The purpose of this study was to determine whether a proprietary xeno-free synthetic culture surface could be used to aid in the production and subsequent retinal-specific differentiation of clinical-grade induced pluripotent stem cells (iPSCs). It was found that Synthemax cell culture surfaces provide an ideal surface for the xeno-free production, culture, and differentiation of adult somatic cell-derived iPSCs. These findings demonstrate the potential utility of these surfaces for the production of clinical-grade retinal neurons for transplantation and induction of retinal regeneration.
Keywords: Induced pluripotent stem cells, Stem cell culture, Retina, Reprogramming, Pluripotent stem cells, Clinical translation
Abstract
The purpose of this study was to determine whether a proprietary xeno-free synthetic culture surface could be used to aid in the production and subsequent retinal-specific differentiation of clinical-grade induced pluripotent stem cells (iPSCs). iPSCs were generated using adult somatic cells via infection with either a single cre-excisable lentiviral vector or four separate nonintegrating Sendai viruses driving expression of the transcription factors OCT4, SOX2, KLF4, and c-MYC. Retinal precursor cells were derived via targeted differentiation of iPSCs with exogenous delivery of dkk-1, noggin, insulin-like growth factor-1, basic fibroblast growth factor, acidic fibroblast growth factor, and DAPT. Phase contrast microscopy, immunocytochemistry, hematoxylin and eosin staining, and reverse transcription-polymerase chain reaction were used to determine reprogramming efficiency, pluripotency, and fate of undifferentiated and differentiated iPSCs. Following viral transduction, cells underwent prototypical morphological changes resulting in the formation of iPSC colonies large enough for manual isolation/passage at 3–4 weeks postinfection. Both normal and disease-specific iPSCs expressed markers of pluripotency and, following transplantation into immune-compromised mice, formed teratomas containing tissue comprising all three germ layers. When subjected to our established retinal differentiation protocol, a significant proportion of the xeno-free substrate-derived cells expressed retinal cell markers, the number of which did not significantly differ from that derived on traditional extracellular matrix-coated dishes. Synthetic cell culture substrates provide a useful surface for the xeno-free production, culture, and differentiation of adult somatic cell-derived iPSCs. These findings demonstrate the potential utility of these surfaces for the production of clinical-grade retinal neurons for transplantation and induction of retinal regeneration.
Introduction
In humans, terminally differentiated cells of the outer retina (photoreceptors and retinal pigmented epithelium [RPE]) lack the capacity for significant regeneration. As such, treatment of retinal degenerative diseases, such as retinitis pigmentosa (RP) and age-related macular degeneration (AMD), will likely require cell replacement strategies.
With the advent of the induced pluripotent stem cell (iPSC), autologous transplantation as a means to treat retinal degenerative disease is now possible. It was recently shown by several groups, including our own, that iPSCs generated from dermal fibroblasts have the ability to differentiate into retinal photoreceptor precursors [1–4]. When transplanted into retinal degenerative hosts, these cells have been shown to give rise to mature rod and cone photoreceptor cells that integrate within the dystrophic retina, form synapses with host bipolar cells, and induce a partial restoration of electrophysiological and anatomical correlates of retinal function [1]. Although these findings establish proof-of-principle for the use of autologous iPSCs for the treatment of retinal degenerative disease, in these studies cells were generated and differentiated in the presence of contaminating mouse feeder cells and/or animal-derived extracellular matrix molecules. Exposure of cell lines to undefined animal-derived products is undesirable, especially if the cell line in question is to be used for human therapy. Although iPSC technology has great potential for patient-specific cell-based therapy, xeno-free derivation, expansion, and differentiation will ultimately be required. The purpose of this study was to determine whether a proprietary xeno-free synthetic culture surface (Synthemax cell culture surface; Corning Life Sciences, Acton, MA, http://www.corning.com) could be used to aid in the production and subsequent retinal-specific differentiation of clinical-grade iPSCs.
Materials and Methods
Ethics Statement
All experiments were conducted with the approval of the University of Iowa Animal Care and Use Committee (Animal Welfare Assurance no. 1009184) and the University of Iowa Internal Review Board (IRB no. 200202022) and were consistent with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research and the Treaty of Helsinki.
Animals
Adult 4–6-week-old 129SVJ mice (Jackson Laboratory, Bar Harbor, ME, http://www.jax.org) were used as fibroblast donors; adult 4–6-week-old severe combined immunodeficient (SCID) mice (Jackson Laboratory) were used as transplant recipients for assessment of teratoma formation. Mice were housed in a pathogen-free barrier facility.
