This study determined the effects of MSC2-based therapies on an inflammation-linked painful diabetic peripheral neuropathy (pDPN) mouse model. Findings indicate that MSC2-based therapy is a new anti-inflammatory treatment to consider in the management of pDPN.
Keywords: Adult stem cells, Bone marrow stromal cells, Cellular therapy, Diabetes, Neuropathy, Mesenchymal stem cells, Marrow stromal stem cells, Immunotherapy
Abstract
Mesenchymal stem cells (MSCs) are very attractive candidates in cell-based strategies that target inflammatory diseases. Preclinical animal studies and many clinical trials have demonstrated that human MSCs can be safely administered and that they modify the inflammatory process in the targeted injured tissue. Our laboratory developed a novel method that optimizes the anti-inflammatory effects of MSCs. We termed the cells prepared by this method MSC2. In this study, we determined the effects of MSC2-based therapies on an inflammation-linked painful diabetic peripheral neuropathy (pDPN) mouse model. Streptozotocin-induced diabetic mice were treated with conventionally prepared MSCs, MSC2, or vehicle at three specific time points. Prior to each treatment, responses to radiant heat (Hargreaves) and mechanical stimuli (von Frey) were measured. Blood serum from each animal was collected at the end of the study to compare levels of inflammatory markers between the treatment groups. We observed that MSC2-treated mice had significant improvement in behavioral assays compared with the vehicle and MSC groups, and moreover these responses did not differ from the observations seen in the healthy wild-type control group. Mice treated with conventional MSCs showed significant improvement in the radiant heat assay, but not in the von Frey test. Additionally, mice treated with MSC2 had decreased serum levels in many proinflammatory cytokines compared with the values measured in the MSC- or vehicle-treated groups. These findings indicate that MSC2-based therapy is a new anti-inflammatory treatment to consider in the management of pDPN.
Introduction
Diabetic peripheral neuropathy (DPN) is a complication of diabetes that affects an estimated 50% of patients with the disease [1, 2]. Additionally, 10%–26% of patients with DPN will have painful diabetic peripheral neuropathy (pDPN) [3–6]. The complaints vary but are often described as burning, cold, crushing, cramping, or tingling [7]. Most concerning is the increased sensitivity to non-nociceptive stimuli, where any external pressure causes excruciating pain. Patients with pDPN have a decreased quality of life and work productivity, and increased health care costs [8].
Although the cause of DPN is multifactorial, inflammation is considered a major contributor to the process [9]. Specifically, low-grade inflammatory reactions are triggered secondary to the oxidative stress generated by reactive oxygen species [10]. Additionally, inflammatory markers such as interleukin (IL)-6, IL-2, and tumor necrosis factor-α (TNF-α) are elevated in hyperglycemia, suggesting that a chronic, low-grade inflammatory state exists in patients with DPN [11–14]. It has been observed that DPN patients with higher plasma and mRNA levels of TNF-α have a greater risk of developing pDPN [12, 15, 16]. Moreover, compared with patients who have painless DPN, those with pDPN have been shown to have higher levels of C-reactive protein, which increases in the blood with any inflammatory process [17].
Current medicinal therapies for pDPN include tricyclic antidepressants, anticonvulsants, opioids, serotonin and norepinephrine reuptake inhibitors, and topical agents [18]. However, none of these therapies directly target the inflammatory milieu, most have untoward side effects, and there is little consensus on the optimal regimen [18], with less than 30% of patients receiving adequate pain relief [19]. Clearly, new therapies for the treatment of pDPN are needed. Recently, mesenchymal stem cell (MSC)-based therapies for inflammatory diseases have received significant attention. This interest is due to the fact that MSCs can be safely infused, selectively home to inflamed tissue to modify the inflammatory processes, have not been shown to have adverse effects, and can be derived from allogeneic as well as autologous donors [20–23]. The therapeutic benefit achieved by the MSCs in these instances is now believed to be by short-term (hours to days) paracrine and juxtacrine modulation of immune responses rather than by long-term (days to months) engraftment of the MSCs to the injured site [20–24]. Furthermore, recent reports of their use in murine models of DPN have shown encouraging results [25, 26]. However, current methods for the preparation of adult MSCs used in cell-based therapy consist of mixed, poorly defined cell populations with inconsistent clinical effects [20–23]. Our laboratory has established a methodology that results in a consistent, uniform, anti-inflammatory mesenchymal stem cell population (MSC2) for use in cell-based therapies [27, 28].
Because of the promising initial studies of conventional MSC therapies in rodent models of diabetic disease [25, 26], we hypothesized that a greater reduction in the symptoms of pDPN would be seen with the use of anti-inflammatory MSC2. After induction of diabetes with streptozotocin (STZ) and prior to receiving the cell therapy, the mice were evaluated for pDPN using two established behavioral assays [29, 30]. Following STZ-induced diabetes, animals were randomized to receive treatments of conventional MSCs, MSC2, or vehicle at three different time points. At sacrifice, blood serum was obtained to examine levels of inflammatory cytokines and chemokines. Animals treated with MSC2 showed statistically significant improvement in behavioral assays compared with animals treated with conventional MSCs or vehicle. These findings suggest that MSC2 are an effective, new anti-inflammatory cell-based therapy that warrants further study in the treatment of pDPN.
Materials and Methods
Animals
The animal protocols and experiments performed in this study were approved by the Institutional Animal Care and Use Committee at Tulane University. All animal use was also in accordance with the National Institutes of Health's guidelines. Six-week-old male C57BL/6J mice that had undergone treatment with STZ and 8-week-old male C57BL/6J mice that had not received STZ were purchased from the Jackson Laboratory (Bar Harbor, ME, http://www.jax.org). The protocol used at the Jackson Laboratory to induce diabetes in C57BL/6J (B6) mice entails the use of 6–8-week-old mice. Prior to treatment all mice are weighed and have glucose levels determined. The STZ regimen is low-dose, and the mice receive 50 mg of STZ per kg for 5 consecutive days via intraperitoneal injection. The mice are moved to clean cages 24 hours after the last injection and then observed until 16 days after the first injection. They are then weighed and have their blood glucose levels determined prior to shipment to the investigator. Upon receipt, the STZ-treated and wild-type animals were housed five mice per cage as appropriate on a 12/12-hour light/dark cycle under pathogen-free conditions with free access to mouse chow and water. After 1 week of acclimation, blood samples were drawn from the tail vein for glucose measurements with a standard commercially available glucometer (ReliOn; Arkray USA, Inc., Minneapolis, http://www.arkrayusa.com). At the end of the study, blood was extracted via cardiac puncture for blood glucose and cytokine/chemokine measurements. Animals were weighed prior to onset of treatment and at the end of the study. At least five mice were used in each experimental group.
Behavioral Testing
Diabetic animals and wild-type control mice underwent baseline behavioral assays on the day prior to injection with placebo, MSCs, or MSC2. Baseline testing occurred at 4 weeks after STZ treatment, whereas the second, third, and fourth rounds of behavioral testing occurred at 6, 8, and 10 weeks post-STZ treatment, respectively. A minimum of an hour for recovery was provided between the two types of behavioral tests.
To assess thermal withdrawal threshold of the hind paws, mice were placed in a clear acrylic box on a glass plate and acclimated for 20 minutes prior to testing. The Hargreaves apparatus [30] was used to provide a heat source to the middle of each hind paw and to obtain a measurement of withdrawal latency (seconds). The heat source was set at an intensity of 25, with a cut-off time of 20 seconds in order to prevent thermal injury to the footpad. Each hind paw was measured three times, and if the latency varied between the three measurements by 4 seconds or more, then a fourth recording was undertaken. Five minutes elapsed between testing trials. Withdrawal latency measurements from each hind paw were then averaged, and the mean of the two scores was used as a composite score for each mouse to reflect the symmetrical nature of the peripheral neuropathy. Experimenters were blind to treatment groups.
