Human adult olfactory neural progenitors (hONPs) can differentiate along several neural lineages in response to morphogenic signals in vitro. This study engrafted cells modified by the most efficient transfection paradigm for dopaminergic neural restriction and pretransfected controls into a unilateral neurotoxin, 6-hydroxydopamine-induced parkinsonian rat model. The results suggest that human adult olfactory epithelial-derived progenitors represent a unique autologous cell type with promising potential for future use in a cell-based therapy for patients with Parkinson's disease.
Keywords: Adult stem cells, Autologous, Autologous stem cell transplantation, Cell transplantation, Cellular therapy, Parkinson's disease, Stem cell-microenvironment interactions, Transcription factors
Abstract
Human adult olfactory epithelial-derived neural progenitors (hONPs) can differentiate along several neural lineages in response to morphogenic signals in vitro. A previous study optimized the transfection paradigm for the differentiation of hONPs to dopaminergic neurons. This study engrafted cells modified by the most efficient transfection paradigm for dopaminergic neural restriction and pretransfected controls into a unilateral neurotoxin, 6-hydroxydopamine-induced parkinsonian rat model. Approximately 35% of the animals engrafted with hONPs had improved behavioral recovery as demonstrated by the amphetamine-induced rotation test, as well as a corner preference and cylinder paw preference, over a period of 24 weeks. The pre- and post-transfected groups produced equivalent responses, indicating that the toxic host environment supported hONP dopaminergic differentiation in situ. Human fibroblasts used as a cellular control did not diminish the parkinsonian rotational deficits at any point during the study. Increased numbers of tyrosine hydroxylase (TH)-positive cells were detected in the engrafted brains compared with the fibroblast-implanted and medium-only controls. Engrafted TH-positive hONPs were detected for a minimum of 6 months in vivo; they were multipolar, had long processes, and migrated beyond their initial injection sites. Higher dopamine levels were detected in the striatum of behaviorally improved animals than in equivalent regions of their nonrecovered counterparts. Throughout these experiments, no evidence of tumorigenicity was observed. These results support our hypothesis that human adult olfactory epithelial-derived progenitors represent a unique autologous cell type with promising potential for future use in a cell-based therapy for patients with Parkinson's disease.
Introduction
Parkinson's disease (PD) is a worldwide neurodegenerative disease whose incidence increases with population longevity [1]. PD is characterized by extensive loss of dopaminergic (DA) neurons in the substantia nigra [2]. Although drugs help relieve the symptoms and potential therapeutic strategies for PD are in clinical trials [3], no cure is available. The major treatment for PD is oral l-3,4-dihydroxyphenylalanine (l-DOPA) [4], the precursor of dopamine that can cross the blood-brain barrier [5]. l-DOPA becomes less effective for two reasons: (a) during the progression of the disease, the neurons become less sensitive to the drug [6], and (b) l-DOPA does not prevent or rescue the DA neurons from degeneration [7]. Deep brain stimulation can benefit PD patients as a component of a medical regimen [8, 9], although the results are variable.
Alternative therapeutic approaches suggest that cell replacement may have significant merit [10, 11]. Studies using cell grafts obtained from pooled fetal ventral mesencephalic dopaminergic neurons pioneered this approach [12, 13]. Improvements were achieved after the engraftment, but they were accompanied by dyskinesia [14, 15]. Stem cells represent an alternative replacement population because of their unlimited capacity for self-renewal and potential for differentiation depending on their origin and the microenvironmental conditions [16, 17]. Mouse and porcine embryonic stem cells (ESCs) have been used in replacement strategies for the treatment of PD animal models, and behavioral improvements have been observed [18, 19]. However, a source of human cells is essential for clinical trials. Dopaminergic lineage-restricted human embryonic stem cells (hESCs) provide relief of parkinsonian symptoms when transplanted into a rodent model of PD but frequently result in teratomas [20, 21]. Human induced pluripotent stem cells (hiPSCs) may also be a promising resource [22–24]. hiPSCs can be obtained from somatic cells and reprogrammed to an embryonic-like state [25]. hiPSCs are similar to hESCs in morphology, proliferation, surface antigens, gene expression, epigenetic status of pluripotent cell-specific genes, and telomerase activity [26]. Transplantation of hiPSCs has been shown to diminish the parkinsonian behavioral deficits in rodent models of PD; unfortunately in many cases, similar to their embryonic counterparts, the engrafted population formed tumors containing tissues from the three germ layers [22, 25]. Collectively, the studies outlined above provide proof of concept that cell replacement is a viable therapeutic strategy for the treatment of individuals with PD. What is needed is a cell source to replace lost or damaged DA neurons that is stable, will not be tumorigenic, and eliminates the ethical concerns associated with embryonic tissues.
The olfactory epithelium (OE) is a unique source for neural progenitors that can be harvested by minimally invasive endoscopic nasal surgery [27]. Furthermore, since no demonstrable olfactory deficits result from OE biopsy, the tissue could be used to generate an autologous progenitor population from patients with PD [27]. An autologous source provides total histocompatibility and eliminates the need for immunosuppressive therapy, as well as long waiting lists for available donors. Methods for the isolation and culture of a neurosphere forming population of neural progenitors have been developed [28]. More than 150 patient-specific cell lines of human adult olfactory neural progenitors (hONPs) have been established from cultures of adult OE isolated from cadavers [28] and patients undergoing endoscopic sinus surgery [27]. These lines have been cryopreserved. The donor's age or sex and the cells' time in culture has no effect on the cells' functional capacities.
These progenitors produce, without genetic modulation, the broad trophic support required for ameliorating motor neuron degeneration. They have the capacity to differentiate into motor neurons and to synthesize and release key trophic factors including glial cell line-derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF), nerve growth factor, fibroblast growth factor-β, neurotrophin (NT)-3, NT-4, vascular endothelial growth factor, and ciliary neurotrophic factor (CNTF) [29, 30]. They also exhibit high levels of the neural crest marker peripherin [28]. Furthermore, through the production of neurotrophic factors, these progenitors, following their engraftment into the spinal cord, have been shown to rescue distant, axotomized neurons in the red nucleus of the brain from retrograde atrophy [31]. These neurotrophins may influence their environment such that the patient's endogenous stem population may be modulated, which in turn could replace lost neurons [32]. In addition, they promote rubrospinal tract axonal regeneration, which demonstrates that once engrafted into the spinal cord they can exert their influence over considerable distances into the brain [31, 33].
hONPs have the potential to differentiate along several neural lineages, dependent on environmental signals in vitro [34, 35]. Therefore, with a proper local microenvironment, the hONPs can differentiate to DA neurons [34]. It has been reported that the genes Pitx3 and Nurr1 are essential for the survival and differentiation of DA neurons in the striatum [36–38]. Both transcription factors function as dopaminergic promoters in chick, mouse, rat, and human embryonic cells [39–42]. They also participate in dopaminergic restriction and dopamine production of hONPs in vitro [43]. Furthermore, Pitx3 and Nurr1 were found to induce TH expression synergistically [23, 44]. In those studies, the hONPs were transfected with Pitx3 and Nurr1, after which they remained tyrosine hydroxylase (TH)-positive following 4 months of selection. After 6 months of cryostorage, pIRES-Pitx3-Nurr1 (IPN)-transfected hONPs retain their ability to produce and release dopamine and therefore have the distinct potential to serve as a stable population for cell therapy for PD [43]. Pretransfected and post-transfected hONPs have equivalent capacity to produce neurotrophins, including BDNF, CNTF, GDNF, NT-3, etc., which are important for the survival and function of DA neurons [23, 45, 46]. Recent studies indicate that these neurotrophins can optimize the microenvironment of damaged areas and induce endogenous stem cells to replace or rescue degenerating neurons [47, 48]. Therefore, genetically modified hONPs, as well as the nontransfected hONPs, may have a dual ability to serve as replacements for dead or dysfunctional dopaminergic neurons and provide protective permissive microenvironments that can rescue dying or damaged neurons from further degeneration while simultaneously having the potential to activate endogenous progenitors.
This study evaluated the function of IPN-transfected and pretransfected hONPs in a unilateral 6-hydroxydopamine (6-OHDA)-lesioned rat model of PD [49], which is widely used in the study of PD [24, 50, 51]. Human fibroblasts served as a cellular control, whereas the culture medium alone was a vehicle control. In the pilot studies two different toxin injection sites, the medium forebrain bundle [52] and the striatum [50], were evaluated. Both models performed equivalently; the striatum was selected as the injection site for the data shown in this report. Several different hONP lines, doses, and vehicle volumes were transplanted into the animals in the initial studies. The hONP lines were equivalent. Therefore, the optimized paradigm with a single hONP line was used in the experiments.
