This study found that lithium stimulates neural stem and progenitor cell proliferation and differentiation following cranial irradiation while also reducing oligodendrocyte loss in the dentate gyrus of juvenile mice. These results indicate that lithium might be beneficial in reducing post-therapy late effects in patients receiving cranial radiotherapy and that blocking inflammation in this context may not be as advantageous as previously suggested.
Keywords: Adult stem cells, Irradiation, Tissue regeneration, Neural differentiation, Oligodendrocytes
Abstract
Radiation-induced brain injury occurs in many patients receiving cranial radiation therapy, and these deleterious effects are most profound in younger patients. Impaired neurocognitive functions in both humans and rodents are associated with inflammation, demyelination, and neural stem cell dysfunction. Here we evaluated the utility of lithium and a synthetic retinoid receptor agonist in reducing damage in a model of brain-focused irradiation in juvenile mice. We found that lithium stimulated brain progenitor cell proliferation and differentiation following cranial irradiation while also preventing oligodendrocyte loss in the dentate gyrus of juvenile mice. In response to inflammation induced by radiation, which may have encumbered the optimal reparative action of lithium, we used the anti-inflammatory synthetic retinoid Am80 that is in clinical use in the treatment of acute promyelocytic leukemia. Although Am80 reduced the number of cyclooxygenase-2-positive microglial cells following radiation treatment, it did not enhance lithium-induced neurogenesis recovery, and this alone was not significantly different from the effect of lithium on this proinflammatory response. Similarly, lithium was superior to Am80 in supporting the restoration of new doublecortin-positive neurons following irradiation. These data suggest that lithium is superior in its restorative effects to blocking inflammation alone, at least in the case of Am80. Because lithium has been in routine clinical practice for 60 years, these preclinical studies indicate that this drug might be beneficial in reducing post-therapy late effects in patients receiving cranial radiotherapy and that blocking inflammation in this context may not be as advantageous as previously suggested.
Introduction
Cranial irradiation is used in the treatment of primary and metastatic central nervous system tumors and prophylactically to prevent cranial relapse in high-risk acute lymphoblastic leukemia and lung cancer. In many instances subacute effects including behavioral changes, sleepiness, and late delayed cognitive effects manifested as lowered intelligence quotients and reduced learning and memory performances are experienced following these treatments [1]. The severity of these side effects is increased with higher doses, larger brain volumes, and a younger age at treatment [2, 3]. A range of cellular alterations have been proposed to be responsible for these debilitating side effects identified in rodents, primates, and humans, such as neural stem and progenitor cell (NSPC) loss, demyelination, and brain inflammation.
NSPCs play a critical role in the renewal of adult brain cells in germinative areas involved in learning and memory: the dentate gyrus (DG) in the hippocampus and the subventricular zone (SVZ)/olfactory bulb system [4–6]. Indeed, loss of NSPCs is associated with impairment of cognitive function following irradiation in both rodents and humans [7, 8]. As a strategy to minimize the effects of irradiation on brain cells, we considered interventions that might promote neurogenesis and repair after radiation injury. Lithium has been in clinical use for 60 years and remains a treatment of choice for bipolar disorder [9]. Experimental evidence supports the idea that lithium can be used efficiently in the treatment of irradiation-induced brain damage [10, 11]. In contrast to a previous study [11], we used lithium as a postradiotherapy treatment to prevent sensitizing endogenous NSPCs to radiation. However, the efficiency of lithium treatment within this context may have potentially been limited by inflammation induced following irradiation [7, 12]. We therefore investigated whether the concomitant use of lithium and inflammatory suppression might achieve an optimal neural cell recovery following cranial irradiation. We focused on the use of a clinically used drug that could be dually applied both to achieve an anticancer therapeutic effect and to reduce inflammation. One such candidate molecules is the synthetic retinoid Am80 (tamibarotene), because it is a promising drug in regard to cancer treatment and has been used already to reduce brain inflammation. Indeed Am80 is clinically approved for relapsed or refractory acute promyelocytic leukemia (APL) and has the advantage over all-trans-retinoic acid to be more stable and to drive the differentiation of APL cells [13]. Importantly, Am80 has been recently shown to reduce brain inflammation following hemorrhage and to promote behavioral recovery [14]. We found here that Am80 can be used to efficiently reduce irradiation-induced microglial cell activation, which mediates inflammation, but that this provided no additional benefit beyond the lithium-alone-induced recovery of neurogenesis.
Materials and Methods
Animal and Experimental Treatment
Juvenile C57Bl/6 male 3–5-week-old mice were used in this study. All of the experiments were conducted according to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. For cranial irradiation, the mice were placed after anesthesia on polypropylene plastic beds and irradiated using the 9 MeV electron beam from a Varian clinical linear accelerator delivering a single dose of 8 Gy. To achieve this, the mouse bodies excluding their heads were protected by a precisely machined Perspex (E-Plas, Tullamarine, VIC, Australia, http://www.eplas.com.au) sheet and a 3-mm lead shield. In rodents, a single dose of 8 Gy produces long-term NSPC proliferation and neurogenesis impairment [15] and is equivalent to a fractionated dose of 20 Gy in 2-Gy doses. The calculation was done as follows: effective dose (2 Gy per fraction) = total dose (α/β + fraction size)/(α/β + 2), so in this instance the effective dose = 8(2 + 8)/(2 + 2) = 20. The α/β ratio describes the tissue tolerance, and an α/β value of 2 is used for brain tissue [16].
For lithium treatment (LiCO3; Sigma-Aldrich, St. Louis, http://www.sigmaaldrich.com), the mice were fed with control chow or lithium chow (0.5%) for 1 or 4 weeks. This protocol as tested by us produced serum lithium levels of 0.9 ± 0.01 mM in mice, within the range of therapeutic concentrations used in humans (0.5–1.2 mM). For Am80 treatment and combination treatment, the mice were fed with Am80 chow (10 mg/kg [17]). To assess cell proliferation in vivo, mice received a 5-bromo-2′-deoxyuridine (BrdU) intraperitoneal (IP) injection (100 mg/kg) 1 hour before sacrifice to label cells engaged in the S phase. For newborn cell number and differentiation experiments, the mice received a BrdU IP injection (100 mg/kg) twice daily for 3 days after 1 week of lithium treatment and were housed for 3 additional weeks of lithium treatment, during which the newborn cells differentiated.
Quantitative Reverse Transcription-Polymerase Chain Reaction
RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) according to manufacturer's instructions. Digestion of genomic DNA was performed by the addition of RNase-free DNase 1 (1 unit per μl; Promega, Madison, WI, http://www.promega.com). Superscript III Reverse Transcriptase (Invitrogen) was used for first-strand cDNA synthesis according to the manufacturer's instructions. Quantitative reverse transcription-polymerase chain reactions (qRT-PCRs) were conducted using an ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com). For one reaction, 8 μl of cDNA (1:10 dilution) was combined with 10 μl of SyBr Green PCR Master Mix (Applied Biosystems) and 200 nM sense and antisense oligonucleotides (Geneworks, Hindmarsh, SA, Australia, http://www.geneworks.com.au) and amplified using temperatures of 50°C for 2 minutes and 95°C for 15 minutes. These initial steps were followed by 45 cycles of 95°C for 15 seconds and 60°C for 1 minutes. Expression of all genes was normalized to β2-microglobulin housekeeping gene expression. No template controls and dissociation curve analysis were used to ensure the specific amplification of the transcripts. Primer sequences were designed against the cDNA sequence of target genes and were as follows: β2-microglobulin: reverse, GTCTTGGGCTCGGCC, and forward, TTCACCCCCACTGAGACT; TNFα: reverse, ACAAGCAGGAATGAGAAGAGG, and forward, CGTGGAACTGGCAGAAGAG; Cyclin D1: reverse, GTGCGTTGTGCGGTAGCAGG, and forward, GCATCTACACTGACAACTCTATC; c-myc, reverse, CTCTGCACACACGGCTCTTC, and forward, GCTGTAGTAATTCCAGCGAGAGACA; interleukin-6 (IL6): reverse, GAAGTAGGGAAGGCCGTGG, and forward, CTGCAAGAGACTTCCATCCAGTT; and IL6-Rα: reverse, GCAACACCGTGAACTCCTTTG, and forward, GACCTGGGACCCGAGTTACTA.