Patient-Derived Cells
Skin biopsies were collected from patients after informed consent was obtained and were used for the generation of fibroblasts and/or keratinocytes (isolation performed as described previously [1, 5, 6]). For some experiments, cells were expanded from a large collection that has been obtained from patients with known inherited diseases of the photoreceptor cells (Batten disease, retinitis pigmentosa, Leber congenital amaurosis, and Stargardt disease), as assessed at the University of Iowa Department of Ophthalmology and Visual Sciences. Iris pigment epithelial cells (IPEs) were cultured from a 94-year-old human donor eye as described previously [7]. Eyes were obtained and IPEs cultured within 5 hours of death.
iPSC Generation
iPSCs were generated from adult mouse and human tissues via infection with either a single cre-excisable lentiviral vector (plasmid 20328; Addgene, Cambridge, MA, http://www.addgene.org) or four separate Sendai viruses (CytoTune; Life Technologies, Rockville, MD, http://www.lifetech.com) each of which was designed to drive expression of the transcription factors OCT4, SOX2, KLF4, and c-MYC. Fibroblasts, IPEs, and keratinocytes plated on six-well tissue culture plates were infected at a multiplicity of infection of 1–5. At 12–16 hours postinfection, cells were washed and fed with fresh growth medium (fibroblasts: minimal essential medium-α, 10% KnockOut Serum Replacement [KSR] [Invitrogen, Carlsbad, CA, http://www.invitrogen.com], 1% primocin [InvivoGen, San Diego, CA, http://www.invivogen.com]; IPE: Dulbecco's modified Eagle's medium [DMEM] F-12 medium, 15% KSR [Invitrogen], 10 ng/ml human recombinant pigment epithelium-derived factor [SinoBio, Beijing, China, http://www.sinobiological.com], 1% primocin [InvivoGen]; keratinocytes: Epilife medium with keratinocyte supplement [Invitrogen], 1% primocin [InvivoGen]). At 5 days postinfection, cells were passaged onto six-well Synthemax cell culture dishes at a density of 100,000 cells per well and fed every day with pluripotency medium (DMEM F-12 medium [Gibco, Grand Island, NY, http://www.invitrogen.com], 15% knockout serum replacement [Gibco], 0.0008% β-mercaptoethanol [Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com], 1% 100× nonessential amino acids [NEAA] [Gibco], 1 × 106 units/l of leukemia inhibitory factor [LIF] [mouse] [ESGRO; Millipore, Billerica, MA, http://www.millipore.com] or 100 ng/ml basic fibroblast growth factor [bFGF] and 10 ng/ml pigment epithelium-derived factor [human] [R&D Systems Inc., Minneapolis, MN, http://www.rndsystems.com], and 1% penicillin/streptomycin [Gibco]). At 3 weeks after viral transduction, iPSC colonies were picked, passaged, and clonally expanded on fresh Synthemax plates for further experimentation. During reprogramming and maintenance of pluripotency, cells were cultured at 5% CO2, 5% O2, and 37°C.
iPSC Differentiation
To maintain pluripotency, adult-derived iPSCs were cultured in LIF-containing (mouse) or bFGF-containing (human) pluripotency medium. To initiate differentiation, iPSCs were removed from the culture substrate via manual passage using Stem Passage manual passage rollers (Invitrogen), resuspended in embryoid body (EB) medium (DMEM F-12 medium [Gibco] containing 10% knockout serum replacement [Gibco], 2% B27 supplement [Gibco], 1% N2 supplement [Gibco], 1% l-glutamine [Gibco], 1% 100× NEAA [Gibco], 1% penicillin/streptomycin [Gibco], 0.2% Fungizone [Gibco], 1 ng/ml noggin [R&D Systems], 1 ng/ml Dkk-1 [R&D Systems], 1 ng/ml insulin-like growth factor-1 [IGF-1] [R&D Systems], and 0.5 ng/ml bFGF [R&D Systems]), and plated at a density of ∼50 cell clusters per cm2 in ultralow-adhesion culture plates (Corning). Cell clusters were cultured for 5 days as indicated above, after which the EBs were removed, washed, and plated at a density of 25–30 EBs per cm2 in fresh differentiation medium 1 (DMEM F-12 medium [Gibco], 2% B27 supplement [Gibco], 1% N2 supplement [Gibco], 1% l-glutamine [Gibco], 1% 100× NEAA [Gibco], 10 ng/ml noggin [R&D Systems], 10 ng/ml Dkk-1 [R&D Systems], 10 ng/ml IGF-1 [R&D Systems], and 1 ng/ml bFGF [R&D Systems]) in six-well Synthemax culture plates. Cultures were fed every other day for 10 days with differentiation medium 1. For the following 6 days, cultures were fed with differentiation medium 2 (differentiation medium 1 + 10 μM of the Notch signaling