Abstract
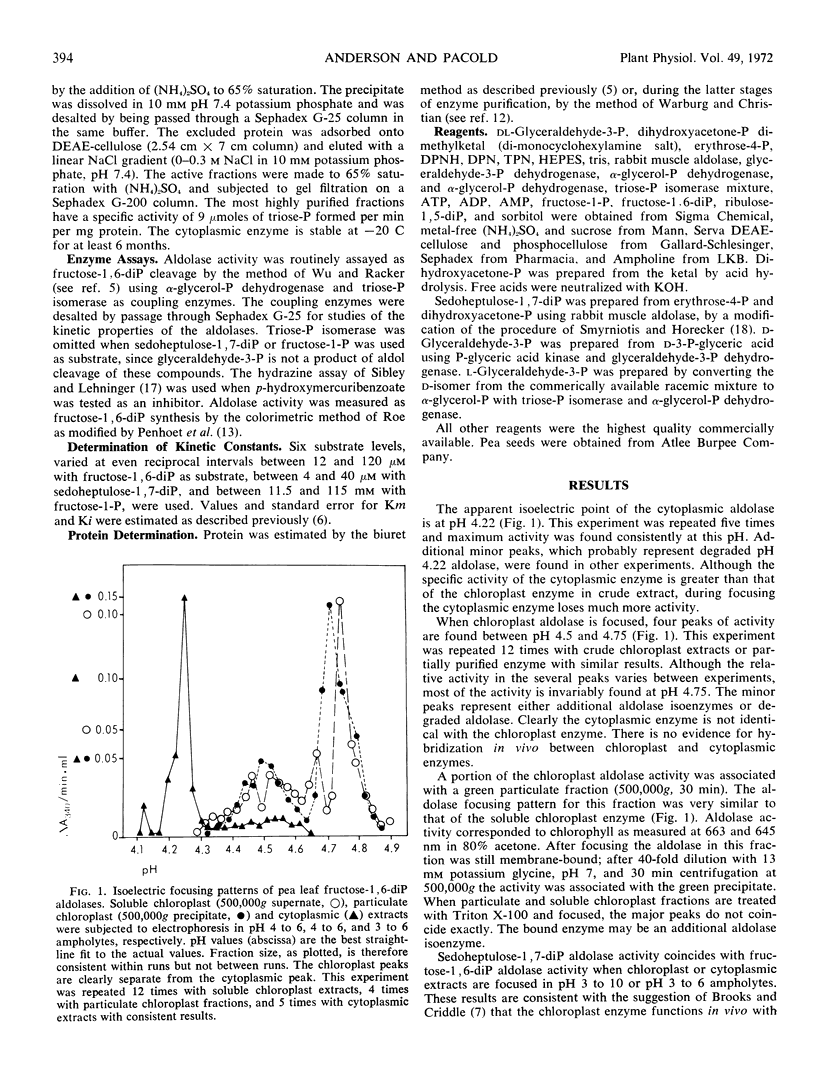
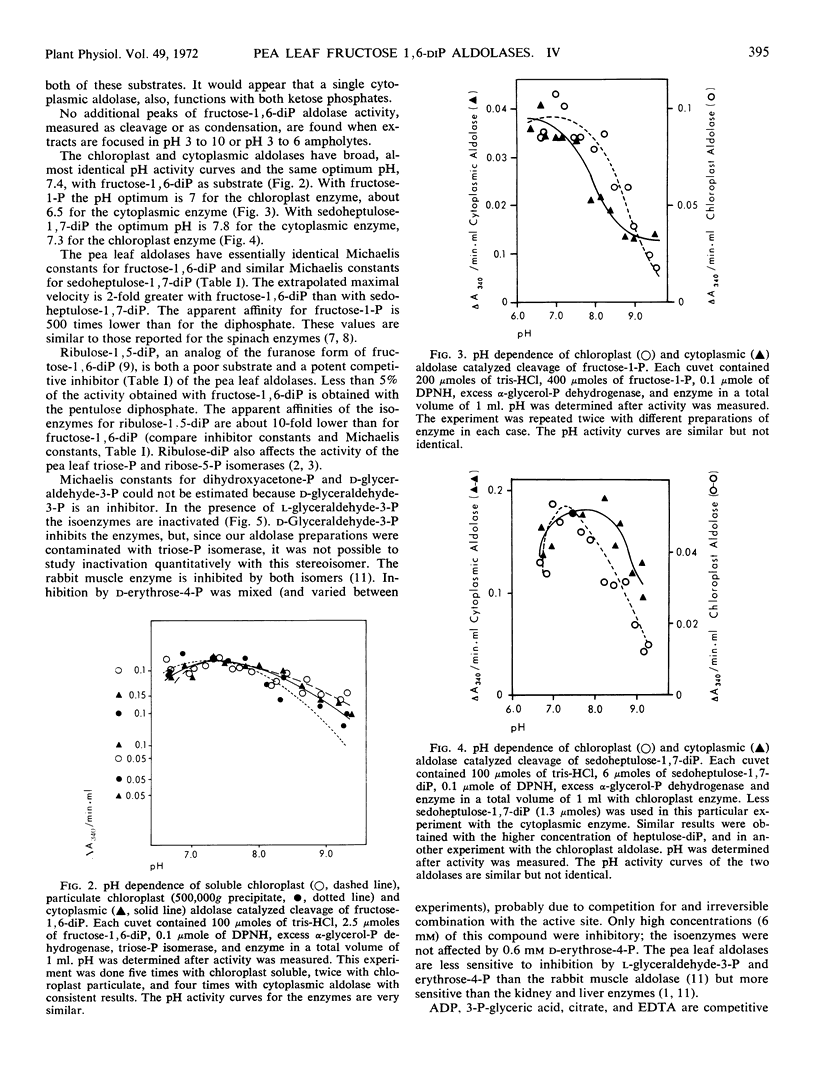
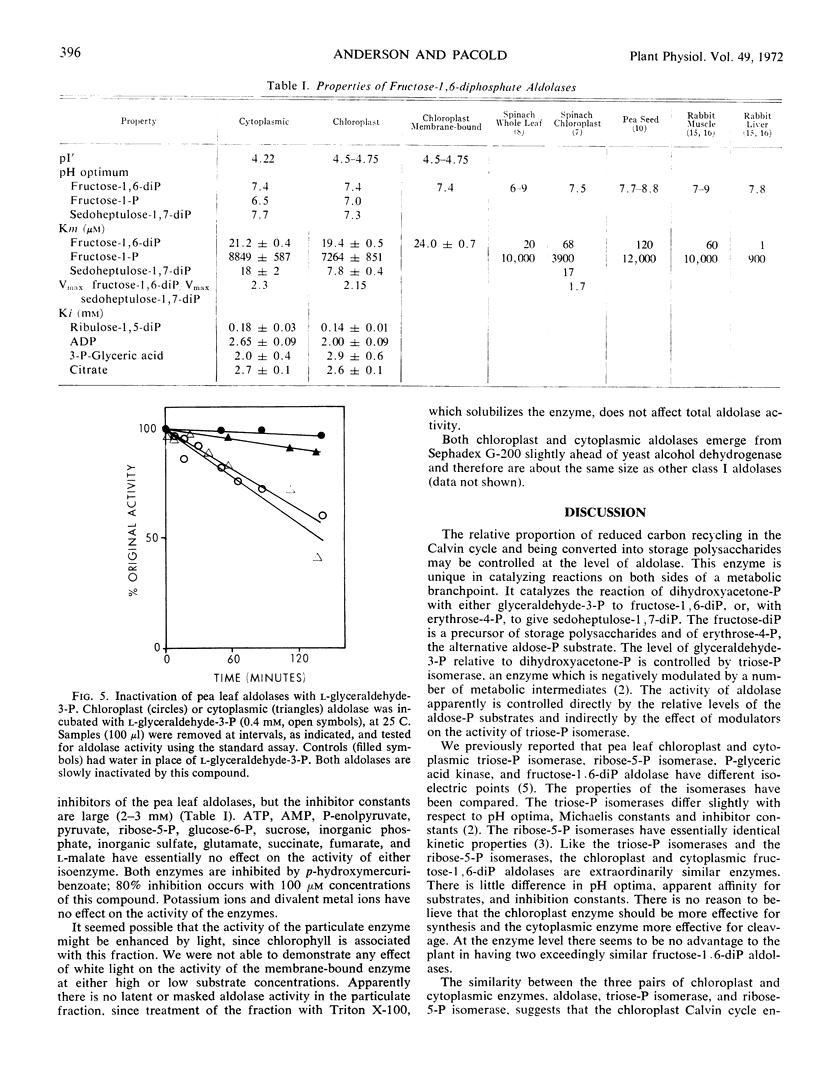
Several peaks of aldolase activity are found in the isoelectric focusing pattern of pea (Pisum sativum) leaf chloroplast extracts. One peak, separated by 0.5 pH unit from the major chloroplast aldolase peak, is found when cytoplasmic extracts are focused. The chloroplast and cytoplasmic enzymes have a pH 7.4 optimum with fructose 1,6-diphosphate. The Michaelis constant for fructose-1,6-diphosphate is 19 μM for the chloroplast, 21 μM for the cytoplasmic enzyme, and for sedoheptulose 1,7-diphosphate, 8 μM for the chloroplast enzyme, 18 μM for the cytoplasmic enzyme. Both enzymes are inhibited by d-glyceraldehyde 3-phosphate and by ribulose 1,5-diphosphate. The similarity in the catalytic properties of the isoenzymes suggests that both enzymes have an amphibolic role in carbon metabolism in the green leaf.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alarcon O., Gonzalez F., Flores H., Marcus F. Isolation of crystalline pig kidney aldolase B. Biochim Biophys Acta. 1971 Feb 10;227(2):460–463. doi: 10.1016/0005-2744(71)90077-5. [DOI] [PubMed] [Google Scholar]
