Developing a durable material with the potential to function with child growth will eliminate the need for reoperation and significantly reduce morbidity and mortality in some types of congenital heart defects. Tissue-engineered vascular grafts offer the ability to drastically improve morbidity and mortality and drastically improve the quality of life of patients after congenital heart defect surgery.
Keywords: Tissue engineering, Stem cells, Cardiac surgery, Congenital heart defects
Abstract
In surgical repair for heart or vascular disease, it is often necessary to implant conduits or correct tissue defects. The most commonly used graft materials to date are (a) artificial grafts; (b) autologous tissues, such as pericardium and saphenous vein; (c) allografts; and (d) xenografts. However, none of these four options offer growth potential, and all are associated with varying levels of thrombogenicity and susceptibility to infection. The lack of growth potential of these four options is particularly important in pediatric cardiac surgery, where patients will often outgrow their vascular grafts and require additional operations. Thus, developing a material with sufficient durability and growth potential that will function as the child grows older will eliminate the need for reoperation and significantly reduce morbidity and mortality of some types of congenital heart defects. Vascular tissue engineering is a relatively new field that has undergone enormous growth over the last decade. The goal of vascular tissue engineering is to produce neovessels and neo-organ tissue from autologous cells using a biodegradable polymer as a scaffold. The most important advantage of tissue-engineered implants is that these tissues can grow, remodel, rebuild, and respond to injury. Once the seeded autologous cells have deposited an extracellular matrix and the original scaffold is biodegraded, the tissue resembles and behaves as native tissue. When tissue-engineered vascular grafts are eventually put to use in the clinical arena, the quality of life in patients after surgery will be drastically improved.
Introduction
It is common in cardiovascular surgery for surgeons to encounter patients with defects or altered anatomy that requires use of biocompatible materials for repair [1–3]. The need for prosthetic materials is particularly important in congenital heart disease (CHD). Pediatric cardiac disease remains a significant health problem and affects nearly 1% of all live births. Without surgical treatment, many congenital heart defects can lead to cyanosis, volume overload, and congestive heart failure. At the present time, the most common type of material used to repair congenital heart defects is biocompatible synthetic polymers, such as Dacron (E.I. du Pont de Nemours and Company, Wilmington, DE, http://www.dupont.com) or Gore-Tex (W.L. Gore & Associates, Inc., Newark, DE, http://www.goremedical.com). However, these materials are associated with numerous short- and long-term complications, including stenosis, thromboembolization, calcium deposition, and infection [4–6]. Perhaps most importantly for pediatric cardiothoracic applications, these materials lack growth potential. Therefore, it is common for patients to outgrow their grafts, thus requiring reoperation, which is associated with significantly higher rates of mortality and mobility than first-time sternotomies [7, 8]. Early and midterm results for implanted grafts composed of synthetic materials are variable, with published 5-year patency rates between 65% and 90% in pediatric cardiac surgery; long-term follow-up demonstrated graft failure rates between 70% and 100% at 10–15 years [9]. Thus, despite significant advances in surgical techniques for CHD which have occurred over the last 30 years, finding an ideal vascular conduit remains a challenge.
Commonly used graft materials to date include (a) artificial grafts (e.g., expanded polytetrafluoroethylene [ePTFE; Gore-Tex], polytetrafluoroethylene [Teflon; E.I. du Pont de Nemours and Company], or polyethylene terephthalate [Dacron]), (b) autologous tissue (e.g., pericardium or saphenous vein), (c) allografts, and (d) xenografts.
Biologic grafts, like autologous tissues, allografts, and xenografts, are advantageous because of decreased rates of thromboembolic events, but they have been observed to have shorter durability because of calcific degradation and secondary graft failure compared with artificial grafts [10–12]. Addressing the limitations of currently used graft materials would lead to a reduction in morbidity and mortality of children born with congenital heart disease and a concomitant improvement in their postoperative quality of life. The ideal vascular conduit would have the following attributes: handling characteristics adequate for surgical implantation, low rates of thromboembolic events, resistance to infection, and growth potential. One method for creating the ideal vascular conduit is to bioengineer tissue using pluripotent or multipotent stem cells, thereby creating a tissue-engineered vascular graft (TEVG).
