This study examined the effects and signaling pathways associated with the actions of 1α,25-dihydroxyvitamin D3 (VD3) on dermal papilla cells (DPCs). Results suggest that VD3 may promote functional differentiation of DPCs and be useful in preserving the hair follicle-inductive capacity of cultured DPCs for hair regeneration therapies.
Keywords: Cell culture, Cell signaling, Cellular therapy, Clinical translation, Gene expression, Signal transduction, Stem cell-microenvironment interactions, Tissue regeneration
Abstract
Dermal papilla cells (DPCs) have the potential to induce differentiation of epithelial stem cells into hair, and Wnt signaling is deeply involved in the initiation process. The functional limitation of expanded adult DPCs has been a difficult challenge for cell-based hair regrowth therapy. We previously reported that 1α,25-dihydroxyvitamin D3 (VD3) upregulates expression of transforming growth factor (TGF)-β2 and alkaline phosphatase (ALP) activity, both features of hair-inducing human DPCs (hDPCs). In this study, we further examined the effects and signaling pathways associated with VD3 actions on DPCs. VD3 suppressed hDPC proliferation in a dose-dependent, noncytotoxic manner. Among the Wnt-related genes investigated, Wnt10b expression was significantly upregulated by VD3 in hDPCs. Wnt10b upregulation, as well as upregulation of ALPL (ALP, liver/bone/kidney) and TGF-β2, by VD3 was specific in hDPCs and not detected in human dermal fibroblasts. Screening of paracrine or endocrine factors in the skin indicated that all-trans retinoic acid (atRA) upregulated Wnt10b gene expression, although synergistic upregulation (combined atRA and VD3) was not seen. RNA interference with vitamin D receptor (VDR) revealed that VD3 upregulation of Wnt10b, ALPL, and TGF-β2 was mediated through the genomic VDR pathway. In a rat model of de novo hair regeneration by murine DPC transplantation, pretreatment with VD3 significantly enhanced hair folliculogenesis. Specifically, a greater number of outgrowing hair shafts and higher maturation of regenerated follicles were observed. Together, these data suggest that VD3 may promote functional differentiation of DPCs and be useful in preserving the hair follicle-inductive capacity of cultured DPCs for hair regeneration therapies.
Introduction
Dermal papilla cells (DPCs) have follicle-inductive ability through interactions with neighboring epithelial components. Various trichogenic assays, using epithelial and mesenchymal cells, have indicated the therapeutic potential of folliculogenesis [1–8]. However, no successful clinical trials have yet been reported. One major reason for this is that human DPCs (hDPCs) lose their hair-inductive capacity after long-term culture [5, 9, 10]. Optimization of culture media and related methods to efficiently expand hDPCs while maintaining their hair-inductive capacity has been a major challenge and is a prerequisite for future cell-based therapies for alopecia [11]. Findings reported in past literature suggest steps that need to be taken toward successful mitigation of the DPC expansion problem. One step is the use of conditioned medium obtained from epidermal keratinocyte culture (keratinocyte-conditioned medium [KCM]) [12]. KCM or coculture with epidermal keratinocytes has been also shown to stimulate proliferation of mesenchymal cells, including DPCs [12, 13]. In addition, bone morphogenetic protein-6 [14] and basic fibroblast growth factor [15] were reported to aid in DPC expansion cultures for preserving the hair-inductive capacity.
The Wnt/β-catenin signaling pathway leads to placode formation during embryogenesis [16] and activates downstream the transcription factor/lymphoid enhancer-binding factor 1 transcription complex. This complex is activated in both the epidermis and dermis during hair development [17]. Wnt-3a-treated DPCs have a higher capacity to induce hair formation in engraftment assays [18]. Although they were not used as an in vitro pretreatment for cultured DPCs, Wnt-7a and Wnt-10b have been shown to promote hair folliculogenesis in in vivo assays [19, 20]. Other strategies to preserve the hair-inductive properties of DPCs include growing them in three-dimensional aggregates [15, 21] and culturing them together with keratinocytes on extracellular matrix substrates [22] in order to mimic the in vivo microenvironment [11].
We previously examined the global gene signatures of hDPCs and human dermal fibroblasts (hDFs) by microarray analysis. This work revealed that transforming growth factor (TGF)-β2, known to be required for folliculogenesis [23], was one of the genes specifically upregulated in hDPCs [24]. TGF-β2 expression in hDPCs decreased after long-term culture but was elevated by KCM and was shown to correlate with the hair-inductive capacity of DPCs. We also found that among paracrine factors from keratinocytes, 1α,25-dihydroxyvitamin D3 (VD3; also known as calcitriol) upregulated TGF-β2 gene expression in hDPCs. VD3, likewise, upregulated alkaline phosphatase (ALP) activity [24], which is used as an index for the hair-inductive capacity of DPCs [14, 25, 26].
VD3, the most biologically active form of vitamin D, is a final synthetic product generated in kidney by 1α-hydroxylase [27] and is systemically distributed in an endocrine manner (and thus sometimes classified as vitamin D hormone) [28, 29]. Vitamin D is classically known to maintain calcium and bone homeostasis, but VD3 also has been found to regulate, directly and/or indirectly, 0.8%–5% of all genes [30] and to be involved in various biological actions, such as apoptosis, suppression of cell growth, promotion of differentiation, and immunomodulation [28, 31]. Recently, much attention has been paid to the preventive or regressive effects of VD3 on cancers, including colorectal, breast, prostate, renal, ovarian, blood, and skin cancers [32, 33].
Previous reports have described the effects of vitamin D on hair follicles. Topical pretreatment of VD3 enhanced hair regrowth in a mouse model of chemotherapy-induced alopecia [34]. Nuclear vitamin D receptor (VDR)-null-mutant mice were reported to develop alopecia and poor whiskers, although they had normal hair until weaning after birth [35, 36]. This suggests that such mice can develop a normal first coat of hair but cannot regulate postnatal hair cycles [30, 37]. In humans, mutations in the VDR coding gene are known to cause hereditary vitamin D-resistant rickets with alopecia [38, 39]. However, detailed effects of VD3 on DPCs have not been elucidated. In this study, we focused on the biological effects of VD3 on DPCs. Specifically, we tested whether VD3 may be useful in preserving or promoting the hair-inductive capacity of DPCs in expansion cultures and, ultimately, in developing a reliable cell-based therapy for hair regeneration.
Materials and Methods
Human and Rat DPC and Dermal Fibroblast Cultures
Human scalp skin samples with hair and periauricular facial skin were obtained from patients undergoing facelift surgery. Informed consent was obtained from patients using a protocol approved by the ethical committee of the University of Tokyo School of Medicine. Dermal papillae were isolated from the hair follicles under a stereomicroscope using a needle and fine forceps. The isolated tissue was then attached to scratched scars made on a bottom of a plastic culture dish containing Dulbecco's modified Eagle's medium (DMEM) (Gibco, Carlsbad, CA, http://www.invitrogen.com) supplemented with 10% fetal bovine serum (FBS). After 2 weeks of explant culture, hDPCs were subcultivated with the same medium, followed by cryopreservation at −80°C. DPCs at passages 3–4 were used for each experiment. For preparation of murine DPCs (mDPCs), whisker follicles of male Fisher 344 rats (6 weeks old) were used, and mDPCs were obtained with the same method as described for hDPCs.
For preparation of hDFs and murine dermal fibroblasts (mDFs), human facial skin and glabrous rat sole skin of F344 rats were excised with a 4-mm-diameter round scalpel. The round piece of skin was incubated in phosphate-buffered saline plus 1,000 U/ml dispase (Dispase II; EIDIA, Tokyo, Japan, http://www.eidia.co.jp/en) at 37°C for 20 minutes to separate the epidermis from the dermis. Then, the explant culture was performed by attaching the separated dermis to the bottom of a plastic culture dish. The remainder of the procedure was the same as described for hDPCs.
Reagents
The supplements given to hDPC cultures, as paracrine or endocrine factors, were acidic fibroblast growth factor (Peprotech, Rocky Hill, NJ, http://www.peprotech.com), basic fibroblast growth factor (Peprotech), interleukin (IL)-1β (Thermo Scientific [formerly Endogen], Rockford, IL, http://www.thermoscientific.com), IL-6 (Peprotech), IL-8 (Wako Chemical, Osaka, Japan, http://www.wako-chem.co.jp/english), vascular endothelial growth factor (Wako), platelet-derived growth factor-BB (Wako), nerve growth factor (Sigma-Aldrich, St. Louis, http://www.sigmaaldrich.com), heparin-binding epidermal growth factor-like growth factor (Peprotech), macrophage inflammatory protein-3α (R&D Systems Inc., Minneapolis, http://www.rndsystems.com), monocyte chemotactic protein-1 (R&D Systems), insulin-like growth factor-1 (Sigma-Aldrich), epithelial cell-derived neutrophil-activating peptide-78 (Wako), growth-related oncogene-α (Wako), VD3 (LKT Laboratories, St. Paul, MN, http://www.lktlabs.com), cholesterol sulfate (Sigma-Aldrich), all-trans retinoic acid (atRA) (BIOMOL International LP, Hamburg, Germany, http://www.biomol.com), 17β-estradiol (Cayman Chemicals, Ann Arbor, MI, http://www.caymanchem.com), dihydrotestosterone (BIOMOL), and ketoconazole (KCZ) (Tokyo Chemical Industry, Tokyo, Japan, http://www.tcichemicals.com). All reagents were diluted in phosphate-buffered saline or ethanol to 1,000-fold the final working concentration and stored in aliquots at −20°C. TEI-9647 (TEI) was a kind gift from Teijin Pharma, Ltd. (Tokyo, Japan, http://www.teijin-pharma.co.jp/english/index.html).