Immunohistochemistry
The mice were deeply anesthetized, and brain tissues were fixed via transcardiac perfusion with 4% paraformaldehyde. The brains were processed for paraffin embedding and sectioning. For BrdU labeling and detection, after dewaxing and rehydration, 6-μm brain sections were subjected to 2 N HCl treatment for 30 minutes at 37°C. Endogenous peroxidase activities were quenched with 3% H2O2 in PBST (phosphate-buffered saline containing 0.1% Triton X-100) for 10 minutes at room temperature (RT). The sections were incubated with mouse anti-BrdU antibody (1/400; DakoCytomation, Glostrup, Denmark, http://www.dakocytomation.com) at RT for 1 hour, rinsed in PBS, and incubated in ImmPress reagent (Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com) for 30 minutes at RT. Staining for BrdU was visualized using diaminobenzidine and H2O2. The sections were then counterstained in Mayer's hematoxylin, dehydrated, and coverslipped in Depex medium. For 2′,3′-cyclic nucleotide-3′-phosphohydrolase (CNP) and cyclooxygenase-2 (COX-2) staining, the HCl incubation step was omitted, and sections were incubated in mouse anti-CNP antibody (1/500; Abcam, Cambridge, U.K., http://www.abcam.com) at RT for 1 hour or in rabbit anti-COX-2 antibody (1/300; Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com) overnight at 4°C after pressure cooker antigen retrieval in EDTA (1 mM) buffer. For coimmunofluorescence staining for BrdU and neuron-specific enolase (NSE) or glial fibrillary acidic protein (GFAP), following pressure cooker antigen retrieval in citrate buffer (10 mM) followed by 2 N HCl treatment for BrdU, the sections were incubated overnight at 4°C in appropriate secondary antibody mixture: mouse anti-BrdU antibody (1/100; DakoCytomation) and rabbit anti-NSE antibody (1/100; Chemicon, Temecula, CA, http://www.chemicon.com) or rabbit anti-GFAP antibodies (1/200; DakoCytomation) for adjacent sections. All of the sections were then washed, and secondary detection was performed using Alexa Fluor 594 goat anti-mouse IgG (for BrdU or CNP) or Alexa Fluor 488 goat anti-rabbit IgG (for GFAP or NSE). The sections were then mounted in a fluorescent mounting medium (DakoCytomation).
Cell Quantitation and Statistical Analysis
Positive stained cells were scored in one-in-five series of three coded sections sampled from three areas throughout the rostral extent of the DG under high power magnification. DG areas were quantified using SPOT software (SPOT Imaging Solutions, Diagnostic Instruments, Inc., Sterling Heights, MI, http://www.spotimaging.com). The volume of DG was determined according to Shi and collaborators [18] by multiplying areas by the thickness of the sections. Cell densities were calculated by dividing cell numbers by the volume. Double-stained cells were analyzed on 0.3-μm optical sections using confocal microscopy.
Behavioral Experiments
Behavioral studies were performed on two separate cohorts of mice, the first of which included 10 control and 10 irradiated mice, at 4 weeks after irradiation; the second cohort included 8 control and 7 irradiated mice, at 8 weeks after radiation. Investigators carrying out the experiments were blinded to the treatment of the mice. The open field is an open arena (40 × 40 × 30 cm) with a clear floor and walls and no distinguishing features. No visual cues were placed within or around the arena. The apparatus was placed on a table under a ceiling-mounted camera. Over three sequential days, the mice were singly placed in the center of the arena and left to explore for 30 minutes, thus determining habituation to the locomotor environment. The Spontaneous Motor Activity Recording & Tracking (SMART) video tracking system (San Diego Instruments, Inc., San Diego, http://www.sandiegoinstruments.com) was used to track the locomotor activity and analyze the total distance traveled (cm) in 5-minute time bins, as well as session totals. The Morris water maze consisted of a circular pool with a submerged platform 1 cm below the water surface [19]. The water was made opaque with nontoxic white paint and kept at 23 ± 2°C. The pool was divided into four equal quadrants (based on compass locations: north, south, east, and west). The experimental room contained various three-dimensional cues (door, computer, desk, curtain, flower, a stuffed toy, and a red striped placard, which served as external visual cues). Internal two-dimensional cues were placed at the north, south, east, and west cardinal points. Subject movements were recorded with a video camera positioned above the pool and attached to the ceiling. The data were analyzed using the SMART Video Tracking System. The program was used to measure the latency to locate the submerged platform, time spent in the target quadrant, platform crossings, time spent in the platform area, and platform proximity on probe trial days. Days 1 and 2 were the cue-associated learning phase (control procedure), in which the mice were trained for 2 days in two daily sessions; each session consisted of four 1-minute trials. The mice were randomly placed in different start positions and allowed 60 seconds to locate the platform indicated by a flag (12 cm visible above the water surface). The platform location was varied with each trial. The mice were left on the platform for 15 seconds after successful navigation; if a mouse failed to locate the platform after 60 seconds, it was led to the platform by a trailing finger. Days 3–5 were the spatial learning phase (acquisition phase), which was undertaken after the cue-associated learning phase. Spatial learning was completed over 3 days with mice undergoing eight trials per day in two sessions: each session consisted of four 1-minute trials. The mice were placed in different start positions and given 60 seconds to locate the submerged platform. The submerged platform location was randomized for each mouse; the location of the platform was kept constant for individual mice across subsequent spatial learning trials. After successful navigation to the platform, the mice were left for 15 seconds or manually placed on the platform if they were unable to locate the platform after 60 seconds. On day 6, a probe trial was conducted to check spatial reference memory. The platform was removed, and the mice were allowed to explore for 60 seconds. They were individually placed in the position furthest from (diametrically opposed to) the previous location of the submerged platform. For days 7–10, a reversal learning phase was performed 1 day after the spatial probe trial had been completed. The location of the submerged platform was moved to the diametrically opposed quadrant of the pool for each mouse. The same procedure was then followed as described for the spatial learning phase.
Statistical Analysis
The data presented are the means ± SEM from at least three different mice. One-way analysis of variance with the Bonferroni post test or the Newman-Keuls post test was performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, Inc., San Diego, http://www.graphpad.com).