In 1998, we reported surgical implantation of TEVGs in lambs, in which scaffolds were constructed with autologous myofibroblasts and endothelial cells that were seeded in vitro onto a synthetic biodegradable graft made from polyglycolic acid (PGA) fiber. This study was the first to demonstrate the creation of a vascular conduit using autologous cells that ultimately yielded a viable structure [13]. This study was an important breakthrough in the nascent field of vascular tissue engineering. Noishiki et al. performed a similar experiment in which bone marrow-derived cells were seeded onto an ePTFE scaffold and implanted in a canine aorta, with adequate endothelialization noted [14]. In 2003, we published a study of seeding bone marrow cells (BMCs) onto biodegradable scaffolds as canine inferior vena cava (IVC) interposition grafts with subsequent development of neovessel [15]. This study demonstrated the safety and feasibility of seeding BMCs onto biodegradable scaffolds, which were then used as preclinical data for the first human clinical trial conducted in Japan investigating TEVG use in children with congenital heart defects. Since then, we have worked to optimize our tissue-engineering techniques, with the goal being further translation from the bench to the bedside [16].
Scaffold Materials
The scaffold material used for TEVG creation should be biodegradable and nonimmunogenic, and it must have a porosity and microstructure that allow for cell attachment. Polymers of PGA, polylactic acid, and poly ε-caprolactone are the three most commonly used materials for scaffolds in vascular tissue engineering [17]. Selection of scaffold material depends on a variety of factors, including biocompatibility, biomechanical properties, and rate of biodegradation. Choosing a scaffold material is a critical step in designing successful constructs for vascular engineering.
Decellularized biologic materials have also been used with some success. Groups have decellularized human and porcine vessels and subsequently seeded the cell-less scaffold with autologous cells [18]. One drawback of this technique is risk of provoking an immunologic response with xenotransplantation of decellularized matrices. Additionally, the chemicals used to decellularize the biologic vessel can affect the material properties of the scaffold.
Seeding and Cells
Various techniques for seeding of cells to create TEVGs have been developed, including static, dynamic, magnetic, vacuum, electrostatic, and centrifugal seeding [19]. Traditional cell seeding involves manual pipetting of cells directly onto a graft and is known as static cell seeding [19]. Vacuum seeding in a specially designed chamber allows for rapid, operator-independent, and self-contained cell seeding; this technique is efficient and promises improved standardization of cell seeding [20].
When designing a TEVG, the decision of what type of cell to use is crucial and important for long-term success. Cell seeding of scaffolds is important, because seeding promotes development of neotissue and leads to long-term graft patency [21–23].
So far, a variety of cell types have been investigated as cell sources for TEVG [24, 25]. The earliest published tissue-engineered vascular grafts were composed of autologous endothelial and smooth muscle cells seeded onto a scaffold. Results were promising, with good patency rates [26]. However, harvesting of endothelial and smooth muscle cells is invasive and their use requires long-term incubation, thus increasing the risk of contamination and precluding its use in the clinical arena.
For a cell source, we use bone marrow-derived mononuclear cells (BM-MNCs). The traditional approach for isolating MNCs involves Ficoll centrifugation, which separates cells on the basis of mass. Recently, a specially designed filter was developed that separates cells on the basis of size and offers the ability to perform MNC isolation using a closed system [20, 27]. Using flow cytometry analysis, we analyzed a cell subpopulation of MNCs. Endothelial precursor cells (EPCs) made up 0.0004% of total MNCs, and mesenchymal stem cells made up 0.001% of total MNCs [27]. Peripheral blood-derived MNCs (p-MNCs) have also been used in vascular tissue engineering [28], but we prefer BM-MNCs because of a higher proportion of EPCs in BM-MNCs than in p-MNCs.
Recently, embryonic stem (ES) cells and induced pluripotent stem (iPS) cells have garnered enthusiasm as cell sources for vascular tissue engineering because of their use and success in regenerative medicine [29–32]. Whereas ES and iPS cells have only recently been used clinically, initial results have been hampered by reports of teratoma and teratocarcinoma formation [33–35]. Thus, more studies are needed before ES and iPS cells are used in the seeding of TEVGs.
In 2001, we began the first study in humans investigating the use of TEVGs in congenital heart surgery in Japan after approval by the ethics committee at Tokyo Women's Medical University [6, 36–38]. The tissue-engineered vascular grafts were composed of a woven fabric of PGA and ε-caprolactone or l-lactide and were seeded with autologous BM-MNCs. The following inclusion criteria were used for patient selection: (a) elective surgery, (b) age younger than 30 years, (c) full understanding of the risks and benefits of the procedure by the patient or parent(s), and (d) minimal extracardiac disease burden.