Materials and Methods
Cell Preparations
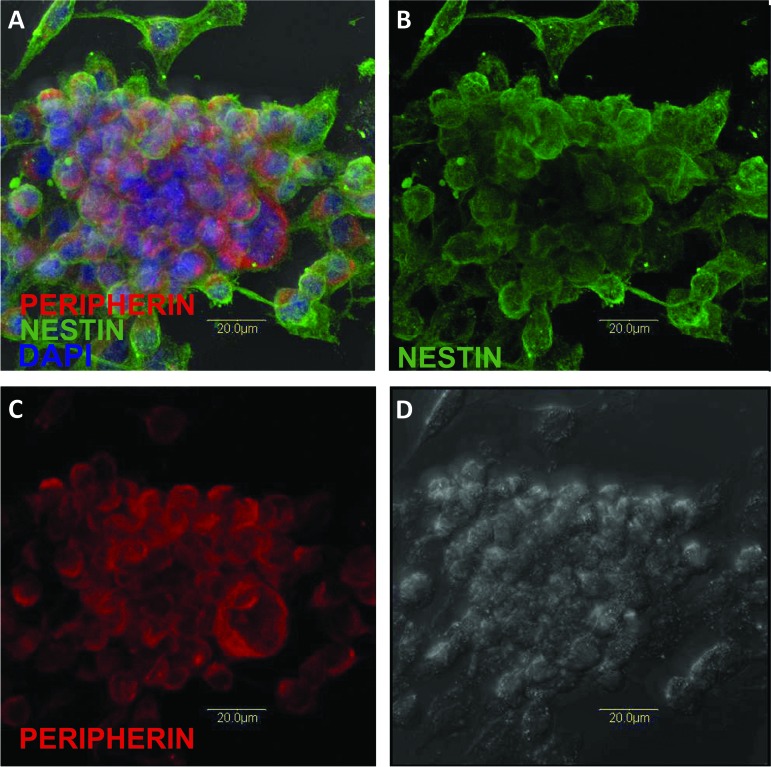
PIRES-Pitx3-Nurr1-transfected and pretransfected hONPs from the same patient-specific cell line were thawed and cultured in minimal essential medium with 10% heat-inactivated fetal bovine serum (Gibco, Grand Island, NY, http://www.invitrogen.com) (10% OE) for 1 week, as described previously [28, 43]. Cryopreserved hONPs retain their viability and progenitor nature. Figure 1A–1C demonstrates the immunoreactivity of previously frozen hONPs to nestin, a well-known neural stem cell marker, as well as their neural crest origin, as shown by substantial perinuclear peripherin reactivity. After recovery from cryopreservation and dilution of dimethyl sulfoxide, these cells divided every 18–20 hours and at low density maintained their perikaryal integrity as they settled down on a solid substrate (Fig. 1D); as their concentration increased, they formed neurospheres (Fig. 1). Human skin fibroblasts (crl-1836; American Type Culture Collection, Manassas, VA, http://www.atcc.org) were cultured under identical conditions. The hONPs were adapted to serum-free growth medium via serial dilution of serum every day for 3 days until the cells were placed in DFBNM (Dulbecco's modified Eagle's medium/F-12 medium supplemented with 1% B27 and 0.5% N2 and 100 μg/ml gentamicin; Gibco). Cells were detached and suspended in DFBNM, and viability was analyzed using 0.4% trypan blue stain (15250; Gibco) according to the manufacturer's protocol. Only viable cells were counted, and cells at a concentration of 2,500 cells per microliter were prepared and kept on ice until engraftment.
Figure 1.
Following 6 months of cryopreservation, human adult olfactory neural progenitors retained their viability and expressed their neural progenitor nature. They formed neurospheres that detached from the substratum and became free-floating; occasional cells expressing a more neuronal-like phenotype could be seen. (A): Merged confocal image demonstrating the presence of the neural stem cell marker-filament nestin (green); the neural crest intermediate filament peripherin (red); and 4′,6-diamidino-2-phenylindole, which highlights nuclear DNA (blue). (B): Nestin only. (C): Peripherin only (red). (D): Cellular integrity is evident with this image.
Animal Model and Cell Transplantation
All animal care and surgical interventions were in accordance with the University of Louisville's Public Health Service Policy on Humane Care and Use of Laboratory Animals and with the approval of the university's Institutional Animal Care and Use Committee and Institutional Biosafety Committee. The endoscopic biopsy of the olfactory epithelium was approved by the University Institutional Review Board.
Establishment of Parkinsonian Rat Model
Female Sprague-Dawley rats (200–250 g; Charles River Laboratories, Wilmington, MA, http://www.criver.com) were maintained under a 12-hour light/dark cycle with constant temperature and humidity. Food and water were available ad libitum. Twenty-four hours prior to surgery, animals were weighed and assigned identification numbers.
The animals were anesthetized using ketamine (Hospira Inc., Lake Forest, IL, http://www.hospira.com)/xylazine (Ben Venue Laboratories, Bedford, OH, http://www.benvenue.com) at 37.7 and 5 mg/kg, respectively. After anesthesia and 30 minutes before lesion, desipramine (D3900; 25 mg/kg i.m.; Sigma-Aldrich, St. Louis, http://www.sigmaaldrich.com) was given to protect noradrenergic neurons from 6-OHDA toxicity, and pargyline (P8013; 50 mg/kg i.m.; Sigma-Aldrich) was given to inhibit endogenous monoamine oxidase. A prophylactic dose of general antibiotics and penicillin (100,000 units/kg i.m.; Butler Schein Animal Health, Dublin, OH, http://www.butlerschein.com) was administered to prevent infection.
Animal hair was shaved, and the skin was prepared with a Betadine solution (Purdue Pharma L.P., Stamford, CT, http://www.purduepharma.com) at the surgical site. Animals were mounted in a stereotaxic apparatus, and the scalp was opened. A small burr hole was made in the skull. For striatum injection, 28 μg of free base 6-OHDA hydrochloride (H116; Sigma-Aldrich) was dissolved immediately before use in 8 μl of sterile saline containing 0.01% ascorbic acid and injected into the right striatum at the coordinates given by the brain atlas of Paxinos and Watson [53]. Four injection sites were used to ensure broad and uniform distribution of the neurotoxin; they were located as follows from the bregma: anterior-posterior (AP): −1.3 mm, lateral (L): 2.6 mm, depth (D): 5 mm; AP: −0.4 mm, L: 3.0 mm, D: 5 mm; AP: 0.4 mm, L: 4.2 mm, D: 5 mm; and AP: 1.3 mm, L: 4.5 mm, D: 5 mm. Two microliters of 6-OHDA solution was dispensed into each point with a 31-gauge needle at a rate of 2 μl/minute. The needle was left in place for an additional 2 minutes to prevent backflow and then slowly removed. The hole was filled with a piece of gel foam, and the scalp was closed. Five milliliters of 0.9% saline was given to counteract any fluid/blood loss. Penicillin (100,000 units/kg i.m.; Butler Schein Animal Health) and buprenorphine (0.02 mg/kg i.m.; Bedford Laboratories, Bedford, OH, http://www.bedfordlabs.com) were administrated for postoperative care for an additional 2 days postsurgery.
Assignment of Experimental Groups
The rotation test (below) was the standard behavioral assessment used to evaluate animals. Grouping of the qualified rats (≥6 rotations per minute on average, based on a total of 542 ± 270 turns for 45 minutes) was performed before engraftment. In all experiments, animals were distributed to one of five groups with equivalent rotational means. Three groups received unilateral (right, same side with 6-OHDA diffusion) cell transplantation (IPN [n = 39], pretransfected hONPs [n = 30], human fibroblast [n = 7]), and one group received a vehicle injection (n = 30). A separate group was designed as a sham group and did not receive any additional treatment except for cyclosporine throughout 24 weeks post-toxin (n = 14).
