor that the essential function should be exclusive to those cells.
Additionally, the restrictive application of these regulatory criteria would lead us to the conclusion that BM in the framework of allogeneic hematopoietic transplant is an ATMP, because the graft itself is used looking not only for hematopoiesis restoration but also for its graft-versus-leukemia effect, essential for the success of BMT. It is evident that, in the donor, BM cells do not recognize foreign allogeneic antigens present in the residual tumor cells of the recipient; therefore, according to the EMA/CAT criteria, we would be dealing with an ATMP. This is more obvious than in the case of the use of BM cells to exploit their physiological function of enhancing neovascularization in ischemic tissues.
The relevance of this debate regarding the classification of BM-MNCs as a whole and CD133+ cells in particular, when intended to enhance neovascularization in ischemic diseases as medicinal products or cellular transplantation (as they are considered when they are used to restore hematopoiesis), resides in its important economic and organizational consequences (Fig. 1). If BM-MNCs and CD133+ cells are considered medicinal products, then the higher requirements for processing BM (e.g., in terms of quality controls or infrastructures: laboratory compliant with good manufacturing practice vs. tissue establishment usually located inside BMT-authorized hospitals) have an important economic impact that cannot be justified in terms of patient safety. BMT is performed in immunocompromised patients frequently receiving allogeneic BM-MNCs infused into the central venous system. It does not seem justified to increase the quality requirements when infusing BM-MNCs used for neovascularization in immunocompetent patients receiving their own BM.
Figure 1.
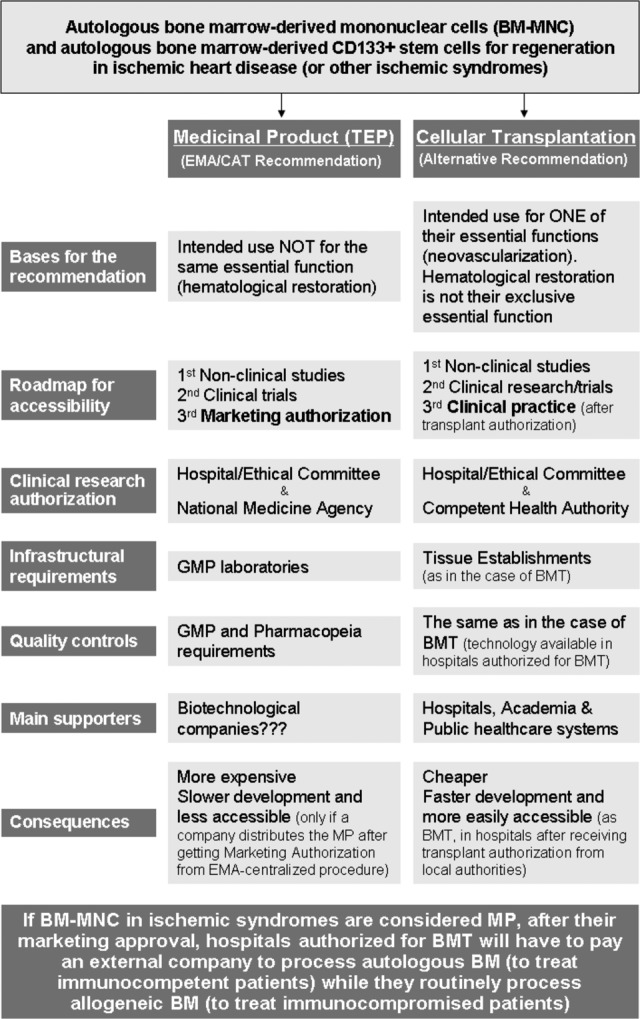
Differences and consequences of considering bone marrow-derived mononuclear cells and bone marrow-derived CD133+ cells as advanced therapy medicinal products or cellular transplantation. Abbreviations: BM, bone marrow; BM-MNC, bone marrow-derived mononuclear cells; BMT, bone marrow transplantation; CAT, Committee for Advanced Therapies; EMA, European Medicines Agency; GMP, good manufacturing practice; MP, medicinal product; TEP, tissue-engineered product.