Results
NSPC Proliferation Is Dampened in the DG Following Brain-Focused Irradiation and Is Associated with Subtle Cognitive Impairment in Juvenile Mouse
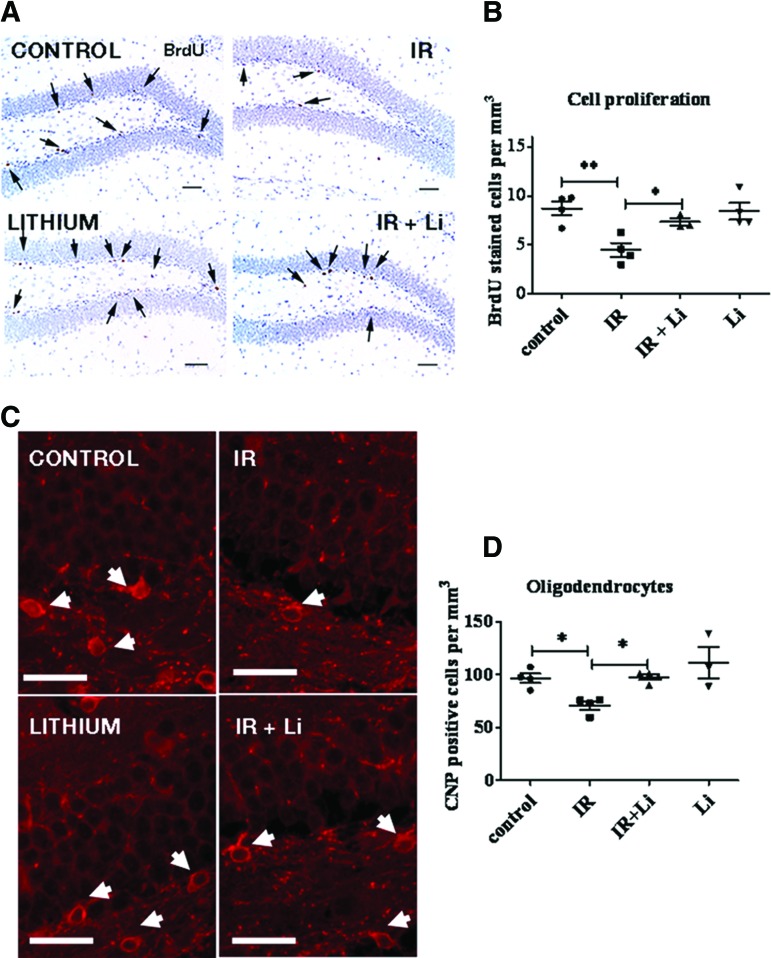
To ensure that the radiation treatment regimen we were testing produced a demonstrable effect on the proliferation of NSPCs in the DG of juvenile mouse, control and irradiated groups were injected with the thymidine analog BrdU 9 days following 8-Gy cranial ionizing radiation exposure. Immunohistochemical detection of cells that have incorporated BrdU into their DNA while progressing through the S phase at the time of the injection showed that irradiation led to a significant reduction in the number of proliferating cells in the DG (Fig. 1A, 1B) of irradiated mice compared with untreated control mice. To investigate whether irradiation in our model also produces a loss in oligodendrocytes, we quantified the number of CNP-positive (CNP+) oligodendrocytes as shown in Figure 1C. Scoring of CNP+ cell bodies showed that irradiation induced a significant reduction in the number of CNP+ oligodendrocytes in the DG of irradiated mice compared with control (Fig. 1D).
Figure 1.
Short-term lithium treatment significantly reverses irradiation-induced decrease in neural stem and progenitor cell (NSPC) proliferation and protects oligodendrocytes in vivo in the dentate gyrus (DG). Experiments were performed using four groups of mice: (a) control, (b) irradiated, (c) lithium-treated, and (d) irradiated and lithium-treated. The mouse groups were fed with control or lithium chow for 1 week starting 2 days after radiation to avoid interfering with cell death and repair. All of the groups were injected with BrdU 1 hour before sacrifice to label NSPCs engaged in the S phase. (A): Hematoxylin-stained coronal sections through the DG showing proliferating BrdU+ cells typically located in the subgranular zone. Lithium increased the number of proliferating cells in the DG, thus reversing irradiation-induced loss of BrdU+ cells. (B): BrdU+ cell quantitation showed that lithium induced a significant increase in the number of BrdU+ cells following irradiation compared with irradiated mice. (C): Oligodendrocytes were identified by immunohistochemical staining for CNP. Typical CNP staining was observed in oligodendrocytes processes and around the cell bodies. Irradiation reduced the number CNP+ cell bodies in the DG (white arrows), whereas lithium blocked this effect. Scale bars = 100 μm. (D): Quantitation of the number of CNP+ cells showed that irradiation produced a significant reduction in the number of oligodendrocytes, whereas lithium restored their numbers to control levels. The data shown are the means ± SEM. *, p < .05. Abbreviations: BrdU, 5-bromo-2′-deoxyuridine; IR, irradiation; CNP, 2′,3′-cyclic nucleotide-3′-phosphohydrolase.
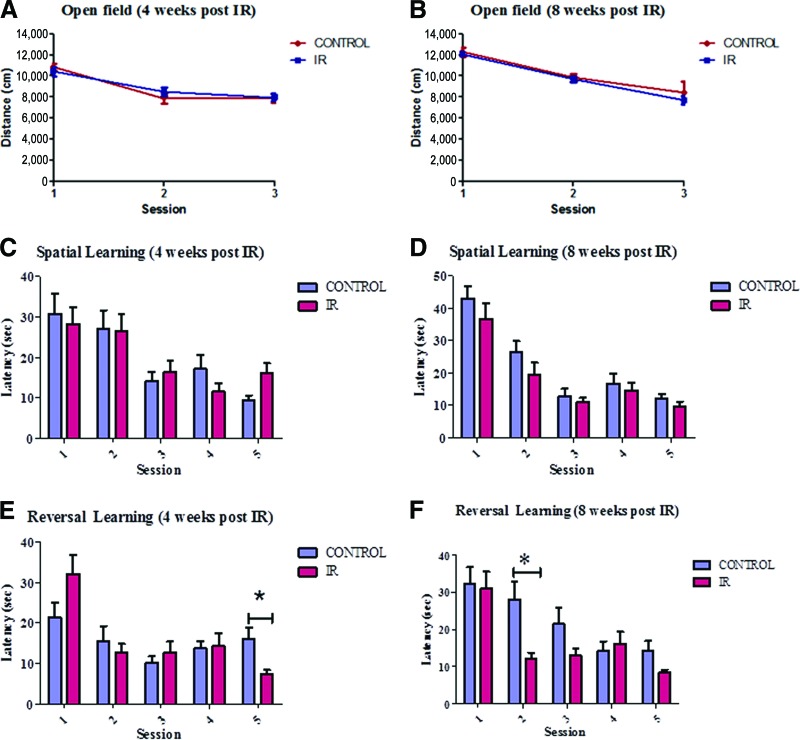
We then evaluated whether these cellular alterations were correlated with learning and memory abilities changes in juvenile mice in our paradigm at 4 and 8 weeks after irradiation (Fig. 2). This allowed the assessment of both subacute and delayed (late) cognitive impairments as seen in whole brain irradiation treated patients. To establish measures of these abilities we used the Morris water maze (MWM) as used by others to assess spatial learning related to hippocampal function. An open field paradigm was used prior to challenging mice in MWM to assess locomotor and exploratory activity over 3 successive days. A robust habituation to the context was observed throughout the period of habituation, such that mice from all treatment groups were significantly less active by day 3 of habituation (Fig. 2A, 2B). Additionally, during each of the 3 days, control or irradiated mice demonstrated similar patterns of locomotor activity.
Figure 2.
A single dose of 8 Gy produces subtle differences in learning and memory ability as assessed using the Morris water maze (MWM). We used the open-field paradigm to assess locomotor and exploratory activity prior to testing the mice in the MWM. (A, B): The mice were first tested in a small open field, and their locomotor behavior was monitored over 3 successive days. A robust habituation was observed in both irradiated and control mice, 4 (A) and 8 (B) weeks after radiation. Hippocampus-related learning and memory function was assessed using the MWM. During five training sessions, a significantly decreased latency to find the platform across sessions was found in both irradiated and control groups 4 (C) and 8 (D) weeks after radiation. The mice were subsequently reassessed for spatial learning to determine apparent neural liability in target reacquisition. Subtle differences were observed between group at 4 weeks after radiation (E) (session 5) and 8 weeks after radiation (F) (session 2). The data shown are the means ± SEM. *, p < .05. Abbreviation: IR, irradiation.
Hippocampus-related learning and memory function were then tested in the MWM. Spatial learning was assessed by training mice to locate a submerged platform. The mice were placed in different start positions and given 60 seconds to locate the submerged platform. The submerged platform location was randomized for each mouse. The location of the platform was kept constant for individual mice across subsequent spatial learning trials. After successful navigation to the platform, the mice were left for 15 seconds or manually placed on the platform if they were unable to locate the platform after 60 seconds. During five training sessions, irradiated and control mice alike demonstrated a significantly decreased latency to find the platform across sessions (Fig. 2C, 2D). The mice were subsequently reassessed for spatial learning to determine apparent neural liability in target reacquisition. Mice from both groups quickly reattained the new location of the platform as shown by decreased latency. Here a significant difference in latency between control and irradiated mice was identified. Indeed irradiated mice tended to have a decreased latency after the first session. In groups assessed 4 weeks after irradiation, a difference was seen at session 5 (Fig. 2E). More consistent latency differences were identified between groups assessed 8 weeks after irradiation (Fig. 2F). Altogether our results suggest that a single dose of 8 Gy produces a subtle difference in learning and memory ability as assessed using MWM.