inhibitor DAPT [Calbiochem, Gibbstown, NJ, http://www.emdbiosciences.com]). For the following 12 days, cultures were fed with differentiation medium 3 (differentiation medium 2 + 2 ng/ml of acidic fibroblast growth factor [R&D Systems]). Mouse differentiation cultures were ended following this 12-day period, whereas human differentiations continued for an additional 60 days in differentiation medium 4 (DMEM F-12 medium [Gibco], 2% B27 supplement [Gibco], 1% N2 supplement [Gibco], 1% l-glutamine [Gibco], 1% 100× NEAA [Gibco]). (A depiction of this protocol is presented in supplemental online Fig. 1, termed differentiation paradigm 1.) Although the recombinant proteins used in the above-described protocol were species-specific, they were derived using various strains of bacteria or animal cell lines (i.e., Dkk1: Spodoptera frugiperda, Sf 21 [baculovirus] derived; noggin, mouse myeloma cell line, NS0 derived; IGF1, Escherichia coli derived; bFGF, E. coli derived) as such a differentiation protocol using these molecules could not truly be classified as xeno-free. In light of this, a completely xeno-free differentiation paradigm (termed differentiation paradigm 2), in which the recombinant proteins noggin, Dkk-1, IGF-1, and bFGF were removed from the above-described medium, was tested (differentiation paradigms tested are shown in supplemental online Fig. 1).
Histology
Teratomas were fixed in 10% formalin for 24 hours prior to dehydration and mounting in paraffin wax (VWR, Radnor, PA, https://us.vwr.com). Samples were sectioned at 6 μm, and hematoxylin and eosin staining was performed as per standard protocols.
Immunostaining
Cells were fixed in a 4% paraformaldehyde solution and immunostained as described previously [1]. Briefly, cells/tissues were incubated overnight at 4°C with antibodies targeted against either mouse SSEA1 (MA1-16907; Thermo Fisher, Waltham, MA, http://www.fishersci.com), human Tra-1-81 (MAB4381; Millipore), Tra-1-60 (Stemgent, Stain Alive, 09-0068), glial fibrillary acidic protein (GFAP) (MAB360; Millipore), or α-smooth muscle actin (αSMA) (ab5694; Abcam, Cambridge, MA, http://www.abcam.com) for teratoma formation or biotinylated-OTX2 (BAF1979; R&D Systems), recoverin (AB5585; Millipore), NF200 (AB1989; Millipore), and Brn3B (ab56026; Abcam) for retinal differentiation. Subsequently, Cy2- or Cy3-conjugated secondary antibodies were used (Jackson Immunoresearch Laboratories, West Grove, PA, http://www.jacksonimmuno.com), and the samples were analyzed using confocal microscopy. Microscopic analysis was performed such that exposure time, gain, and depth of field remained constant between experimental conditions.
Cell Counting
Cell counts were performed by counting the total number of cells expressing the protein of interest in the differentiated population (taken 200 μm outside of the originally plated embryoid bodies) at 90 days postdifferentiation. In each case counts were performed using 10 microscopic fields from each of three experimental repeats. As such, statistical analysis was based on counts from 30 microscopic fields.
RNA Isolation and Reverse Transcription-Polymerase Chain Reaction
Total RNA was extracted from undifferentiated D0 and differentiated D33 iPSCs using the RNeasy Mini-kit (Qiagen, Valencia, CA, http://www.qiagen.com) following the provided instructions. Briefly, cells were lysed and homogenized, and ethanol was added to adjust binding conditions. Samples were spun using RNeasy spin columns and washed, and RNA was eluted using RNase-free water. One microgram of RNA was reverse transcribed into cDNA using the random hexamer (Invitrogen) priming method and Omniscript reverse transcriptase (Qiagen). All polymerase chain reactions (PCRs) were performed in a 40-μl reaction containing 1× PCR buffer, 1.5 mM MgCl2, 0.2 mM dNTPs, 100 ng of DNA, 1.0 U of AmpliTaq Gold (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com), and 20 pmol of each gene-specific primer. All cycling profiles incorporated an initial denaturation temperature of 94°C for 10 minutes through 35 amplification cycles (30 seconds at 94°C, 30 seconds at the annealing temperature of each primer, and 1 minute at 72°C) and a final extension at 72°C for 10 minutes. PCR products were separated by electrophoresis on 2% agarose gels (Invitrogen). Gene-specific primers (Invitrogen) are given in supplemental online Table 1.