Informed consent was obtained from the patient or parent(s) before proceeding. Between September 2001 and December 2004, 25 patients underwent an extracardiac total cavopulmonary connection (EC-TCPC) with TEVG used as a conduit (Table 1). Mean patient age at the time of TEVG implantation was 5.5 years, and average patient body weight was 19.5 kg. Anticoagulation and antiplatelet therapy with warfarin and aspirin was started 2 days postoperatively and continued for 3–6 months. Patients were followed postoperatively in a multidisciplinary clinic. Graft patency and cardiac function were monitored with transthoracic echocardiography, multislice computed tomography (CT), angiography, or magnetic resonance imaging (MRI) angiography.
Table 1.
Patient status after tissue-engineered vascular graft implantation
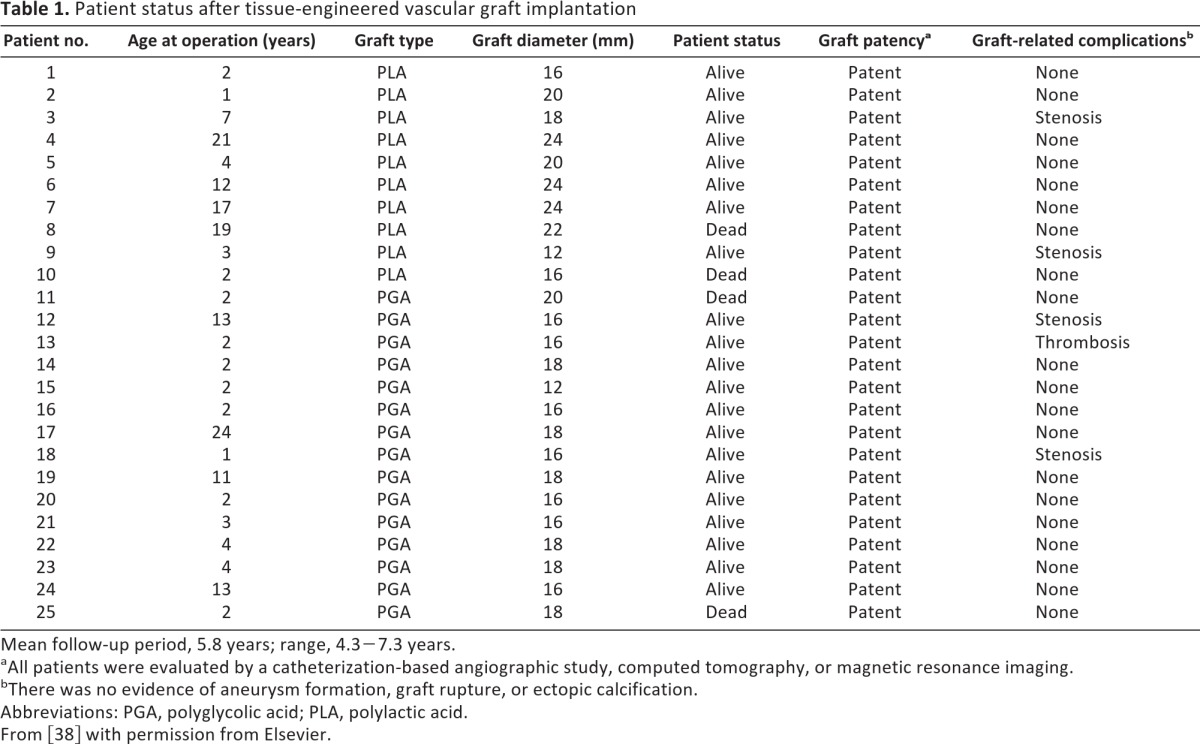
Mean follow-up period, 5.8 years; range, 4.3−7.3 years.
aAll patients were evaluated by a catheterization-based angiographic study, computed tomography, or magnetic resonance imaging.
bThere was no evidence of aneurysm formation, graft rupture, or ectopic calcification.
Abbreviations: PGA, polyglycolic acid; PLA, polylactic acid.
From [38] with permission from Elsevier.
At 1 month post-TEVG implantation, all patients were alive and symptom-free. Imaging at this time point with angiography, ultrasonography, or CT demonstrated 100% TEVG patency with no evidence of stenosis, thrombosis, or aneurysmal dilatation.