Proliferation Assay for hDPCs
To examine the proliferative effect of VD3, hDPCs were seeded at a density of 1 × 104 cells per well in a 24-well plate and cultured in DMEM/10% FBS supplemented with VD3 (0, 1, 10, or 100 nM). After 1, 2, 3, 5, and 7 days of culture, the number of cells was counted using a cell counter (Sunlead Glass Corp., Saitama, Japan, http://sunleadglass.com). 5-Bromo-2′-deoxyuridine (BrdU) incorporation was quantified using a cell proliferation enzyme-linked immunosorbent assay BrdU kit (Roche Diagnostics, Mannheim, Germany, http://www.roche-applied-science.com). Human DPCs were seeded at a density of 3 × 103 cells per well in a 96-well plate. After 24 hours of BrdU incorporation, the intensity of chemiluminescence was measured with a plate reader (DTX 880 Multimode Detector; Beckman Coulter, Fullerton, CA, http://www.beckmancoulter.com) according to the manufacturer's instructions.
Cell Death Assay for hDPCs
Human DPCs were seeded at a density of 2 × 104 cells per 35-mm dish and cultured for 5 days. Cells were stained with Hoechst 33342 (Dojindo, Kumamoto, Japan, http://www.dojindo.co.jp), Annexin V-fluorescein isothiocyanate (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com), and propidium iodide (PI) (Sigma-Aldrich). This staining distinguished three types of nucleated cells: viable hDPCs (Hoechst+/Annexin−/PI−), apoptotic hDPCs (Hoechst+/Annexin+/PI−), and necrotic hDPCs (Hoechst+/Annexin+/PI+). The number of cells of each population type was counted using a ×8 objective lens, using four randomly selected fields per dish.
Real-Time Reverse Transcription-Polymerase Chain Reaction
After hDPCs were seeded at 3 × 103 cells per cm2 and cultured until subconfluence (5–7 days), total RNA was isolated from each sample using the RNeasy Mini Kit (Qiagen, Valencia, CA, http://www.qiagen.com), followed by a reverse transcription reaction step. Quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR) was performed with the ABI 7700 sequence detection system and SYBR Green PCR master mix (both from Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com). Primer sequences used are shown in supplemental online Table 1. Expression levels were calculated by the comparative CT method using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an endogenous reference gene, or are shown as a ratio compared with GAPDH.
Immunocytochemical Staining
For immunocytochemical staining, human DPCs were seeded at a density of 2,000 cells per well in an eight-well chamber slide (Lab-Tek; Nalge Nunc International, Roskilde, Denmark, http://www.nalgenunc.com) and cultured in DMEM containing 10% FBS, supplemented with VD3 (0, 10, or 100 nM). After incubation for 5 days, cells were fixed in 4% paraformaldehyde for 1 minute and washed in phosphate-buffered saline for 5 minutes. Cells were then incubated with 5% goat serum at room temperature for 30 minutes and incubated with anti-Wnt10b antibody (dilution, 1:100; R&D Systems) at room temperature for 60 minutes. Isotype antibody (normal goat immunoglobulin G [IgG]) was used as a negative control. For staining visualization, a secondary antibody, Alexa Fluor 546-conjugated rabbit anti-goat IgG (dilution, 1:200; Molecular Probes, Eugene, OR, http://probes.invitrogen.com), was used. Counterstaining was performed with Hoechst 33342.
Retroviral Short Hairpin RNA Against Endogenous VDR
Five sets of sense and antisense oligos targeting human VDR mRNA were designed and purchased from Operon Biotechnologies (Huntsville, AL, http://www.operon.com). Oligos with random sequences were used as negative controls. Each set of oligos was annealed to make double-stranded DNA and ligated into a multicloning site of the pSIREN retroQ ZsGreen vector (Clontech, Mountain View, CA, http://www.clontech.com). The sense sequences of six sets of oligos are shown in supplemental online Table 2. To prepare retroviral media, short hairpin RNA (shRNA) vectors were transfected into a platinum retrovirus expression system, amphotropic (Cell Biolabs, San Diego, CA, http://www.cellbiolabs.com), according to the manufacturer's instructions. Human DPCs (passage 2) were infected in the viral media for 8 hours. After subcultivating, ZsGreen fluorescence-positive cells were sorted by a flow cytometric cell sorter (FACSAria; BD Biosciences) and further expanded for gene expression analyses.
Western Blotting
To examine which sequence interfered with mRNA most effectively, each shRNA vector and flag-tagged human VDR-expressing vector (pVDR; a kind gift from Dr. Eiji Ochiai, Teijin Pharma Ltd.) was cotransfected into the HEK293 cell line. Transfection efficiency of shRNA was evaluated by ZsGreen fluorescence. HEK293 cells were prepared in a 60-mm dish with DMEM/10% FBS and transiently transfected with both pVDR and shRNA vectors. Transfection was performed using Lipofectamine LTX and PLUS regent (Invitrogen, Carlsbad, CA, http://www.invitrogen.com). Three days after transfection, whole cell lysate was collected using radioimmunoprecipitation assay buffer (Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com). Protein concentration was determined using the bicinchoninic acid protein assay kit (Pierce, Rockford, IL, http://www.piercenet.com), and equal amounts of protein (10 μg) were loaded into each sample lane of a sodium dodecyl sulfate polyacrylamide gel electrophoresis gel. The resolved proteins were transferred to a polyvinylidene fluoride membrane (Bio-Rad, Hercules, CA, http://www.bio-rad.com), followed by immunostaining using rabbit anti-flag antibody (dilution: 1:1,000; Sigma-Aldrich) and horseradish peroxidase-conjugated goat anti-rabbit IgG (dilution 1:5,000; R&D Systems).
In Vivo Assay for De Novo Hair Folliculogenesis
All animal experiments were performed with approval from the Institutional Animal Care and Use Committee at the University of Tokyo. A transplantation method of DPCs termed the hemivascularized sandwich (HVS) method, which we recently reported [8], was used for de novo regeneration of hair follicles. Briefly, a circular split-thickness of rat's glabrous sole skin (8-week-old F344 rat) was cut off with a round scalpel and digested to separate the epidermis from the dermal compartment. The epidermis was replaced on the remaining deep dermis, in the donor hole, after a cultured DPC construct (a cell sheet fragment of rat DPCs) was grafted.
For preparation of the DPC construct, mDPCs (passage 2) derived from F344 rat whiskers were seeded at 4,000 cells per well in a 96-well plate and cultured in DMEM/10% FBS. After 7 days of culture, expanded mDPCs reached confluence. For preparation of the control construct, mDFs (passage 2) derived from F344 rat hairless sole skin were used. VD3 (100 nM) was then supplemented for 0, 24, or 72 hours into the DPC culture before transplantation (n = 8 for each group), whereas VD3 was used for 0 or 72 hours in the dermal fibroblast (DF) culture. The mDPC and mDF sheets were scraped off the 96-well plate with a rubber scraper. One fragment of the mDPC or mDF sheet was used as a graft for the HVS method.
Histological Evaluation and Scoring of Regenerated Hair Follicles
Eight weeks after grafting the mDPC sheets or mDF sheets, rat sole skin was harvested and subjected to histological examination. Skin samples were embedded in O.C.T. compound (Tissue-Tek; Sakura Finetek, Tokyo, Japan, http://www.sakura.com), frozen in liquid nitrogen, and cut into 10-μm-thick sections. Every other section was processed for hematoxylin and eosin staining, and the remaining sections were stained with Hoechst 33342 (Dojindo). Stained sections were observed and photographed under a fluorescence microscope (BioZero; Keyence, Tokyo, Japan, http://www.keyence.com).
Hair follicle regeneration was evaluated histologically and classified into eight stages of developmental hair follicle maturation, according to a previously described method [40]. In addition, follicles were divided in each stage into regulated or dysregulated follicles, as we have previously reported [8] to classify regenerated follicles. In contrast to regenerated follicles, showing normal developmental stages, dysregulated regenerated follicles showed atypical morphology, including multiply fused follicles.
Inhibition of Wnt Signaling with Wnt-Inhibitory Factor-1
Wnt inhibitory factor-1 (Wif-1) (R&D Systems) was used to inhibit Wnt signaling, although Wif-1 does not specifically inhibit Wnt10b effects. Wif-1 (0, 10, 100, and 1,000 nM) was added to culture media containing VD3 (100 nM) for 48 hours to examine whether VD3-induced upregulation of ALPL (ALP, liver/bone/kidney) and TGF-β2 gene expression in hDPCs is mediated by Wnt signaling.