Results
Dermal fibroblasts, isolated from adult mouse and human skin samples, were expanded on Synthemax cell culture surfaces and used for iPSC generation. Approximately 2–3 weeks following lentiviral transduction, small, morphologically distinct cell clusters could be detected. Two to 3 weeks later, these clusters expanded into clearly defined iPSC colonies (Fig. 1A, 1E) that were mechanically dissected from the underlying fibroblast layer. Each isolated colony was dissociated into 150–200-μm square cell clusters and cultured in individual wells of a six-well Synthemax cell culture plate. Each well was maintained as a separate clonally expanded line for 10 passages prior to analysis. At passage 10, well-defined densely packed colonies consisting of cells with a high nucleus to cytoplasm ratio were present (Fig. 1B, 1F). These colonies expressed alkaline phosphatase (typically used as a marker of successful reprogramming, Fig. 1C, 1G), as well as the pluripotency markers SSEA1 (mouse; Fig. 1D) and Tra-1-81 (human; Fig. 1H).
Figure 1.

Feeder-free derivation of induced pluripotent stem cell (iPSC) lines from adult mouse and human dermal fibroblasts. (A–H): Microscopic analysis of mouse (A–D) and human (E–H) iPSCs generated and cultured on Synthemax cell culture surfaces (Synthemax-iPSC). At 3–4 weeks after viral transduction, embryonic stem cell-like iPSC colonies were identified (A, E). iPSC colonies isolated, subcultured, and expanded for 10 passages on Synthemax cell culture surfaces maintained a pluripotent morphology (B, F) and expressed alkaline phosphatase (C, G) and the pluripotency markers SSEA1 ([D], mouse) and Tra-1-81 ([H], human). Scale bars = 400 μm. Abbreviation: P, passage.
To further test pluripotency, reverse transcription (RT)-PCR and teratoma assays were performed. RT-PCR analysis revealed that both mouse (Fig. 2A) and human (Fig. 2B) iPSCs, generated and expanded on Synthemax cell culture surfaces, expressed the pluripotency markers Nanog, SOX2, c-MYC, and KLF4. Teratomas, generated via i.m. injection of 2.5 × 106 undifferentiated iPSCs (Fig. 3A, 3C, 3E, mouse; Fig. 3B, 3D, 3F, human) into immune-deficient (SCID) mice, were excised, fixed, paraffin-embedded, and sectioned. Histologic analysis of these tumors revealed tissue specific to each of the three embryonic germ layers (Fig. 3A, 3B, neural rosettes, neuroepithelia: ectoderm; Fig. 3C, 3D, chondrocytes: mesoderm; Fig. 3E, 3F, glandular epithelium: endoderm). Similarly, immunohistochemical staining revealed GFAP-positive neural epithelium/rosettes (Fig. 3G, 3H) and αSMA-positive vascular structures (Fig. 3I, 3J).
Figure 2.

Reverse transcription-polymerase chain reaction (RT-PCR) analysis of Synthemax-induced pluripotent stem cell (iPSC) potency. (A, B): RT-PCR analysis of undifferentiated mouse (A) and human (B) iPSCs for expression of the pluripotency genes Nanog, SOX2, c-MYC, and KLF4.
Figure 3.