To test for behavioral responses to non-noxious mechanical stimuli [29], the mice were placed in an individual cage with a wire mesh floor and acclimated for 20 minutes. The mechanical withdrawal threshold was measured by applying a series of eight calibrated von Frey filaments (0.02, 0.04, 0.07, 0.16, 0.4, 1, 2, and 6 gauge; Ugo Basile, Collegeville, PA, http://www.ugobasileusa.com) to the mid-plantar surface of the foot. Both hind paws were tested, with a gap of 5 minutes between the testing. Monofilaments were applied until a positive sign for pain behavior, such as paw flinching or brisk withdrawal, was elicited. If a positive pain response was observed, the next monofilament tested was of the next lower force. In cases where there was not a response to a monofilament, the next monofilament to be tested was the one of next greater force. In the absence of a pain response at 6 gauge, no further testing was undertaken. The threshold force required to elicit withdrawal of the paw was determined by using the up-down method [29]. Results from both hind paws were averaged, and the mean of the two scores was used as the composite score in data analysis. Experimenters were blind to treatment groups.
Mesenchymal Stem Cell Preparations
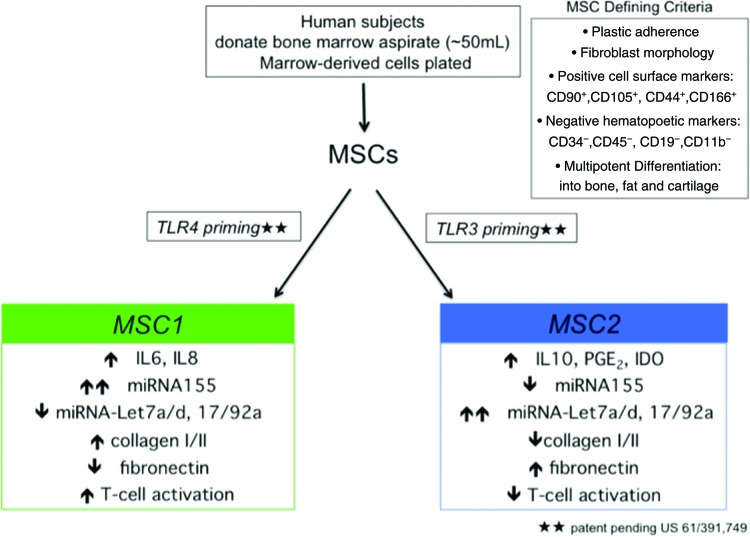
Primary human MSCs (hMSCs) were obtained from our collaborators at the Tulane University Center for Gene Therapy. Additionally, hMSCs were obtained from Lonza (Walkersville, MD, http://www.lonza.com) to ensure variability of the starting cell population and to independently confirm that findings were consistent and not unique to a single donor pool, as previously described [31]. The hMSCs from both of these sources are considered exempt from requiring approval from an institutional review board since they are obtained without any identifiers. Two male donors and one female donor ranging in age from 35 to 43 years old from both sources were used in this study. All of the MSC donor preparations from these sources were tested for hematopoietic stem cell markers by the sources and in our laboratory. All of the MSC preparations used in this study were less than 1% positive for CD34 and CD45. MSCs of a passage number no greater than 4 were used in all the experiments to maintain consistency. Additionally, no fewer than three different unrelated donor MSC pools were tested in all experiments. MSC2 preparation was carried out as previously described and as shown in Figure 1 ([27, 28]). A consistent anti-inflammatory effect by MSC2 therapy has been observed in several animal models of disease [32] (R.S. Waterman, S.L. Henkle, and A.M. Betancourt, manuscript submitted for publication).
Figure 1.
Preparation and characteristics of the MSC1 and MSC2 phenotypes. Short-term and low-level priming of TLR4 (left side) and TLR3 (right side) led to the induction of heterogeneous human MSC preparations into a proinflammatory MSC1 phenotype or an anti-inflammatory MSC2 phenotype (adapted from [27, 32]). Abbreviations: IDO, indolamine 2,3-dioxygenase; IL, interleukin; miRNA, microRNA; MSC, mesenchymal stem cells; MSC2, anti-inflammatory mesenchymal stem cells; PGE2, prostaglandin E2; TLR, Toll-like receptor.
Cell-Based Therapy
The day following testing, mice were brought to the procedure room and allowed to acclimate for 20 minutes. Mice then received an intraperitoneal injection of conventionally prepared MSCs, MSC2, or vehicle (0.5–1 × 106 cells in 0.5 ml of Hanks' balanced salt solution per mouse) depending on treatment group assignment. No anesthesia was required for these procedures.
Bio-Plex Assays
Blood was collected at necropsy via cardiac puncture from each animal, and the serum was analyzed with murine-specific Bio-Plex cytokine assays (Bio-Plex Pro Mouse Cytokine, catalog nos. M60-009RDPD and MD0-00000EL; Bio-Rad, Hercules, CA, http://www.bio-rad.com) following the manufacturer's instructions [27]. Pooled blood samples from three independent experiments were run in triplicate.
Histological Analysis
At the end of the animal experiments, animals were euthanized, and the hind paws were cut off at the knee joint for histological analysis. The skin was removed, and hind paws were fixed in tubes containing 4% formalin. Fixed and washed mouse hind paws were transferred to labeled tissue embedding and processing cassettes. Decalcification was accomplished by placing the cassettes within a beaker filled with decalcification solution (0.15% [wt/vol] EDTA, pH 7.5). The cassettes with the hind paws were transferred to a 2-liter beaker and placed at 4°C on a rotator with constant stirring. Decalcification proceeded for 4–6 weeks with weekly changes of solution. Subsequently, cassettes with hind paws were transferred to 70% ethanol, embedded in paraffin, and sectioned (5 mM) prior to staining with hematoxylin and eosin (H&E).
Statistical Analysis
Results were averaged for each group, and values were expressed as mean ± SEM. Data were evaluated using unpaired t test or one-way analysis of variance followed by the Tukey-Kramer post hoc test when necessary. A p value of less than .05 was considered statistically significant. Data analysis was performed with JMP 9.0.1 software (SAS Institute, Inc., Cary, NC, http://www.sas.com).
Results
MSC-Based Therapies Did Not Affect Blood Glucose Levels or Body Weight of STZ-Diabetic Induced Mice
There were no significant differences in average blood glucose among the MSC, MSC2, and vehicle control groups for the duration of the study (p = .21 prior to treatment; p = .84 at study completion), and the diabetic state was maintained among the STZ-treated mice throughout the experiment (Table 1). Thus, the prestudy level for the wild-type control group was 159 ± 3 mg/dl, and for all STZ-treated groups it was 372 ± 36.4 mg/dl. The poststudy glucose level for the wild-type control group was 159.5 ± 11.5 mg/dl, and for all STZ-treated it was 486.73 ± 38.67 mg/dl. By day 40, the mean blood glucose of the MSC2-treated mice was 498.6 ± 37.8 mg/dl, the mean blood glucose of the MSC-treated mice was 495 ± 23.7 mg/dl, and the mean blood glucose of the vehicle treatment group was 466.6 ± 61.2 mg/dl (Table 1). The mean glucose from day 1 to day 40 increased 20.0%, 15.8%, and 35.7% in the vehicle, MSC, and MSC2 treatment groups, respectively.
Table 1.