Midterm results at 1 year after implantation were notable for one patient who was diagnosed on routine radiography with partial mural thrombosis and was subsequently treated with warfarin. One patient with hypoplastic left heart syndrome died 6 months after TEVG implantation of congestive heart failure resulting from severe tricuspid regurgitation; at autopsy, the TEVG was noted to be intact.
Long-term results at 4 years after implantation demonstrated no instances of graft-related mortality. Additionally, no cases of aneurysm formation, graft rupture, or ectopic calcification have been identified using routine radiographic TEVG surveillance. An additional three patients died after TEVG implantation at long-term follow-up (total: four deaths postimplantation). Importantly, surveillance imaging was performed on all four patients who died in the months before expiration—radiographic studies in each patient demonstrated a patent TEVG. Six patients (24%) had asymptomatic graft narrowing noted on routine surveillance imaging. Four of those six patients underwent successful balloon angioplasty, including one patient who required repeat balloon angioplasty and stent placement in the stenosed segment of the TEVG.
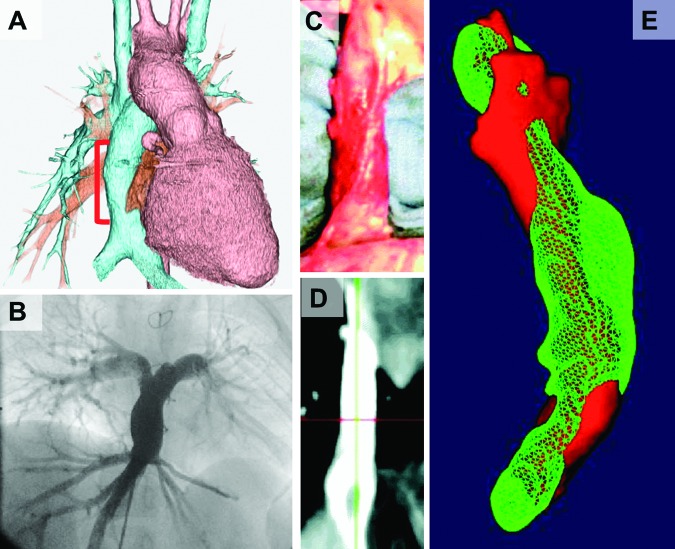
This first human clinical trial clarified that our TEVG functioned well without aneurismal change or graft rupture. Three important observations from that study are as follows. First, asymptomatic graft stenosis developed in six patients with smaller diameter (<18 mm) conduits (four of whom required and were successfully treated with angioplasty and/or stenting). Second, there were no reported thromboembolic, hemorrhagic, or infectious complications; no grafts required explantation or replacement. Third, there was significant growth of the TEVG by serial imaging (Fig. 1A, 1B). These data support the overall feasibility and safety of this technology.
Figure 1.
Imaging results after transplantation in human and in sheep. (A, B): Three-dimensional (3D) computed tomography angiogram (A) and angiography (B) of tissue-engineered vascular graft (TEVG) seeded with bone marrow-derived mononuclear cells as an extracardiac total cavopulmonary connection conduit after implantation in clinical trial. There was no stenosis, aneurysmal dilatation, or ectopic calcification noted. (C, D): Ex vivo photo (C) and two-dimensional image with magnetic resonance angiography (D) of TEVG at 6 months after inferior vena cava implantation in sheep. (E): 3D-rendered image of the outside surface of TEVG at 1 month (red) and 6 months (green) after rigid alignment of the surfaces. Mean TEVG luminal volume growth was 126.9 ± 9.9% over 6 months compared with mean growth for the right pulmonary artery over the same period, being 140 ± 12%. From [40] with permission from Wolters Kluwer Health.
Despite the overall success of the human clinical study performed in Japan investigating TEVG, we deemed it necessary to further investigate and delineate the mechanisms that govern transition from a seeded scaffold to a neovessel, with the goal being to ultimately modulate TEVG remodeling to prevent stenosis. Thus, we pivoted and went from the clinic back to the bench.