- Anderson L. E., Advani V. R. Chloroplast and cytoplasmic enzymes: three distinct isoenzymes associated with the reductive pentose phosphate cycle. Plant Physiol. 1970 May;45(5):583–585. doi: 10.1104/pp.45.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E. Chloroplast and cytoplasmic enzymes. 3. Pea leaf ribose 5-phosphate isomerases. Biochim Biophys Acta. 1971 Apr 14;235(1):245–249. doi: 10.1016/0005-2744(71)90052-0. [DOI] [PubMed] [Google Scholar]
- Anderson L. E. Chloroplast and cytoplasmic enzymes. II. Pea leaf triose phosphate isomerases. Biochim Biophys Acta. 1971 Apr 14;235(1):237–244. doi: 10.1016/0005-2744(71)90051-9. [DOI] [PubMed] [Google Scholar]
- Anderson L. E., Fuller R. C. Photosynthesis in Rhodospirillum rubrum. IV. Isolation and characterization of ribulose 1,5-diphosphate carboxylase. J Biol Chem. 1969 Jun 25;244(12):3105–3109. [PubMed] [Google Scholar]
- Fluri R., Ramasarma T., Horecker B. L. Purification and properties of fructose diphosphate aldolase from spinach leaves. Eur J Biochem. 1967 Mar;1(1):117–124. doi: 10.1007/978-3-662-25813-2_19. [DOI] [PubMed] [Google Scholar]
- Gray G. R., Barker R. Studies on the substrates of D-fructose 1,6-diphosphate aldolase in solution. Biochemistry. 1970 Jun 9;9(12):2454–2462. doi: 10.1021/bi00814a010. [DOI] [PubMed] [Google Scholar]
- Hatz C., Leuthardt F. Isolation and characterization of fructose diphosphate aldolase from Pisum sativum. Biochim Biophys Acta. 1967 Jul 11;139(2):460–468. doi: 10.1016/0005-2744(67)90049-6. [DOI] [PubMed] [Google Scholar]