Another important consequence of considering BM-MNCs and CD133+ cells as medicinal products is the longer time it will take for these treatments to become available to the patients. Because of the experimental nature of the use of BM-MNCs in ischemic syndromes, independently of their consideration as a cell transplant or medicinal product, both nonclinical and clinical studies must be performed to demonstrate their safety and efficacy. Nevertheless, after completing clinical research, if BM-MNCs and CD133+ cells are considered medicinal products, patients will be able to access these treatments only after a company obtains marketing authorization, whereas if they are considered cell transplantation, hospitals could offer these procedures as they offer BMT with transplant authorization.
Furthermore, the consideration of these treatments as medicinal products would force BMT-authorized hospitals into a contradictory situation. Depending on the patient, hospitals will be able to process the BM (for BMT in immunocompromised patients receiving an allogeneic product) or will be obliged to send it to a company for processing with the attendant costs (for immunocompetent patients receiving their own cells). Therefore, BMT-authorized hospitals will have to pay for a process that they routinely perform, using the same technology, for patients at higher risk.
The potential fear of damaging companies, or even the industry as a whole, by considering BM-MNCs and CD133+ cells as transplants is unjustified. If we look at the sponsors of clinical trials using BM-MNCs or CD133+ cells (search the ClinicalTrials.gov Web site [http://www.clinicaltrials.gov] using “autologous graft” as the search term, “interventional studies” as the type of study, and “CD133 cells” or “bone marrow mononuclear cells” as the intervention), we find that these are hospitals, universities, research centers, or foundations, with the sole exception of a company that sells the technology for cell separation and the CD133 isolation kit used by hospitals authorized for BMT. This means that the development of these products is being carried out mainly in the context of nonprofit organizations. This makes sense if we bear in mind that there is no manufacturing procedure involved in their processing, only very simple technology already available to hospitals authorized to perform BMT.
Finally, the classification of BM-MNCs and CD133+ cells is of crucial importance for European public health care systems if they are willing to incorporate these therapies into clinical practice [48]. If hospitals, with no commercial interest, are not allowed to offer this treatment as a service (after its safety and efficacy have been proven), they will have to support the higher costs involved. This is hardly the best moment to waste public funds on something that public health care systems have been doing for more than 50 years without safety problems for patients at higher risk.
Conclusion
The EMA/CAT has very recently classified BM-MNCs and CD133+ stem cells as ATMP (specifically TEP) when intended for regeneration through stem cell-induced angiogenesis in ischemic heart tissue on the basis that the products are not intended to be used for the same essential function (hematological restoration). Although these scientific recommendations are not binding, they should be reviewed in light of the organizational and economic consequences, the lower risks compared with allogeneic BMT, and the scientific knowledge acquired over the last few years about the mechanisms of postnatal neovascularization.
We consider that BM-MNCs and CD133+ stem cells are not an ATMP, because the procedure to obtain them includes only manipulations considered nonsubstantial and because, when intended for the repair of ischemic tissues, they are used for the same essential function in the recipient as in the donor. Because of the fact that BM-MNCs, and especially CD133+ cells, as endothelial progenitors have a fundamental determining physiological role in the processes of postnatal neovascularization, when these cells are used therapeutically for vascular restoration in ischemic tissues, they are carrying out nothing other than their physiological function. When we use BM-MNCs, looking for hematopoietic restoration (one of their essential functions), they are not considered a medicinal product; therefore, the same criteria should apply when we use BM-MNCs for neovascularization, as we are using them for one of their own functions, which is the same in the recipient as in the donor.
The consideration of BM-MNCs and CD133+ stem cells as medicinal products instead of as cellular transplantation when we intend to use them to enhance neovascularization has important consequences at all levels, requiring deeper reflection from the EMA/CAT in collaboration with scientific societies. If the consideration of those products as ATMPs does not change, this will have a very negative impact not only on European public health services and on patients who will have to wait longer and pay more for their treatments but also in that we will all have to support the higher costs involved. The legal framework does allow for a more flexible interpretation, taking into account the scientific evidence, and our society deserves this.
