Chronic obstructive pulmonary disease (COPD) is characterized by a progressive and not fully reversible airflow limitation caused by chronic small airway disease and lung parenchymal destruction. Clinically available drugs improve airflow obstruction and respiratory symptoms but cannot cure the disease. Recently, a method for the direct isolation of individual cell types from human lung has been developed, and fingerprints of each cell type in COPD lungs can be analyzed. Research using this technique combined with the recently discovered lung endogenous stem-progenitor populations will give a better understanding about the fate of COPD lung cells and provide a future for cell-based therapy to treat this intractable disease.
Keywords: Lung, Respiratory tract, Stem cell, Clinical trials, Adult stem cells, Cellular therapy
Abstract
Chronic obstructive pulmonary disease (COPD) is becoming a major cause of death worldwide. COPD is characterized by a progressive and not fully reversible airflow limitation caused by chronic small airway disease and lung parenchymal destruction. Clinically available drugs improve airflow obstruction and respiratory symptoms but cannot cure the disease. Slowing the progressive lung destruction or rebuilding the destroyed lung structure is a promising strategy to cure COPD. In contrast to small animal models, pharmacological lung regeneration is difficult in human COPD. Maturation, aging, and senescence in COPD lung cells, including endogenous stem cells, may affect the regenerative capacity following pharmacological therapy. The lung is a complex organ composed of more than 40 different cell types; therefore, detailed analyses, such as epigenetic modification analysis, in each specific cell type have not been performed in lungs with COPD. Recently, a method for the direct isolation of individual cell types from human lung has been developed, and fingerprints of each cell type in COPD lungs can be analyzed. Research using this technique combined with the recently discovered lung endogenous stem-progenitor populations will give a better understanding about the fate of COPD lung cells and provide a future for cell-based therapy to treat this intractable disease.
Introduction
The lung is a complex three-dimensional organ that is composed of more than 40 different cell types. Gas exchange is the most important function of the lung; therefore, the lung is primarily composed of millions of alveoli surrounded by a capillary network (Fig. 1A). The alveolar surface is covered with alveolar type I and II epithelial cells and is open to air. Toxic reagents from outside, such as air pollution, cigarette smoke, and pathogens, can easily reach airways, and some of them can reach alveoli. Such harmful stresses damage and injure bronchial and alveolar epithelial cells. These damaged epithelia should be repaired or replaced rapidly to maintain lung homeostasis, but lung cell turnover is generally slow compared with that of other organs that face the outside, such as the skin and intestine. This repair capacity of the bronchial and alveolar epithelia influences the resolution after lung inflammation.
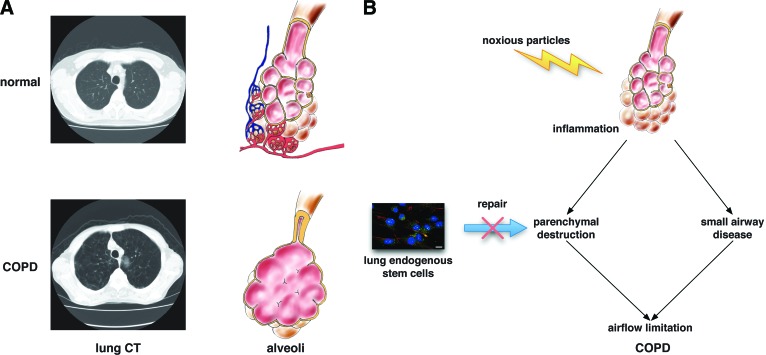
Figure 1.
Structural changes and pathogenesis of COPD. (A): Alveolar structure in normal lung and COPD lung. The lung is composed of millions of alveoli surrounded by a capillary network. Alveolar destruction and small airway obstruction are seen in COPD lung. (B): Mechanisms of airflow limitation in COPD. Insufficient repair capacity of lung endogenous stem cells may cause the alveolar destruction. Abbreviations: COPD, chronic obstructive pulmonary disease; CT, computed tomography.