Characteristics of treatment groups (mean ± SE) used in this study
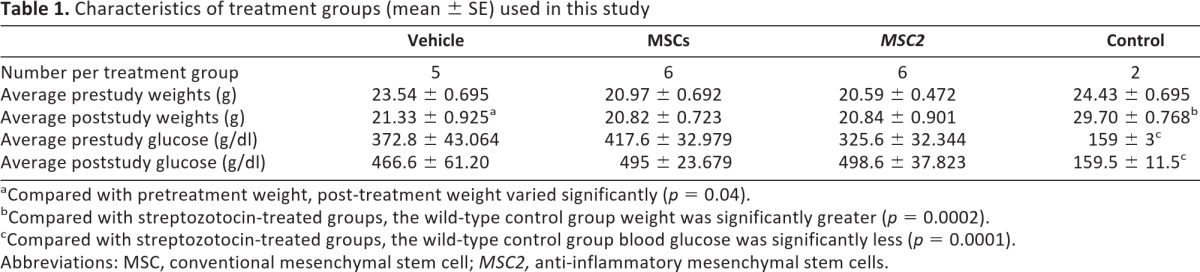
aCompared with pretreatment weight, post-treatment weight varied significantly (p = 0.04).
bCompared with streptozotocin-treated groups, the wild-type control group weight was significantly greater (p = 0.0002).
cCompared with streptozotocin-treated groups, the wild-type control group blood glucose was significantly less (p = 0.0001).
Abbreviations: MSC, conventional mesenchymal stem cell; MSC2, anti-inflammatory mesenchymal stem cells.
In contrast, there was a statistically significant difference between the blood glucose levels of the wild-type control group (from 159 ± 3 to 159.5 ± 11.5 mg/dl) and the STZ-treated mice (both at the beginning and at the end of the study; p = .0001). The average blood glucose of the STZ-induced diabetic mice rose over the course of the study regardless of which treatment they received, whereas the average blood glucose of the control mice was nearly the same by the study's end (Table 1). Blood glucose levels of 250 mg/dl or above were considered diabetic [33].
Average weights of the animals treated with STZ did not vary significantly (p = .89) regardless of treatment. Compared with the wild-type control animals, the STZ diabetic mice showed a distinct difference in weights (p = .0002) at the end of the study. The STZ-induced diabetic mice that received treatment with vehicle lost weight over the course of the study (p = .04). There were no changes prestudy versus poststudy in the weights of the STZ-induced diabetic mice that received MSC or MSC2 treatments (Table 1).
MSC-Based Therapies Did Not Affect Mortality or Morbidity
There was no premature mortality or morbidity during the study due to the cell-based therapy or any other experimental conditions. Furthermore, necropsy of all of the animals at the end of the experiments revealed no macroscopic pathology to any of the major organs. The hearts, lungs, spleen, kidneys, and livers of each animal group appeared normal. The hind paws were harvested, fixed, and decalcified prior to histological analyses of fixed sections. Few differences were noted among these upon H&E staining. There were regions that appeared to have greater inflammatory infiltrates in control over the MSC- or MSC2-treated groups (supplemental online Figure 1).
MSC2 Therapy Dramatically Improved the Symptoms of pDPN in STZ-Diabetic Induced Mice Compared with MSC Therapy or Vehicle
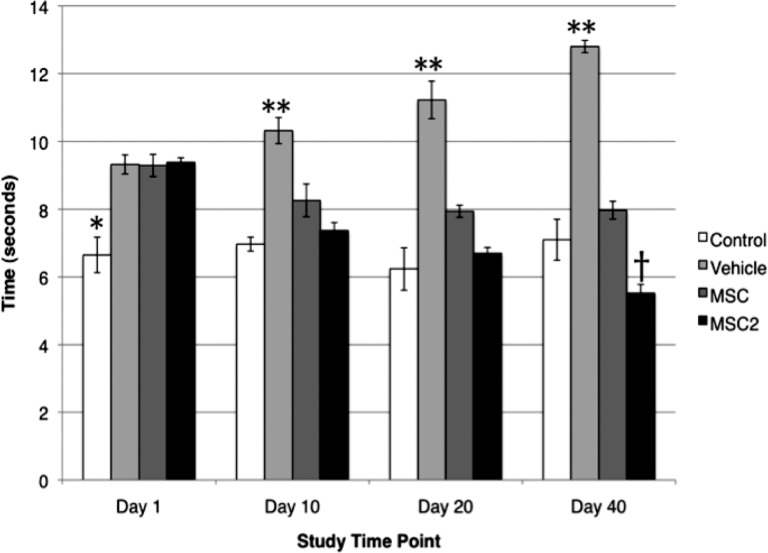
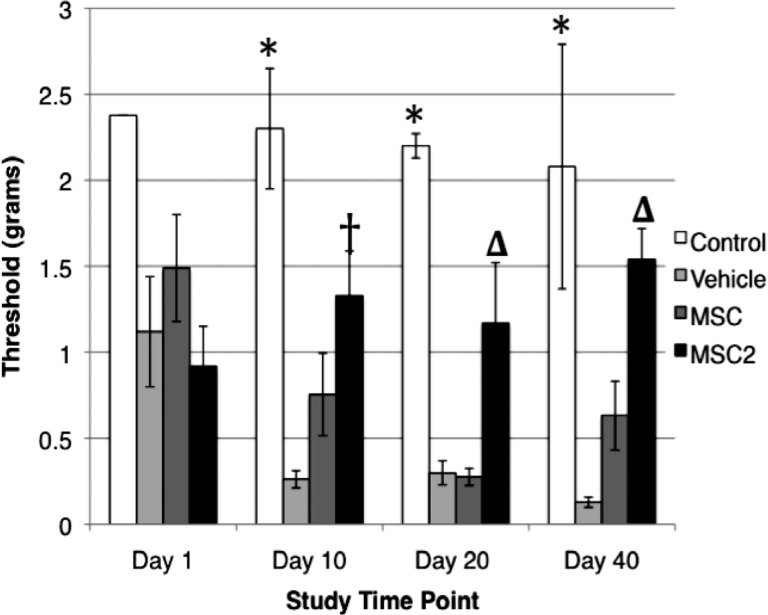
The symptoms of pDPN were assessed by using the established behavioral assays that evaluate mechanical allodynia and heat hypoalgesia [29, 30]. In the present study, MSC2 treatment significantly improved the behavioral assays in STZ-induced diabetic mice more than the conventional MSC therapy. At day 40, MSC2-treated mice had a 41.2% improvement in heat hypoalgesia (Fig. 2) and a 40.3% improvement in mechanical allodynia from baseline testing (Fig. 3). During the same period of time, the MSC-treated animals had a 14.2% improvement in heat hypoalgesia (Fig. 2). In contrast, no improvement was observed in mechanical allodynia in the MSC group (Fig. 3).
Figure 2.
Results of thermal sensitivity assays (mean ± SE). *, At baseline, streptozotocin-treated groups had significantly higher thresholds than the wild-type control (p < .001). **, At days 10, 20, and 40, the control (n = 2), MSC (n = 6), and MSC2 (n = 6) groups had significantly lower thresholds compared with the vehicle group (n = 5) (p < .001). †, At day 40, MSC2 varied significantly from MSC (p < .0001) and control (p < .018). Abbreviations: MSC, conventional mesenchymal stem cells; MSC2, anti-inflammatory mesenchymal stem cells.
Figure 3.
Results of mechanical sensitivity assays (mean ± SE). *, At days 10, 20, and 40, the MSC (n = 6) and vehicle (n = 5) groups had significantly greater sensitivity to mechanical stimuli than the wild-type control (n = 2) (p < .01). †, At day 10, the MSC2 (n = 6) group was significantly different from the vehicle (n = 5) group (p = .02). Δ, Compared with MSC or vehicle, MSC2 had significantly less sensitivity to mechanical stimuli (p = .02 and p = .34, respectively, on day 20; p = .019 and p = .0007, respectively, on day 40). MSC2 did not vary significantly from the wild-type control at any of the tested time points. Abbreviations: MSC, conventional mesenchymal stem cells; MSC2, anti-inflammatory mesenchymal stem cells.