Cell Transplantation
The rats were anesthetized, prepared, and mounted for the surgery; a hole was made in each skull. On the basis of an initial pilot series of studies, the minimal dose that produced a maximal response was determined to be 15,000 cells in a total volume of 6 μl. Higher concentrations did not produce greater responses. In fact, concentrations of greater than 50,000 per 6 μl were inhibitory. The concentration used in this study was substantially below the widely used levels of other cell types. However, hONPs maintain a high level of viability following their engraftment, whereas many other cell types undergo dramatic reductions in number in the host environment. To ensure that the cells were evenly distributed in the neurotoxin-treated area, the cells were implanted into the striatum of the animal in three specific locations (AP: −0.8 mm, L: 2.8 mm, D: 5 mm; AP: 0 mm, L: 3.6 mm, D: 5 mm; AP: 0.8 mm, L: 4.4 mm, D: 5 mm), which were strategically placed between the four neurotoxin injection sites. The injection at each point was administered for 1 minute, and the syringe was allowed to remain in place for an additional 2 minutes before it was slowly withdrawn to prevent reflux of the solution. At the surgery site, the skin was sutured and 5 ml of 0.9% saline was given intradermally. Penicillin (100,000 units/kg i.m.; Butler Schein Animal Health) and buprenorphine (0.02 mg/kg i.m.; Bedford Laboratories) were administered for postoperation care for an additional 2 days after surgery. Cyclosporine (Bedford Laboratories) was injected intramuscularly at a dosage of 10 mg/kg body weight every other day for at least 10 weeks starting from the day of transplantation. All cellular control and nonengrafted animals received the same dosage of cyclosporine at the same frequency.
Behavioral Analysis
Amphetamine-Induced Rotation Test
Three weeks after injection of 6-OHDA, the rats were stimulated with amphetamine (3.0 mg/kg i.p., 3.0 mg/ml, 0.1 ml/100 g × rat; A5880; Sigma-Aldrich). A determination of the number of rotations began 15 minutes after drug injection to allow for diffusion and continued for 45 minutes (recorded in a 3 × 15-minute pattern). Only rats that rotated six or more turns per minute (for a total of 270 rotations in 45 minutes) were used for the remainder of the experiment. Those rats were assigned randomly into control or cell engrafted groups. All groups were coded after cell engraftment; the rotation test was performed once every 2 weeks starting from the third week through 6 months. Decoding occurred at the conclusion of the study.
Complementary Behavioral Tests
Corner Test.
Rats were placed in a right-angle corner with the forelimbs raised off the floor. The direction the animal turned to leave the corner was recorded for eight consecutive times. The number of left turns out of eight trials was summarized and averaged for each group.
Limb-Use Asymmetry (Cylinder) Test.
Rats were placed in a clear cylinder (30 cm tall by 22 cm in diameter). The number of wall contacts made by their forelimbs (left, right, or both) was recorded for 3 minutes. The ratio of left/right usage was calculated and averaged for each group.
These tests were performed once before the neurotoxin injection for baseline and were continued once every week starting from the second week post-treatment until 6 months after cell transplantation or medium administration.
Immunohistochemistry
Animals were deeply anesthetized with ketamine/xylazine administered intraperitoneally and perfused transcardially with a phosphate-buffered saline wash followed by a 4% buffered paraformaldehyde (P-6148; Sigma-Aldrich) fixative. Removed brains were cryoprotected overnight in a 4% buffered paraformaldehyde followed by 20% sucrose (S9378; Sigma-Aldrich) at 4°C. The striatum of each animal was located and dissected with a brain cutting block and mounted in frozen O.C.T. compound (Sakura Finetek, Torrance, CA, http://www.sakura.com). Coronal sections (12 μm) were cut with a sliding microtome (CM3050S; Leica, Heerbrugg, Switzerland, http://www.leica.com). Sections were reacted with an antibody against TH (monoclonal; Sigma-Aldrich) using the ABC method (Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com). Some sections were used for double labeling of TH expression and anti-human nuclear localization (MAB 1281; Chemicon, Temecula, CA, http://www.chemicon.com). Nuclear DNA staining was achieved with 1:500 4′,6-diamidino-2-phenylindole (Molecular Probes, Eugene, OR, http://probes.invitrogen.com).
Dopamine Assay
The rats were terminated with an overdose of ketamine/xylazine 4 months after cell or medium injection, brains were removed, and each animal's striatum was dissected with a brain slicer and lysed in RIPA buffer (R0278; Sigma-Aldrich) with a tissue grinder (101020; Kimble Chase, Vineland, NJ, http://www.kimble-chase.com) in the presence of protease inhibitor (P8340; Sigma-Aldrich). Total protein of each sample was determined, and dopamine production was analyzed quantitatively with a dopamine enzyme immunoassay kit (Dopamine EIA; Immuno-Biological Laboratories Co., Takasaki, Gunma, Japan, http://www.ibl-japan.co.jp) according to the manufacturer's protocol.
Statistical Analysis
All data are presented as means with error bars equivalent to the SD of the mean. The analysis was performed using GraphPad software. Student's t test was used for comparisons and determination of significant differences between groups. Significance was considered to exist at p > .05.
Results
The Effect of Cell Engraftment on Behavioral Activity
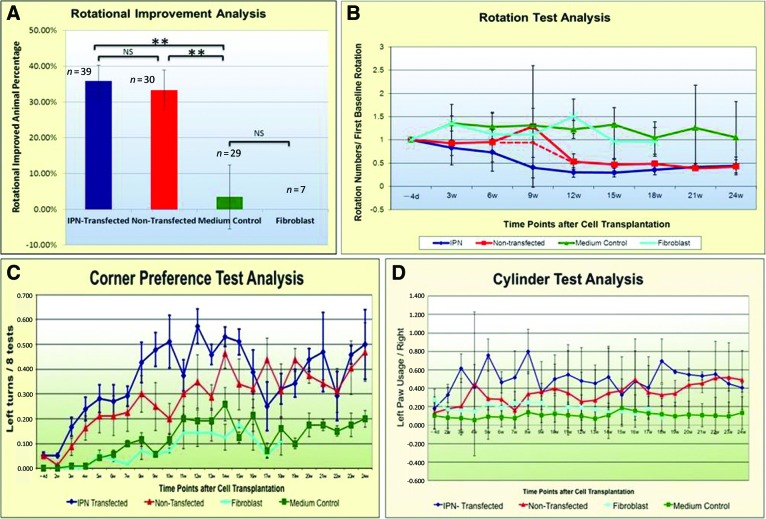
Based on a series of six individual equivalent experiments, animals receiving hONPs had reduced behavioral deficits following the engraftment of IPN-transfected and the matched pretransfected cells into the striatum of animals 3 weeks after 6-OHDA. In contrast, in a single experiment that was initiated after the first series, animals transplanted with the medium or human fibroblasts remained unchanged from neurotoxin-treated controls. Six months after engraftment and 27 weeks after the 6-OHDA, approximately 36% (14 of 39) of the engrafted animals in the IPN-transfected group exhibited at least a 50% rotational reduction (behavioral recovery). IPN transfection initiated the dopaminergic differentiation of the hONPs and resulted in improvement that was detected 6 weeks post-transplantation. In contrast, 33% (10 of 30) of the pretransfected hONPs also exhibited 50% rotational reduction, although the improvement required a longer postengraftment period. The IPN engrafted animals improved in week 6 postengraftment, whereas the pretransfected animals did not show improvement until 12 weeks. At the conclusion of these experiments (27 weeks), no significant difference in level or degree of recovery was detected between pretransfected and post-transfected groups (p > .05); the improved rotation levels were reduced to 43% of the initial levels in both groups. In a single additional experiment that was the last in the series also summarized in Figure 2, human fibroblasts were used as a cellular control to further evaluate the specific role of the hONPs in cell replacement therapy. This experiment was undertaken after the other preceding experiments, which were performed in a double-blinded fashion, were decoded, and surprisingly, it was determined that the pretransfected and the IPN transfect were found to produce equivalent responses. Animals receiving the fibroblasts had no improvement for the duration of the experiment (18 weeks postengraftment), which was significantly different from the IPN-transfected or pretransfected hONP groups (p < .05). There was no difference between the medium control or fibroblast-engrafted animals (p > .05); neither group improved throughout the treatment. In contrast, a significant difference was observed between the hONP (pre- and/or post-transfected) animals and the medium-only control and/or fibroblast-engrafted groups (p < .01) (Fig. 2A, 2B).
Figure 2.
Effect of engraftment on rotation. (A): Engraftment with human adult olfactory neural progenitors (transfected and nontransfected) reduced rotation compared with medium or fibroblasts (p < .01). (B): Effect of time on rotation. No differences were found between fibroblast and medium groups. (C, D): Corner and cylinder analyses were equivalent to the rotation. (B–D): Error bars indicate SD. Abbreviations: d, days; IPN, pIRES-Pitx3-Nurr1; NS, not significant; w, weeks.