Acknowledgments
We greatly appreciate the collaboration and critical review carried out by Prof. Jose Maria Perez Pomares from the University of Malaga and Dr. Rosario Mata from the Andalusian Initiative for Advanced Therapies.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
Author Contributions
N.C. and C.H.: conception and design, provision of study material or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; L.R.: administrative support, provision of study material or patients, collection and/or assembly of data, collaboration in the conception and design of the figure.
References
- 1.Thomas ED, Lochte HL, Jr., Lu WC, et al. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491–496. doi: 10.1056/NEJM195709122571102. [DOI] [PubMed] [Google Scholar]
- 2.Martin-Rendon E, Brunskill S, Dorée C, et al. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2008:CD006536. doi: 10.1002/14651858.CD006536.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Suárez de Lezo J, Herrera C, Pan M, et al. Regenerative therapy in patients with a revascularized acute anterior myocardial infarction and depressed ventricular function. Rev Esp Cardiol. 2007;60:357–365. [PubMed] [Google Scholar]
- 4.Schächinger V, Erbs S, Elsässer A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 5.Williams AR, Trachtenberg B, Velazquez DL, et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: Functional recovery and reverse remodeling. Circ Res. 2011;108:792–796. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adler DS, Lazarus H, Nair R, et al. Safety and efficacy of bone marrow-derived autologous CD133+ stem cell therapy. Front Biosci (Elite Ed) 2011;3:506–514. doi: 10.2741/e265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walter DH, Krankenberg H, Balzer JO, et al. Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: A randomized-start, placebo-controlled pilot trial (PROVASA) Circ Cardiovasc Interv. 2011;4:26–37. doi: 10.1161/CIRCINTERVENTIONS.110.958348. [DOI] [PubMed] [Google Scholar]
- 8.Idei N, Soga J, Hata T, et al. Autologous bone-marrow mononuclear cell implantation reduces long-term major amputation risk in patients with critical limb ischemia: A comparison of atherosclerotic peripheral arterial disease and Buerger disease. Circ Cardiovasc Interv. 2011;4:15–25. doi: 10.1161/CIRCINTERVENTIONS.110.955724. [DOI] [PubMed] [Google Scholar]
- 9.Scientific recommendation on classification of advanced therapy medicinal products. EMA/920913/2011. [Accessed December 10, 2011]. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Report/2011/11/WC500118208.pdf.
- 10.Scientific recommendation on classification of advanced therapy medicinal products. EMA/921674/2011. [Accessed December 10, 2011]. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Report/2011/11/WC500118207.pdf.
- 11.Shi Q, Rafii S, Wu MH, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- 12.Jackson KA, Majka SM, Wang H, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Challen G, Little M. A side order of stem cells: The SP phenotype. Stem Cells. 2006;24:3–12. doi: 10.1634/stemcells.2005-0116. [DOI] [PubMed] [Google Scholar]
- 14.Salem HK, Thiemermann C. Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kucia MJ, Wysoczynski M, Wu W, et al. Evidence that very small embryonic-like stem cells are mobilized into peripheral blood. Stem Cells. 2008;26:2083–2092. doi: 10.1634/stemcells.2007-0922. [DOI] [PubMed] [Google Scholar]
- 16.Ji KH, Xiong J, Hu KM, et al. Simultaneous expression of Oct4 and genes of three germ layers in single cell-derived multipotent adult progenitor cells. Ann Hematol. 2008;87:431–438. doi: 10.1007/s00277-008-0470-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park C, Ma YD, Choi K. Evidence for the hemangioblast. Exp Hematol. 2005;33:965–970. doi: 10.1016/j.exphem.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto Y, Suyama T, Yashita T, et al. Bone marrow subpopulations contain distinct types of endothelial progenitor cells and angiogenic cytokine-producing cells. J Mol Cell Cardiol. 2007;43:627–635. doi: 10.1016/j.yjmcc.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Kucia M, Reca R, Jala VR, et al. Bone marrow as a home of heterogeneous populations of non-hematopoietic stem cells. Leukemia. 2005;19:1118–1127. doi: 10.1038/sj.leu.2403796. [DOI] [PubMed] [Google Scholar]