The matrix is another key component of the lung that is required to properly maintain its function. The lung alveolar structure is similar to a sponge: thin walls built like a labyrinth and filled with air. Being filled with air is one of the unique characteristics of the lung compared with other solid organs, and it makes cell migration more difficult. Unless the structure is destroyed, damaged alveolar epithelia can be replaced with migrated progenitor cells. However, once the proper alveolar architecture is destroyed, progenitors cannot by themselves rebuild the appropriate functional lung structure, and a force from the parenchyma provided from elastic fibers [1] is needed to regenerate the alveolar wall (Fig. 2). During lung growth and regeneration, alveolar septation (alveolarization) combined with parenchymal growth is necessary. Primary lung structure development is completed before birth; however, the number of alveoli increases even after birth throughout childhood and adolescence (postnatal alveolarization) [2]. Postlobectomy and postpneumonectomy alveolarization (compensatory lung growth) is observed in children [3] and experimental animal models [4–6]. These results suggest that the potency of dynamic alveolar reconstruction is higher than generally expected. However, it is not clear whether adult and aged lungs have the same potential for alveolar reconstruction.
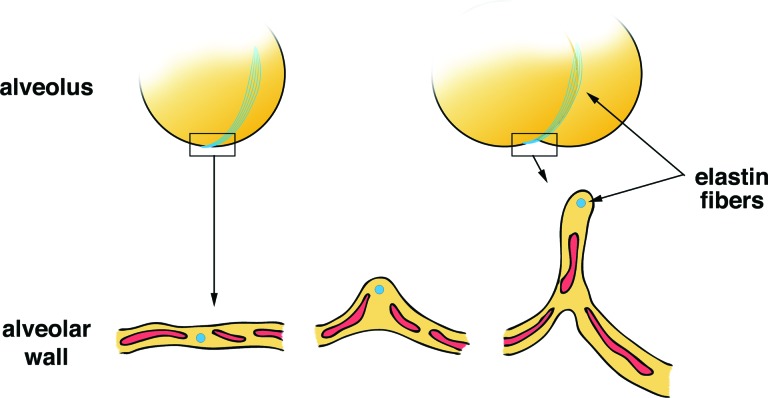
Figure 2.
Conceptual model of new alveolarization. The presence of key proteins in the extracellular matrix is required for lung regeneration. Elastic fibers made by extracellular matrices, such as elastin, surround alveoli. These fibers support the alveolar septation.
Chronic obstructive pulmonary disease (COPD) is a common disease and has a major impact on morbidity and mortality worldwide [7]. Chronic and amplified inflammation induced by the inhalation of noxious particles, mainly cigarette smoke, is the primary pathogenesis of COPD. Genetic factors and aging effects are also involved in disease development. COPD is characterized by progressive airflow limitation, resulting in chronic respiratory failure [7]. This air flow limitation is caused by chronic small airway disease and lung parenchymal destruction (emphysema) (Fig. 1B). Slowing progressive lung destruction or rebuilding the destroyed lung structure is a promising strategy to cure COPD. However, repairing parenchymal destruction is challenging because the regenerative capacity of adult and aged lungs is believed to be limited. In this review, a history of lung regeneration studies, recent and growing knowledge about lung endogenous stem cells and clinical prospects for treating COPD with regenerative approaches are discussed.
Pharmacologic Approach
Several reagents could promote lung regeneration in animal lung emphysema models [8–13]. Retinoic acid (RA), an active metabolite of vitamin A, is the most extensively studied reagent. RA has a variety of roles in lung development and alveologenesis [14], including embryonic branching morphogenesis [15], the production of alveolar elastic fibers [16], and elastin synthesis [17].
RA reverses anatomic and functional lung destruction in rat and mouse pulmonary emphysema models [8, 18]. However, the capacity of RA-induced lung regeneration is different among the emphysema models. Aging is one of the causes of this discrepancy. Small animals, such as rodents, have a better capacity for lung regeneration because their somatic growth continues throughout their life span.