- Lai C. Y., Martinez-de Dretz G., Bacila M., Marinello E., Horecker B. L. Labeling of the active site of aldolase with glyceraldehyde 3-phosphate and erythrose 4-phosphate. Biochem Biophys Res Commun. 1968 Mar 27;30(6):665–672. doi: 10.1016/0006-291x(68)90564-0. [DOI] [PubMed] [Google Scholar]
- Penhoet E. E., Kochman M., Rutter W. J. Molecular and catalytic properties of aldolase C. Biochemistry. 1969 Nov;8(11):4396–4402. doi: 10.1021/bi00839a026. [DOI] [PubMed] [Google Scholar]
- Rapoport G., Davis L., Horecker B. L. The subunit structure of the fructose diphosphate aldolase from spinach leaf. Arch Biochem Biophys. 1969 Jun;132(1):286–293. doi: 10.1016/0003-9861(69)90364-6. [DOI] [PubMed] [Google Scholar]
- Rutter W. J., Rajkumar T., Penhoet E., Kochman M., Valentine R. Aldolase variants: structure and physiological significance. Ann N Y Acad Sci. 1968 Jun 14;151(1):102–117. doi: 10.1111/j.1749-6632.1968.tb11881.x. [DOI] [PubMed] [Google Scholar]
- SMYRNIOTIS P. Z., HORECKER B. L. The preparation of sedoheptulose diphosphate. J Biol Chem. 1956 Feb;218(2):745–752. [PubMed] [Google Scholar]
- Willard J. M., Gibbs M. Role of Aldolase in Photosynthesis. II Demonstration of Aldolase Types in Photosynthetic Organisms. Plant Physiol. 1968 May;43(5):793–798. doi: 10.1104/pp.43.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]