The baseline times of withdrawal for wild-type control and vehicle-, MSC-, and MSC2-treated groups were 6.7 ± 0.53, 9.3 ± 0.28, 9.3 ± 0.33, and 9.4 ± 0.14 seconds, respectively, when evaluating thermal sensitivity [30] (Fig. 2). These results reflected a statistically significant higher threshold in the STZ-treated animals than control animals (p < .001). Over the course of the study, the vehicle group continued to have higher thresholds that were significantly greater than the wild-type control, MSCs, or MSC2 on days 10, 20, and 40 (p < .001). By day 40, the MSC2-treated mice had significantly lower thresholds than the vehicle (p < .001), MSC (p < .0001), and wild-type control (p < .018) groups (Fig. 2).
To assess behavioral responses to non-noxious mechanical stimuli, von Frey monofilaments were used [29]. All of the STZ-induced diabetic mice initially displayed reduced behavioral responses to a range of von Frey monofilaments compared with the wild-type control (vehicle 1.1 ± 0.71, MSCs 1.5 ± 0.76, MSC2 0.92 ± 0.58, wild-type control 2.38 ± 0 g). However, the difference was not found to be significant (Fig. 3). By day 10, significant differences were evident between the vehicle and MSC groups compared with the wild-type control (p = .0017, p = .014, respectively), and a significant difference remained between these groups through day 40 (p = .0007 for vehicle compared with wild-type control, p = .0078 for MSCs compared with wild-type control). At day 10, there was no observed difference between MSC2 and the wild-type control (p = .157), and this trend continued through to day 40 (p = .544). The MSC group's withdrawal threshold was 0.23 ± 0.12 g compared with 1.17 ± 0.79 g in the MSC2 group (p = .02) on day 20, and 0.64 ± 0.50 g versus 1.54 ± 0.41 g in the MSC2-treated mice (p = .0194) on day 40.
Serum Assays Indicated a Trend Toward Lower Levels of Proinflammatory Factors and Greater Levels of Anti-Inflammatory Factors in MSC2-Treated STZ Diabetic Mice
The secreted immune modulating factors in the different treatment arms were profiled by cytokine and chemokine assays of serum at day 40. We determined the concentration of 28 factors and observed a trend of lower proinflammatory and greater anti-inflammatory cytokine and chemokine levels in the sera of MSC2-treated diabetic mice compared with that of the diabetic mice treated with MSCs (Table 2; a complete list is included in supplemental online Table 1).
Table 2.
Results of Bio-Plex assay of mouse blood serum for immune modulating factors
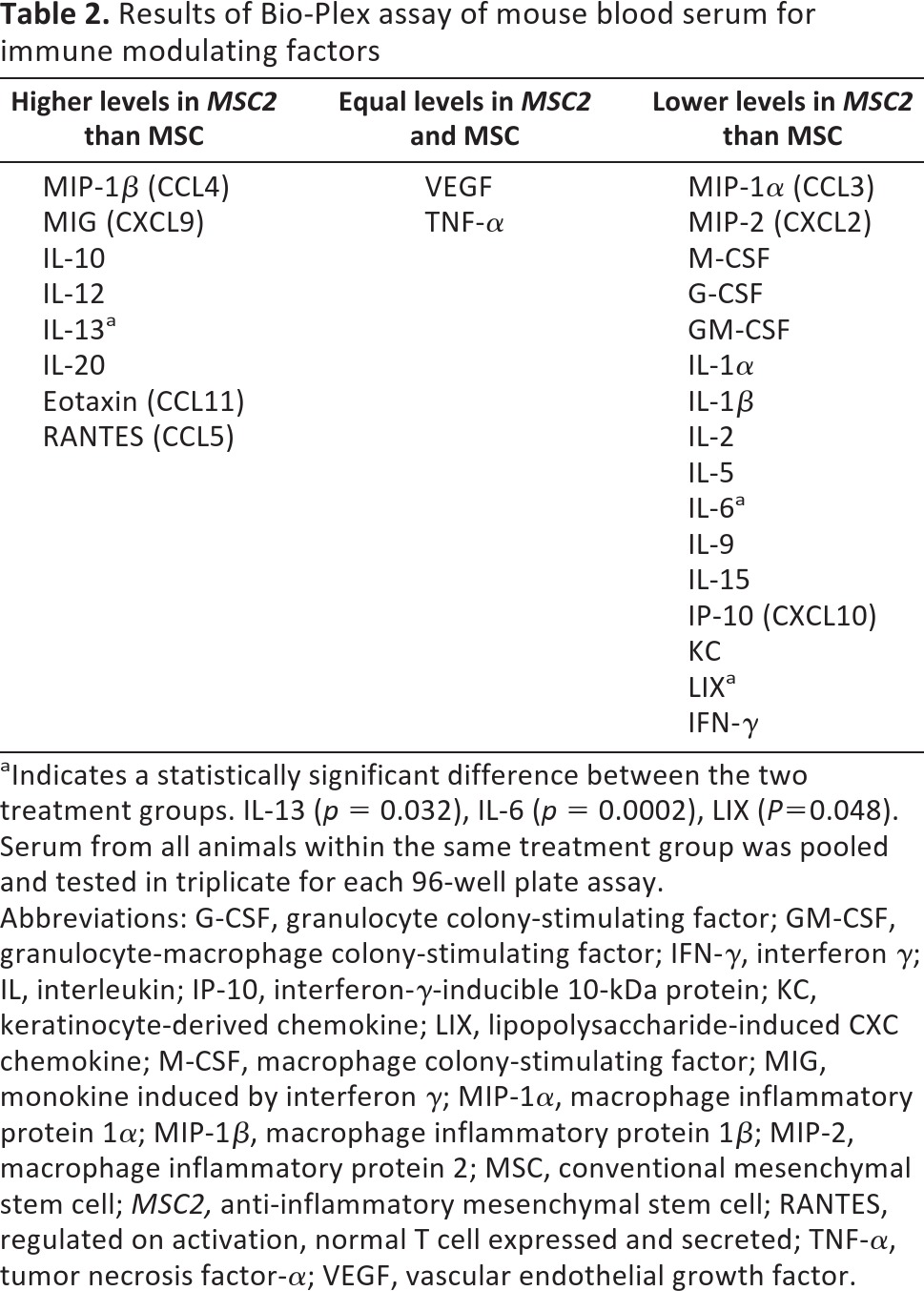
aIndicates a statistically significant difference between the two treatment groups. IL-13 (p = 0.032), IL-6 (p = 0.0002), LIX (P=0.048). Serum from all animals within the same treatment group was pooled and tested in triplicate for each 96-well plate assay.
Abbreviations: G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon γ; IL, interleukin; IP-10, interferon-γ-inducible 10-kDa protein; KC, keratinocyte-derived chemokine; LIX, lipopolysaccharide-induced CXC chemokine; M-CSF, macrophage colony-stimulating factor; MIG, monokine induced by interferon γ; MIP-1α, macrophage inflammatory protein 1α; MIP-1β, macrophage inflammatory protein 1β; MIP-2, macrophage inflammatory protein 2; MSC, conventional mesenchymal stem cell; MSC2, anti-inflammatory mesenchymal stem cell; RANTES, regulated on activation, normal T cell expressed and secreted; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
The results showed that a subset of the factors in the serum differed significantly between MSC-treated and MSC2-treated diabetic mice. The mean for the proinflammatory cytokine IL-6 was 2.2 ± 1.47 pg/ml in the MSC2-treated diabetic mice compared with a mean of 26.5 ± 1.47 pg/ml in the MSCs-treated mice (p = .0002). The results showed that at day 40 the proinflammatory lipopolysaccharide-induced CXC chemokine was significantly reduced in the diabetic mice receiving MSC2 (4,584.3 ± 272.11 pg/ml) compared with diabetic mice receiving MSC treatment (6,649.9 ± 272.11 pg/ml). In addition, the median concentration of the anti-inflammatory IL-13 in the serum of the animals was significantly higher (p = .032) in the MSC2-treated group (204.3 ± 19.74 pg/ml) compared with the median concentration of the MSC-treated mice (38.2 ± 19.74 pg/ml).