Statistical Analysis
Data were expressed as mean ± SEM. The unpaired Student's t test was used to compare data between two groups. Comparisons of more than two groups were done by analysis of variance with the Bonferroni correction. A value of p < .05 was considered significant.
Results
VD3 Effects on Proliferation of Cultured hDPCs
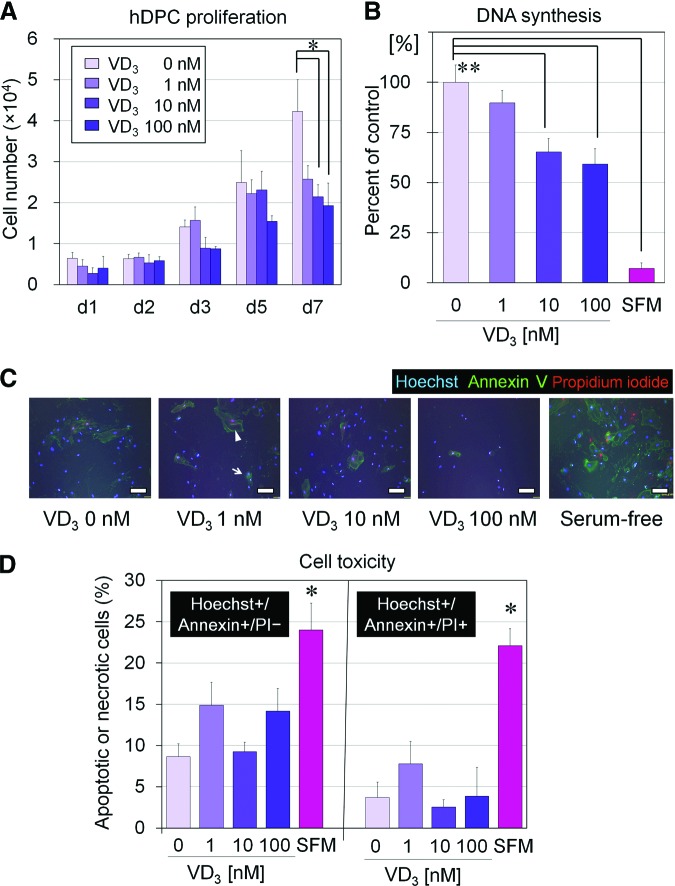
During the 7 days of culture, the number of expanded hDPCs was smaller in VD3-supplemented media (1, 10, and 100 nM) than in the control media, and the difference was statistically significant on day 7 (Fig. 1A). BrdU incorporation assay confirmed the suppression of hDPC proliferation by VD3, with significant suppression observed at 10 and 100 nM (Fig. 1B). To assess whether the proliferation-suppressive effect of VD3 resulted from a cytotoxic effect on hDPCs, immunocytochemical staining for apoptosis (Hoechst+/Annexin+/PI−) and necrosis (Hoechst+/Annexin+/PI+) was performed (Fig. 1C, 1D). Representative images of an apoptotic cell and a necrotic cell are shown in Figure 1C by the arrow and arrowhead, respectively. As expected, serum-free medium induced significantly higher percentages of apoptotic and necrotic cells compared with VD3-free serum-containing medium. Importantly, there were no significant differences among apoptotic (or necrotic) cells in VD3-free medium versus VD3-containing media, regardless of VD3 concentration. Thus, the proliferation-suppressive effect of VD3 did not result from a cytotoxic effects of VD3 on hDPCs.
Figure 1.
VD3 effects on proliferation of cultured hDPCs. (A): Proliferation of hDPCs cultured for 1–7 days (d1, d2, d3, d5, and d7) in Dulbecco's modified Eagle's medium (DMEM)/10% fetal bovine serum (FBS) supplemented with various concentrations of VD3 (0, 1, 10, or 100 nM) (n = 3). *, Significant differences from VD3-free (0 nM) medium (p < .05). (B): DNA synthesis (5-bromo-2′-deoxyuridine incorporation) compared from hDPCs cultured in DMEM/10% FBS in the presence of various concentration of VD3 (0, 1, 10, or 100 nM) or in DMEM/SFM (n = 4). **, Significant differences from the control (VD3-free [0 nM] serum-containing medium) (p < .01). (C): Representative images of immunocytochemical staining of cultured hDPCs with Hoechst 33342, Annexin V, and PI. Arrow and arrowhead show representative images of an apoptotic cell and a necrotic cell, respectively. Scale bars = 50 μm. (D): Percentage of Hoechst+/Annexin+/PI− (apoptotic) and Hoechst+/Annexin+/PI+ (necrotic) cells cultured in media with each VD3 concentration. SFM showed a significantly higher apoptotic or necrotic cell number compared with VD3-free medium. However, there were no significant differences among hDPCs cultured with different VD3 doses (n = 4). *, Significant difference from VD3-free medium (p < .05). Abbreviations: d, day; hDPC, human dermal papilla cell; PI, propidium iodide; SFM, serum-free medium; VD3, 1α,25-dihydroxyvitamin D3.
VD3 Effects on Wnt-Related Gene Expression in Cultured hDPCs
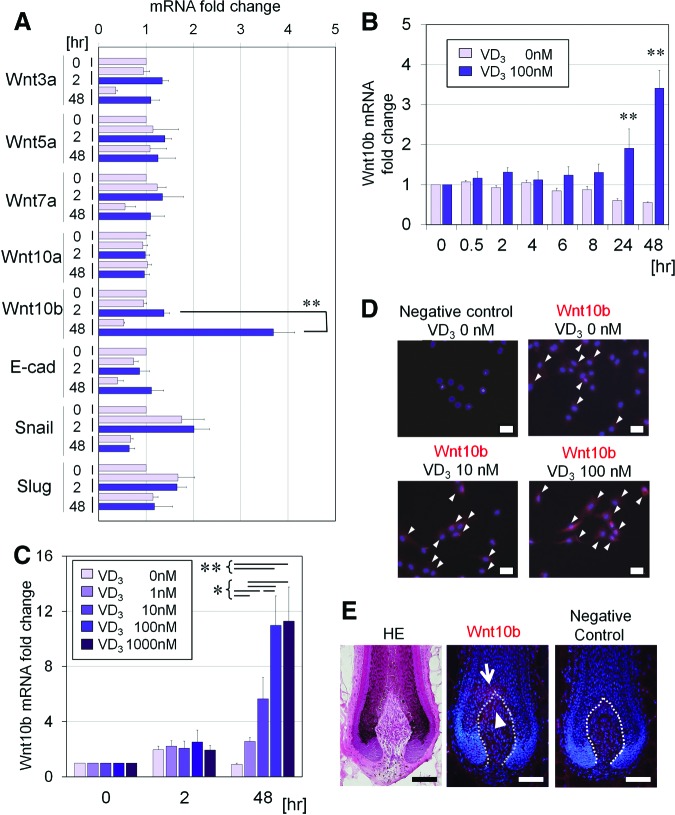
We investigated VD3 effects on expression of various Wnt-related genes (Wnt3a, Wnt5a, Wnt7a, Wnt10a, Wnt10b, E-cadherin, snail, and slug). Only Wnt10b was significantly upregulated by VD3 treatment compared with the control treatment (Fig. 2A). Kinetic analysis showed that Wnt10b mRNA was upregulated after 24 hours of culture (Fig. 2B) and in a dose-dependent manner up to 100 nM (Fig. 2C). Immunocytochemical staining after 3 days of treatment with VD3 confirmed that Wnt10b protein expression in hDPCs was also upregulated in a dose-dependent manner (Fig. 2D).
Figure 2.
Effect of VD3 on expression of Wnt-related genes in cultured human dermal papilla cells (hDPCs). (A): Gene expression of Wnt3a, Wnt5a, Wnt7a, Wnt10a, Wnt10b, E-cad, snail, and slug was examined in hDPCs cultured in Dulbecco's modified Eagle's medium (DMEM)/10% fetal bovine serum (FBS) for 0, 2, or 48 hours, with or without VD3 (100 nM). Data are shown as fold change compared with baseline (n = 4). **, Significant differences between pairs (p < .01). (B): Kinetic analysis (0, 2, 4, 6, 8, 24, or 48 hours) of Wnt10b mRNA relative expression in hDPCs cultured in DMEM/10% FBS, supplemented with or without VD3 (100 nM). Data are shown as fold changes compared with the baseline expression (n = 4). **, Significant differences between conditions (with and without VD3), at each incubation time point (p < .01). (C): Dose-dependent effects (0, 1, 10, 100, or 1,000 nM) of VD3 on Wnt10b mRNA expression, in hDPCs cultured for 0, 2, or 48 hours (n = 4). Significant differences between groups, at each incubation period, are shown as * (p < .05) or ** (p < .01). (D): Representative images of immunocytochemical staining for Wnt10b in hDPCs cultured with various concentrations of VD3 (0, 10, or 100 nM) for 3 days. Goat IgG was used as a negative control. Arrowheads indicate Wnt10b protein expression. Scale bars = 50 μm. (E): Representative serial sections of the intact human scalp hair follicles. Shown are HE staining (left), immunohistochemical staining for Wnt10b (middle), and negative control with goat IgG (right). Wnt10b was expressed in the top portion of human dermal papilla (arrowhead) and the adjacent hair matrix (arrow). Scale bars = 100 μm. Abbreviations: E-cad, E-cadherin; HE, hematoxylin/eosin; VD3, 1α,25-dihydroxyvitamin D3.