Lithium Promotes Neurogenesis and Prevents Oligodendrocytes Loss Following Cranial Irradiation in the DG In Vivo
The effect of lithium treatment on NSPC proliferation in the DG with or without cranial irradiation was then investigated. Accordingly mice were fed control or lithium chow for 1 week and received a 1-hour pulse of BrdU to assess the number of proliferating cells in the subgranular layer of the DG where NSPCs reside. This protocol achieved lithium levels in serum of 0.9 ± 0.01 mM in mice within the range of therapeutic concentrations used in humans (0.5–1.2 mM). Immunohistochemistry (IHC) detection of BrdU+ cells suggested that lithium treatment increased the proportion of cells engaged in the S phase in the DG following irradiation (Fig. 1A). Quantitation of the number of these cells showed that lithium significantly reversed the effect of irradiation on NSPC proliferation in the DG of irradiated mice (Fig. 1B). These results indicate that DG NSPCs can be stimulated by lithium treatment following cranial irradiation.
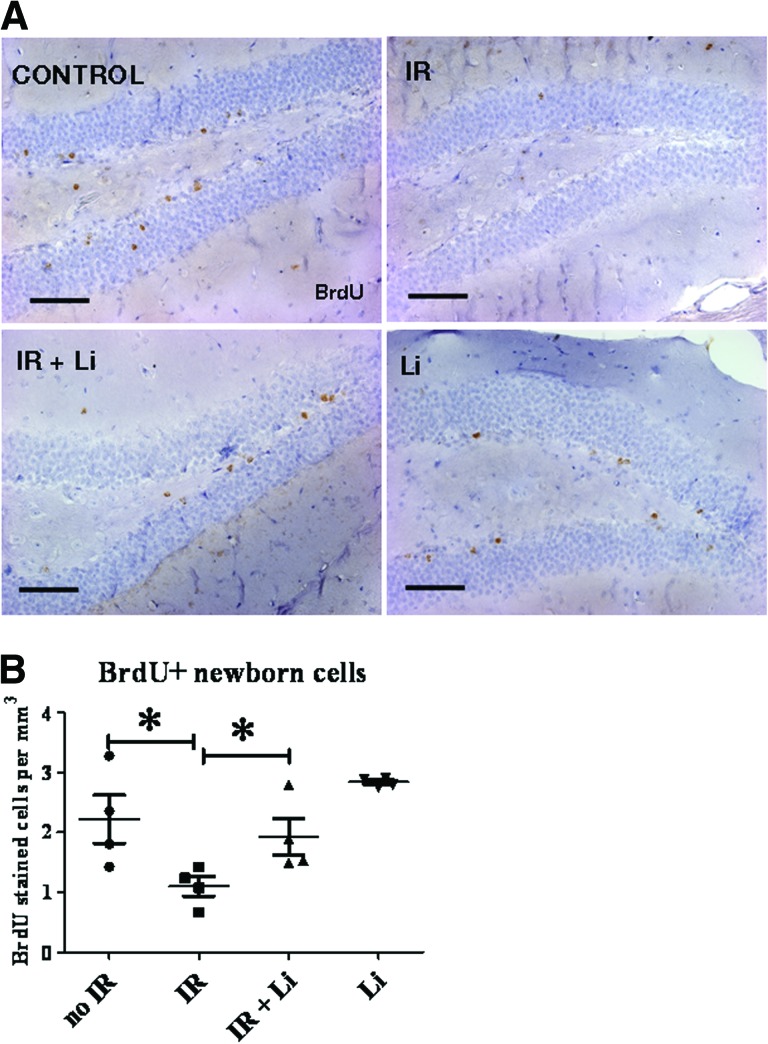
To investigate whether the increase in NSPC proliferation translated into an increased number of newly generated cells, mice were injected with BrdU twice daily for 3 days 1 week following irradiation, and the number of BrdU+ newborn cells was quantified 3 weeks later (4 weeks after radiation). Using this protocol, proliferating cells are no longer detectable because of BrdU dilution as a result of successive rounds of proliferation. As shown in Figure 3A, BrdU+ cells were typically seen in the granular layer, in the hilus, and occasionally in the molecular layer of the DG. BrdU+ cell quantitation (Fig. 3B) showed that irradiation produced a 50% decrease in the number of newborn cells after 3 weeks, whereas lithium induced a significant recovery in their number. These data support the view that lithium treatment enhances NSPC proliferation and neural cell birth.
Figure 3.
Lithium increases the number of newborn cells in the dentate gyrus (DG) following brain-focused irradiation. The mice received BrdU injections twice daily for 3 days 1 week after radiation to label newly generated cells, and the number of surviving newborn cells was assessed after 3 additional weeks. The mice were fed with lithium or control chow for a total of 4 weeks. (A): Newly generated cells were detected by immunohistochemistry for BrdU on coronal sections through the DG. BrdU+ cells were typically seen in the granular layer, in the hilus, and occasionally in the molecular layer of the DG. Irradiation induced a reduction of the number of BrdU+ cell, and lithium reversed this effect. (B): BrdU+ quantitation showed that irradiation produced a 50% decrease in the number of newborn cells after 3 weeks, whereas lithium induced significant recovery in their number. The data shown are the means ± SEM. Scale bar = 100 μm. *, p < .05. Abbreviations: IR, irradiation; BrdU, 5-bromo-2′-deoxyuridine.
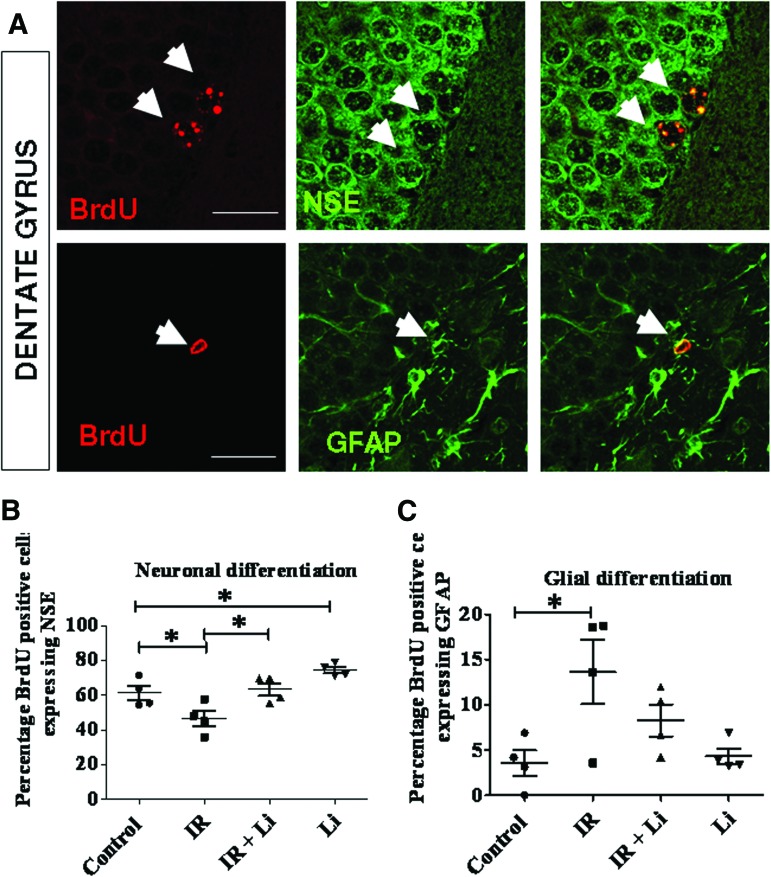
Next, we investigated whether lithium enhances the differentiation of newly generated cells into neurons in vivo. The mice were injected with BrdU twice daily for 3 days 1 week following irradiation, and the fate of BrdU+ cells was determined using coimmunostaining for BrdU and specific markers for neurons (NSE) or astrocyte marker GFAP (Fig. 4A). Using immunofluorescence and confocal microscopy, we found that lithium enhanced differentiation toward the neuronal lineage in lithium-treated mice compared with control mice following cranial irradiation as shown by BrdU and NSE costaining (Fig. 4A, 4B). In contrast, lithium treatment did not significantly affected glial differentiation as shown by BrdU and GFAP colabeling (Fig. 4C).