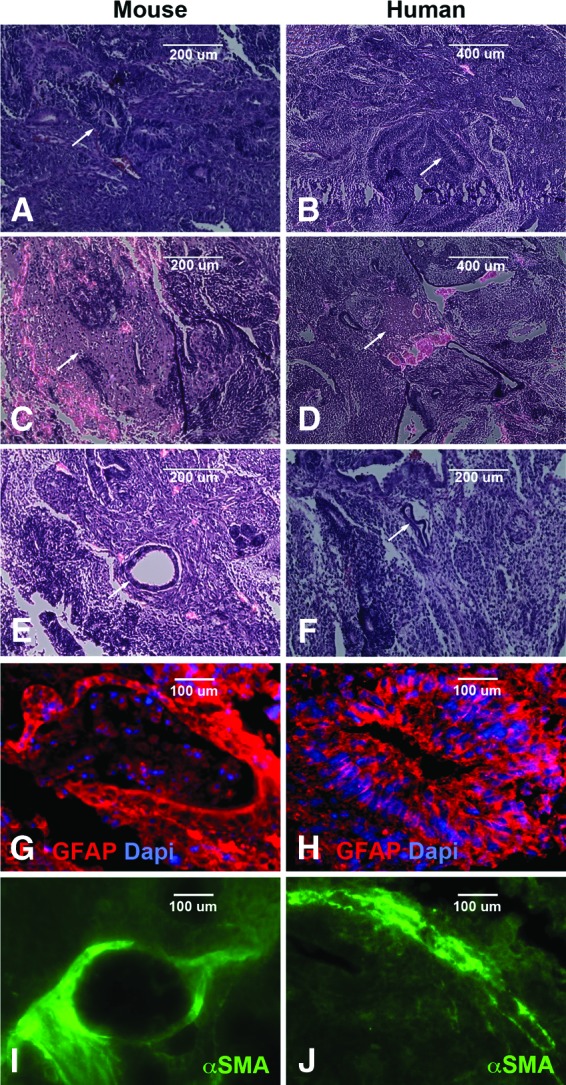
Microscopic analysis of Synthemax-induced pluripotent stem cell (iPSC) potency. (A–F): Histological analysis of Synthemax-iPSC generated teratomas for production of cells/tissues specific to ectodermal (A, B), mesodermal (C, D), and endodermal (E, F) germ layers. (G–J): Immunocytochemical analysis of Synthemax-iPSC generated teratomas with antibodies targeted against GFAP (ectodermal [G, H]) and αSMA, (mesodermal [I, J]). Arrows indicate location of example tissues. Scale bars = 200 μm (A, C, E, F), 400 μm (B, D), and 100 μm (G–J). Abbreviations: Dapi, 4′,6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic protein; αSMA, α-smooth muscle actin.
To demonstrate the utility of the Synthemax cell culture surface for production of patient-specific iPSCs, dermal fibroblasts, isolated from human patients with four distinctly different molecularly confirmed retinal degenerative diseases (RP, Stargardt disease, Leber congenital amaurosis, and Batten disease), were expanded on Synthemax cell culture surfaces and targeted for iPSC generation. As described above, iPSC colonies, initially detected at approximately 2–3 weeks post-viral transduction, were isolated and clonal expanded as described above. At passage 10, well-defined densely packed colonies consisting of cells with a high nucleus-to-cytoplasm ratio were present (Fig. 4A–4D). Following transplantation into immune-compromised mice, teratomas containing tissue specific to each of the three embryonic germ layers were identified (Fig. 4E–4H, 4Q–4T, ectoderm; Fig. 4I–4L, 4U–4X, mesoderm; Fig. 4M–4P, endoderm).
Figure 4.
Feeder-free derivation of induced pluripotent stem cell (iPSC) lines from human dermal fibroblasts isolated from patients with retinal degenerative disease. (A–D): Microscopic analysis of human retinal disease-specific iPSCs generated and expanded for 10 passages on Synthemax cell culture surfaces. (E–P): Histological analysis of retinal disease-specific iPSC-derived teratomas for production of cells/tissues specific to ectodermal (E–H), mesodermal (I–L), and endodermal (M–P) germ layers. (Q–X): Immunocytochemical analysis of retinal disease-specific iPSC derived teratomas with antibodies targeted against GFAP (ectodermal [Q–T]) and αSMA (mesodermal [U–X]). Scale bars = 400 μm (A–D) and 200 μm (Q–X). Abbreviations: BD, Batten disease; Dapi, 4′,6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic protein; LCA, Leber congenital amaurosis; RP, retinitis pigmentosa; SMA, α-smooth muscle actin; STG, Stargardt disease.
For eye diseases such as AMD, production of patient-specific iPSCs will require reprogramming of cells isolated from older individuals. As it is well known that donor age significantly affects cellular reprogrammability (i.e., dermal fibroblast isolated from older donors are more difficult to reprogram then those isolated from young individuals), targeting other cell types that are more readily reprogrammed, such as keratinocytes, may be required. Likewise, to be truly clinically relevant, it may also be beneficial for iPSCs to not only be produced on a xeno-free culture surface but to be generated using integration-free technologies.