VD3 Effects on Gene Expression of 24-Hydroxylase, ALPL, and TGF-β2 in Cultured hDPCs
VD3 is catabolized into an inactive form, calcitric acid, by 24-hydroxylase (24(OH)ase). The promoter of human 24(OH)ase has a vitamin D-responsive element (VDRE), and its expression is directly upregulated via the VDR pathway in an autocrine manner [27, 30]. Through this negative feedback system, vitamin D limits its own biologic effects. In our study, we confirmed that gene expression of 24(OH)ase in hDPCs was upregulated exponentially by VD3 during the 48 hours of observation (supplemental online Fig. 1A). Likewise, gene expression of ALPL and TGF-β2, both related to the hair-inductive capacity of hDPCs [24], was upregulated by VD3 treatment at 24–48 hours (supplemental online Fig. 1B, 1C). This suggested that VD3 indirectly upregulated ALPL and TGF-β2 mRNA expression. We further examined whether the VD3-induced upregulation of ALPL and TGF-β2 gene expression in hDPCs was mediated by Wnt signals. Inhibition of Wnt signals by Wif-1 did not affect the upregulation of ALPL and TGF-β2 gene expression, suggesting that the effects of VD3 are independent of Wnt signaling (supplemental online Fig. 2).
Comparison of VD3 Effects on Wnt10b, ALPL, and TGF-β2 Gene Expression Between hDPCs and hDFs
We further investigated the difference in the influential effects of VD3 on the gene expression of Wnt10b, ALPL, and TGF-β2 between hDPCs and hDFs. At baseline, Wnt10b, ALPL, and TGF-β2 mRNAs were expressed in hDPCs, approximately 6, 350, and 15 times as high as in hDFs, respectively (Fig. 3). After a 48-hour incubation with VD3, the gene expression of Wnt10b, ALPL, and TGF-β2 was significantly upregulated in hDPCs, whereas VD3 upregulated only ALPL gene expression in hDFs. Thus, the effects of VD3 on the gene expression of Wnt10b and TGF-β2 appeared to be specific to hDPCs, and this fact may characterize hDPCs that have hair-inductive ability.
Figure 3.
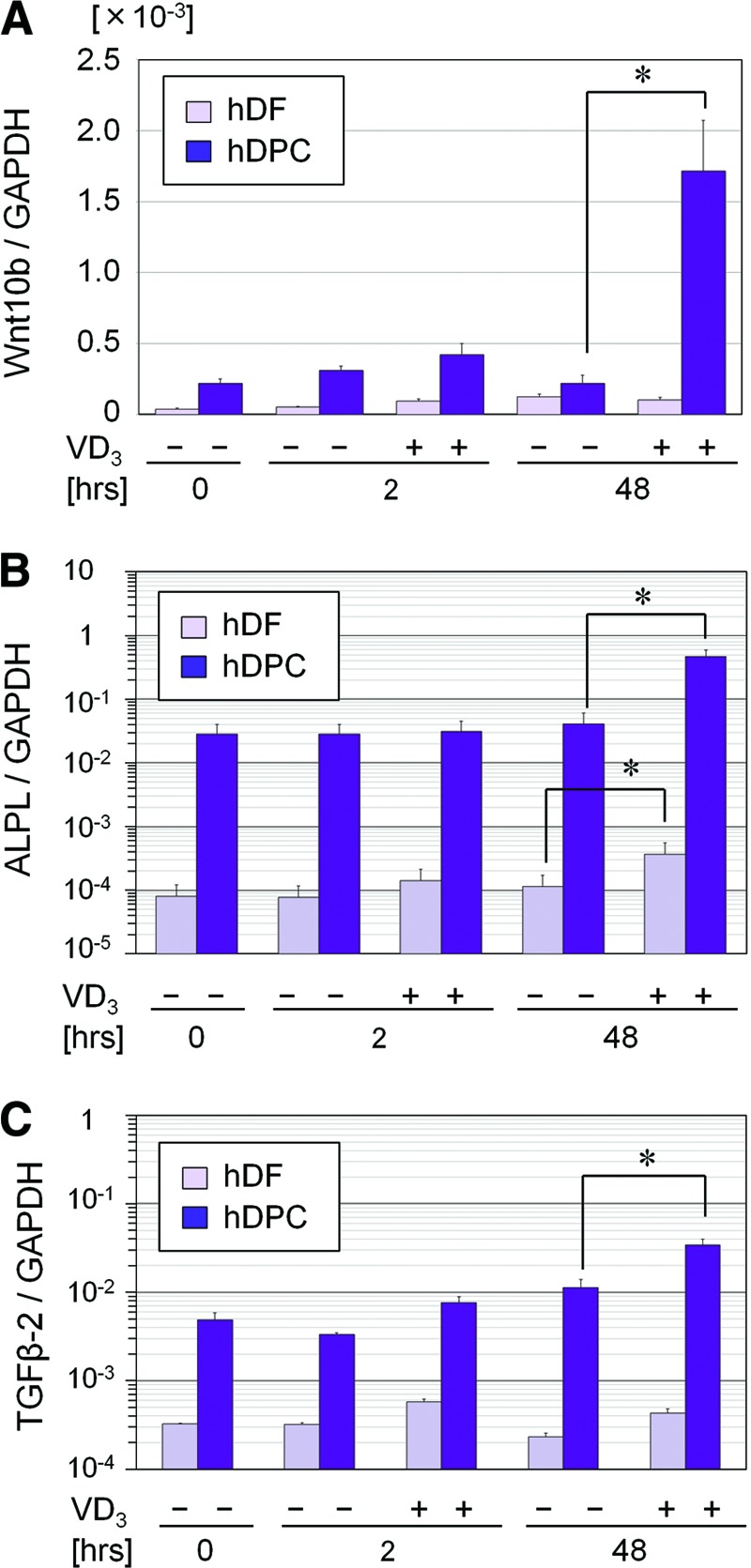
VD3 effects on gene expression of Wnt10b, ALPL, and TGF-β2 in hDPCs and hDFs. Gene expression of Wnt10b (A), ALPL (B), and TGF-β2 (C) was examined in both hDPCs and hDFs cultured in Dulbecco's modified Eagle's medium/serum-free medium, supplemented with or without VD3 (100 nM) for 0, 2, or 48 hours. Data are shown as the ratio of the respective gene expression to GAPDH mRNA expression (n = 4). *, Significant difference between pairs (p < .05). DPCs showed much higher expression of the three genes and upregulated expression of Wnt10b and TGF-β2 after a 48-hour incubation with VD3. Abbreviations: ALPL, ALP, liver/bone/kidney; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; hDF, human dermal fibroblast; hDPC, human dermal papilla cell; TGF, transforming growth factor; VD3, 1α,25-dihydroxyvitamin D3.
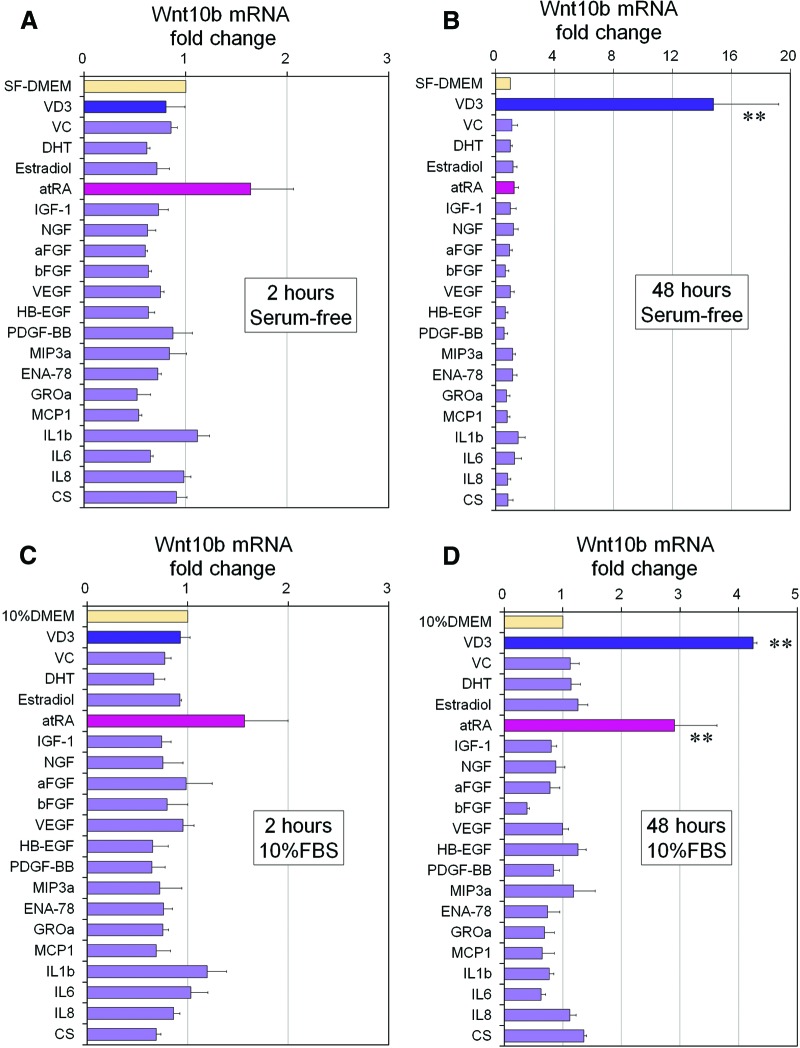
Influence of Paracrine or Endocrine Factors on Wnt10b mRNA Expression in hDPCs
To examine whether other skin-related paracrine or endocrine factors affect Wnt10b mRNA expression in hDPCs, 20 such factors were examined in culture, with or without serum, in an effort to find an optimized culture medium for DPC expansion. Results from serum-free medium cultures showed that VD3 alone remarkably promoted Wnt10b mRNA expression at 48 hours of culture, whereas atRA upregulated its expression at 2 hours (Fig. 4A, 4B). Here, atRA may have directly, but temporarily, promoted Wnt10b. In serum-containing media, almost the same result was obtained at 2 hours of culture (Fig. 4C), whereas not only VD3 but also atRA showed a promoting effect on Wnt10b mRNA expression at 48 hours (Fig. 4D). These results suggested that atRA and serum synergistically influence Wnt10b mRNA expression.