The corner preference and cylinder tests were performed for comparison with the rotational results. Normal animals tended to turn left and right equivalently in the corner test, whereas those receiving the unilateral 6-OHDA turned toward the toxin-injected side (the right side, in this study) much more frequently than to the left. In contrast, the corner preference test demonstrated that those animals with diminished rotations had reduced preference and turned to both the left and right when faced into a corner. Animals with no rotational improvement similarly had an overwhelming preference to turn to the right. In contrast, the fibroblast-engrafted and the medium-only control animals continued their preference for the right, turning to toward the right seven times more than to the left (Fig. 2C). In the cylinder climbing test, normal animals used their right and left front paws equally against the glass wall as they reached for the top of the cylinder, whereas the toxin-injured animals had a strong preference to use their left. In this study, the animals with improved rotational deficits also had improved cylinder tests; their left/right paw usage increased from 10% to 40% over 24 weeks following engraftment, which was three times as great as the human fibroblast transplanted or medium-only treated controls (Fig. 2D; supplemental online Movie 1).
Transplanted hONPs Promote TH Expression in the Toxin-Injured Sites
Unilateral treatment of the striatum with the 6-OHDA destroyed all TH-positive cells after 3 weeks, as demonstrated by the lack of immunoreactivity in the area of treatment. No detectable spontaneous recovery of TH-positive cells occurred throughout 24 weeks postneurotoxin, nor was TH-positive immunoreactivity observed in any toxin-treated region of the sham-operated controls (sham group, Fig. 3A–3D), nor was it detected in the human fibroblast or medium-only groups (Fig. 3G, 3H). The left sides of the brains, which did not receive the toxin, expressed TH in the striatum, whereas the right-side striatum did not exhibit any TH regeneration over 24 weeks after the toxin injection, nor was it restored in the cellular or medium controls. In contrast, both the pre- and post-transfected hONP-engrafted animals, which improved in the behavior tests, exhibited greater TH expression in sections of their striatum compared with the controls (Fig. 3E, 3F).
Figure 3.
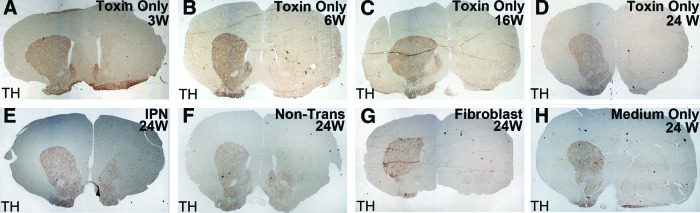
Effect of unilateral 6-hydroxydopamine on striatal dopaminergic neurons. No TH reactivity occurred in the lesioned striatum (right side) over 24 weeks (A–D). Dopaminergic neurons were found in the lesion sites of animals engrafted with transfected and nontransfected human adult olfactory neural progenitors (E, F); medium- and fibroblast-administered controls lacked dopaminergic neurons (G, H). Localization is shown as described in the text. Magnification, ×4.5. Abbreviations: IPN, pIRES-Pitx3-Nurr1; Non-Trans, nontransfected; TH, tyrosine hydroxylase; W, weeks.
Survival of hONPs After Engraftment
Twenty-four weeks after engraftment, dopaminergic neurons were detected in the toxin-injured striatum of the hONP transplanted animals (Fig. 4A, 4B). Two groups of dopaminergic neurons could be distinguished on the basis of reactivity to human nuclear antigen. Many neurons were double-labeled, reflecting their human origin. However, some of the dopaminergic neurons did not react with the human antigen, suggesting either that these neurons resulted from an endogenous population of stem cells or that they reflect rescued damaged host neurons. Intact, engrafted TH-positive hONPs resembled multipolar neurons with long processes that frequently passed out of the plane of focus (Fig. 4C, 4D). Furthermore, TH-positive processes were found well beyond the injection sites (Fig. 4E, 4F). TH-positive perikarya and processes were detected 800 μm away from the implantation sites. Approximately 35% of the animals that received either the pre- or post-transfected hONPs were partially recovered. A quantitative study demonstrated differences in the number of TH-positive cells between recovered and nonrecovered transfected animals (p < .05) and between prerecovered and the pretransfected nonrecovered animals (p < .05). No significant differences were observed between the recovered transfected and recovered pretransfected animals (p < .05). Similarly, no differences were found between the nonrecovered animals of these two groups (p > .05) (Table 1). No TH-positive cells were observed in the toxin-injured striatum of the human fibroblast animals, the medium-only animals, or the sham controls. Human fibroblasts were not detected 4 months after transplantation.
Figure 4.
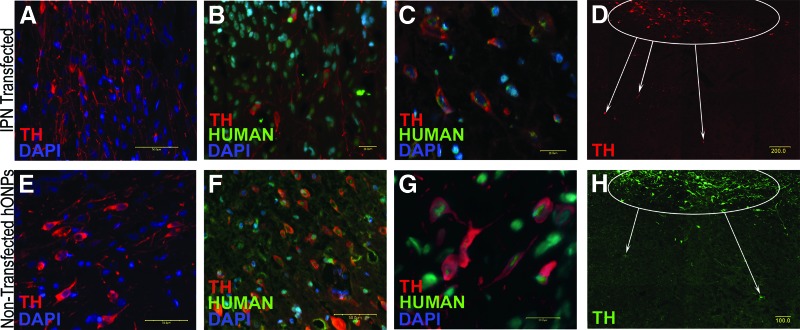
Immunolocalization of engrafted hONPs in the 6-hydroxydopamine-lesioned striatum. IPN-transfected hONPs (A–D) and the pretransfected hONPs (E–G) were intact and TH-positive 24 weeks after the engraftment. High-magnification microscopy revealed that the transfected and nontransfected hONPs had TH-positive processes (C, G), which were observed beyond the injection sites (D, F). (H): Pretransfected hONPs; lower power image equivalent to (D) demonstrating similar migration from the site of engraftment as in (D) for the transfected cells. Note that not all of the TH-positive cells double-labeled with human nucleus antigen, reflecting the multifaceted action of hONPs (cell rescue and endogenous stem cell differentiation). Scale bars = 20.0 μm (B, C, G), 50.0 μm (A, E, F), 100.0 μm (H), and 200.0 μm (D). Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; hONP, human adult olfactory neural progenitor; IPN, pIRES-Pitx3-Nurr1; TH, tyrosine hydroxylase.
Table 1.
Significance (p value) between engraftment groups
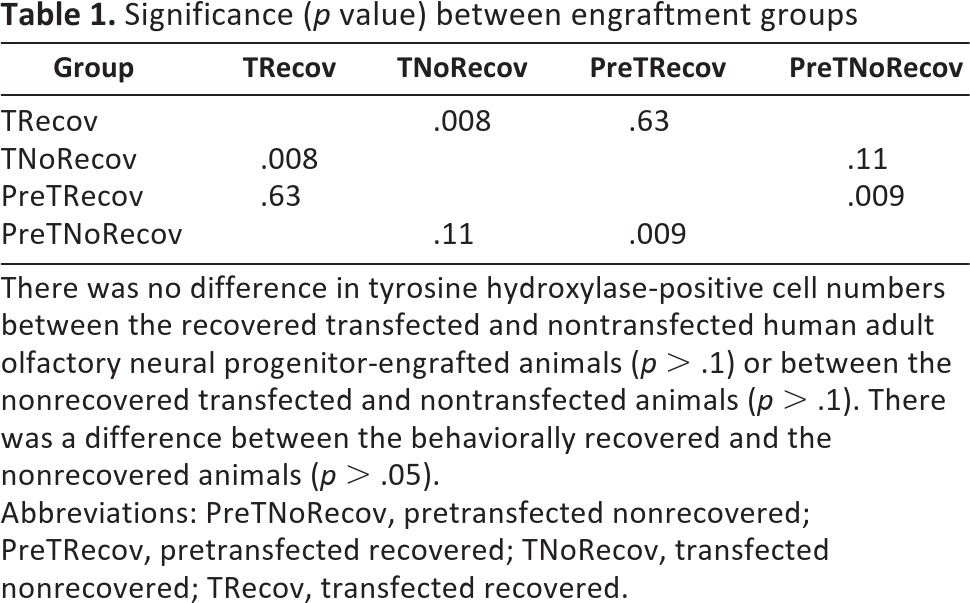
There was no difference in tyrosine hydroxylase-positive cell numbers between the recovered transfected and nontransfected human adult olfactory neural progenitor-engrafted animals (p > .1) or between the nonrecovered transfected and nontransfected animals (p > .1). There was a difference between the behaviorally recovered and the nonrecovered animals (p > .05).