Figure 4.
Lithium increases the percentage of newborn cells that differentiate into neurons under basal conditions and following irradiation but does not influence glial cell differentiation. (A): To assess newborn cell fate in the dentate gyrus (DG), sections were costained for BrdU and NSE to identify neurons or for BrdU and GFAP to identify astrocytes (white arrows) and were analyzed by confocal microscopy. (B): Results of confocal analyses revealed that irradiation significantly reduced neuronal differentiation, whereas long-term lithium treatment significantly enhanced neuronal differentiation at a basal level and following irradiation in the DG. (C): Irradiation led to an increased production of glia (gliosis), whereas lithium did not have any significant effect on glial differentiation. The data shown are the means ± SEM. Scale bars = 50 μm. *, p < .05; **, p < .01. Abbreviations: BrdU, 5-bromo-2′-deoxyuridine; NSE, neuron-specific enolase; GFAP, glial fibrillary acidic protein; IR, irradiation.
To address the effects of irradiation and lithium treatment on demyelination, we quantified the number of differentiated oligodendrocytes identified by CNP IHC. Interestingly, we found that lithium restored the number of CNP+ oligodendrocytes following irradiation (Fig. 1C, 1D). These results suggest that lithium treatment stimulates NSPC proliferation and neuronal differentiation and reduces the loss of oligodendrocytes following cranial irradiation.
Lithium Treatment Increases Cyclin D1 Expression in the DG Following Irradiation
To investigate the molecular mechanism by which lithium stimulates NSPC proliferation, we evaluated the action of lithium on another population of NSPCs. In this instance NSPCs were isolated from the SVZ [20]. Indeed, in contrast to DG NSPCs, NSPCs derived from the SVZ can be efficiently expanded using the neurosphere assays (supplemental online Fig. 1A). NSPCs were plated at a density of 104 cells per milliliter into 96-well plates in the presence or absence of 0.5 or 1 mM lithium. This exposure is within the therapeutic range for humans (0.5–1.2 mM lithium). Neurosphere growth was assessed using [3H]thymidine incorporation for 24 hours. Under both nonirradiated and irradiated conditions, 0.5 and 1 mM lithium increased neurosphere growth (supplemental online Fig. 1B). Quantitation confirmed a doubling in incorporated radioactivity in lithium-treated cultures compared with untreated cultures under both nonirradiated and irradiated conditions (supplemental online Fig. 1C). To rule out the possibility that [3H]thymidine incorporation was occurring as a consequence of the repair process, we have also quantify neurosphere growth by viability assay using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (supplemental online Fig. 1D). We found that, in accord with the [3H]thymidine incorporation experiment, adding 0.5 or 1 mM lithium increased neurosphere growth under both nonirradiated and irradiated conditions. Altogether these data suggest that lithium stimulates neurosphere cell proliferation.
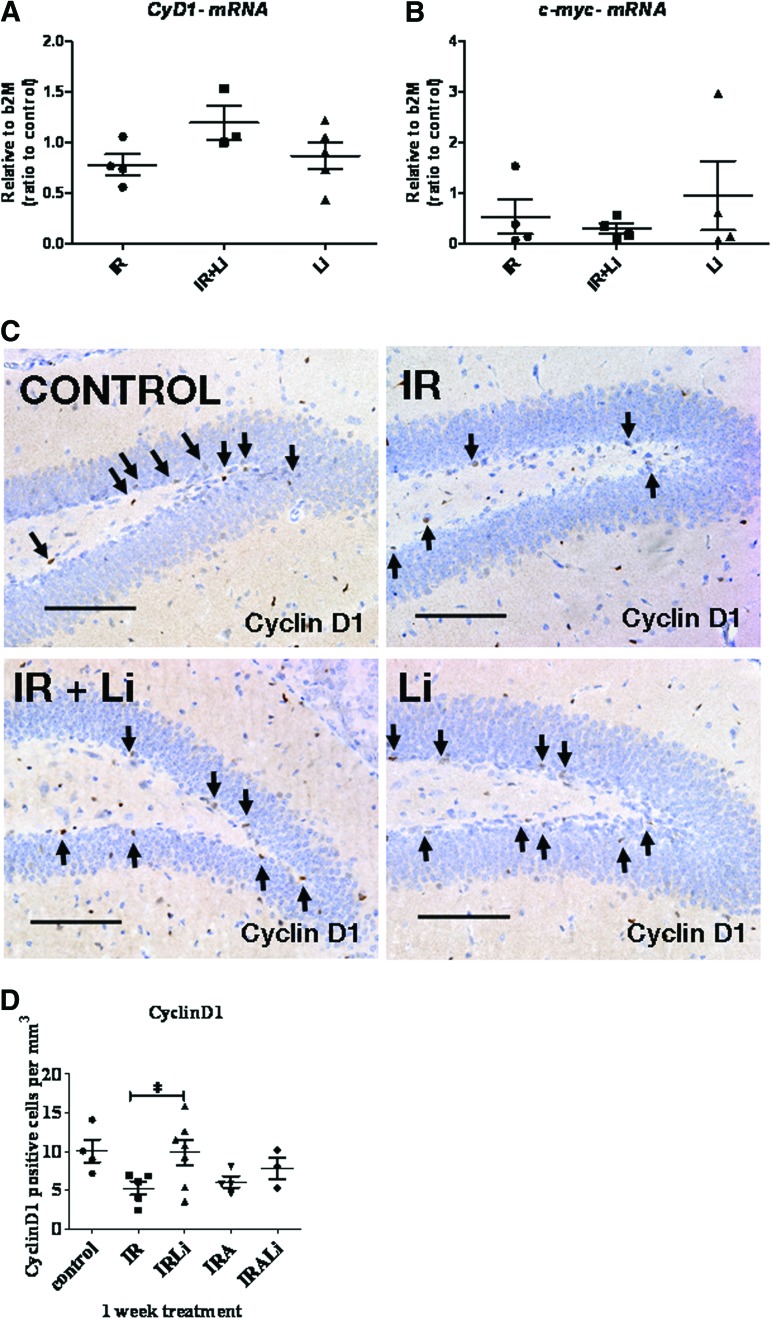
Because lithium as been shown to activate the β-catenin-mediated signaling pathway, we measured the expression of one of its key target genes, Cyclin D1, at the mRNA level using qRT-PCR (supplemental online Fig. 1E). Neurospheres were incubated in 1 mM lithium for 24 hour after radiation to allow changes in transcription. Under these conditions we found that Cyclin D1 (supplemental online Fig. 1E) was upregulated by lithium treatment after radiation in NSPCs derived from SVZ. We then investigated whether our findings in SVZ derived NSPCs translated to DG NSPCs in vivo. The levels of Cyclin D1 and c-Myc mRNA, another β-catenin target gene, were measured in the hippocampus following 1 week of lithium treatment after irradiation using qRT-PCR (Fig. 5). Our results show that lithium treatment for 1 week following irradiation did not induce any significant change in either Cyclin D1 (Fig. 5A) or c-Myc mRNA expression level (Fig. 5B). However, IHC for Cyclin D1 presented in Figure 5C and 5D shows that CyclinD1+ cells are more abundant in lithium-treated neurogenic regions compared with untreated after brain irradiation. This is likely to be a more sensitive measure of β-catenin target gene expression in the DG.
Figure 5.
Effect of lithium treatment on Cyclin D1 and c-Myc expression following irradiation in vitro and in vivo. (A, B): Cyclin D1 (A) and c-Myc (B) mRNA expression was measured in the hippocampus by quantitative reverse transcription-polymerase chain reaction 1 week following brain-focused irradiation. (C): Immunohistochemistry (IHC) detection of Cyclin D1 was performed on brain sections obtained from control, irradiated, and lithium-treated mice. Photographs taken from stained coronal sections show that Cyclin D1+ cells were more abundant in lithium-treated samples compared with untreated samples following irradiation. Scale bars = 100 μm. (D): Quantitation of IHC for Cyclin D1. Lithium treatment restored the number of Cyclin D1+ cells to control levels following irradiation. Am80 treatment did not change the number of Cyclin D1+ cells. The data shown are the means ± SEM. *, p < .05. Abbreviations: CyD1, Cyclin D1; IR, irradiation; IRA, irradiation and lithium; b2M, β2-microglobulin.