Figure 4.
Influence of keratinocyte-conditioned medium components and other factors on Wnt10b mRNA expression in human dermal papilla cells (hDPCs). (A, B): Wnt10b mRNA expression of hDPCs cultured in SF-DMEM with supplementation of each factor for 2 (A) or 48 (B) hours. Data are shown as fold change compared with control medium (SF-DMEM) (n = 3). **, Significant differences from control medium (p < .01). (C, D): Also shown is Wnt10b mRNA expression of hDPCs cultured in DMEM/10% FBS with supplementation of each factor for 2 (C) or 48 (D) hours. Data are shown as fold change compared with control medium (10% DMEM) (n = 3). **, Significant differences from control media (p < .01). Supplements and concentrations used were as follows: aFGF (100 ng/ml), bFGF (100 ng/ml), IL-1β (100 ng/ml), IL-6 (100 ng/ml), IL-8 (100 ng/ml), VEGF (100 ng/ml), PDGF-BB (100 ng/ml), NGF (100 ng/ml), HB-EGF (100 ng/ml), MIP (100 ng/ml), MCP (100 ng/ml), IGF (100 ng/ml), ENA (100 ng/ml), GROa (100 ng/ml), VD3 (100 nM), VC (100 μM), CS (100 μM), atRA (10 nM), 17β-estradiol (10 nM), and DHT (10 nM). Abbreviations: aFGF, acidic fibroblast growth factor; atRA, all-trans retinoic acid; bFGF, basic fibroblast growth factor; CS, cholesterol sulfate; DHT, dihydrotestosterone; ENA-78, epithelial cell-derived neutrophil-activating peptide-78; FBS, fetal bovine serum; GROa, growth-related oncogene-α; HB-EGF, heparin-binding epidermal growth factor-like growth factor; IGF-1, insulin-like growth factor 1; IL, interleukin; MCP1, monocyte chemotactic protein 1; MIP3a, macrophage inflammatory protein-3α; NGF, nerve growth factor; PDGF-BB, platelet-derived growth factor-BB; SF-DMEM, serum-free Dulbecco's modified Eagle's medium; VC, ascorbic acid; VD3, 1α,25-dihydroxyvitamin D3; VEGF, vascular endothelial growth factor.
Combined Effects of VD3 and atRA, KCZ, or TEI on Wnt10b mRNA Expression in hDPCs
We further examined the possible interactions between VD3 and atRA, KCZ, or TEI, because these three agents were previously reported to interact with VD3. atRA significantly upregulated Wnt10b gene expression at 4–6 hours but not at 48 hours (supplemental online Fig. 3A), whereas a synergistic enhancement with atRA and VD3 (100 nM) was not clearly detected. KCZ neither affected Wnt10b mRNA expression in hDPCs nor suppressed VD3-induced upregulation of Wnt10b in vitro (supplemental online Fig. 3B), although it was previously reported that VD3 signaling in hDPCs is stimulated by KCZ in vivo [41]. TEI is a vitamin D3 analog that acts as an agonist of VDR-mediated genomic actions in rodent cells and as an antagonist of them in human cells [42, 43]. The extent of the antagonism has been shown to depend on the cell taxonomy and culture conditions [44]. Our quantitative RT-PCR results using VD3 and TEI showed that TEI neither enhanced nor suppressed Wnt10b mRNA expression in hDPCs (supplemental online Fig. 3C).
Knockdown Assay of VDR with Short Hairpin RNAs
To examine whether VD3-induced upregulation of Wnt10b, ALPL, and TGF-β2 genes was mediated through a VDR-VDRE transduction pathway, five shRNA vectors for knocking down VDR were designed and prepared (supplemental online Table 1). Western blotting revealed that shRNAs 1, 3, 4, and 5 successfully suppressed human VDR protein expression, although vector 2 did not (Fig. 5A). Both shRNAs 1 and 3, as well as a negative control shRNA, were selected for further use. Since the transfection efficacy by lipofection was quite low in hDPCs (data not shown), retroviral infection was used for inserting shRNA vectors into hDPCs. After retroviral transfection, hDPCs expressing ZsGreen were sorted by flow cytometry, and infected cells were collected (supplemental online Fig. 4). Suppression of VDR-mediated signaling by shRNAs 1 and 3 was confirmed by quantitative RT-PCR for VDR-signal target gene 24(OH)ase (supplemental online Fig. 5).
Figure 5.
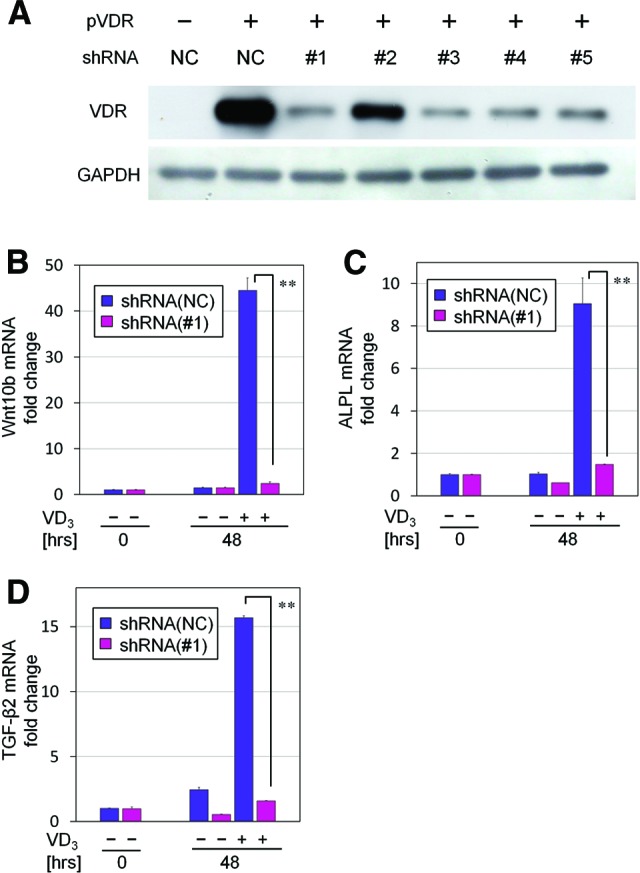
Knockdown effects of prepared shRNAs on VDR expression. (A): Each designed shRNA (negative control and shRNAs 1–5) was inserted into the cloning site of pSIREN-retroQ ZsGreen vectors. Both pVDR and an individual knockdown vector were cotransfected into HEK293 cells according to the combinations shown in the list. Western blotting revealed the knockdown efficacy of each shRNA on VDR protein expression. VDR expression was well knocked down by shRNAs 1, 3, 4, and 5. (B–D): Quantitative real-time polymerase chain reaction for gene expression of Wnt10b (B), ALPL (C), and TGF-β2 (D). Human dermal papilla cells cultured in serum-free Dulbecco's modified Eagle's medium, with or without the supplementation of VD3 (100 nM), were treated with NC or VDR knocking down shRNA 1 for 48 hours (n = 4). Data are shown as fold changes compared with the baseline expression at 0 hours. **, Significant differences between pairs (p < .01). Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NC, negative control short hairpin RNA; pVDR, flag-tagged human vitamin D receptor-expressing vector; shRNA, short hairpin RNA; TGF, transforming growth factor; VD3, 1α,25-dihydroxyvitamin D3; VDR, vitamin D receptor.
Treatment with shRNA 1 significantly suppressed VD3-mediated upregulation of Wnt10b mRNA (Fig. 5B), which was previously observed at 48 hours (Fig. 2B). In addition, upregulated expression of ALPL and TGF-β2 mRNA by VD3 was also significantly suppressed by treatment with shRNA 1 (Fig. 5C, 5D). These results were also confirmed by independent assays using shRNA 3 (data not shown), suggesting that upregulated gene expression of Wnt10b, ALPL, and TGF-β2 by VD3 was mediated by a VDR genomic signaling pathway.