Abbreviations: PreTNoRecov, pretransfected nonrecovered; PreTRecov, pretransfected recovered; TNoRecov, transfected nonrecovered; TRecov, transfected recovered.
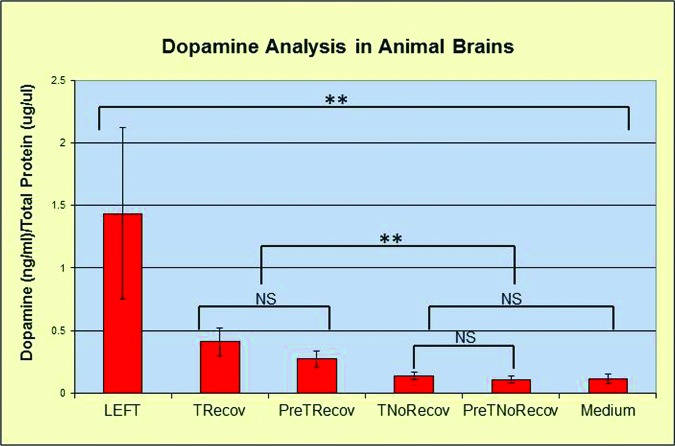
Analysis of the Dopamine Levels
Dopamine enzyme immunoassay was used 4 months after toxin injury to determine the dopamine levels in the striatum sections of half brains (HB). The left HBs that never received 6-OHDA contained 1.44 ± 0.68 dopamine (pg)/total protein (mg) (D/TP), which was 14 times as great as that of the toxin-injured and medium-only-injected HBs (0.11 ± 0.04 D/TP). The recovered pretransfected and post-transfected tissue had D/PT levels of 0.27 ± 0.07 and 0.41 ± 0.11 (pg/mg), respectively. The D/PT levels of pretransfected and post-transfected nonrecovered HBs averaged 0.11 ± 0.03 and 0.13 ± 0.03 (pg/mg), respectively. The improved animals exhibited higher levels of dopamine compared with the nonrecovered or the medium-only controls. The dopamine levels in the left side (untreated) brains were significantly higher than those of all the right sides. There was no significant difference between the transfected recovered and nontransfected recovered. The dopamine levels in the treated brains of recovered and nonrecovered animals were statistically different from each other (Fig. 5).
Figure 5.
Dopamine analysis. The left, untreated side of the brain retained the highest dopamine level and was different from all right side levels. The difference was significant between the recovered and nonrecovered animals (**, p < .05). There was no difference (p > .1, NS) between TRecov and PreTRecov animals or between TNoRecov and PreTNoRecov animals. Abbreviations: NS, not significant; PreTNoRecov, nontransfected nonrecovered; PreTRecov, nontransfected recovered; TNoRecov, transfected nonrecovered; TRecov, transfected recovered.
Discussion
Although PD is characterized by the degeneration of dopaminergic neurons in the substantia nigra, the etiology and cure remain unknown [2]. Pharmacological agents transiently relieve symptoms, losing their effectiveness with prolonged use [54, 55]. Substantial proof-of-concept studies, aimed at developing a cell replacement therapy, are ongoing [10, 11]. Early attempts included the transplantation of embryonic tissues such as mesencephalic tissue [56, 57], fetal nigral cells [58, 59], or ventral midbrains [60]. With the relief of parkinsonian symptoms, these trials raised concerns. (a) There was a lack of donor tissue; each patient receiving this surgery required 3–5 fetal brains [56], which increased the risk of bacterial or viral infection. (b) Even with well-experienced surgical teams, the outcomes were unreliable. (c) Dyskinesia occurred in a significant proportion of patients [14, 15]. Therefore, an alternate source for dopaminergic cells has been a topic under study [61, 62]. Stem cells have potential for cell-replacement therapy for PD because of their capacity for self-renewal and ability to differentiate into other cell types [62]. Human ESCs were among the first populations used in a PD model, and significant decreases in rotation tests from pretransplantation levels following transplantation were reported [20, 63, 64]. Several recent studies have focused on induced pluripotent stem cells (iPSCs) derived from human fibroblasts [25, 26], human or rat primordial germ cells [65–67], or mammalian embryos [68, 69]. These cells promoted behavioral recovery when transplanted into parkinsonian animal models [24]. However, like ESCs, in many cases (50% or more) they generated teratomas within 6–9 weeks [22, 70, 71]. These studies used relatively high numbers of cells for engraftment with a range of 100,000 to 2 million cells per treatment.
The long-term goal of the present study was to find a stable, nontumorigenic cell source that could be used in a cell-based therapy for Parkinson's disease. Methods to isolate and expand neural progenitors from human adult olfactory epithelium were developed [36]. The tissue was obtained via endoscopic biopsy of the olfactory mucosa in the nasal cavity without invasive surgery or significant injury to the donor. They were cultured for 8–12 weeks until the progenitors (hONPs) were obtained as previously described [27, 59]. The use of hONPs that can be obtained from the patient and then returned to the patient would eliminate ethical concerns, as well as the need for immunosuppressive agents, since they would be autologous. Previous studies demonstrated that hONPs can differentiate into neurons in response to their local environment [72, 73].
The hONPs were lineage restricted to dopaminergic neurons by transfection with Nurr1 and Pitx3, which worked synergistically in this process [43]. Furthermore, hONPs produce a variety of neurotrophic factors in vitro [35] and in vivo following their engraftment [74]. Recent studies have shown that hONPs act as biological minipumps, releasing neurotrophins that create a permissive regenerative environment [74]. Furthermore, the released neurotrophins, including BDNF, CNTF, GDNF, and NT-3, have been shown to be crucial in the recovery of primate and rodent models of Parkinson's disease [12, 75]. The pretransfected hONPs produce these essential neurotrophins, including BDNF and NT-3, even in a serum-enriched medium [35]. This study demonstrated that transfection of hONPs did not alter neurotrophin production. Therefore, genetically modified and unmodified hONPs can serve as replacements for the dead, dying, or dysfunctional dopaminergic neurons while simultaneously having the potential to provide a protective permissive microenvironment to rescue dying or damaged neurons from further degeneration and enhance the endogenous progenitor populations. Recent studies strongly suggest that engrafted neural progenitors may be a most promising way to introduce neurotrophins into the central nervous system (CNS) as a therapy for PD [76]. Engrafted hONPs have a dual mechanism: they synthesize and release dopamine and enhance the deteriorating, nonpermissive environment created by the 6-OHDA with neurotrophins. The presence of both human reactive and nonreactive DA neurons following 26 weeks of hONP engraftment supports this conclusion. In contrast, the fibroblast-engrafted controls and those animals that received medium only had no improvement, reflecting the hostile environment created by 6-OHDA. The amphetamine-induced rotation test was used; it has been widely used to evaluate this parkinsonian model [77, 78]. Corner preference and vertical climbing data supported the rotational studies. Furthermore, when the rotations were reduced to half of the starting level, the rat was operationally considered partially recovered. We predicted that animals that received IPN cells would recover behaviorally, whereas the pretransfected hONPs implanted animals would not recover, or would have less recovery, because the pretransfected hONPs produced less dopamine than the transfected hONPs in vitro. However, 36% of the animals that received IPN-transfected hONPs improved. Surprisingly, 33% of the animals engrafted with pretransfected hONPs exhibited reduced rotation numbers equivalent to those engrafted with the IPN-transfected cells. There was no difference in the final level of recovery between the two groups, although the pretransfected cell-engrafted animals exhibited rotational reductions 6 weeks later than the IPN-transfected progenitor-implanted animals. This might reflect the time required for the pretransfected hONPs to differentiate into dopaminergic neurons and/or to affect the local microenvironment and stimulate endogenous stem cells to form dopaminergic neurons. Trophic factors act neuroprotectively in Parkinson's disease models [79, 80]. Therefore, hONPs are likely to have a dual role: “cellular protection” in addition to cellular replacement for the dopaminergic cells.
Partial behavioral recovery has been reported in primate Parkinson's models in response to the human neural stem cell transplantation; these animals exhibited improvement during a 60-day period after stem cell transplantation [12, 81]. These authors suggested that the parkinsonian primate CNS may benefit from replacement of degenerating DA neurons by differentiated human stem cells, and/or the trophic, protective, and guidance effects of stem cell-derived progeny, which is consistent with our results.