To evaluate both the technique of seeding BM-MNCs onto a PGA scaffold and the growth potential of these TEVGs, we used a large-animal (ovine) model, in which we implanted TEVGs as IVC interposition grafts [39]. All implanted TEVGs at 6 months were patent and increased in luminal volume as measured by difference in pixel summation in magnetic resonance angiography at 1 month and 6 months. The volume of seeded TEVGs at 6 months averaged 126.9 ± 9.9% of their volume at 1 month (Fig. 1C–1E). MRI demonstrated no evidence of aneurysmal change. These TEVGs resembled the native IVC histologically and had comparable amounts of collagen (157.9 ± 26.4 μg/mg), elastin (186.9 ± 16.7 μg/mg), and glycosaminoglycan (9.7 ± 0.8 μg/mg) to the native vein. Immunohistochemical staining and Western blot analysis demonstrated that Ephrin-B4, a marker of normal venous development, was observed at 6 months after implantation. These results reiterated that TEVGs seeded with BM-MNCs had the ability to grow and form venous neotissue without major complications.
To further understand and characterize our model for TEVGs, we developed a unique mouse model, whereby TEVGs with a luminal diameter of <1 mm are implanted as abdominal IVC and aortic interposition grafts [40, 41]. Below are some observations from our mouse studies [42].
BM-MNC Seeded onto TEVGs Improve Graft Patency by Decreasing Rate and Degree of Neointimal Hyperplasia
Using serial ultrasound and micro-CT angiography, we were able to demonstrate that seeded TEVGs had higher patency rates than unseeded TEVGs. TEVG occlusion in unseeded grafts developed gradually, as demonstrated by the formation of collateral blood vessels. Histological evaluation of the TEVGs demonstrated that the mechanism of graft failure was stenosis [42].
After Implantation, There Is Rapid Loss of Seeded Cells Within TEVGs, with <1% of Seeded Cells Present at 1 Week Postsurgery
There are few reports that describe the role of seeded cells after TEVG implantation. We reported data in 2010 that suggested that seeded cells do not directly contribute to the cellularity of developing TEVGs [43]. We tracked seeded cells in TEVG after implantation and found that the number of seeded cells within the graft decreased dramatically in the first few days after implantation. By 1 week postimplantation, nearly all the originally seeded cells were absent from the TEVG. These results imply that seeded cells play a significant role in promoting cellular migration and ingrowth from circulating cells and adjacent cells; however, seeded cells do not differentiate and populate the neovessel, as outlined in the traditional tissue-engineering paradigm. As such, more work regarding the role of seeded cells must be done to clarify their role in transition from TEVGs to new vessels.
Inflammation-Mediated Processes by Macrophage/Mononuclear Cells Are Crucial for Neovessel Formation and Affect Development of Stenosis
In order to assess the role of macrophages in TEVGs, we used liposomal clodronate, a compound that depletes macrophages. We found that macrophages are critical for TEVG patency and long-term function after implantation. However, excessive macrophage infiltration leads to neointimal hyperplasia and, thereby, a stenotic or occluded graft. Conversely, complete inhibition of macrophage infiltration using liposomal clodronate blocks neotissue formation and inhibits vascular repair [43, 44].
Thus, from our studies, we have developed a framework by which we believe seeded scaffolds transform into neovessels, which is as follows. After surgical implantation of a seeded scaffold, BM-MNCs release chemokines, such as monocyte chemoattractant protein-1, which attract circulating monocytes into the scaffold, where they ultimately differentiate into macrophages. These macrophages then release a variety of chemokines, cytokines, and growth factors, such as platelet-derived growth factor and vascular endothelial growth factor. Macrophage-derived signaling molecules induce ingrowth and migration of adjacent smooth muscle cells and endothelial cells. Macrophages, fibroblasts, and smooth muscle cells then begin extracellular matrix (ECM) deposition, whereas scaffold degradation occurs simultaneously. The ECM remodels as scaffold degradation continues. At some point, the biomechanical properties of the neovessel are no longer determined by scaffold characteristics and instead are related to collagen and elastin content [37]. Finally, the seeded TEVG has completely transformed into a neovessel with cellular, biomechanical, and physiologic characteristics similar to that of a native vessel.
In August 2011, we began the first U.S. Food and Drug Administration-approved human clinical trial in the U.S. investigating TEVG use in children with congenital heart defects [45, 46]. This trial is being performed at Yale University and is titled “A Pilot Study Investigating the Clinical Use of Tissue Engineered Vascular Grafts in Congenital Heart Surgery. ” The inclusion criteria used for patient selection are as follows: (a) patients who are candidates for EC-TCPC for completion of the modified Fontan procedure, (b) elective surgery, (c) age younger than 30 years, (d) full understanding of the risks and benefits of the procedure by the patient or parent(s)/legal guardian, and (e) minimal extracardiac disease burden.