Reduced Inflammation Is Not Required to Allow Increased Neurogenesis Following Brain-Focused Irradiation in Juvenile Mice In Vivo
Irradiation-induced inflammation and subsequent gliosis is considered to functionally impair neurogenesis following irradiation [12]. We thus postulated that the suppression of inflammation might improve the stimulatory effect of lithium on neurogenesis following whole-brain irradiation in juvenile mice. With the aim of maximizing recovery, we introduced a combinatorial approach using lithium plus the synthetic retinoid Am80 (tamibarotene) [13, 14, 21] to reduce inflammation. In these experiments, the mice were fed with Am80 chow (10 mg/kg) as previously described [17].
Initially we investigated the effect of Am80 on brain inflammation following irradiation by measuring the mRNA level of proinflammatory molecules. Therefore IL6, IL6-Rα, and TNFα mRNA expression were measured in the hippocampus of juvenile mice by qRT-PCR 1 and 4 weeks following irradiation. Interestingly we found that only TNFα mRNA (supplemental online Fig. 2A, 2B) but not IL6 mRNA (supplemental online Fig. 2C, 2D) or IL6-Rα mRNA (supplemental online Fig. 2E, 2F) expression was elevated in irradiated mice compared with unirradiated control mice only after 1 week. However, Am80 treatment over 4 weeks did not significantly reduce TNFα mRNA expression following irradiation.
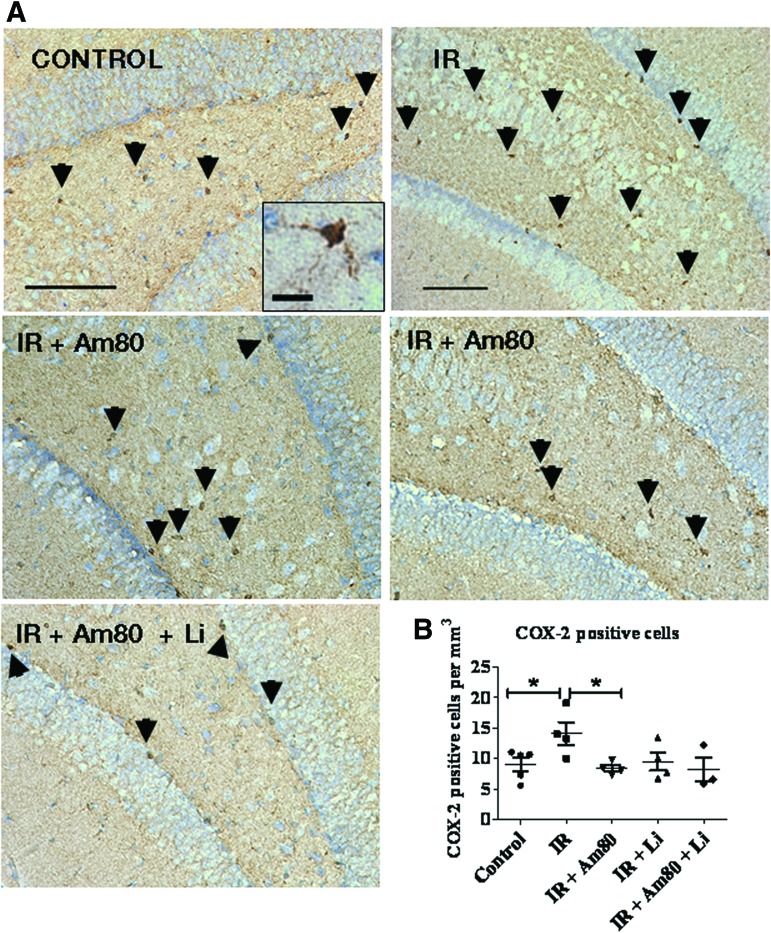
Although we endeavored to isolate the hippocampal neurogenic regions for mRNA analysis, we suspected that the cells responsible for the production of these proinflammatory mediators are in the minority of the cell total. Accordingly we were concerned that any effects of Am80 might be overwhelmed by the presence of the total tissue content. Therefore to directly visualize the effect of irradiation and drug treatment on microglial activation, IHC for COX-2 was used (Fig. 6A, 6B). Indeed activated microglial cells are known to be the source of TNFα following irradiation [22, 23] and to robustly express the inflammatory molecule COX-2. Using this strategy to evaluate inflammation, we were able to demonstrate that the number of microglial cells expressing COX-2 after irradiation is significantly increased in the DG, and when mice are treated with Am80 for 4 weeks, this irradiation-induced increase is efficiently blocked. When lithium or lithium plus Am80 was administrated, the number of COX-2+ cells in the irradiated tissue was not different from the unirradiated control group. We then asked whether the two drug interventions had an effect on neurogenesis.
Figure 6.
Effect of 4 weeks of lithium and Am80 treatment on the number of cyclooxygenase-2-positive (COX-2+) microglial cells in the dentate gyrus (DG) following brain-focused irradiation. (A): Immunohistochemistry for COX-2 was performed to assess the effect of Am80 on brain inflammation following cranial irradiation. Typical COX-2+ microglia cells (as shown in the inset) were seen in the hilus, subgranular, and granular layer of the DG (black arrowheads). (B): Positive COX-2 microglia cells were scored to show that their number was significantly elevated following brain-focused irradiation. Administration of Am80 for 4 weeks significantly blocked this effect. Scale bars = 100 μm and scale bar = 20 μm for inset. The data shown are the means ± SEM. *, p < .05. Abbreviation: IR, irradiation.
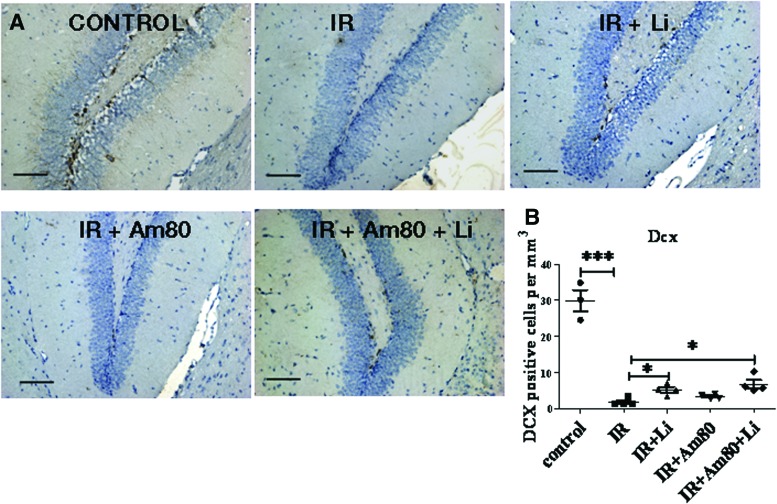
To specifically quantify the number of new neurons, we used the immature neuronal marker doublecortin (DCX), which has been shown to reflect the level of neurogenesis [24]. Quantitation of DCX+ cells demonstrated that lithium significantly increased the number of immature neurons following brain-focused irradiation (Fig. 7A, 7B). By contrast Am80 treatment alone did not increase the number of DCX-stained neuroblasts. Furthermore we found that when we used lithium and Am80 in combination, they were no more efficient in increasing the number of neuroblasts than lithium alone. This was an important experiment in establishing that inflammation was not alone in impeding neurogenesis or that it perturbs the positive action of lithium.
Figure 7.
Effect of lithium and Am80 treatment on neurogenesis following brain-focused irradiation. (A): Immunohistochemistry for DCX, a marker for neuroblasts, was used to assess the effect of drug treatment on neurogenesis. (B): Quantitation showed that DCX+ cells were significantly decreased by cranial irradiation when assessed after 4 weeks, whereas lithium treatment for 4 weeks after radiation significantly increased DCX+ cell number compared with irradiated mice but did not revert to control levels. Am80 did not have any significant effect on DCX+ cell number, and concomitant administration with lithium did not produce any additive effect. Scale bars = 100 μm. The data shown are the means ± SEM. *, p < .05; **, p < .01; ***, p < .001. Abbreviations: IR, irradiation; Dcx or DCX, doublecortin.