On the basis of the animal studies, a double-blind placebo-controlled clinical trial using RA was performed in moderate-to-severe COPD patients [19, 20]. Although the oral administration of RA modulates the protease/antiprotease balance in COPD patients [21], no statistical change is observed in lung function or density of computed tomography (CT) images. A clinical trial with an active γ-selective retinoid agonist in patients with α-1 antitrypsin deficiency (the REPAIR study) did not demonstrate a significant benefit [22]. Another trial using the retinoid agonist in COPD patients (the TESRA study) has been completed, and potential benefits in selected patients were suggested [23].
Other reagents, such as hepatocyte growth factor (HGF), also demonstrate promising effects on lung regeneration [10, 11]. However, these growth factors often induce tumor growth. Because the risk of lung cancer is much higher in COPD patients, clinical trials using such reagents are difficult.
Another issue concerning the regenerative approach in COPD lungs is that most COPD lungs are aged and matured. Because aging or senescence in COPD lung cells, including endogenous stem cells, may affect the regenerative capacity by pharmacological therapy, cell-based analyses in COPD lungs are needed to determine whether aging or senescence is a problem in pharmacological lung regeneration.
Lung Growth
Volume reduction surgery in patients with pulmonary emphysema increases the residual volume and improves the symptoms, although this procedure has not commonly been performed in recent years [24, 25]. Promoting compensatory lung growth after volume reduction surgery could be a promising strategy to improve the outcome of COPD patients.
In experimental animal models, the degree of compensatory lung growth differs among species and varies with age. Small animals have a better and more rapid capacity for compensatory growth than larger animals. For example, the weight of the remaining lung doubles within 14 days after pneumonectomy in rats [26], whereas a period of 28 days is needed in rabbits [27] and a 5-month period is needed in dogs [4]. This result suggests that life span may affect the speed of compensatory lung growth. Age is another important factor in the ability of lung regrowth. In the adult dog lung, compensatory lung growth is slow and incomplete, but extensive lung resection in an immature dog stimulates rapid and vigorous compensatory growth, resulting in complete normalization of lung function at maturity [5]. This result suggests that compensatory lung growth is maturity-dependent. These issues should be considered in lungs with COPD.
Another key factor of compensatory lung growth is mechanical stress. Stretch stimulation on lung cells induces cAMP expression [26], cell proliferation [28], growth factor production [29], and changes in gene expression, such as early growth response gene-1 [30]. Positive airway pressure induces cell proliferation and extracellular matrix remodeling [31]. The increased blood flow and shear stress in pulmonary capillaries also induce endothelial cell growth and septal remodeling [32]. The free space in the thoracic cavity produced by volume reduction surgery provides mechanical stress to the remaining lung tissue and may promote its growth.
Shigemura et al. performed lung volume reduction surgery in rats and then covered the cut edge of the remaining lung tissue with a polyglycolic acid felt sheet that was coated with cultured adipose tissue-derived stromal cells [33]. After the surgery, alveolar regeneration was accelerated in the area covered by the sheet. HGF that was secreted from the adipose tissue-derived stromal cells played a role in this accelerated lung regrowth after the surgery. This new strategy may improve the outcome of volume reduction surgery for emphysema patients.
Lung Endogenous Stem Cells
In contrast to the increasing reports of mouse lung stem cells [34, 35], knowledge about the endogenous stem/progenitor population of human lung tissue was limited until recently [36, 37]; therefore, the role of these progenitor populations in COPD has not been studied or identified as a target for therapy. Recently, several candidates for human lung stem/progenitor cells have been reported [38, 39]. Analyzing the repair capacity and epigenetic modification of these progenitor populations will provide new understanding about COPD development and a new therapeutic strategy. Furthermore, endogenous progenitors might be a good target for drug discovery.