The other observed differences between the MSC2-treated and MSC-treated anti-inflammatory markers were more modest (Tables 1, 2). IL-1α levels were lowest in the MSC2 treatment arm (170.1 ± 83.0 pg/ml), and this differed significantly from vehicle values (1,262.8 ± 83.0 pg/ml; p = .0041) but not from values observed in the MSC-treated group (605.4 ± 83.0 pg/ml; p = .16). The levels of IL-1β were lowest in the MSC2 group (3.4 ± 1.34 pg/ml) and were significantly different from levels observed in the vehicle group (23.5 ± 1.34 pg/ml; p = .0021), but they were not significantly different in the MSC group (8.7 ± 1.34 pg/ml; p = .298). When comparing levels of IL-2, the diabetic animals receiving MSC2 had significantly lower amounts (0.1 ± 1.33 pg/ml) of this cytokine than those receiving vehicle (13.0 ± 1.33 pg/ml; p = .001), but the decrease was not significant compared with the MSC treatment group (7.0 ± 1.33 pg/ml; p = .17). Although granulocyte colony-stimulating factor levels were lowest in the group treated with placebo (82.8 ± 19.97 pg/ml), levels seen in the MSC2 arm (252.3 ± 19.97 pg/ml) were still lower than those seen in the MSC arm (344.1 ± 19.97 pg/ml). Interferon-γ (IFN-γ) levels were lower in the mice treated with MSC2 (4.9 ± 1.87 pg/ml) than in those treated with MSCs (5.6 ± 1.87 pg/ml); however, these findings were not significant (p = .989). Additionally, the following mean serum factor levels were lower in MSC2-treated diabetic mice than the MSC-treated diabetic mice: macrophage inflammatory protein (MIP)-2, granulocyte-macrophage colony-stimulating factor, IL-5, IL-9, IL-15, IFN-γ-inducible 10-kDa protein, keratinocyte-derived chemokine, MIP-1α, and macrophage colony-stimulating factor (Table 3; supplemental online Table 1).
Table 3.
Results of Bio-Plex assay of mouse blood serum immune modulating factors where vehicle-treated levels of proinflammatory markers were greater than with the conventional MSC or MSC2 treatments
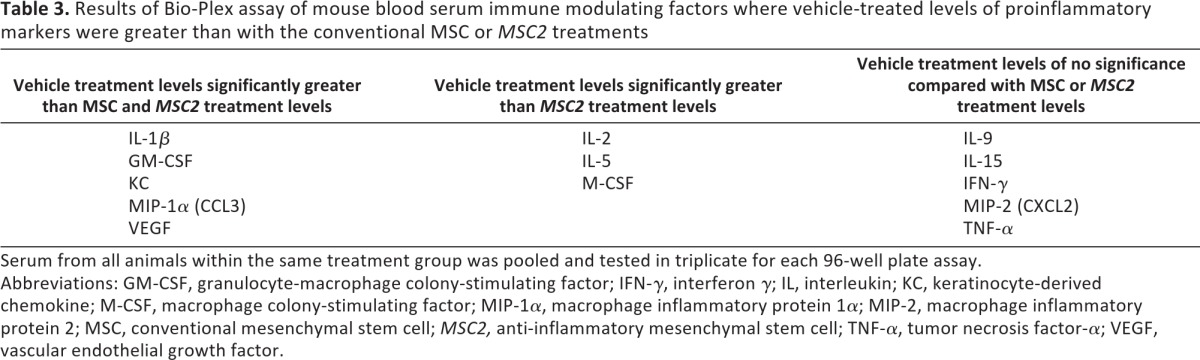
Serum from all animals within the same treatment group was pooled and tested in triplicate for each 96-well plate assay.
Abbreviations: GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon γ; IL, interleukin; KC, keratinocyte-derived chemokine; M-CSF, macrophage colony-stimulating factor; MIP-1α, macrophage inflammatory protein 1α; MIP-2, macrophage inflammatory protein 2; MSC, conventional mesenchymal stem cell; MSC2, anti-inflammatory mesenchymal stem cell; TNF-α, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
The majority of the anti-inflammatory mediators were increased in the MSC2-treated diabetic mice compared with the MSC-treated diabetic mice. Specifically, IL-10 was 28.8 ± 3.0 pg/ml in the MSC2 group compared with 20.6 ± 3.0 in the MSC group (p = .53). Although mean levels of IL-12 did not significantly vary among the three treatment regimens, the highest level of the cytokine was found in the MSC2 group (35.6 ± 2.0 pg/ml) compared with the MSC (28.5 ± 2.0 pg/ml) and vehicle (31.3 ± 2.0 pg/ml) groups. Higher mean levels of IL-20 were also observed in the MSC2-treated diabetic mice (12.5 ± 1.7 pg/ml). The lowest levels of IL-20 were found in the vehicle group (1.1 ± 1.71 pg/ml), whereas the mean level of IL-20 was approximately half of the MSC2 value in the MSC group (6.8 ± 1.71 pg/ml) (supplemental online Table 1).
A few of the mean levels of cytokines and chemokines that have proinflammatory effects were greater in the MSC2-treated arm compared with the mice treated with MSCs. Macrophage inflammatory protein 1β (MIP-1β) mean levels were greatest in the MSC2 group (38.7 ± 3.0 pg/ml). Comparatively, mean levels of MIP-1β were 20.0 ± 3.0 pg/ml in the vehicle group and 18.3 ± 3.0 pg/ml in the MSC group. The mean levels of monokine induced by IFN-γ were also the highest in the MSC2-treated diabetic mice (399.7 ± 18.7 pg/ml), with lower mean levels found in the MSC-treated diabetic mice (308.03 ± 18.7 pg/ml) and vehicle-treated mice (208.0 ± 18.7 pg/ml). The cytokine RANTES (regulated on activation, normal T-cell expressed and secreted) had a greater mean in the MSC2 group (21.9 ± 1.0 pg/ml) compared with the MSC group (18.4 ± 1.0 pg/dl). Although levels of eotaxin were greater in the MSC2-treated diabetic mice (52.3 ± 27.23 pg/ml) than the MSC-treated diabetic mice (357.8 ± 27.23 pg/ml), these levels did not vary significantly (p = .073).
Levels of IL-3, IL-4, and IL-7 were undetectable in the blood serum in all three treatment groups. TNF-α and vascular endothelial growth factor (VEGF) levels were also undetectable in the blood serum of the MSC- and MSC2-treated diabetic mice, but low levels were found in the vehicle group (TNF-α, 0.73 ± 0.21 pg/ml; VEGF, 9.2 ± 0.44 pg/ml) (supplemental online Table 1).
Discussion
The present results indicate that the MSC2-treated diabetic mice had significant improvement in symptoms of pDPN compared with the vehicle and to MSC treatment groups when evaluated for tactile allodynia and thermal hypoalgesia (Figs. 2, 3). These observations, along with the changes found in immune modulating factors from the blood sera of the MSC2-treated diabetic mice, suggest that the anti-inflammatory properties of the MSC2-based therapy most likely contributed to the improvements in the pDPN symptoms of the STZ-induced diabetic mice. These findings provide strong evidence for exploring MSC2-based therapy as an improved treatment option for pDPN.