Discussion
Our data demonstrate that lithium treatment promotes NSPC proliferation and neuronal differentiation and reduces oligodendrocyte loss in the DG of juvenile mice in a model of brain-focused irradiation in vivo. This model reproduces the deleterious effect of radiotherapy as seen in humans including NSPC loss, inflammation, and demyelination. These alterations are proposed to underpin the occurrence of subacute and delayed cognitive side effects observed in patients who are receiving whole brain irradiation during the course of cancer treatment. We also investigated the use of the synthetic retinoid Am80 with the prospect of achieving an optimal recovery in combination with lithium treatment.
NSPCs are extremely radiosensitive cells, and irradiation has been shown to produce long-lasting impairments in neurogenesis in vivo [25]. In rodents, lithium has been shown to exert a protective effect against ischemia [26–28] and neurodegeneration in mouse models of Alzheimer [29] and Huntington [30, 31] diseases. In parallel, lithium positively influences neurogenesis in the DG [32, 33], and lithium pretreatment prior to irradiation improves cognitive performance of mice [11]. Our findings suggest that lithium reverses radiation-induced NSPC dysfunction by promoting neuronal progenitor proliferation and differentiation. These data are in line with studies showing that lithium increases neuronal differentiation [34, 35]. To take advantage of the stimulatory effect of lithium on neurogenesis following whole-brain irradiation, we tested whether we could enhance its action by blocking inflammation. To this end we combined lithium with a clinically used drug with the underlying idea that we could achieve both anticancer therapeutic effect and amelioration of radiotherapy side effects. With this in mind, we have focused our attention on the synthetic retinoid Am80 (tamibarotene), which is a potent drug clinically approved for relapsed or refractory APL and which has been recently shown to reduce brain inflammation following hemorrhage and to promote behavioral recovery [14]. Our results indicate that Am80 can be used to achieve inhibition of irradiation-induced microglial activation as shown by COX-2 immunohistological staining, but this did not benefit neurogenesis following irradiation. This result is in accord with our result using an IL6-null mouse-irradiation model, which showed that genetic loss of IL-6 prevented the lithium beneficial effect on cell birth in the DG as shown by BrdU pulse/chase experiment (supplemental online Fig. 3) compared with that seen with wild-type mice. In further support of these observations, there is an increasing body of evidence suggesting that inflammation has beneficial effects on brain recovery in experimental and clinical traumatic brain injury [36], neurodegenerative diseases [37], and ischemia [38]. Thus it has been suggested that activated microglia may help in promoting optimal recovery [39].
Collectively our data show that lithium promotes NSPC proliferation and commitment to the neuronal lineage following irradiation. They also provide proof of principle for the use of lithium in the clinic in the context of promoting postirradiation neurogenesis and tissue repair. Our data also reveal that lithium assists in reducing oligodendrocyte loss induced by brain irradiation, similar to its ability to promote Schwann cell proliferation in the peripheral nervous system [40] and its protective effect in an in vivo model of induced allergic encephalomyelitis [41]. These data imply that lithium promotes normal neuronal function by ensuring proper myelination following irradiation. The question remains whether the use of lithium provides a functional behavioral recovery. Accordingly we have assessed the effect of irradiation using the MWM paradigm. Studies from the literature report either reduced abilities [11, 42, 43] or no difference [44] using this behavioral test. In this work we have found that the apparent latency to find the platform is modestly reduced in irradiated compared with nonirradiated groups in the reversal form of the MWM where the submerged platform location is changed to the quadrant diametrically opposed to that held previously. One explanation we favor is that previous training has influenced the response of the unirradiated mice [45]. The reversal tests are run 24 hours after a probe trial when the platform is removed and the mice have to swim with no escape. Therefore the control group may have learned that the platform is not present and thus offers a reduced stimulation. Indeed, in forced swimming experiments a reduced mobility reflects a relatively successful coping strategy for energy conservation [46]. However, the end point of the experiment was not different between groups in term of time spent in the target quadrant (not shown), platform crossings (not shown), or time spent in the platform area (not shown). Because these differences were subtle and certainly not sufficiently large enough, we did not pursue further investigation of the behavioral effects of lithium treatment following irradiation. Moreover, lithium has previously been shown to improve cognitive performances of irradiated mice when used as a pretreatment [11]. In our study we used lithium as a postirradiation treatment with the view that it would be more acceptable in a clinical setting. Indeed administering lithium to patients as a pretreatment might be damaging because it is likely to induce NSPC to proliferate prior to radiotherapy. Indeed, quiescent NSPCs are somewhat protected from irradiation, whereas when induced into cycle they are potentially more vulnerable to radiation.
Conclusion
We have found here that lithium stimulates NSPC proliferation and differentiation following cranial irradiation while also reducing oligodendrocyte loss in the DG of juvenile mice. Importantly our data have served as a trigger for a phase I clinical trial in lung cancer patients who are receiving whole-brain prophylactic radiotherapy to prevent the occurrence of cranial metastases. We thus believe the beneficial cellular and molecular changes generated using lithium as described here add hope that patients receiving craniotherapy during the course of cancer management might receive improved neurogenic recovery post-treatment.
Acknowledgments
We thank Dr. A. Peace for performing serum lithium testing in mice. This work was funded in part by the National Health and Medical Research Council of Australia and Cure Cancer Australia. R.G.R., J.M., and M.E. were supported by the National Health and Medical Research Council funding. J.M. was supported in part by Cancer Australia/Cure Cancer Australia, and D.D. was supported by the Cooperative Research Centre for Biomedical Imaging Development.