Alveolar Epithelial Progenitor Cells
The alveolar space is covered with alveolar type I and type II epithelial cells. Type I cells are flattened and cover 95% of the total surface area of the alveoli. Type II cells are cuboidal and secrete surfactant protein to maintain the surface tension of the alveoli. In contrast to their small footprint on the alveolar surface, the number of type II cells is much greater than that of type I cells. Type II cells are believed to be progenitors of type I cells. Type II cell impairment was observed in COPD lungs [40, 41]. However, progenitors for type II cells in human lungs have not previously been reported.
Recently, alveolar epithelial progenitor cells (AEPCs) were isolated from adult human lungs [38]. AEPCs have an epithelial phenotype with a mesenchymal stem cell character. According to a microarray analysis, AEPCs share many genes in common with type II cells and mesenchymal stem cells, which suggests an overlapping phenotype with both the alveolar epithelium and the mesenchyme in these cells. AEPCs were present in alveolar type II cell hyperplasias. The transitional phenotype of AEPCs between the epithelium and mesenchyme suggests that these cells act as lung endogenous stem cells in lung tissue repair. Mesenchymal properties, such as antiapoptotic activity and motility, may allow a functional epithelial progenitor to become involved in alveolar repair in COPD lungs.
c-kit-Positive Human Lung Stem Cells
Kajstura et al. reported that c-kit-positive and lineage-negative cells in adult human lungs demonstrated a stem cell phenotype, and they called these cells human lung stem cells (hLSCs) [39]. hLSCs can differentiate into not only epithelial cells but also mesenchymal and endothelial lineages in injured mouse lungs.
c-Kit is a transmembrane tyrosine kinase receptor, and its expression has been detected in fetal lung development [42, 43]. Binding to its ligand, a stem cell factor, promotes cell proliferation and differentiation [44]. Lindsey et al. determined that c-kit was associated with the development of spontaneous airspace enlargement [45], suggesting its role in COPD.
It is not yet clear whether the naive hLSCs within human lungs have the same capacity as stem cells in situ. The stemness of the hLSCs may be acquired with the cell culture conditions in vitro. Therefore, the presence and characteristics of the hLSCs within human lungs are still under discussion [46–48]. Furthermore, the role of hLSCs in the pathogenesis of COPD is not yet clear.
Cell Therapy
Cell therapies using various stem cells have been extensively evaluated. The lung is one of the easiest organs in which to instill exogenous cells because cells can be applied through both the airway and circulation. In addition, most of the intravenously instilled cells are trapped within the pulmonary circulation; therefore, the efficacy of cell delivery is naturally high.
Mesenchymal stem cells (MSCs) are the most extensively evaluated candidates for clinical cell-based therapy. Many clinical trials using MSCs have been registered and are ongoing. Autologous MSCs are easily isolated from the bone marrow and other tissues. MSCs are expected to reduce inflammation and promote the repair process. These beneficial effects are thought to be based on the ability of MSCs to modulate the immune system and their capacity to produce growth factors and cytokines [49], such as keratinocyte growth factor, HGF, and prostaglandin E2.
Because of these anti-inflammatory effects, a phase II clinical trial using MSCs has been performed in moderate and severe COPD patients [50]. The trial successfully demonstrated the safety of cell therapies using MSCs and some reduction in the inflammatory response in COPD patients but did not show any beneficial effects on lung function. Additional studies, especially in early-stage COPD patients, are needed.
Endothelial progenitor cells (EPCs) have a potential to repair damaged endothelia, and they are another candidate for the cell therapy. Clinical trials using autologous EPCs were conducted in patients with pulmonary hypertension [51]. Because endothelial dysfunction and fewer circulating EPCs are observed in COPD patients [52, 53], repair of damaged vasculature using EPCs could be a good strategy to treat COPD.