Notable exclusion criteria include need for graft diameter <18 mm or >24 mm, the presence of pacemaker, pulmonary vascular resistance >4 um2 (u = Wood's units), and the presence of moderate-to-severe atrioventricular valve regurgitation. At the time of manuscript submission, one TEVG implantation has been performed. At 6 months after implantation, the TEVG is functioning well with no radiographic evidence of aneurysmal change. Most importantly, the patient is doing well and is asymptomatic.
As we have outlined in this review, the field of vascular tissue engineering has undergone enormous growth over the last decade. Much of the focus in vascular tissue engineering and in regenerative medicine has been on optimizing cell type, cellular function, and scaffold materials. Further understanding of cellular processes, such as isolating the chemokines that BM-MNCs release to promote adjacent cellular migration and ingrowth, can allow for a second-generation TEVG to be engineered, which improves on initial successes [44]. Over the coming years, a variety of emerging biotechnological techniques will be refined and will lead to further growth in this field. New generation scaffolds may be seeded with nanoparticles containing cytokines, chemokines, or cell clusters; scaffolds may be custom-made using electrospinning; stenosis may be prevented by targeted release of molecular modulators of tissue ingrowth; and neovessel formation may be accelerated by implantation of proangiogenic factors within the scaffold, which are released as scaffolds undergo biodegradation [47]. Over the coming years, it is also likely that further refinement of BM-MNC isolation will occur, allowing for more optimal cell seeding of TEVG. Moreover, advances in use of iPS and ES cells in other subfields of regenerative medicine will certainly benefit vascular tissue engineering. Finally, it is inevitable that TEVGs will be used in more clinical applications, such as arteriovenous grafts, coronary artery bypass grafts, and peripheral artery bypass grafts.
Conclusion
The field of vascular tissue engineering has found a successful clinical application in pediatric cardiothoracic surgery, where use of TEVG offers the ability to drastically improve morbidity and mortality after surgery for congenital heart defects. Further study will allow for optimization of TEVG development and will expand opportunities for clinical application.
Acknowledgments
We have received grant support from Gunze Ltd. and Pall Corp.
Author Contributions
H.K.: conception and design, manuscript writing; M.W.M.: manuscript writing; C.K.B. and T.S.: final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Petrossian E, Reddy VM, Collins KK, et al. The extracardiac conduit Fontan operation using minimal approach extracorporeal circulation: Early and midterm outcomes. J Thorac Cardiovasc Surg. 2006;132:1054–1063. doi: 10.1016/j.jtcvs.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Lin P, Yao Q, et al. Development of small-diameter vascular grafts. World J Surg. 2007;31:682–689. doi: 10.1007/s00268-006-0731-z. [DOI] [PubMed] [Google Scholar]
- 3.Twine CP, McLain AD. Graft type for femoro-popliteal bypass surgery. Cochrane Database Syst Rev. 2010:CD001487. doi: 10.1002/14651858.CD001487.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Giannico S, Hammad F, Amodeo A, et al. Clinical outcome of 193 extracardiac Fontan patients: The first 15 years. J Am Coll Cardiol. 2006;47:2065–2073. doi: 10.1016/j.jacc.2005.12.065. [DOI] [PubMed] [Google Scholar]
- 5.Petrossian E, Reddy VM, McElhinney DB, et al. Early results of the extracardiac conduit Fontan operation. J Thorac Cardiovasc Surg. 1999;117:688–696. doi: 10.1016/S0022-5223(99)70288-6. [DOI] [PubMed] [Google Scholar]
- 6.Shinoka T, Breuer C. Tissue-engineered blood vessels in pediatric cardiac surgery. Yale J Biol Med. 2008;81:161–166. [PMC free article] [PubMed] [Google Scholar]