Author Contributions
J.M.: manuscript writing, conception and design, collection and/or assembly of data, data analysis and interpretation, financial support, final approval of manuscript; C.S.M., D.D., E.L., and J.H.: collection and/or assembly of data, data analysis and interpretation; S.L.: collection and/or assembly of data; K.S.: provision of study material and manuscript writing; M.E.: conception and design, provision of study material; D.M.A. and G.W.: manuscript writing, conception and design, final approval of manuscript; J.L.S.: manuscript writing, conception and design, collection and/or assembly of data, data analysis and interpretation, final approval of manuscript; R.G.R.: manuscript writing, conception and design, financial support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Hertzberg H, Huk WJ, Ueberall MA, et al. CNS late effects after ALL therapy in childhood. Part I: Neuroradiological findings in long-term survivors of childhood ALL—An evaluation of the interferences between morphology and neuropsychological performance. The German Late Effects Working Group. Med Pediatr Oncol. 1997;28:387–400. doi: 10.1002/(sici)1096-911x(199706)28:6<387::aid-mpo1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 2.Riva D, Giorgi C. The neurodevelopmental price of survival in children with malignant brain tumours. Childs Nerv Syst. 2000;16:751–754. doi: 10.1007/s003810000332. [DOI] [PubMed] [Google Scholar]
- 3.Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: Current knowledge and future directions. Int J Radiat Oncol Biol Phys. 1995;31:983–998. doi: 10.1016/0360-3016(94)00550-8. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez-Buylla A, Lim DA. For the long run: Maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 5.Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eriksson PS, Perfilieva E, Bjork-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 7.Monje ML, Vogel H, Masek M, et al. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol. 2007;62:515–520. doi: 10.1002/ana.21214. [DOI] [PubMed] [Google Scholar]
- 8.Monje ML, Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16:129–134. doi: 10.1097/01.wco.0000063772.81810.b7. [DOI] [PubMed] [Google Scholar]
- 9.Fountoulakis KN, Vieta E, Sanchez-Moreno J, et al. Treatment guidelines for bipolar disorder: A critical review. J Affect Disord. 2005;86:1–10. doi: 10.1016/j.jad.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Rowe MK, Chuang DM. Lithium neuroprotection: Molecular mechanisms and clinical implications. Expert Rev Mol Med. 2004;6:1–18. doi: 10.1017/S1462399404008385. [DOI] [PubMed] [Google Scholar]
- 11.Yazlovitskaya EM, Edwards E, Thotala D, et al. Lithium treatment prevents neurocognitive deficit resulting from cranial irradiation. Cancer Res. 2006;66:11179–11186. doi: 10.1158/0008-5472.CAN-06-2740. [DOI] [PubMed] [Google Scholar]
- 12.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 13.Sanda T, Kuwano T, Nakao S, et al. Antimyeloma effects of a novel synthetic retinoid Am80 (tamibarotene) through inhibition of angiogenesis. Leukemia. 2005;19:901–909. doi: 10.1038/sj.leu.2403754. [DOI] [PubMed] [Google Scholar]
- 14.Matsushita H, Hijioka M, Hisatsune A, et al. A retinoic acid receptor agonist Am80 rescues neurons, attenuates inflammatory reactions, and improves behavioral recovery after intracerebral hemorrhage in mice. J Cereb Blood Flow Metab. 2011;31:222–234. doi: 10.1038/jcbfm.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukuda A, Fukuda H, Swanpalmer J, et al. Age-dependent sensitivity of the developing brain to irradiation is correlated with the number and vulnerability of progenitor cells. J Neurochem. 2005;92:569–584. doi: 10.1111/j.1471-4159.2004.02894.x. [DOI] [PubMed] [Google Scholar]
- 16.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 17.Shudo K, Kagechika H, Yamazaki N, et al. A synthetic retinoid Am80 (tamibarotene) rescues the memory deficit caused by scopolamine in a passive avoidance paradigm. Biol Pharm Bull. 2004;27:1887–1889. doi: 10.1248/bpb.27.1887. [DOI] [PubMed] [Google Scholar]
- 18.Shi Y, Chichung Lie D, Taupin P, et al. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature. 2004;427:78–83. doi: 10.1038/nature02211. [DOI] [PubMed] [Google Scholar]
- 19.Vorhees CV, Williams MT. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malaterre J, Mantamadiotis T, Dworkin S, et al. c-Myb is required for neural progenitor cell proliferation and maintenance of the neural stem cell niche in adult brain. Stem Cells. 2008;26:173–181. doi: 10.1634/stemcells.2007-0293. [DOI] [PubMed] [Google Scholar]
- 21.Kawahara K, Nishi K, Suenobu M, et al. Oral administration of synthetic retinoid Am80 (tamibarotene) decreases brain beta-amyloid peptides in APP23 mice. Biol Pharm Bull. 2009;32:1307–1309. doi: 10.1248/bpb.32.1307. [DOI] [PubMed] [Google Scholar]
- 22.Hwang SY, Jung JS, Kim TH, et al. Ionizing radiation induces astrocyte gliosis through microglia activation. Neurobiol Dis. 2006;21:457–467. doi: 10.1016/j.nbd.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 23.de Oliveira AC, Candelario-Jalil E, Langbein J, et al. Pharmacological inhibition of Akt and downstream pathways modulates the expression of COX-2 and mPGES-1 in activated microglia. J Neuroinflammation. 2012;9:2. doi: 10.1186/1742-2094-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Couillard-Despres S, Winner B, Schaubeck S, et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- 25.Monje ML, Mizumatsu S, Fike JR, et al. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 26.Bian Q, Shi T, Chuang DM, et al. Lithium reduces ischemia-induced hippocampal CA1 damage and behavioral deficits in gerbils. Brain Res. 2007;1184:270–276. doi: 10.1016/j.brainres.2007.09.054. [DOI] [PubMed] [Google Scholar]
- 27.Yan XB, Hou HL, Wu LM, et al. Lithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemia. Neuropharmacology. 2007;53:487–495. doi: 10.1016/j.neuropharm.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki T, Han F, Shioda N, et al. Lithium-induced activation of Akt and CaM kinase II contributes to its neuroprotective action in a rat microsphere embolism model. Brain Res. 2006;1108:98–106. doi: 10.1016/j.brainres.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Engel T, Goni-Oliver P, Gomez de Barreda E, et al. Lithium, a potential protective drug in Alzheimer's disease. Neurodegener Dis. 2008;5:247–249. doi: 10.1159/000113715. [DOI] [PubMed] [Google Scholar]
- 30.Sarkar S, Krishna G, Imarisio S, et al. A rational mechanism for combination treatment of Huntington's disease using lithium and rapamycin. Hum Mol Genet. 2008;17:170–178. doi: 10.1093/hmg/ddm294. [DOI] [PubMed] [Google Scholar]
- 31.Wei H, Qin ZH, Senatorov VV, et al. Lithium suppresses excitotoxicity-induced striatal lesions in a rat model of Huntington's disease. Neuroscience. 2001;106:603–612. doi: 10.1016/s0306-4522(01)00311-6. [DOI] [PubMed] [Google Scholar]
- 32.Chen G, Rajkowska G, Du F, et al. Enhancement of hippocampal neurogenesis by lithium. J Neurochem. 2000;75:1729–1734. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- 33.Kim JS, Chang MY, Yu IT, et al. Lithium selectively increases neuronal differentiation of hippocampal neural progenitor cells both in vitro and in vivo. J Neurochem. 2004;89:324–336. doi: 10.1046/j.1471-4159.2004.02329.x. [DOI] [PubMed] [Google Scholar]
- 34.Su H, Chu TH, Wu W. Lithium enhances proliferation and neuronal differentiation of neural progenitor cells in vitro and after transplantation into the adult rat spinal cord. Exp Neurol. 2007;206:296–307. doi: 10.1016/j.expneurol.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 35.Wexler EM, Geschwind DH, Palmer TD. Lithium regulates adult hippocampal progenitor development through canonical Wnt pathway activation. Mol Psychiatry. 2008;13:285–292. doi: 10.1038/sj.mp.4002093. [DOI] [PubMed] [Google Scholar]
- 36.Cederberg D, Siesjo P. What has inflammation to do with traumatic brain injury? Childs Nerv Syst. 2010;26:221–226. doi: 10.1007/s00381-009-1029-x. [DOI] [PubMed] [Google Scholar]
- 37.Amor S, Puentes F, Baker D, et al. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia W, Han J, Huang G, et al. Inflammation in ischaemic brain injury: Current advances and future perspectives. Clin Exp Pharmacol Physiol. 2010;37:253–258. doi: 10.1111/j.1440-1681.2009.05279.x. [DOI] [PubMed] [Google Scholar]
- 39.Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics. 2010;7:366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshino JE, DeVries GH. Effect of lithium on Schwann cell proliferation stimulated by axolemma- and myelin-enriched fractions. J Neurochem. 1987;48:1270–1277. doi: 10.1111/j.1471-4159.1987.tb05657.x. [DOI] [PubMed] [Google Scholar]
- 41.Levine S, Saltzman A. Inhibition of experimental allergic encephalomyelitis by lithium chloride: Specific effect or nonspecific stress? Immunopharmacology. 1991;22:207–213. doi: 10.1016/0162-3109(91)90045-z. [DOI] [PubMed] [Google Scholar]
- 42.Fan Y, Liu Z, Weinstein PR, et al. Environmental enrichment enhances neurogenesis and improves functional outcome after cranial irradiation. Eur J Neurosci. 2007;25:38–46. doi: 10.1111/j.1460-9568.2006.05269.x. [DOI] [PubMed] [Google Scholar]
- 43.Rola R, Raber J, Rizk A, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Madsen TM, Kristjansen PE, Bolwig TG, et al. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 45.McIlwain KL, Merriweather MY, Yuva-Paylor LA, et al. The use of behavioral test batteries: Effects of training history. Physiol Behav. 2001;73:705–717. doi: 10.1016/s0031-9384(01)00528-5. [DOI] [PubMed] [Google Scholar]
- 46.West AP. Neurobehavioral studies of forced swimming: The role of learning and memory in the forced swim test. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14:863–877. doi: 10.1016/0278-5846(90)90073-p. [DOI] [PubMed] [Google Scholar]