Painful diabetic peripheral neuropathy is a complication of diabetes, and the mechanisms that produce this state have not yet been fully explained [10–16]. Because of recent reports implicating inflammatory mechanisms in pDPN [15–17, 34–36], pursuing the potential of new therapeutics with anti-inflammatory properties is a logical step. MSC-based therapy is increasingly viewed as an anti-inflammatory therapy as a result of its reported effects in more than 100 clinical trials and numerous preclinical investigations in models of human disease [37, 38]. Although it was originally expected that the delivered MSCs would home to the site of injury and instigate repair by engraftment at the site, the therapeutic role of MSCs is increasingly attributed to local and systemic immune modulation rather than direct tissue repair following their incorporation. Conventional MSCs used in the treatment of murine models of diabetic peripheral neuropathy have provided encouraging results [25, 26]. Despite these successes, one limitation of MSC-based therapies is that current methods for the preparation of these adult-derived cells yield a mixed pool of undefined cells that precludes consistency in preparation and clinical effect [20–23].
Our laboratory has established a novel methodology that results in a consistent, uniform, anti-inflammatory MSC2 population for use in cell-based therapies [27, 28]. In the present study, we found that whereas conventional MSCs predictably attenuated the immune response in an STZ-induced diabetic murine model, MSC2 treatment did so to a greater extent (Tables 2, 3). Additionally, treating pDPN with the cell-based anti-inflammatory MSC2 yielded even greater improvement in the symptoms of pDPN than conventional MSC-based therapy (Figs. 2, 3).
In line with other studies, the C57BL/6J STZ-treated mice that we studied developed tactile allodynia and heat hypoalgesia [39–43]. These are features of insensate neuropathy, are seen in advanced cases of DPN, and correlate with pDPN [44]. Throughout the study, all STZ-treated mice remained diabetic (Table 1). Given the improvement seen in the diabetic animals treated with cell-based therapy despite uncontrolled glucose, better results may be expected in a model where strict glucose control is maintained.
In this study, the animals that received conventional MSC treatment showed significant improvement in thermal hypoalgesia but not in tactile allodynia. Other studies have shown improvements in animal models of neuropathy after treatment with MSCs, but they used different strains of animals and measured endpoints that varied from ours [25, 26]. Additionally, the MSCs used in the above referenced studies were autologous, whereas in our study we used allogeneic human MSCs, which may help explain the difference in behavioral responses. Nonetheless, we were surprised that the conventional MSC-treated mice did not show significant improvement in the evaluation for non-noxious mechanical stimuli. This finding could be due to the type of nerve fibers tested in each of the behavioral assays, as well as study conditions. The von Frey assay for mechanical allodynia stimulates large myelinated A-β fibers sensitive to light touch and small unmyelinated C fibers involved in the pain response [45]. In the Hargreaves assay for thermal hypoalgesia, pain- and temperature-sensing C and A-δ fibers are stimulated [45–48]. At day 40 of our study, the conventional MSC-treated mice demonstrated improvement in mechanical allodynia after decreases from baseline on days 10 and 20.
Early preclinical and clinical successes of various experimental MSC-based therapies such as these are driving a quick translation of these cells for the treatment of various human diseases. However, the limitation of MSC-based studies comes from the fact that these cells are poorly defined, and there has not been a systematic study to compare the different immune modulating activities elicited by distinct MSC preparations derived from different tissue sources, cell expansion protocols, or hosts, and given for each indication [22, 49, 50]. We expect that there will be therapeutic differences driven by use of autologous versus allogeneic MSC cell sources that are important to identify. For instance, we anticipate that although a general immune suppressive state is supported by both allogeneic and autologous MSC-based therapies, there will be differences in the secreted bioactive factors and immune cells behind the effect that are essential to understand. Indeed, an immune host must recognize a self cell as being different from a non-self cell, and there are vital clues in this distinction that remain to be learned.
Similar to our study, other laboratories have shown that preclinical diabetic-induced rodent models have increased levels of proinflammatory markers [51–54]. For example, in STZ-treated rats, elevated levels of IL-1β, IL-6, and TNF-α were found in spinal cord tissue [51], whereas increased IL-6, cyclooxygenase-2, inducible nitric oxide synthase, and TNF-α were found in their blood [52]. Many of the inflammatory markers we evaluated in this study have been measured in painful disease states [15–17, 51, 52]; therefore, their attenuation likely contributed to the improvement in the symptoms of pDPN seen in the present results.
Levels of the anti-inflammatory cytokine IL-10 were greater in the MSC2 treatment arm compared with the MSC group. IL-10 is known to decrease levels of IL-1, IL-6, and TNF-α [55]. This cytokine does not have a long duration, and by modifying IL-10 with polyethylene glycol, Soderquist et al. have shown that the therapeutic half-life and magnitude of the anti-inflammatory cytokine can be increased [56]. Because the half-life of IL-10 is short, the time interval for measurement may not have been optimal for this investigation. Since IL-10 does influence a number of proinflammatory cytokines, it is reasonable to speculate that IL-10 involvement in the inflammatory process contributed to the effects seen with the cell-based therapies.
The proinflammatory cytokine IL-6, which can be decreased by the anti-inflammatory cytokine IL-10, was found to be significantly less expressed in the MSC2-treated compared with the MSC-treated mice (supplemental online Table 1). IL-6 plays a significant role in pain, and after sciatic nerve injury in an animal model, IL-6 receptors are found to be upregulated [57]. In addition, IL-6 is seen in greater concentration at the surgical sites of elective surgeries that are considered severe [58, 59].
IL-1α and IL-1β were also both lower in the MSC2-treated mice compared with the MSC-treated mice (supplemental online Table 1). IL-1β has been implicated in chronic pain conditions and is elevated in the cerebral spinal fluid of chronic pain patients [60, 61]. Even though a statistically significant difference was not observed with regard to IL-1α or IL-1β between the two cell-based therapies, the overall dampened immune state of the MSC2-treated mice may have been a factor in the improvement in their behavioral assays.
At the time point when the immune factors were measured, both TNF-α and VEGF were undetectable in the sera of the MSC- and MSC2-treated animals. However, the vehicle control group had detectable levels of both VEGF and TNF-α. It has been reported that patients with higher whole blood mRNA levels are at increased risk for developing pDPN [12, 15, 16, 62]. In vitro studies have shown that VEGF may contribute to diabetic neuropathy [63], and in STZ-diabetic induced rats, increased expression of VEGF has been reported in the sciatic nerve and dorsal root ganglia [64]. The observed undetectable serum levels of VEGF and TNF-α in both the stem cell-treated groups are in agreement with previous studies and may be due to the time interval of measurement or as a result of the stem cells' ability to modulate the immune system [20–23]. Importantly, as mentioned above, a systematic follow-up approach will determine optimal dosing and timing of the MSC-based therapies to improve on these and other pDPN parameters, which may help identify critical host immune components.
Although findings in this study were promising, it must be noted that applying the observations of this STZ-induced diabetic model as a clinical therapy may be difficult as the unknown effects of STZ may influence the study. The severe hyperglycemia induced in the mice is not typically seen in humans for such long periods, so extrapolating results of this study to humans must be done only with caution. Finally, since the etiology of diabetic neuropathy appears to be multifactorial, and as a single intervention may not be sufficient to treat the disease, a multitargeted approach to treatment is most likely necessary.
Conclusion
In conclusion, our results demonstrated beneficial therapeutic effects on pDPN by the MSC2-based therapy. Moreover, our results showing greater modification of the inflammatory state and vast improvement of symptoms argue for the continued investigation of MSC2-based therapy in such diseases. Further evaluation of the potential of MSC2 to be developed as an off-the-shelf product for the improved treatment of pDPN should follow this study.
Acknowledgments
We thank Dr. J. Zadina and his laboratory for the use of equipment and support during this experiment. This study was supported by a grant from the Foundation for Anesthesiology Education and Research (to R.S.W.). A.M.B. was supported by National Institutes of Health Grant 1P20RR20152-01, Department of Defense OC073102 Concept Award, and research support from the Tulane Cancer Center and the Center for Stem Cell Research and Regenerative Medicine.