Implantation of Fetal Lung Tissue or Stem Cells
Kenzaki et al. implanted fetal lung tissue fragments into adult rat lungs [54]. The implanted lung tissue was connected to the pulmonary circulation, and its alveolar spaces were opened. However, lung fragments obtained from adult rats did not expand after implantation [54]. These observations suggest that premature lung cells and/or growth factors produced from premature cells are key elements for lung regrowth.
Andrade et al. implanted Gelfoam sponges supplemented with fetal rat lung cells into adult rat lungs [55]. The cells inside the implanted sponges formed an alveolar-like structure with neovascularization. The Gelfoam sponges degraded several months after implantation. Although these approaches are experimental and have ethical problems, recent advances in induced pluripotent stem cells may provide reliability in these approaches.
Clinical Prospects for Treating COPD
At this stage, most of the regenerative approaches are experimental and cannot provide completely repaired or restored impaired lung function in COPD patients. We need several breakthroughs in rebuilding a three-dimensional organ architecture and identifying lung stem cell populations involved in COPD development. In the meantime, pragmatic approaches to treat COPD patients with regenerative medicine include (a) using a stem cell sheet after volume reduction surgery to promote regrowth in the remaining lung, and (b) cell therapy using autologous MSCs.
Conclusion
The challenges of lung regeneration have made clear what we know and what we do not know about lungs. The lack of knowledge about the role of lung endogenous stem cells and functional changes in lung cells in COPD limits the development of lung regenerative therapy. The recent discovery of several candidates for lung endogenous stem cells [38, 39] and a new isolation technique for human lung cells [56] will give a better understanding of the COPD lungs, and those fundamentally different approaches will open a new paradigm for future regenerative therapies for COPD patients.
Acknowledgments
This work was supported by Grant 22390163 from the Japan Society for the Promotion of Science (to H.K.).
Author Contributions
H.K.: conception and design, financial support, manuscript writing.
Disclosure of Potential Conflicts of Interest
The author indicates no potential conflicts of interest.
References
- 1.Schittny JC, Mund SI, Stampanoni M. Evidence and structural mechanism for late lung alveolarization. Am J Physiol Lung Cell Mol Physiol. 2008;294:L246–L254. doi: 10.1152/ajplung.00296.2007. [DOI] [PubMed] [Google Scholar]
- 2.Narayanan M, Owers-Bradley J, Beardsmore CS, et al. Alveolarization continues during childhood and adolescence: New evidence from 3He magnetic resonance. Am J Respir Crit Care Med. 2012;185:186–191. doi: 10.1164/rccm.201107-1348OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakajima C, Kijimoto C, Yokoyama Y, et al. Longitudinal follow-up of pulmonary function after lobectomy in childhood: Factors affecting lung growth. Pediatr Surg Int. 1998;13:341–345. doi: 10.1007/s003830050334. [DOI] [PubMed] [Google Scholar]
- 4.Hsia CC, Herazo LF, Fryder-Doffey F, et al. Compensatory lung growth occurs in adult dogs after right pneumonectomy. J Clin Invest. 1994;94:405–412. doi: 10.1172/JCI117337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeda S, Hsia CC, Wagner E, et al. Compensatory alveolar growth normalizes gas-exchange function in immature dogs after pneumonectomy. J Appl Physiol. 1999;86:1301–1310. doi: 10.1152/jappl.1999.86.4.1301. [DOI] [PubMed] [Google Scholar]
- 6.Ding B-S, Nolan DJ, Guo P, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147:539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roisin R. Global Strategy for the Diagnosis, Management and Prevention of COPD. [Accessed April 28, 2010]. Available at http://www.goldcopd.com.