Transplantation of Cultured mDPCs or mDFs Pretreated with VD3 for Hair Folliculogenesis in Mice
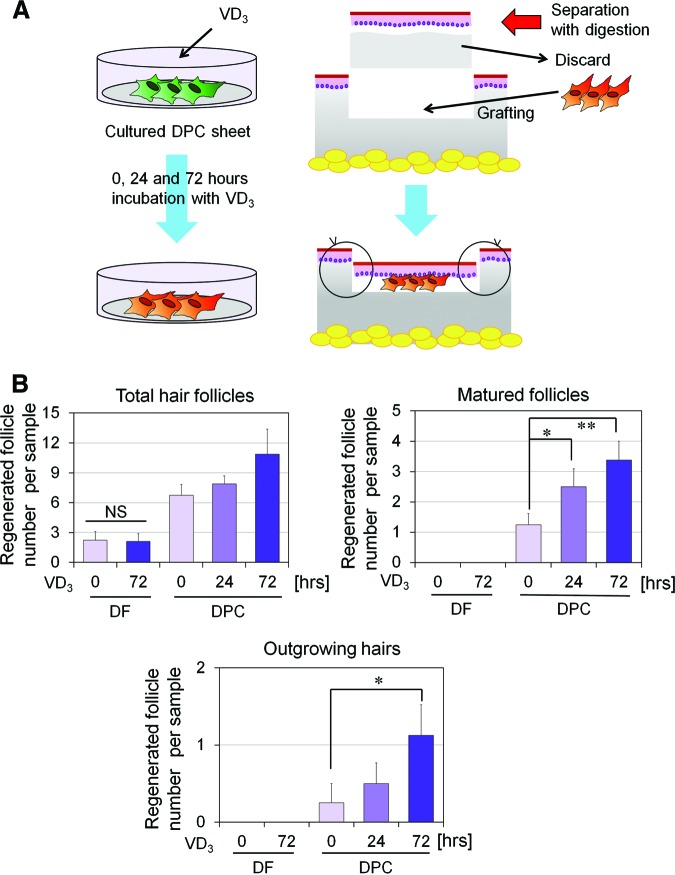
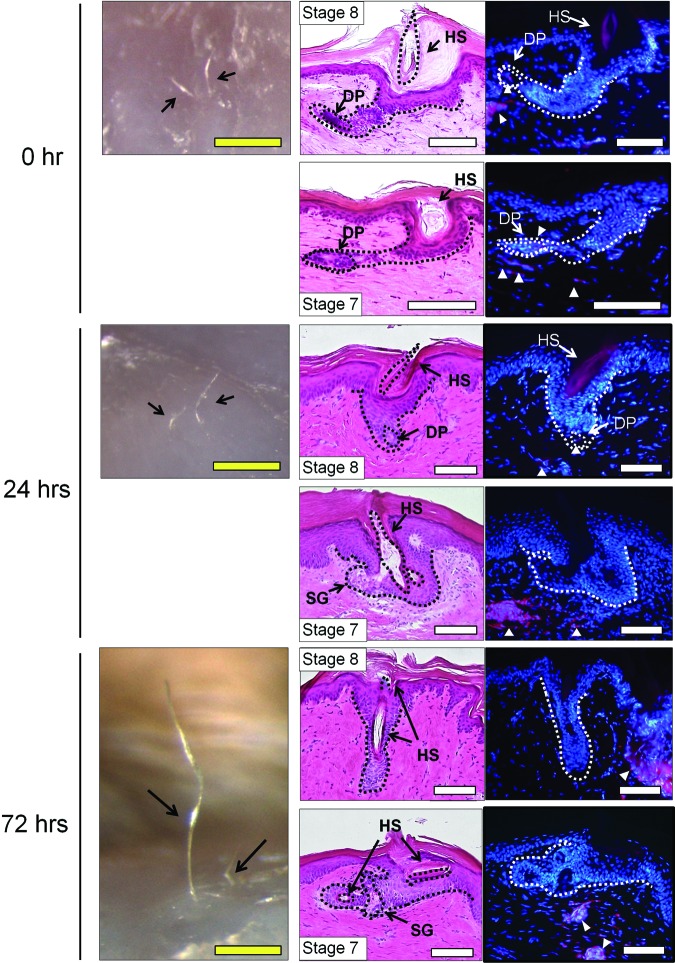
We further examined the therapeutic effect of VD3 on the hair-inductive capacity of cultured mDPCs in vivo. Since VD3 not only promoted expression of hair induction-related genes (supplemental online Figs. 1B, 1C, 2B) but also suppressed proliferation of DPCs (Fig. 1A, 1B), we performed VD3 treatment after cultured DPCs reached confluence. A cell sheet of mDPCs was incubated with VD3 (100 nM) for a short period of time (0, 24, or 72 hours) just before transplantation. As a control, a cell sheet of mDFs was incubated with VD3 (100 nM) for 0 or 72 hours (Fig. 6). The cultured mDPC and mDF sheet fragments were grafted into rat sole skin using the HVS method [8]. In this method, the DPC construct is expected to interact with epithelial stem cells in the epidermis, harvested from a nonhairy area for neogenesis of hair follicles (Fig. 6A). Macroscopic and histological evaluations of regenerated hair follicles are summarized in supplemental online Table 3, and representative histological images are shown in Figure 7. Hair folliculogenesis was observed in all samples from the three groups of DPCs. In contrast, the DF constructs induced a very small number of hair follicles, and the number and maturity were not enhanced by VD3 incubation time. High-stage hair development, such as stage 7 or 8, was seen in each group of DPCs, and a hair shaft and/or sebaceous glands were detected in well-matured follicles (Fig. 7). The total number of DPC-induced hair follicles increased with increased VD3 incubation time, although the difference did not reach statistical significance (Fig. 6B, top left). Regenerated follicles of stages 6–8 (matured follicles) from DPCs significantly increased in number when pretreated with VD3 (Fig. 6B, top right), whereas DF constructs induced no matured hair follicles. Moreover, the number of outgrowing hairs, defining the ultimate maturation of the regenerated follicle, induced by DPCs was significantly greater in the 72-hour pretreatment group: nine regenerated hairs in a total of eight samples, versus four hairs in the 24-hour pretreatment group and two hairs in the no-pretreatment group (Fig. 6B, bottom). These results suggested that VD3, which upregulates the gene expression of Wnt10b as well as ALPL and TGF-β2 in DPCs, may be useful in expansion cultures of DPCs targeting a cell-based therapy for hair regeneration.
Figure 6.
Hair folliculogenesis induced by transplantation of murine DPCs (mDPCs) or murine DFs (mDFs) pretreated with VD3 for 0, 24, or 72 hours. (A): Hair regeneration experiment using transplantation of a cultured mDPC construct in F344 rats. Cultured cell sheets of mDPCs (or mDFs as a control) were prepared and treated with VD3 for 0, 24, or 72 hours. After VD3 pretreatment, the mDPC (or mDF) sheet was fragmented, and the small fragment was transplanted into the nonhairy sole skin of F344 rats, using the hemivascularized sandwich method. Split-thickness skin (150–300 μm in thickness) was sliced off and digested with dispase, to separate the epidermis from the dermal compartment. The dermal compartment was discarded, and the epidermis was replaced on the remaining deep dermis after a cultured mDPC or mDF sheet fragment was grafted. Eight weeks after the transplantation, hair folliculogenesis was evaluated. (B): Hair regeneration induced by mDPC or mDF transplantation. Hair folliculogenesis of each sample was histologically evaluated (detailed data are summarized in supplemental online Table 3). Regenerated hair follicles were classified by maturity into stages 1–8. The numbers of total regenerated hair follicles (top left), matured (stage 6–8) follicles (top right), and outgrowing hairs (bottom) are shown. Significant differences among groups are shown as * (p < .05) or ** (p < .01). Abbreviations: DF, dermal fibroblast; DPC, dermal papilla cell; NS, not significant; VD3, 1α,25-dihydroxyvitamin D3.
Figure 7.
Representative macroscopic and histological images of regenerated hair follicles via transplantation of murine dermal papilla cells (mDPCs) pretreated with 1α,25-dihydroxyvitamin D3 (VD3) for 0, 24, or 72 hours. Left column shows representative macroscopic views 8 weeks after transplantation of an mDPC sheet fragment pretreated with VD3 for 0 (top), 24 (center), and 72 (bottom) hours. Arrows indicate hair regrowth on the skin. Middle and right columns show histological serial sections stained with hematoxylin/eosin (middle) or Hoechst 33342 (right). Two representative samples for each group are shown. Arrowheads indicate DiI-labeled grafted mDPCs. Scale bars = 500 μm (yellow), 100 μm (white). Abbreviations: DP, dermal papilla; HS, hair shaft; SG, sebaceous gland.