Other studies using human embryonic [81] or adult [82] stem cells in the 6-OHDA rat model also reported some behavioral recovery of the parkinsonian deficits following transplantation. It was suggested that the engrafted human stem cells may be protective against the toxicity from the 6-OHDA in rats' striatum. Studies with an alternate, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease have shown that genetically modified GDNF-secreting progenitors increase TH-positive cells and reduce behavioral deficits [76]. These reports support the hypothesis that the behavioral improvement in the hONP-engrafted animals may be the result of two separate but complementary actions: the trophin-enriched protective microenvironment and/or the replacement and rescue of dopaminergic neurons.
Control groups demonstrated that the recovery was specifically due to the engrafted hONPs rather than the toxin-lesioned environment alone, with the medium or with the injection of non-hONP cell types. A sham group was included with the same dosage and location of 6-OHDA injection and evaluated for the entire 27 weeks. Immunohistochemistry determined that no DA neurons were restored in the sham, medium, or cellular control groups. No TH expression was detected in any of these control groups, indicating that the microenvironment of the lesioned sites can only support DA neuron development induced by the hONPs. Human fibroblasts were not detected 18 weeks after the engraftment, which further demonstrates the specific stability and viability of the engrafted hONP population. Therefore, the hONPs represent a unique cell type for cell-mediated therapy of PD with the ability to differentiate into dopaminergic neurons and perhaps to stimulate the microenvironment for host stem cell activation in situ. In contrast, in the recovered animals (transfected and nontransfected engrafted) hONPs survived and remained TH-positive for the duration of the experiment (a minimum of 24 weeks after transplantation), indicating their long-term potential to provide dopamine- and neurotrophin-rich environments. Furthermore, the TH-positive hONPs were observed 800 μm from their initial engraftment site, demonstrating that hONPs can migrate in the local environment of the 6-OHDA-lesioned striatum, which is essential to cell integration. Similar results were reported by other groups using hESCs and iPSCs [82–85], which survived and promoted improvement in the behavior tests in the animals with parkinsonian symptoms. Bjugstad et al. [82] reported that the neural stem cells isolated from the human fetal telencephalon migrated along the nigrostriatal pathway 4 months after transplantation in an adult monkey MPTP lesion. More cells were detected along the pathway 3 months postengraftment, demonstrating that the engrafted population did not remain entirely in the injection site but migrated along pathways [82]. Studies with iPSCs engrafted into a MPTP-lesioned mouse model demonstrate migration from the site of transplantation in the striatum to the lesion site, localizing in the substantia nigra [83]. Collectively these studies are in agreement with the present study and demonstrate cell migration after transplantation, although different species and models were used.
It has been reported that the PD models that were engrafted with ESCs or iPSCs eventually developed teratomas and died within 6 weeks for ESCs [56, 70, 71] and 7–9 weeks for iPSCs [17, 79]. In the present study, no tumor formation was detected 24 weeks after engraftment, suggesting that hONPs likely represent a more stable lineage-committed population and thus are more suited for cell replacement therapy for PD. Furthermore, the hONPs can be cryostored in liquid nitrogen without loss of viability for future engraftment should serial treatments become necessary [43].
Dopamine EIA was applied to detect the dopamine level in animal brains. It has been shown that the brains of parkinsonian models have decreased dopamine levels [86]. In the hONP-engrafted brains, the dopamine levels in the behaviorally improved animals were higher than in animals without improvement, suggesting that the hONPs were functional and that the recovery was partially the result of the increased dopamine levels. Others report that intracranial transplantation with iPSCs resulted in improved striatal concentrations of dopamine in the behaviorally recovered MPTP mouse model as measured by high-performance liquid chromatography [83], which further supports the likelihood that the observed behavioral improvement was the result of the increased dopamine, as well as neurotrophin support provided by the engrafted hONPs.
Conclusion
hONPs may represent an ideal population for cell therapy for PD because of their capacity to survive, produce dopamine, and provide neurotrophic support in the toxin-lesioned environment, as well as the neurotoxic environment of the parkinsonian brain. Furthermore, hONPs have a significant advantage since they can be harvested from the patient's olfactory epithelium without highly invasive surgery and thus represent an autologous cell source, where the patient is both the donor and the recipient. This benefit eliminates the need for waiting lists for histocompatable donors, as well as the use of immunosuppressive agents typically applied following cell engraftment. Finally, these studies demonstrate that genetic engineering (transfection) is not required for an effective dopaminergic formation but that the microenvironment of the substantia nigra can modulate hONPs to become functional, stable, dopamine-releasing cells that may offer long-term survival following their engraftment without tumor formation. Future studies will be required to optimize engraftment parameters and to determine whether hONPs have a positive action in patients with Parkinson's disease.
Acknowledgments
We thank Debbie Black, Chris Ekstrom, Ian Fentie, Jodi Hallgren, Yu Lin, and Ying Song for their technical expertise and assistance. This work was supported by the Dishman Family Foundation and by a grant from RhinoCyte, Inc. (to F.R.).
Author Contributions
M.W.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; C.L.: collection and/or assembly of data, data analysis and interpretation, background studies; F.R.: conception and design, financial support, administrative support, provision of study material or patients, data analysis and interpretation, manuscript writing, final approval of manuscript, background studies.
Disclosure of Potential Conflicts of Interest
C.L. and F.R. helped found RhinoCyte, Inc., in 2005 and have a financial interest in the company. RhinoCyte provides the University of Louisville with a grant for F.R.'s research.
References
- 1.Savica R, Rocca WA, Ahlskog JE. When does Parkinson disease start? Arch Neurol. 2010;67:798–801. doi: 10.1001/archneurol.2010.135. [DOI] [PubMed] [Google Scholar]
- 2.Hornykiewicz O. Parkinson's disease: From brain homogenate to treatment. Fed Proc. 1973;32:183–190. [PubMed] [Google Scholar]
- 3.Vidailhet M. Movement disorders in 2010: Parkinson disease—symptoms and treatments. Nat Rev Neurol. 2011;7:70–72. doi: 10.1038/nrneurol.2010.216. [DOI] [PubMed] [Google Scholar]
- 4.Bidet-Ildei C, Pollak P, Kandel S, et al. Handwriting in patients with Parkinson disease: Effect of l-DOPA and stimulation of the sub-thalamic nucleus on motor anticipation. Hum Mov Sci. 2011;30:783–791. doi: 10.1016/j.humov.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Hornykiewicz O. Dopamine in the basal ganglia. Its role and therapeutic implications (including the clinical use of l-DOPA) Br Med Bull. 1973;29:172–178. doi: 10.1093/oxfordjournals.bmb.a070990. [DOI] [PubMed] [Google Scholar]
- 6.Callaway E. Gene therapy offers hope for Parkinson's disease. Nature. 10.1038/news.2011.167. [Google Scholar]