Author Contributions
R.S.W.: financial support, conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; J.M.: study design, data interpretation, manuscript writing, final approval of manuscript; B.D.N.: study design, manuscript writing, final approval of manuscript; A.E.S.: data collection, manuscript writing; S.A.S.: collection and assembly of data; A.M.B.: financial support, study design, collection and assembly of data, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
A.M.B. is the inventor of the patent-pending “Mesenchymal Stem Cells and Related Therapies” (MSC1 and MSC2 methodology), US patent pending 61/391 749.
References
- 1.Argoff CE, Cole BE, Fishbain DA, et al. Diabetic peripheral neuropathic pain: Clinical and quality-of-life issues. Mayo Clin Proc. 2006;81:S3–S11. doi: 10.1016/s0025-6196(11)61474-2. [DOI] [PubMed] [Google Scholar]
- 2.Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: A statement by the American Diabetes Association. Diabetes Care. 2005;28:956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 3.Berger A, Dukes EM, Oster G. Clinical characteristics and economic costs of patients with painful neuropathic disorders. J Pain. 2004;5:143–149. doi: 10.1016/j.jpain.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Davies M, Brophy S, Williams R, et al. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29:1518–1522. doi: 10.2337/dc05-2228. [DOI] [PubMed] [Google Scholar]
- 5.Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain. 2002;18:350–354. doi: 10.1097/00002508-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Van Acker K, Bouhassira D, De Bacquer D, et al. Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab. 2009;35:206–213. doi: 10.1016/j.diabet.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Baron R, Tolle TR, Gockel U, et al. A cross-sectional cohort survey in 2100 patients with painful diabetic neuropathy and postherpetic neuralgia: Differences in demographic data and sensory symptoms. Pain. 2009;146:34–40. doi: 10.1016/j.pain.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 8.daCosta DiBonaventura M, Cappelleri JC, Joshi AV. A longitudinal assessment of painful diabetic peripheral neuropathy on health status, productivity, and health care utilization and cost. Pain Med. 2011;12:118–126. doi: 10.1111/j.1526-4637.2010.01012.x. [DOI] [PubMed] [Google Scholar]
- 9.Vincent AM, Callaghan BC, Smith AL, et al. Diabetic neuropathy: Cellular mechanisms as therapeutic targets. Nat Rev Neurol. 2011;7:573–583. doi: 10.1038/nrneurol.2011.137. [DOI] [PubMed] [Google Scholar]
- 10.Dominiczak MH. Obesity, glucose intolerance and diabetes and their links to cardiovascular disease. Implications for laboratory medicine. Clin Chem Lab Med. 2003;41:1266–1278. doi: 10.1515/CCLM.2003.194. [DOI] [PubMed] [Google Scholar]
- 11.Bailey CJ. Treating insulin resistance: Future prospects. Diab Vasc Dis Res. 2007;4:20–31. doi: 10.3132/dvdr.2007.002. [DOI] [PubMed] [Google Scholar]
- 12.Kampoli AM, Tousoulis D, Briasoulis A, et al. Potential pathogenic inflammatory mechanisms of endothelial dysfunction induced by type 2 diabetes mellitus. Curr Pharm Des. 2011;17:4147–4158. doi: 10.2174/138161211798764825. [DOI] [PubMed] [Google Scholar]
- 13.Niehoff AG, van Haeften TW, Onland-Moret NC, et al. C-reactive protein is independently associated with glucose but not with insulin resistance in healthy men. Diabetes Care. 2007;30:1627–1629. doi: 10.2337/dc06-2531. [DOI] [PubMed] [Google Scholar]
- 14.Sjöholm A, Nyström T. Endothelial inflammation in insulin resistance. Lancet. 2005;365:610–612. doi: 10.1016/S0140-6736(05)17912-4. [DOI] [PubMed] [Google Scholar]
- 15.Purwata TE. High TN. F-alpha plasma levels and macrophages iNOS and TNF-alpha expression as risk factors for painful diabetic neuropathy. J Pain Res. 2011;4:169–175. doi: 10.2147/JPR.S21751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uçeyler N, Rogausch JP, Toyka KV, et al. Differential expression of cytokines in painful and painless neuropathies. Neurology. 2007;69:42–49. doi: 10.1212/01.wnl.0000265062.92340.a5. [DOI] [PubMed] [Google Scholar]
- 17.Doupis J, Lyons TE, Wu S, et al. Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. J Clin Endocrinol Metab. 2009;94:2157–2163. doi: 10.1210/jc.2008-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Argoff CE, Backonja MM, Belgrade MJ, et al. Consensus guidelines: Treatment planning and options. Diabetic peripheral neuropathic pain. Mayo Clin Proc. 2006;81:S12–S25. doi: 10.1016/s0025-6196(11)61475-4. [DOI] [PubMed] [Google Scholar]
- 19.Barrett AM, Lucero MA, Le T, et al. Epidemiology, public health burden, and treatment of diabetic peripheral neuropathic pain: A review. Pain Med. 2007;8(suppl 2):S50–S62. doi: 10.1111/j.1526-4637.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 20.Bunnell BA, Betancourt AM, Sullivan DE. New concepts on the immune modulation mediated by mesenchymal stem cells. Stem Cell Res Ther. 2010;1:34. doi: 10.1186/scrt34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): Controversies, myths, and changing paradigms. Mol Ther. 2009;17:939–946. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salem HK, Thiemermann C. Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer NG, Caplan AI. Mesenchymal stem cells: Mechanisms of inflammation. Annu Rev Pathol. 2011;6:457–478. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- 24.von Bahr L, Batsis I, Moll G, et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicate limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012 doi: 10.1002/stem.1118. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Kim BJ, Jin HK, Bae JS. Bone marrow-derived mesenchymal stem cells improve the functioning of neurotrophic factors in a mouse model of diabetic neuropathy. Lab Anim Res. 2011;27:171–176. doi: 10.5625/lar.2011.27.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibata T, Naruse K, Kamiya H, et al. Transplantation of bone marrow-derived mesenchymal stem cells improves diabetic polyneuropathy in rats. Diabetes. 2008;57:3099–3107. doi: 10.2337/db08-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waterman RS, Tomchuck SL, Henkle SL, et al. A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS One. 2010;5:e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Betancourt AM. Mesenchymal stem cells and related therapies. US patent pending 61/391 749. 2011 Oct 11; inventor; Administrators of the Tulane Educational Fund, assignee.
- 29.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 30.Hargreaves K, Dubner R, Brown F, et al. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 31.Tomchuck SL, Zwezdaryk KJ, Coffelt SB, et al. Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells. 2008;26:99–107. doi: 10.1634/stemcells.2007-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Betancourt AM, Waterman RS. Biswas S, editor. The role of mesenchymal stem cells in the tumor microenvironment. Tumor Microenvironment and Myelomonocytic Cells. InTech. 2012. [Accessed March 1, 2012]. Available at: http://www.intechopen.com/books/tumor-microenvironment-and-myelomonocytic-cells/the-role-of-mesenchymal-stem-cells-in-the-tumor-microenvironment.