- 8.Massaro GD, Massaro D. Retinoic acid treatment abrogates elastase-induced pulmonary emphysema in rats. Nat Med. 1997;3:675–677. doi: 10.1038/nm0697-675. [DOI] [PubMed] [Google Scholar]
- 9.Ishizawa K, Kubo H, Yamada M, et al. Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett. 2004;556:249–252. doi: 10.1016/s0014-5793(03)01399-1. [DOI] [PubMed] [Google Scholar]
- 10.Ishizawa K, Kubo H, Yamada M, et al. Hepatocyte growth factor induces angiogenesis in injured lungs through mobilizing endothelial progenitor cells. Biochem Biophys Res Commun. 2004;324:276–280. doi: 10.1016/j.bbrc.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 11.Hegab AE, Kubo H, Yamaya M, et al. Intranasal HGF administration ameliorates the physiologic and morphologic changes in lung emphysema. Mol Ther. 2008;16:1417–1426. doi: 10.1038/mt.2008.137. [DOI] [PubMed] [Google Scholar]
- 12.Murakami S, Nagaya N, Itoh T, et al. Adrenomedullin regenerates alveoli and vasculature in elastase-induced pulmonary emphysema in mice. Am J Respir Crit Care Med. 2005;172:581–589. doi: 10.1164/rccm.200409-1280OC. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi S, Nakamura H, Seki M, et al. Reversal of elastase-induced pulmonary emphysema and promotion of alveolar epithelial cell proliferation by simvastatin in mice. Am J Physiol Lung Cell Mol Physiol. 2008;294:L882–L890. doi: 10.1152/ajplung.00238.2007. [DOI] [PubMed] [Google Scholar]
- 14.Massaro GD, Massaro D. Postnatal treatment with retinoic acid increases the number of pulmonary alveoli in rats. Am J Physiol. 1996;270:L305–L310. doi: 10.1152/ajplung.1996.270.2.L305. [DOI] [PubMed] [Google Scholar]
- 15.Malpel S, Mendelsohn C, Cardoso WV. Regulation of retinoic acid signaling during lung morphogenesis. Development. 2000;127:3057–3067. doi: 10.1242/dev.127.14.3057. [DOI] [PubMed] [Google Scholar]
- 16.McGowan S, Jackson SK, Jenkins-Moore M, et al. Mice bearing deletions of retinoic acid receptors demonstrate reduced lung elastin and alveolar numbers. Am J Respir Cell Mol Biol. 2000;23:162–167. doi: 10.1165/ajrcmb.23.2.3904. [DOI] [PubMed] [Google Scholar]
- 17.Liu B, Harvey CS, McGowan SE. Retinoic acid increases elastin in neonatal rat lung fibroblast cultures. Am J Physiol. 1993;265:L430–L437. doi: 10.1152/ajplung.1993.265.5.L430. [DOI] [PubMed] [Google Scholar]
- 18.Kubo H. Lung repair and regeneration: Animal models. In: Polak DJ, editor. Cell Therapy for Lung Disease. London, U.K.: Imperial College Press; 2010. pp. 199–235. [Google Scholar]
- 19.Mao JT, Goldin JG, Dermand J, et al. A pilot study of all-trans-retinoic acid for the treatment of human emphysema. Am J Respir Crit Care Med. 2002;165:718–723. doi: 10.1164/ajrccm.165.5.2106123. [DOI] [PubMed] [Google Scholar]
- 20.Roth MD, Connett JE, D'Armiento JM, et al. Feasibility of retinoids for the treatment of emphysema study. Chest. 2006;130:1334–1345. doi: 10.1378/chest.130.5.1334. [DOI] [PubMed] [Google Scholar]
- 21.Mao JT, Tashkin DP, Belloni PN, et al. All-trans retinoic acid modulates the balance of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in patients with emphysema. Chest. 2003;124:1724–1732. doi: 10.1378/chest.124.5.1724. [DOI] [PubMed] [Google Scholar]
- 22.Stolk J, Stockley RA, Stoel BC, et al. Randomized controlled trial for emphysema with a selective agonist of the gamma type retinoic acid receptor. Eur Respir J. doi: 10.1183/09031936.00161911. [DOI] [PubMed] [Google Scholar]
- 23.Jones PW, Rames D. TESRA (Treatment of Emphysema with a Selective Retinoid Agonist) study results. Am J Respir Crit Care Med. 2011;183:A6418. [Google Scholar]