- 7.Lang AE, Lozano AM. Parkinson's disease. First of two parts. N Engl J Med. 1998;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- 8.Williams A, Gill S, Varma T, et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson's disease (PD surg trial): A randomised, open-label trial. Lancet Neurology. 2010;9:581–591. doi: 10.1016/S1474-4422(10)70093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bronstein JM, Tagliati M, Alterman RL, et al. Deep brain stimulation for Parkinson disease: An expert consensus and review of key issues. Arch Neurol. 2011;68:165. doi: 10.1001/archneurol.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson L, Caldwell MA. Human neural progenitor cell transplants into the subthalamic nucleus lead to functional recovery in a rat model of Parkinson's disease. Neurol Dis. 2007;27:133–140. doi: 10.1016/j.nbd.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Parish CL, Castelo-Branco G, Rawal N, et al. Wnt5a-Treated Midbrain neural stem cells improve dopamine cell replacement therapy in Parkinsonian mice. J Clin Invest. 2008;118:149–160. doi: 10.1172/JCI32273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redmond DE, Jr., Bjugstad KB, Teng YD, et al. Behavioral improvement in a primate Parkinson's model is associated with multiple homeostatic effects of human neural stem cells. Proc Natl Acad Sci USA. 2007;104:12175–12180. doi: 10.1073/pnas.0704091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruff CA, Wilcox JT, Fehlings MG. Cell-based transplantation strategies to promote plasticity following spinal cord injury. Exp Neurol. 2012;235:78–90. doi: 10.1016/j.expneurol.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Lane EL, Bjorklund A, Dunnett SB, et al. Neural grafting in Parkinson's disease unraveling the mechanisms underlying graft-induced dyskinesia. Prog Brain Res. 2010;184:295–309. doi: 10.1016/S0079-6123(10)84015-4. [DOI] [PubMed] [Google Scholar]
- 15.Barker RA, Kuan WL. Graft-induced dyskinesias in Parkinson's disease: What is it all about? Cell Stem Cell. 2010;7:148–149. doi: 10.1016/j.stem.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Xiong N, Zhang Z, Huang J, et al. VEGF-expressing human umbilical cord mesenchymal stem cells, an improved therapy strategy for Parkinson's disease. Gene Ther. 2011;18:394–402. doi: 10.1038/gt.2010.152. [DOI] [PubMed] [Google Scholar]
- 17.Hwang DY, Kim DS, Kim DW. Human ES and iPS cells as cell sources for the treatment of Parkinson's disease: current state and problems. J Cell Biochem. 2010;109:292–301. doi: 10.1002/jcb.22411. [DOI] [PubMed] [Google Scholar]
- 18.Lonardo E, Parish CL, Ponticelli S, et al. A small synthetic cripto blocking peptide improves neural induction, dopaminergic differentiation, and functional integration of mouse embryonic stem cells in a rat model of Parkinson's disease. Stem Cells. 2010;28:1326–1337. doi: 10.1002/stem.458. [DOI] [PubMed] [Google Scholar]
- 19.Yang JR, Liao CH, Pang CY, et al. Directed differentiation into neural lineages and therapeutic potential of porcine embryonic stem cells in rat Parkinson's disease model. Cell Reprogram. 2010;12:447–461. doi: 10.1089/cell.2009.0078. [DOI] [PubMed] [Google Scholar]
- 20.Brederlau A, Correia AS, Anisimov SV, et al. Transplantation of human embryonic stem cell-derived cells to a rat model of Parkinson's disease: Effect of in vitro differentiation on graft survival and teratoma formation. Stem Cells. 2006;24:1433–1440. doi: 10.1634/stemcells.2005-0393. [DOI] [PubMed] [Google Scholar]
- 21.Roy NS, Cleren C, Singh SK, et al. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 22.Chang YL, Chen SJ, Kao CL, et al. Docosahexaenoic acid promotes dopaminergic differentiation in induced pluripotent stem cells and inhibits teratoma formation in rats with Parkinson-like pathology. Cell Transplant. 2012;21:313–332. doi: 10.3727/096368911X580572. [DOI] [PubMed] [Google Scholar]
- 23.Soldner F, Laganiere J, Cheng AW, et al. Generation of isogenic pluripotent stem cells differing exclusively at two early onset Parkinson point mutations. Cell. 2011;146:318–331. doi: 10.1016/j.cell.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hargus G, Cooper O, Deleidi M, et al. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc Natl Acad Sci USA. 2010;107:15921–15926. doi: 10.1073/pnas.1010209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 27.Winstead W, Marshall CT, Lu CL, et al. Endoscopic biopsy of human olfactory epithelium as a source of progenitor cells. Am J Rhinol. 2005;19:83–90. [PubMed] [Google Scholar]
- 28.Roisen FJ, Klueber KM, Lu CL, et al. Adult human olfactory stem cells. Brain Res. 2001;890:11–22. doi: 10.1016/s0006-8993(00)03016-x. [DOI] [PubMed] [Google Scholar]
- 29.Khalyfa A, Buazza M, Qiang H, et al. Gene expression profiling for adult human olfactory neuroepithelial-derived progenitors. Gene Ther Mol Biol. 2007;11:203–216. [Google Scholar]
- 30.Marshall CT, Lu C, Winstead W, et al. The therapeutic potential of human olfactory-derived stem cells. Histol Histopathol. 2006;21:633–643. doi: 10.14670/HH-21.633. [DOI] [PubMed] [Google Scholar]
- 31.Xiao M, Klueber KM, Lu C, et al. Human adult olfactory neural progenitors rescue axotomized rodent rubrospinal neurons and promote functional recovery. Exp Neurol. 2005;194:12–30. doi: 10.1016/j.expneurol.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 32.Lu C, Hallgren JJ, Lin Y, et al. Human olfactory-derived neural progenitors diminish locomotory deficits following spinal cord contusion injury. J Neurodegener Regen. 2011;4:33–50. [Google Scholar]
- 33.Xiao M, Klueber KM, Guo Z, et al. Human adult olfactory neural progenitors promote axotomized rubrospinal tract axonal reinnervation and locomotor recovery. Neurobiol Dis. 2007;26:363–374. doi: 10.1016/j.nbd.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Cai J, Klueber KM, et al. Role of transcription factors in motoneuron differentiation of adult human olfactory neuroepithelial-derived progenitors. Stem Cells. 2006;24:434–442. doi: 10.1634/stemcells.2005-0171. [DOI] [PubMed] [Google Scholar]
- 35.Marshall CT, Guo Z, Lu C, et al. Human adult olfactory neuroepithelial derived progenitors retain telomerase activity and lack apoptotic activity. Brain Res. 2005;1045:45–56. doi: 10.1016/j.brainres.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 36.Haubenberger D, Reinthaler E, Mueller JC, et al. Association of transcription factor polymorphisms Pitx3 and En1 with Parkinson's disease. Neurobiol Aging. 2011;32:302–307. doi: 10.1016/j.neurobiolaging.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Perlmann T, Wallen-Mackenzie A. Nurr1, an orphan nuclear receptor with essential functions in developing dopamine cells. Cell Tissue Res. 2004;318:45–52. doi: 10.1007/s00441-004-0974-7. [DOI] [PubMed] [Google Scholar]
- 38.Maxwell SL, Ho HY, Kuehner E, et al. Pitx3 regulates tyrosine hydroxylase expression in the substantia nigra and identifies a subgroup of mesencephalic dopaminergic progenitor neurons during mouse development. Dev Biol. 2005;282:467–479. doi: 10.1016/j.ydbio.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 39.Courtois ET, Castillo CG, Seiz EG, et al. In vitro and in vivo enhanced generation of human A9 dopamine neurons from neural stem cells by Bcl-Xl. J Biol Chem. 2010;285:9881–9897. doi: 10.1074/jbc.M109.054312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hwang DY, Ardayfio P, Kang UJ, et al. Selective loss of dopaminergic neurons in the substantia nigra of Pitx3-deficient aphakia mice. Brain Res Mol Brain Res. 2003;114:123–131. doi: 10.1016/s0169-328x(03)00162-1. [DOI] [PubMed] [Google Scholar]
- 41.Saucedo-Cardenas O, Kardon R, Ediger TR, et al. Cloning and structural organization of the gene encoding the murine nuclear receptor transcription factor, Nurr1 gene. 1997;187:135–139. doi: 10.1016/s0378-1119(96)00736-6. [DOI] [PubMed] [Google Scholar]
- 42.Katunar MR, Saez T, Brusco A, et al. Ontogenetic expression of dopamine-related transcription factors and tyrosine hydroxylase in prenatally stressed rats. Neurotox Res. 2010;18:69–81. doi: 10.1007/s12640-009-9132-z. [DOI] [PubMed] [Google Scholar]
- 43.Wang M, Lu C, Roisen F, et al. Lineage restriction of adult human olfactory-derived progenitors to dopaminergic neurons. Stem Cell Discov. 2011;1:29–43. [Google Scholar]
- 44.Martinat C, Bacci JJ, Leete T, et al. Cooperative transcription activation by Nurr1 and Pitx3 induces embryonic stem cell maturation to the midbrain dopamine neuron phenotype. Proc Natl Acad Sci USA. 2006;103:2874–2879. doi: 10.1073/pnas.0511153103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pessach IM, Notarangelo LD. Gene therapy for primary immunodeficiencies: Looking ahead, toward gene correction. J Allergy Clin Immunol. 2011;127:1344–1350. doi: 10.1016/j.jaci.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh S, Ahmad R, Mathur D, et al. Neuroprotective effect of BDNF in young and aged 6-OHDA treated rat model of Parkinson disease. Indian J Exp Biol. 2006;44:699–704. [PubMed] [Google Scholar]
- 47.Kassis I, Vaknin-Dembinsky A, Karussis D. Bone marrow mesenchymal stem cells: Agents of immunomodulation and neuroprotection. Curr Stem Cell Res Ther. 2011;6:63–68. doi: 10.2174/157488811794480762. [DOI] [PubMed] [Google Scholar]