- 33.Flodström M, Tyrberg B, Eizirik DL, et al. Reduced sensitivity of inducible nitric oxide synthase-deficient mice to multiple low-dose streptozotocin-induced diabetes. Diabetes. 1999;48:706–713. doi: 10.2337/diabetes.48.4.706. [DOI] [PubMed] [Google Scholar]
- 34.Chopra K, Tiwari V, Arora V, et al. Sesamol suppresses neuro-inflammatory cascade in experimental model of diabetic neuropathy. J Pain. 2010;11:950–957. doi: 10.1016/j.jpain.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Pabreja K, Dua K, Sharma S, et al. Minocycline attenuates the development of diabetic neuropathic pain: Possible anti-inflammatory and anti-oxidant mechanisms. Eur J Pharmacol. 2011;661:15–21. doi: 10.1016/j.ejphar.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 36.Valsecchi AE, Franchi S, Panerai AE, et al. The soy isoflavone genistein reverses oxidative and inflammatory state, neuropathic pain, neurotrophic and vasculature deficits in diabetes mouse model. Eur J Pharmacol. 2011;650:694–702. doi: 10.1016/j.ejphar.2010.10.060. [DOI] [PubMed] [Google Scholar]
- 37.Newman RE, Yoo D, LeRoux MA, et al. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets. 2009;8:110–123. doi: 10.2174/187152809788462635. [DOI] [PubMed] [Google Scholar]
- 38.Waterman RS, Betancourt AM. Treating chronic pain with mesenchymal stem cells: A therapeutic approach worthy of continued investigation. J Stem Cell Res Ther. 2011;S2:1–9. [Google Scholar]
- 39.Cameron NE, Tuck Z, McCabe L, et al. Effect of the hydroxyl radical scavenger, dimethylthiourea, on peripheral nerve tissue perfusion, conduction velocity and nociception in experimental diabetes. Diabetologia. 2001;44:1161–1169. doi: 10.1007/s001250100626. [DOI] [PubMed] [Google Scholar]
- 40.Drel VR, Mashtalir N, Ilnytska O, et al. The leptin-deficient (ob/ob) mouse: A new animal model of peripheral neuropathy of type 2 diabetes and obesity. Diabetes. 2006;55:3335–3343. doi: 10.2337/db06-0885. [DOI] [PubMed] [Google Scholar]
- 41.Drel VR, Pacher P, Vareniuk I, et al. Evaluation of the peroxynitrite decomposition catalyst Fe(III) tetra-mesitylporphyrin octasulfonate on peripheral neuropathy in a mouse model of type 1 diabetes. Int J Mol Med. 2007;20:783–792. [PMC free article] [PubMed] [Google Scholar]
- 42.Ilnytska O, Lyzogubov VV, Stevens MJ, et al. Poly(ADP-ribose) polymerase inhibition alleviates experimental diabetic sensory neuropathy. Diabetes. 2006;55:1686–1694. doi: 10.2337/db06-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevens MJ, Li F, Drel VR, et al. Nicotinamide reverses neurological and neurovascular deficits in streptozotocin diabetic rats. J Pharmacol Exp Ther. 2007;320:458–464. doi: 10.1124/jpet.106.109702. [DOI] [PubMed] [Google Scholar]
- 44.Obrosova IG. Diabetic painful and insensate neuropathy: Pathogenesis and potential treatments. Neurotherapeutics. 2009;6:638–647. doi: 10.1016/j.nurt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beiswenger KK, Calcutt NA, Mizisin AP. Epidermal nerve fiber quantification in the assessment of diabetic neuropathy. Acta Histochem. 2008;110:351–362. doi: 10.1016/j.acthis.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herrmann DN, Griffin JW, Hauer P, et al. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology. 1999;53:1634–1640. doi: 10.1212/wnl.53.8.1634. [DOI] [PubMed] [Google Scholar]
- 47.Muller KA, Ryals JM, Feldman EL, et al. Abnormal muscle spindle innervation and large-fiber neuropathy in diabetic mice. Diabetes. 2008;57:1693–1701. doi: 10.2337/db08-0022. [DOI] [PubMed] [Google Scholar]
- 48.Sinnreich M, Taylor BV, Dyck PJ. Diabetic neuropathies: Classification, clinical features, and pathophysiological basis. Neurologist. 2005;11:63–79. doi: 10.1097/01.nrl.0000156314.24508.ed. [DOI] [PubMed] [Google Scholar]
- 49.Bianco P. Back to the future: Moving beyond “mesenchymal stem cells.”. J Cell Biochem. 2011;112:1713–1721. doi: 10.1002/jcb.23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bianco P, Robey PG, Saggio I, et al. “Mesenchymal” stem cells in human bone marrow (skeletal stem cells): A critical discussion of their nature, identity, and significance in incurable skeletal disease. Hum Gene Ther. 2010;21:1057–1066. doi: 10.1089/hum.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bishnoi M, Bosgraaf CA, Abooj M, et al. Streptozotocin-induced early thermal hyperalgesia is independent of glycemic state of rats: Role of transient receptor potential vanilloid 1(TRPV1) and inflammatory mediators. Mol Pain. 2011;7:52. doi: 10.1186/1744-8069-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar A, Negi G, Sharma SS. JSH-23 targets nuclear factor-kappa B and reverses various deficits in experimental diabetic neuropathy: Effect on neuroinflammation and antioxidant defence. Diabetes Obes Metab. 2011;13:750–758. doi: 10.1111/j.1463-1326.2011.01402.x. [DOI] [PubMed] [Google Scholar]
- 53.Luo L, Dai DZ, Cheng YS, et al. Sildenafil improves diabetic vascular activity through suppressing endothelin receptor A, iNOS and NADPH oxidase which is comparable with the endothelin receptor antagonist CPU0213 in STZ-injected rats. J Pharm Pharmacol. 2011;63:943–951. doi: 10.1111/j.2042-7158.2011.01268.x. [DOI] [PubMed] [Google Scholar]
- 54.Silva DC, Freitas AL, Pessoa CD, et al. Pectin from Passiflora edulis shows anti-inflammatory action as well as hypoglycemic and hypotriglyceridemic properties in diabetic rats. J Med Food. 2011;14:1118–1126. doi: 10.1089/jmf.2010.0220. [DOI] [PubMed] [Google Scholar]
- 55.Fiorentino DF, Zlotnik A, Mosmann TR, et al. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 56.Soderquist RG, Milligan ED, Harrison JA, et al. PEGylation of interleukin-10 for the mitigation of enhanced pain states. J Biomed Mater Res A. 2010;93:1169–1179. doi: 10.1002/jbm.a.32611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cui JG, Holmin S, Mathiesen T, et al. Possible role of inflammatory mediators in tactile hypersensitivity in rat models of mononeuropathy. Pain. 2000;88:239–248. doi: 10.1016/S0304-3959(00)00331-6. [DOI] [PubMed] [Google Scholar]
- 58.Cruickshank AM, Fraser WD, Burns HJ, et al. Response of serum interleukin-6 in patients undergoing elective surgery of varying severity. Clin Sci (Lond) 1990;79:161–165. doi: 10.1042/cs0790161. [DOI] [PubMed] [Google Scholar]
- 59.Holzheimer RG, Steinmetz W. Local and systemic concentrations of pro- and anti-inflammatory cytokines in human wounds. Eur J Med Res. 2000;5:347–355. [PubMed] [Google Scholar]
- 60.Alexander GM, van Rijn MA, van Hilten JJ, et al. Changes in cerebrospinal fluid levels of pro-inflammatory cytokines in CRPS. Pain. 2005;116:213–219. doi: 10.1016/j.pain.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 61.Watkins LR, Maier SF, Goehler LE. Immune activation: The role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain. 1995;63:289–302. doi: 10.1016/0304-3959(95)00186-7. [DOI] [PubMed] [Google Scholar]
- 62.Yamakawa I, Kojima H, Terashima T, et al. Inactivation of TNF-alpha ameliorates diabetic neuropathy in mice. Am J Physiol Endocrinol Metab. 2011;301:E844–E852. doi: 10.1152/ajpendo.00029.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xia P, Aiello LP, Ishii H, et al. Characterization of vascular endothelial growth factor's effect on the activation of protein kinase C, its isoforms, and endothelial cell growth. J Clin Invest. 1996;98:2018–2026. doi: 10.1172/JCI119006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Samii A, Unger J, Lange W. Vascular endothelial growth factor expression in peripheral nerves and dorsal root ganglia in diabetic neuropathy in rats. Neurosci Lett. 1999;262:159–162. doi: 10.1016/s0304-3940(99)00064-6. [DOI] [PubMed] [Google Scholar]