- 24.Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. New Engl J Med. 2003;348:2059–2073. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 25.Naunheim KS, Wood DE, Mohsenifar Z, et al. Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann Thorac Surg. 2006;82:431–443. doi: 10.1016/j.athoracsur.2006.05.069. [DOI] [PubMed] [Google Scholar]
- 26.Nijjar MS, Thurlbeck WM. Alterations in enzymes related to adenosine 3′,5′-monophosphate during compensatory growth of rat lung. Eur J Biochem. 1980;105:403–407. doi: 10.1111/j.1432-1033.1980.tb04514.x. [DOI] [PubMed] [Google Scholar]
- 27.Cowan MJ, Crystal RG. Lung growth after unilateral pneumonectomy: Quantitation of collagen synthesis and content. Am Rev Respir Dis. 1975;111:267–277. doi: 10.1164/arrd.1975.111.3.267. [DOI] [PubMed] [Google Scholar]
- 28.Chess PR, Toia L, Finkelstein JN. Mechanical strain-induced proliferation and signaling in pulmonary epithelial H441 cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L43–L51. doi: 10.1152/ajplung.2000.279.1.L43. [DOI] [PubMed] [Google Scholar]
- 29.Waters CM, Chang JY, Glucksberg MR, et al. Mechanical forces alter growth factor release by pleural mesothelial cells. Am J Physiol. 1997;272:L552–L557. doi: 10.1152/ajplung.1997.272.3.L552. [DOI] [PubMed] [Google Scholar]
- 30.Landesberg LJ, Ramalingam R, Lee K, et al. Upregulation of transcription factors in lung in the early phase of postpneumonectomy lung growth. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1138–L1149. doi: 10.1152/ajplung.2001.281.5.L1138. [DOI] [PubMed] [Google Scholar]
- 31.Berg JT, Fu Z, Breen EC, et al. High lung inflation increases mRNA levels of ECM components and growth factors in lung parenchyma. J Appl Physiol. 1997;83:120–128. doi: 10.1152/jappl.1997.83.1.120. [DOI] [PubMed] [Google Scholar]
- 32.Haworth SG, McKenzie SA, Fitzpatrick ML. Alveolar development after ligation of left pulmonary artery in newborn pig: Clinical relevance to unilateral pulmonary artery. Thorax. 1981;36:938–943. doi: 10.1136/thx.36.12.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shigemura N, Sawa Y, Mizuno S, et al. Induction of compensatory lung growth in pulmonary emphysema improves surgical outcomes in rats. Am J Respir Crit Care Med. 2005;171:1237–1245. doi: 10.1164/rccm.200411-1518OC. [DOI] [PubMed] [Google Scholar]
- 34.Kim CF, Jackson EL, Woolfenden AE, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 35.Chapman HA, Li X, Alexanderet JP, et al. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest. 2011;121:2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blaisdell CJ, Gail DB, Nabel EG. National Heart, Lung, and Blood Institute perspective: Lung progenitor and stem cells—gaps in knowledge and future opportunities. Stem Cells. 2009;27:2263–2270. doi: 10.1002/stem.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McQualter JL, Bertoncello I. Concise review: Deconstructing the lung to reveal its regenerative potential. Stem Cells. 2012;30:811–816. doi: 10.1002/stem.1055. [DOI] [PubMed] [Google Scholar]
- 38.Fujino N, Kubo H, Suzuki T, et al. Isolation of alveolar epithelial type II progenitor cells from adult human lungs. Lab Invest. 2011;91:363–378. doi: 10.1038/labinvest.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kajstura J, Rota M, Hall SR, et al. Evidence for human lung stem cells. New Engl J Med. 2011;364:1795–1806. doi: 10.1056/NEJMoa1101324. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Yokohori N, Aoshiba K, Nagai A. Increased levels of cell death and proliferation in alveolar wall cells in patients with pulmonary emphysema. Chest. 2004;125:626–632. doi: 10.1378/chest.125.2.626. [DOI] [PubMed] [Google Scholar]