- 7.Dearani JA, Danielson GK, Puga FJ, et al. Late follow-up of 1095 patients undergoing operation for complex congenital heart disease utilizing pulmonary ventricle to pulmonary artery conduits. Ann Thorac Surg. 2003;75:399–410. doi: 10.1016/s0003-4975(02)04547-2. discussion 410–391. [DOI] [PubMed] [Google Scholar]
- 8.Holst KA, Dearani JA, Burkhart HM, et al. Risk factors and early outcomes of multiple reoperations in adults with congenital heart disease. Ann Thorac Surg. 2011;92:122–128. doi: 10.1016/j.athoracsur.2011.03.102. discussion 129–130. [DOI] [PubMed] [Google Scholar]
- 9.Homann M, Haehnel JC, Mendler N, et al. Reconstruction of the RVOT with valved biological conduits: 25 years experience with allografts and xenografts. Eur J Cardiothorac Surg. 2000;17:624–630. doi: 10.1016/s1010-7940(00)00414-0. [DOI] [PubMed] [Google Scholar]
- 10.Stark J. The use of valved conduits in pediatric cardiac surgery. Pediatr Cardiol. 1998;19:282–288. doi: 10.1007/s002469900311. [DOI] [PubMed] [Google Scholar]
- 11.Cleveland DC, Williams WG, Razzouk AJ, et al. Failure of cryopreserved homograft valved conduits in the pulmonary circulation. Circulation. 1992;86:II150–II153. [PubMed] [Google Scholar]
- 12.Jonas RA, Freed MD, Mayer JE, Jr., et al. Long-term follow-up of patients with synthetic right heart conduits. Circulation. 1985;72:II77–II83. [PubMed] [Google Scholar]
- 13.Shinoka T, Shum-Tim D, Ma PX, et al. Creation of viable pulmonary artery autografts through tissue engineering. J Thorac Cardiovasc Surg. 1998;115:536–545. doi: 10.1016/S0022-5223(98)70315-0. discussion 545–536. [DOI] [PubMed] [Google Scholar]
- 14.Noishiki Y, Tomizawa Y, Yamane Y, et al. Autocrine angiogenic vascular prosthesis with bone marrow transplantation. Nat Med. 1996;2:90–93. doi: 10.1038/nm0196-90. [DOI] [PubMed] [Google Scholar]
- 15.Matsumura G, Miyagawa-Tomita S, Shin'oka T, et al. First evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. Circulation. 2003;108:1729–1734. doi: 10.1161/01.CIR.0000092165.32213.61. [DOI] [PubMed] [Google Scholar]
- 16.Shinoka T, Imai Y, Matsumura G. Current status of tissue engineering for therapeutic use [in Japanese] Nihon Rinsho. 2001;59:1389–1399. [PubMed] [Google Scholar]
- 17.Lee J, Cuddihy MJ, Kotov NA. Three-dimensional cell culture matrices: State of the art. Tissue Eng Part B Rev. 2008;14:61–86. doi: 10.1089/teb.2007.0150. [DOI] [PubMed] [Google Scholar]
- 18.Quint C, Kondo Y, Manson RJ, et al. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc Natl Acad Sci USA. 2011;108:9214–9219. doi: 10.1073/pnas.1019506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villalona GA, Udelsman B, Duncan DR, et al. Cell-seeding techniques in vascular tissue engineering. Tissue Eng Part B Rev. 2010;16:341–350. doi: 10.1089/ten.teb.2009.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Udelsman B, Hibino N, Villalona GA, et al. Development of an operator-independent method for seeding tissue-engineered vascular grafts. Tissue Eng Part C Methods. 2011;17:731–736. doi: 10.1089/ten.tec.2010.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pawlowski KJ, Rittgers SE, Schmidt SP, et al. Endothelial cell seeding of polymeric vascular grafts. Front Biosci. 2004;9:1412–1421. doi: 10.2741/1302. [DOI] [PubMed] [Google Scholar]
- 22.Cho SW, Lim SH, Kim IK, et al. Small-diameter blood vessels engineered with bone marrow-derived cells. Ann Surg. 2005;241:506–515. doi: 10.1097/01.sla.0000154268.12239.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashi CK, Zhu Y, Yang GY, et al. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc Natl Acad Sci USA. 2007;104:11915–11920. doi: 10.1073/pnas.0704581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber B, Emmert MY, Schoenauer R, et al. Tissue engineering on matrix: Future of autologous tissue replacement. Semin Immunopathol. 2011;33:307–315. doi: 10.1007/s00281-011-0258-8. [DOI] [PubMed] [Google Scholar]
- 25.Dahl SL, Kypson AP, Lawson JH, et al. Readily available tissue-engineered vascular grafts. Sci Transl Med. 2011;3:68ra9. doi: 10.1126/scitranslmed.3001426. [DOI] [PubMed] [Google Scholar]