Discussion
This study showed that VD3 suppressed proliferation of hDPCs in a dose-dependent manner, without inducing cytotoxicity. Among various Wnt-related genes investigated, VD3 significantly upregulated hDPC Wnt10b gene expression, in an indirect manner. VD3 also significantly upregulated hDPC gene expression of two other folliculogenesis-related genes, ALPL and TGF-β2. The dermal papilla and lower dermal sheath around the end bulb of a follicle specifically express ALP activity and have the potential capacity to restore hair growth [25]. TGF-β2 is involved in promotion of the hair placode [16], contributes to anagen induction [45], is highly expressed in hDPCs compared with hDFs, and is suggested to mediate the hair-inductive capacity of DPCs [24]. Wnt10b is known to play crucial roles in fetal development of hair follicles. Specifically, Wnt10b is first expressed strongly in the placode of the epidermis at embryonic days 13.5–14.5 in mice [46, 47], suggesting its possible contribution to the initiation of folliculogenesis. Activation of the Wnt pathway is thought to be the first mesenchymal signal involved in the epithelial-mesenchymal interaction of folliculogenesis [16, 48]. A chamber assay (mix of dissociated competent epithelial and mesenchymal cells) with Wnt10b-producing cells promoted hair folliculogenesis [20]. Intriguingly, hair follicle neogenesis was induced in a Wnt signal-dependent manner in normal mice after skin wounding [19]. This suggested that the activation of Wnt signaling and subsequent stabilization of β-catenin serve as a trigger for the initiation of folliculogenesis and may determine whether activated stem cells in damaged skin differentiate into epidermis or hair. Furthermore, Wnt10b was also shown to enhance hair shaft growth in organ culture of hair follicles [49]. The upregulated expression of Wnt10b in hDPCs induced by VD3 may mean terminal and functional differentiation of proliferating hDPCs, although it has not been verified by this study.
atRA upregulated Wnt10b gene expression in hDPCs directly and temporarily in serum-free medium and prolonged the upregulation in serum-containing medium. It has previously been reported that VDR signaling can be stimulated by the presence of a retinoid X receptor (RXR) ligand (such as 9-cis retinoic acid) in human skin in vivo [41]. A major part of the effects of vitamin D are mediated through its nuclear receptor, VDR [50], which forms a heterodimeric complex with RXR [28, 51]. atRA is one of the natural ligands of RXR and may interfere with the VDR-VDRE signaling pathway. Since VD3 is converted from 25-hydroxyvitamin D3 (25(OH)D3) in the kidney, and serum concentration of VD3 is maintained at 1/1,000 to 1/500 of that of 25(OH)D3 (less than 0.4 nM), which is found at 25–80 ng/ml in healthy subjects [52], the synergistic response of Wnt10b by atRA and serum may possibly be due to serum-contained VD3. KCZ is an imidazole that inhibits cytochrome P-450 enzymatic activity, such as 24(OH)ase [53], and enhances the biological effect of VD3 in human skin [41]. However, synergistic upregulation of Wnt10b was not detected in cultured hDPCs.
Although most physiologic activities of VD3 are mediated via the VDR-VDRE signaling pathway, vitamin D is also known to act via nongenomic pathways, such as transmembrane ion channels [54, 55], and other intracellular signaling pathways [56]. By using RNA interference against VDR, we examined whether upregulation of Wnt10b, ALPL, and TGF-β2 in hDPCs, induced by VD3, was mediated through VDR, and we confirmed that all of these upregulations were exerted via the genomic VDR pathway in hDPCs.
Finally, we examined the potential of VD3 in a cell-based therapy targeting hair follicle regeneration, where cultured DPCs were pretreated with VD3 before transplantation. VD3 pretreatment augmented therapeutic efficiency. Specifically, a greater number of regenerated outgrowing hair shafts were macroscopically detected, and histological evaluation confirmed that the maturation of regenerated follicles in VD3-treated groups was significantly superior to that in the untreated group. Thus, the results suggested that pretreatment with VD3, in expanding cultures, enhances the hair-inductive capacity of DPCs. It is probably important to administer VD3 to DPCs after sufficient confluence has been reached, because VD3 showed a suppressive effect on DPC proliferation. VD3 may enhance functionality of cultured DPCs by promoting its differentiation.
Conclusion
VD3 was shown to upregulate gene expression related to hair inductive capacity of DPCs such as Wnt10b, ALPL, and TGF-β2, although VD3 did not promote proliferation of DPCs. An animal study indicated that pretreatment with VD3, in expanding culture, enhanced the hair regeneration by DPC transplantation to the nonhairy skin. Our results suggest that VD3 may promote functional differentiation of DPCs and be useful in preserving the hair follicle-inductive capacity of cultured DPCs for hair regeneration therapies, although the method of usage should be carefully designed.
Acknowledgments
We thank Dr. Eiji Ochiai (Teijin Pharma Ltd., Tokyo, Japan) for technical supervision of the pVDR experiment and Ayako Kurata for technical assistance. This work was supported by Grants 18591964 and 21791738 from the Japanese Ministry of Education, Culture, Sports, Science, and Technology and by the second Annual Research Award Grant of the Japanese Society of Anti-Aging Medicine.
Author Contributions
N.A.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; K.I.: conception and design, collection and/or assembly of data, data analysis and interpretation; T.C.: collection and/or assembly of data, data analysis and interpretation, provision of study material; R.F., H.Y., and S.I.: data analysis and interpretation; H.K., H.E., and K.D.: collection and/or assembly of data; S.K.: provision of study material, data analysis and interpretation, administrative support; K.Y.: conception and design, data analysis and interpretation, financial support, administrative support, final approval of manuscript, manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Jahoda CA. Induction of follicle formation and hair growth by vibrissa dermal papillae implanted into rat ear wounds: Vibrissa-type fibres are specified. Development. 1992;115:1103–1109. doi: 10.1242/dev.115.4.1103. [DOI] [PubMed] [Google Scholar]
- 2.Jahoda CA, Reynolds AJ, Oliver RF. Induction of hair growth in ear wounds by cultured dermal papilla cells. J Invest Dermatol. 1993;101:584–590. doi: 10.1111/1523-1747.ep12366039. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds AJ, Jahoda CA. Cultured dermal papilla cells induce follicle formation and hair growth by transdifferentiation of an adult epidermis. Development. 1992;115:587–593. doi: 10.1242/dev.115.2.587. [DOI] [PubMed] [Google Scholar]
- 4.Lichti U, Weinberg WC, Goodman L, et al. In vivo regulation of murine hair growth: Insights from grafting defined cell populations onto nude mice. J Invest Dermatol. 1993;101:124S–129S. doi: 10.1111/1523-1747.ep12363165. [DOI] [PubMed] [Google Scholar]
- 5.Weinberg WC, Goodman LV, George C, et al. Reconstitution of hair follicle development in vivo: Determination of follicle formation, hair growth, and hair quality by dermal cells. J Invest Dermatol. 1993;100:229–236. doi: 10.1111/1523-1747.ep12468971. [DOI] [PubMed] [Google Scholar]
- 6.Morris RJ, Liu Y, Marles L, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 7.Qiao J, Philips E, Teumer J. A graft model for hair development. Exp Dermatol. 2008;17:512–518. doi: 10.1111/j.1600-0625.2007.00661.x. [DOI] [PubMed] [Google Scholar]
- 8.Aoi N, Inoue K, Kato H, et al. Clinically applicable transplantation procedure of dermal papilla cells for hair follicle regeneration. J Tissue Eng Regen Med. 2012;6:85–95. doi: 10.1002/term.400. [DOI] [PubMed] [Google Scholar]
- 9.Horne KA, Jahoda CA, Oliver RF. Whisker growth induced by implantation of cultured vibrissa dermal papilla cells in the adult rat. J Embryol Exp Morphol. 1986;97:111–124. [PubMed] [Google Scholar]
- 10.Qiao J, Zawadzka A, Philips E, et al. Hair follicle neogenesis induced by cultured human scalp dermal papilla cells. Regen Med. 2009;4:667–676. doi: 10.2217/rme.09.50. [DOI] [PubMed] [Google Scholar]
- 11.Driskell RR, Clavel C, Rendl M, et al. Hair follicle dermal papilla cells at a glance. J Cell Sci. 2011;124:1179–1182. doi: 10.1242/jcs.082446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inamatsu M, Matsuzaki T, Iwanari H, et al. Establishment of rat dermal papilla cell lines that sustain the potency to induce hair follicles from afollicular skin. J Invest Dermatol. 1998;111:767–775. doi: 10.1046/j.1523-1747.1998.00382.x. [DOI] [PubMed] [Google Scholar]