- 41.Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med. 2006;174:886–893. doi: 10.1164/rccm.200509-1374OC. [DOI] [PubMed] [Google Scholar]
- 42.Chang KT, Rajadurai VS, Walford NQ, et al. Alveolar capillary dysplasia: Absence of CD117 immunoreactivity of putative hemangioblast precursor cells. Fetal Pediatr Pathol. 2008;27:127–140. doi: 10.1080/15513810802077594. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki T, Kubo H, Fujino N, et al. Dynamic and multilineage expression of c-kit in developing human lungs. Am J Respir Crit Care Med. 2010;181:A4898. [Google Scholar]
- 44.Lennartsson J, Jelacic T, Linnekin D, et al. Normal and oncogenic forms of the receptor tyrosine kinase kit. Stem Cells. 2005;23:16–43. doi: 10.1634/stemcells.2004-0117. [DOI] [PubMed] [Google Scholar]
- 45.Lindsey JY, Ganguly K, Brass DM, et al. c-Kit is essential for alveolar maintenance and protection from emphysema-like disease in mice. Am J Respir Crit Care Med. 2011;183:1644–1652. doi: 10.1164/rccm.201007-1157OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 46.Lung stem cells: Looking beyond the hype. Nat Med. 2011;17:788–789. doi: 10.1038/nm0711-788. [DOI] [PubMed] [Google Scholar]
- 47.Anversa P, Kajstura J, Leri A, et al. Tissue-specific adult stem cells in the human lung. Nat Med. 2011;17:1038–1039. doi: 10.1038/nm.2463. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki T, Kondo T, Kubo H. Evidence for human lung stem cells. New Engl J Med. 2011;365:465. doi: 10.1056/NEJMc1106693. author reply 465–466. [DOI] [PubMed] [Google Scholar]
- 49.Salem HK, Thiemermann C. Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osiris. Osiris Therapeutics Reports interim data for COPD stem cell study 2009. [Accessed May 17, 2012]. Available at: http://osir.client.shareholder.com/releasedetail.cfm?ReleaseID=391580.
- 51.Fadini GP, Avogaro A, Ferraccioli G, et al. Endothelial progenitors in pulmonary hypertension: New pathophysiology and therapeutic implications. Eur Respir J. 2010;35:418–425. doi: 10.1183/09031936.00112809. [DOI] [PubMed] [Google Scholar]
- 52.Fadini GP, Schiavon M, Cantiniet M, et al. Circulating progenitor cells are reduced in patients with severe lung disease. Stem Cells. 2006;24:1806–1813. doi: 10.1634/stemcells.2005-0440. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi T, Suzuki S, Kubo H, et al. Impaired endothelial progenitor cell mobilization and colony-forming capacity in chronic obstructive pulmonary disease. Respirology. 2011;16:680–687. doi: 10.1111/j.1440-1843.2011.01959.x. [DOI] [PubMed] [Google Scholar]
- 54.Kenzaki K, Sakiyama S, Kondo K, et al. Lung regeneration: Implantation of fetal rat lung fragments into adult rat lung parenchyma. J Thorac Cardiovasc Surg. 2006;131:1148–1153. doi: 10.1016/j.jtcvs.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 55.Andrade CF, Wong AP, Waddell TK, et al. Cell-based tissue engineering for lung regeneration. Am J Physiol Lung Cell Mol Physiol. 2007;292:L510–L518. doi: 10.1152/ajplung.00175.2006. [DOI] [PubMed] [Google Scholar]
- 56.Fujino N, Kubo H, Ota C, et al. A novel method for isolating individual cellular components from the adult human distal lung. Am J Respir Cell Mol Biol. 2012;46:422–430. doi: 10.1165/rcmb.2011-0172OC. [DOI] [PubMed] [Google Scholar]