- 26.Rashid ST, Salacinski HJ, Fuller BJ, et al. Engineering of bypass conduits to improve patency. Cell Prolif. 2004;37:351–366. doi: 10.1111/j.1365-2184.2004.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hibino N, Nalbandian A, Devine L, et al. Comparison of human bone marrow mononuclear cell isolation methods for creating tissue-engineered vascular grafts: Novel filter system versus traditional density centrifugation method. Tissue Eng Part C Methods. 2011;17:993–998. doi: 10.1089/ten.tec.2011.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Germani A, Di Campli C, Pompilio G, et al. Regenerative therapy in peripheral artery disease. Cardiovasc Ther. 2009;27:289–304. doi: 10.1111/j.1755-5922.2009.00105.x. [DOI] [PubMed] [Google Scholar]
- 29.Lerou PH, Daley GQ. Therapeutic potential of embryonic stem cells. Blood Rev. 2005;19:321–331. doi: 10.1016/j.blre.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Raya A, Rodriguez-Piza I, Aran B, et al. Generation of cardiomyocytes from new human embryonic stem cell lines derived from poor-quality blastocysts. Cold Spring Harbor Symp Quant Biol. 2008;73:127–135. doi: 10.1101/sqb.2008.73.038. [DOI] [PubMed] [Google Scholar]
- 32.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 33.Pera MF. Stem cells: The dark side of induced pluripotency. Nature. 2011;471:46–47. doi: 10.1038/471046a. [DOI] [PubMed] [Google Scholar]
- 34.Zhao T, Zhang ZN, Rong Z, et al. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 35.Okita K, Nagata N, Yamanaka S. Immunogenicity of induced pluripotent stem cells. Circ Res. 2011;109:720–721. doi: 10.1161/RES.0b013e318232e187. [DOI] [PubMed] [Google Scholar]
- 36.Hibino N, McGillicuddy E, Matsumura G, et al. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg. 2010;139:431–436. 436.e1–2. doi: 10.1016/j.jtcvs.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 37.Naito Y, Shinoka T, Duncan D, et al. Vascular tissue engineering: Towards the next generation vascular grafts. Adv Drug Deliv Rev. 2011;63:312–323. doi: 10.1016/j.addr.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Shin'oka T, Imai Y, Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med. 2001;344:532–533. doi: 10.1056/NEJM200102153440717. [DOI] [PubMed] [Google Scholar]
- 39.Brennan MP, Dardik A, Hibino N, et al. Tissue-engineered vascular grafts demonstrate evidence of growth and development when implanted in a juvenile animal model. Ann Surg. 2008;248:370–377. doi: 10.1097/SLA.0b013e318184dcbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez-Soler RI, Brennan MP, Goyal A, et al. Development of a mouse model for evaluation of small diameter vascular grafts. J Surg Res. 2007;139:1–6. doi: 10.1016/j.jss.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 41.Goyal A, Wang Y, Su H, et al. Development of a model system for preliminary evaluation of tissue-engineered vascular conduits. J Pediatr Surg. 2006;41:787–791. doi: 10.1016/j.jpedsurg.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Breuer CK. The development and translation of the tissue-engineered vascular graft. J Pediatr Surg. 2011;46:8–17. doi: 10.1016/j.jpedsurg.2010.09.058. [DOI] [PubMed] [Google Scholar]
- 43.Roh JD, Sawh-Martinez R, Brennan MP, et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci USA. 2010;107:4669–4674. doi: 10.1073/pnas.0911465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hibino N, Yi T, Duncan DR, et al. A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. FASEB J. 2011;25:4253–4263. doi: 10.1096/fj.11-186585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogel G. Tissue engineering: Mending the youngest hearts. Science. 2011;333:1088–1089. doi: 10.1126/science.333.6046.1088. [DOI] [PubMed] [Google Scholar]
- 46.Dolgin E. Taking tissue engineering to heart. Nat Med. 2011;17:1032–1035. doi: 10.1038/nm0911-1032. [DOI] [PubMed] [Google Scholar]
- 47.Mima Y, Fukumoto S, Koyama H, et al. Enhancement of cell-based therapeutic angiogenesis using a novel type of injectable scaffolds of hydroxyapatite-polymer nanocomposite microspheres. PLoS One. 2012;7:e35199. doi: 10.1371/journal.pone.0035199. [DOI] [PMC free article] [PubMed] [Google Scholar]