- 13.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 14.Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543–557. doi: 10.1101/gad.1614408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osada A, Iwabuchi T, Kishimoto J, et al. Long-term culture of mouse vibrissal dermal papilla cells and de novo hair follicle induction. Tissue Eng. 2007;13:975–982. doi: 10.1089/ten.2006.0304. [DOI] [PubMed] [Google Scholar]
- 16.Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118:216–225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- 17.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- 18.Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000;14:1181–1185. [PMC free article] [PubMed] [Google Scholar]
- 19.Ito M, Yang Z, Andl T, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 20.Ouji Y, Yoshikawa M, Shiroi A, et al. Promotion of hair follicle development and trichogenesis by Wnt-10b in cultured embryonic skin and in reconstituted skin. Biochem Biophys Res Commun. 2006;345:581–587. doi: 10.1016/j.bbrc.2006.04.142. [DOI] [PubMed] [Google Scholar]
- 21.Higgins CA, Richardson GD, Ferdinando D, et al. Modelling the hair follicle dermal papilla using spheroid cell cultures. Exp Dermatol. 2010;19:546–548. doi: 10.1111/j.1600-0625.2009.01007.x. [DOI] [PubMed] [Google Scholar]
- 22.Havlickova B, Bíró T, Mescalchin A, et al. A human folliculoid microsphere assay for exploring epithelial-mesenchymal interactions in the human hair follicle. J Invest Dermatol. 2009;129:972–983. doi: 10.1038/jid.2008.315. [DOI] [PubMed] [Google Scholar]
- 23.Foitzik K, Paus R, Doetschman T, et al. The TGF-β2 isoform is both a required and sufficient inducer of murine hair follicle morphogenesis. Dev Biol. 1999;212:278–289. doi: 10.1006/dbio.1999.9325. [DOI] [PubMed] [Google Scholar]
- 24.Inoue K, Aoi N, Yamauchi Y, et al. TGF-beta2 is specifically expressed in human dermal papilla cells and modulates hair folliculogenesis. J Cell Mol Med. 2009;13:4643–4656. doi: 10.1111/j.1582-4934.2009.00739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McElwee KJ, Kissling S, Wenzel E, et al. Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J Invest Dermatol. 2003;121:1267–1275. doi: 10.1111/j.1523-1747.2003.12568.x. [DOI] [PubMed] [Google Scholar]
- 26.Iida M, Ihara S, Matsuzaki T. Hair cycle-dependent change of alkaline phosphatase activity in the mesenchyme and epithelium in mouse vibrissal follicles. Dev Growth Differ. 2007;49:185–195. doi: 10.1111/j.1440-169X.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- 27.Sutton ALM, MacDonald PN. Vitamin D: More than a “bone-a-fide” hormone. Mol Endocrinol. 2003;17:777–791. doi: 10.1210/me.2002-0363. [DOI] [PubMed] [Google Scholar]
- 28.Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 29.Bikle DD. Vitamin D: An ancient hormone. Exp Dermatol. 2011;20:7–13. doi: 10.1111/j.1600-0625.2010.01202.x. [DOI] [PubMed] [Google Scholar]
- 30.Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCullough ML, Bostick RM, Mayo TL. Vitamin D gene pathway polymorphisms and risk of colorectal, breast, and prostate cancer. Annu Rev Nutr. 2009;29:111–132. doi: 10.1146/annurev-nutr-080508-141248. [DOI] [PubMed] [Google Scholar]
- 32.Bikle DD. Vitamin D and the skin. J Bone Miner Metab. 2010;28:117–130. doi: 10.1007/s00774-009-0153-8. [DOI] [PubMed] [Google Scholar]
- 33.Mamede AC, Tavares SD, Abrantes AM, et al. The role of vitamins in cancer: A review. Nutr Cancer. 2011;63:479–494. doi: 10.1080/01635581.2011.539315. [DOI] [PubMed] [Google Scholar]
- 34.Paus R, Schilli MB, Handjiski B, et al. Topical calcitriol enhances normal hair regrowth but does not prevent chemotherapy-induced alopecia in mice. Cancer Res. 1996;56:4438–4443. [PubMed] [Google Scholar]
- 35.Yoshizawa T, Handa Y, Uematsu Y, et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997;16:391–396. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- 36.Li YC, Pirro AE, Amling M, et al. Targeted ablation of the vitamin D receptor: An animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci USA. 1997;94:9831–9835. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakai Y, Kishimoto J, Demay MB. Metabolic and cellular analysis of alopecia in vitamin D receptor knockout mice. J Clin Invest. 2001;107:961–966. doi: 10.1172/JCI11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller J, Djabali K, Chen T, et al. Atrichia caused by mutations in the vitamin D receptor gene is a phenocopy of generalized atrichia caused by mutations in the hairless gene. J Invest Dermatol. 2001;117:612–617. doi: 10.1046/j.0022-202x.2001.01438.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhou Y, Wang J, Malloy PJ, et al. Compound heterozygous mutations in the vitamin D receptor in a patient with hereditary 1,25-dihydroxyvitamin D-resistant rickets with alopecia. J Bone Miner Res. 2009;24:643–651. doi: 10.1359/JBMR.081216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paus R, Müller-Röver S, Van Der Veen C, et al. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J Invest Dermatol. 1999;113:523–532. doi: 10.1046/j.1523-1747.1999.00740.x. [DOI] [PubMed] [Google Scholar]
- 41.Kang S, Li XY, Duell EA, et al. The retinoid X receptor agonist 9-cis-retinoic acid and the 24-hydroxylase inhibitor ketoconazole increase activity of 1,25-dihydroxyvitamin D3 in human skin in vivo. J Invest Dermatol. 1997;108:513–518. doi: 10.1111/1523-1747.ep12289736. [DOI] [PubMed] [Google Scholar]
- 42.Miura D, Manabe K, Ozono K, et al. Antagonistic action of novel 1α,25-dihydroxyvitamin D3-26,23-lactone analogs on differentiation of human leukemia cells (HL-60) induced by 1α,25-dihydroxyvitamin D3. J Biol Chem. 1999;274:16392–16399. doi: 10.1074/jbc.274.23.16392. [DOI] [PubMed] [Google Scholar]
- 43.Ochiai E, Miura D, Eguchi H, et al. Molecular mechanism of the vitamin D antagonistic actions of (23S)-25-dehydro-1α-hydroxyvitamin D3-26,23-lactone depends on the primary structure of the carboxyl-terminal region of the vitamin d receptor. Mol Endocrinol. 2005;19:1147–1157. doi: 10.1210/me.2004-0234. [DOI] [PubMed] [Google Scholar]
- 44.Ozono K, Saito M, Miura D, et al. Analysis of the molecular mechanism for the antagonistic action of a novel 1α,25-dihydroxyvitamin D(3) analogue toward vitamin D receptor function. J Biol Chem. 1999;274:32376–32381. doi: 10.1074/jbc.274.45.32376. [DOI] [PubMed] [Google Scholar]
- 45.Oshimori N, Fuchs E. Paracrine TGF-β signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell. 2012;10:63–75. doi: 10.1016/j.stem.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reddy S, Andl T, Bagasra A, et al. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev. 2001;107:69–82. doi: 10.1016/s0925-4773(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Tomann P, Andl T, et al. Reciprocal requirements for EDA/EDAR/NF-kappaB and Wnt/beta-catenin signaling pathways in hair follicle induction. Dev Cell. 2009;17:49–61. doi: 10.1016/j.devcel.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kratochwil K, Dull M, Farinas I, et al. Lef1 expression is activated by BMP-4 and regulates inductive tissue interactions in tooth and hair development. Genes Dev. 1996;10:1382–1394. doi: 10.1101/gad.10.11.1382. [DOI] [PubMed] [Google Scholar]
- 49.Ouji Y, Yoshikawa M, Moriya K, et al. Effects of Wnt-10b on hair shaft growth in hair follicle cultures. Biochem Biophys Res Commun. 2007;359:516–522. doi: 10.1016/j.bbrc.2007.05.135. [DOI] [PubMed] [Google Scholar]
- 50.Darwish H, DeLuca HF. Vitamin D-regulated gene expression. Crit Rev Eukaryot Gene Expr. 1993;3:89–116. [PubMed] [Google Scholar]
- 51.Lemon BD, Freedman LP. Selective effects of ligands on vitamin D3 receptor- and retinoid X receptor-mediated gene activation in vivo. Mol Cell Biol. 1996;16:1006–1016. doi: 10.1128/mcb.16.3.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kennel KA, Drake MT, Hurley DL. Vitamin D deficiency in adults: When to test and how to treat. Mayo Clin Proc. 2010;85:752–757. doi: 10.4065/mcp.2010.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lehmann B, Sauter W, Knuschke P, et al. Demonstration of UVB-induced synthesis of 1 α,25-dihydroxyvitamin D3 (calcitriol) in human skin by microdialysis. Arch Dermatol Res. 2003;295:24–28. doi: 10.1007/s00403-003-0387-6. [DOI] [PubMed] [Google Scholar]
- 54.Zanello LP, Norman A. 1α,25(OH)2 vitamin D3 actions on ion channels in osteoblasts. Steroids. 2006;71:291–297. doi: 10.1016/j.steroids.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 55.Menegaz D, Barrientos-Duran A, Kline A, et al. 1α,25(OH)2-Vitamin D3 stimulation of secretion via chloride channel activation in Sertoli cells. J Steroid Biochem Mol Biol. 2010;119:127–134. doi: 10.1016/j.jsbmb.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 56.Ellison TI, Dowd DR, MacDonald PN. Calmodulin-dependent kinase IV stimulates vitamin D receptor-mediated transcription. Mol Endocrinol. 2005;19:2309–2319. doi: 10.1210/me.2004-0382. [DOI] [PubMed] [Google Scholar]