- 48.Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders—time for clinical translation? J Clin Invest. 2010;120:29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ungerstedt U, Arbuthnott GW. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 1970;24:485–493. doi: 10.1016/0006-8993(70)90187-3. [DOI] [PubMed] [Google Scholar]
- 50.Danielyan L, Schafer R, von Ameln-Mayerhofer A, et al. Therapeutic efficacy of intranasally delivered mesenchymal stem cells in a rat model of Parkinson disease. Rejuvenation Res. 2011;14:3–16. doi: 10.1089/rej.2010.1130. [DOI] [PubMed] [Google Scholar]
- 51.Rauch F, Schwabe K, Krauss JK. Effect of deep brain stimulation in the pedunculopontine nucleus on motor function in the rat 6-hydroxydopamine Parkinson model. Behav Brain Res. 2010;210:46–53. doi: 10.1016/j.bbr.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Zhang HL, Wu JJ, Ren HM, et al. Therapeutic effect of microencapsulated porcine retinal pigmented epithelial cells transplantation on rat model of Parkinson's disease. Neurosci Bull. 2007;23:137–144. doi: 10.1007/s12264-007-0020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paxinos G, Watson C. 6th ed. Salt Lake City, UT: Academic Press; 2007. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- 54.Lloyd KG, Hornykiewicz O. l-Glutamic acid decarboxylase in Parkinson's disease: Effect of l-DOPA therapy. Nature. 1973;243:521–523. doi: 10.1038/243521a0. [DOI] [PubMed] [Google Scholar]
- 55.Sharpe JA, Rewcastle NB, Lloyd KG, et al. Striatonigral degeneration. Response to levodopa therapy with pathological and neurochemical correlation. J Neurol Sci. 1973;19:275–286. doi: 10.1016/0022-510x(73)90091-9. [DOI] [PubMed] [Google Scholar]
- 56.Freed CR, Greene PE, Breeze RE, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. New Engl J Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 57.Piccini P, Lindvall O, Bjorklund A, et al. Delayed recovery of movement-related cortical function in Parkinson's disease after striatal dopaminergic grafts. Ann Neurol. 2000;48:689–695. [PubMed] [Google Scholar]
- 58.Nikkhah G, Cunningham MG, Jodicke A, et al. Improved graft survival and striatal reinnervation by microtransplantation of fetal nigral cell suspensions in the rat Parkinson model. Brain Res. 1994;633:133–143. doi: 10.1016/0006-8993(94)91532-6. [DOI] [PubMed] [Google Scholar]
- 59.Kordower JH, Freeman TB, Chen EY, et al. Fetal nigral grafts survive and mediate clinical benefit in a patient with Parkinson's disease. Mov Disord. 1998;13:383–393. doi: 10.1002/mds.870130303. [DOI] [PubMed] [Google Scholar]
- 60.Mendez I, Sanchez-Pernaute R, Cooper O, et al. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson's disease. Brain. 2005;128:1498–1510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Daadi MM. Activation and differentiation of endogenous neural stem cell progeny in the rat Parkinson animal model. Methods Mol Biol. 2002;198:265–271. doi: 10.1385/1-59259-186-8:265. [DOI] [PubMed] [Google Scholar]
- 62.Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders: How to make it work. Nat Med. 2004;10(suppl):S42–S50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- 63.Bjorklund LM, Sanchez-Pernaute R, Chung S, et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci USA. 2002;99:2344–2349. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Correia AS, Anisimov SV, Li JY, et al. Stem cell-based therapy for Parkinson's disease. Ann Med. 2005;37:487–498. doi: 10.1080/07853890500327967. [DOI] [PubMed] [Google Scholar]
- 65.Shamblott MJ, Axelman J, Wang S, et al. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci USA. 1998;95:13726–13731. doi: 10.1073/pnas.95.23.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamanaka S, Yamaguchi T, Kobayashi T, et al. Generation of germline-competent rat induced pluripotent stem cells. PLoS One. 2011;6:e22008. doi: 10.1371/journal.pone.0022008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 68.Nichols J, Zevnik B, Anastassiadis K, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 69.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hedlund E, Pruszak J, Ferree A, et al. Selection of embryonic stem cell-derived enhanced green fluorescent protein-positive dopamine neurons using the tyrosine hydroxylase promoter is confounded by reporter gene expression in immature cell populations. Stem Cells. 2007;25:1126–1135. doi: 10.1634/stemcells.2006-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arnhold S, Klein H, Semkova I, et al. Neurally selected embryonic stem cells induce tumor formation after long-term survival following engraftment into the subretinal space. Invest Ophthalmol Vis Sci. 2004;45:4251–4255. doi: 10.1167/iovs.03-1108. [DOI] [PubMed] [Google Scholar]
- 72.Zhang X, Klueber KM, Guo Z, et al. Induction of neuronal differentiation of adult human olfactory neuroepithelial-derived progenitors. Brain Res. 2006:1073–1074:109–119. doi: 10.1016/j.brainres.2005.12.059. [DOI] [PubMed] [Google Scholar]
- 73.Zhang X, Cai J, Klueber KM, et al. Induction of oligodendrocytes from adult human olfactory epithelial-derived progenitors by transcription factors. Stem Cells. 2005;23:442–453. doi: 10.1634/stemcells.2004-0274. [DOI] [PubMed] [Google Scholar]
- 74.Lu C, Hallgren JJ, Lin Y, et al. Human olfactory-derived neural progenitors diminish locomotory deficits following spinal cord contusion injury. J Neurodegener Regen. 2011;4:1–18. [Google Scholar]
- 75.Yoneyama M, Kawada K, Shiba T, et al. Endogenous nitric oxide generation linked to ryanodine receptors activates cyclic GMP/protein kinase G pathway for cell proliferation of neural stem/progenitor cells derived from embryonic hippocampus. J Pharmacol Sci. 2011;115:182–195. doi: 10.1254/jphs.10290FP. [DOI] [PubMed] [Google Scholar]
- 76.Emborg ME, Ebert AD, Moirano J, et al. GDNF-secreting human neural progenitor cells increase, tyrosine hydroxylase and VMAT2 expression in MPTP-treated cynomolgus monkeys. Cell Transplant. 2008;17:383–395. [PubMed] [Google Scholar]
- 77.Nikkhah G, Duan WM, Knappe U, et al. Restoration of complex sensorimotor behavior and skilled forelimb use by a modified nigral cell suspension transplantation approach in the rat Parkinson model. Neuroscience. 1993;56:33–43. doi: 10.1016/0306-4522(93)90559-x. [DOI] [PubMed] [Google Scholar]
- 78.Lei Z, Jiang Y, Li T, et al. Signaling of glial cell line-derived neurotrophic factor and its receptor GFRα1 induce Nurr1 and Pitx3 to promote survival of grafted midbrain-derived neural stem cells in a rat model of Parkinson disease. J Neuropathol Exp Neurol. 2011;70:736–747. doi: 10.1097/NEN.0b013e31822830e5. [DOI] [PubMed] [Google Scholar]
- 79.Kong XY, Cai Z, Pan L, et al. Transplantation of human amniotic cells exerts neuroprotection in MPTP-induced Parkinson disease mice. Brain Res. 2008;1205:108–115. doi: 10.1016/j.brainres.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 80.Torp R, Singh PB, Sorensen DR, et al. Growth factors as neuroprotective treatment in Parkinson disease? [in Norwegian] Tidsskr Nor Laegeforen. 2006;126:899–901. [PubMed] [Google Scholar]
- 81.Redmond DE, Jr., Weiss S, Elsworth JD, et al. Cellular repair in the parkinsonian nonhuman primate brain. Rejuvenation Res. 2010;13:188–194. doi: 10.1089/rej.2009.0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bjugstad KB, Teng YD, Redmond DE, Jr., et al. Human neural stem cells migrate along the nigrostriatal pathway in a primate model of Parkinson's disease. Exp Neurol. 2008;211:362–369. doi: 10.1016/j.expneurol.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolff EF, Gao XB, Yao KV, et al. Endometrial stem cell transplantation restores dopamine production in a Parkinson's disease model. J Cell Mol Med. 2011;15:747–755. doi: 10.1111/j.1582-4934.2010.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu Q, Ma J, Yu L, et al. Grafted neural stem cells migrate to substantia nigra and improve behavior in Parkinsonian rats. Neurosci Lett. 2009;462:213–218. doi: 10.1016/j.neulet.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 85.Svendsen CN, Caldwell MA, Shen J, et al. Long-term survival of human central nervous system progenitor cells transplanted into a rat model of Parkinson's disease. Exp Neurol. 1997;148:135–146. doi: 10.1006/exnr.1997.6634. [DOI] [PubMed] [Google Scholar]
- 86.Santaniello S, Gale JT, Montgomery EB, et al. Modeling the motor striatum under deep brain stimulation in normal and MPTP conditions. Conf Proc IEEE Eng Med Biol Soc. 2010;2010:2065–2068. doi: 10.1109/IEMBS.2010.5626354. [DOI] [PMC free article] [PubMed] [Google Scholar]