- 20.Deb A, Wang S, Skelding KA, et al. Bone marrow-derived cardiomyocytes are present in adult human heart: A study of gender-mismatched bone marrow transplantation patients. Circulation. 2003;107:1247–1249. doi: 10.1161/01.cir.0000061910.39145.f0. [DOI] [PubMed] [Google Scholar]
- 21.Rovó A, Gratwohl A. Plasticity after allogeneic hematopoietic stem cell transplantation. Biol Chem. 2008;389:825–836. doi: 10.1515/BC.2008.103. [DOI] [PubMed] [Google Scholar]
- 22.Maltais S, Perrault LP, Ly HQ. The bone marrow-cardiac axis: Role of endothelial progenitor cells in heart failure. Eur J Cardiothorac Surg. 2011;39:368–374. doi: 10.1016/j.ejcts.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 23.Kollet O, Shivtiel S, Chen YQ, et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poulsom R, Forbes SJ, Hodivala-Dilke K, et al. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001;195:229–235. doi: 10.1002/path.976. [DOI] [PubMed] [Google Scholar]
- 25.Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 26.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt-Lucke C, Rössig L, Fichtlscherer S, et al. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: Proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 28.Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi M, Li TS, Suzuki R, et al. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol. 2006;291:H886–H893. doi: 10.1152/ajpheart.00142.2006. [DOI] [PubMed] [Google Scholar]
- 30.Kubal C, Sheth K, Nadal-Ginard B, et al. Bone marrow cells have a potent anti-ischemic effect against myocardial cell death in humans. J Thorac Cardiovasc Surg. 2006;132:1112–1118. doi: 10.1016/j.jtcvs.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 31.Yin AH, Miraglia S, Zanjani ED, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- 32.Ito Y, Hamazaki TS, Ohnuma K, et al. Isolation of murine hair-inducing cells using the cell surface marker prominin-1/CD133. J Invest Dermatol. 2007;127:1052–1060. doi: 10.1038/sj.jid.5700665. [DOI] [PubMed] [Google Scholar]
- 33.Kordes C, Sawitza I, Müller-Marbach A, et al. CD133+ hepatic stellate cells are progenitor cells. Biochem Biophys Res Commun. 2007;352:410–417. doi: 10.1016/j.bbrc.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 34.Sagrinati C, Netti GS, Mazzinghi B, et al. Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 35.Richardson GD, Robson CN, Lang SH, et al. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci. 2004;117:3539–3545. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- 36.Kuçi S, Wessels JT, Bühring HJ, et al. Identification of a novel class of human adherent CD34-stem cells that give rise to SCID-repopulating cells. Blood. 2003;101:869–876. doi: 10.1182/blood-2002-03-0711. [DOI] [PubMed] [Google Scholar]
- 37.Koutna I, Peterkova M, Simara P, et al. Proliferation and differentiation potential of CD133+ and CD34+ populations from the bone marrow and mobilized peripheral blood. Ann Hematol. 2011;90:127–137. doi: 10.1007/s00277-010-1058-2. [DOI] [PubMed] [Google Scholar]
- 38.Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 39.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 40.Asahara T, Masuda H, Takahasi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 41.Shintani S, Murohara T, Ikeda H, et al. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi T, Kalka C, Mashuda H, et al. Ischemia-and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 43.Crosby JR, Kaminsky WE, Schatteman G, et al. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res. 2000;87:728–730. doi: 10.1161/01.res.87.9.728. [DOI] [PubMed] [Google Scholar]
- 44.Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 45.Kamihata H, Matsubara H, Nishiue T, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands and cytokines. Circulation. 2001;104:1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 46.Gnecchi M, Zhang Z, Ni A, et al. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tongers J, Roncalli JG, Losordo DW. Role of endothelial progenitor cells during ischemia-induced vasculogenesis and collateral formation. Microvasc Res. 2010;79:200–206. doi: 10.1016/j.mvr.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cuende N, Izeta A. Clinical translation of stem cell therapies: A bridgeable gap. Cell Stem Cell. 2010;6:508–512. doi: 10.1016/j.stem.2010.05.005. [DOI] [PubMed] [Google Scholar]