A series of experiments focused on reprogramming of two additional accessible cell types from elderly individuals was performed. As shown in Figure 5, keratinocytes and iris pigmented epithelial cells (derived from a human eye donor) plated on Synthemax cell culture surfaces could both be reprogrammed to pluripotency using the above-described reprogramming protocol (Fig. 5A–5D). In an attempt to generate integration-free iPSCs using xeno-free Synthemax cell culture surfaces three separate reprogramming paradigms (minicircle DNA: SBI SC301A-1; mRNA: Stemgent 00-0071; nonintegrating Sendai virus: CytoTune A1378002), targeting both keratinocytes and IPEs isolated from elderly individuals (68 and 94 years of age, respectively), were attempted. Although we were unsuccessful in generating iPSCs using minicircle and/or mRNA-based approaches regardless of plating conditions (data not shown), iPSC colonies were successfully generated from both keratinocytes (Fig. 5E) and IPEs (Fig. 5H) using nonintegrating Sendai viruses. Following passage and expansion on fresh Synthemax cell culture surfaces, these Sendai virus-generated iPSCs maintained a pluripotent morphology (Fig. 5F, 5I) and expressed markers of pluripotency as determined by RT-PCR (Fig. 5G, 5J).
Figure 5.
Feeder-free derivation of iPSCs from human keratinocytes and IPEs isolated from elderly individuals. (A–D): Phase micrographs of human keratinocytes (A), keratinocyte-derived iPSCs (B), IPEs (C), and IPE-derived iPSCs (D) cultured and generated on Synthemax cell culture surfaces. (E–J): Phase micrographs (E, F, H, I) and reverse transcription-polymerase chain reaction (RT-PCR) analysis (G, J) of keratinocyte-derived (E–G) and IPE-derived (H–J) iPSCs generated on Synthemax cell culture surfaces using nonintegrating Sendai viruses expressing OCT4, SOX2, KLF4, and c-MYC. (K–M): Phase micrograph (K), live Tra-1-60 staining (L), and RT-PCR analysis (M) of IPE-derived iPSCs generated on Synthemax cell culture surfaces using nonintegrating Sendai viruses expressing OCT4 and SOX2. Scale bars = 400 μm. Abbreviations: bp, base pairs; IPE, iris pigment epithelial cell; iPSC, induced pluripotent stem cell.
In the process of performing these experiments, it became evident that reprogramming of IPEs was significantly quicker and more efficient than other cell types tested. In light of this observation, we chose to target IPEs with Sendai viruses driving expression of OCT4 and SOX2 only. As indicated in Figure 5, forced expression of these two factors was sufficient to induce cellular reprogramming of IPEs. That is, at approximately 3–4 weeks posttransduction, morphologically distinct iPSC clusters (Fig. 5K) that expressed the cell surface pluripotency antigen Tra-1-60 (Fig. 5L) could be detected. As with four-factor iPSCs, these cells expressed the pluripotency markers DNA methyltransferase 1, c-MYC, Nanog, SOX2, KLF4, and OCT4 (Fig. 5M). Collectively, these findings demonstrate that Synthemax cell culture surfaces can be successfully used to produce iPSCs using a variety of different cell types and reprogramming methodologies.
To produce retinal neurons for pathophysiologic study of disease and/or subretinal transplantation, our previously published stepwise differentiation protocol, depicted in supplemental online Figure 1, was used [1, 2]. This protocol was designed to maximize the percentage of retinal cells produced. Specifically, it takes into account the role of bone morphogenic protein and Wnt signaling pathway inhibition in neuroectodermal development [8–10], as well as the role of IGF-1 in anterior neural/eye field development [11] and Notch pathway inhibition in photoreceptor cell development [12]. In our previously published studies, iPSCs were differentiated on cell culture surfaces coated with the extracellular matrix molecules collagen, laminin, and fibronectin [1]. These molecules were derived from nonautologous cell and animal sources and as a result would not be suitable for clinical-grade cell production.
To determine whether Synthemax cell culture surfaces could be used to promote cell adhesion and retinal specification, morphologic and RT-PCR analyses were performed on normal/nondiseased mouse and human iPSCs postdifferentiation. Microscopically, clonal areas of differentiation were evident in both mouse (Fig. 6A–6C) and human (Fig. 6D–6F) iPSC cultures. Within differentiated cell clusters, cells morphologically resembling RPE (Fig. 6A, 6D), photoreceptor precursors (Fig. 6B, 6E), and retinal neurons (Fig. 6C, 6F) could be identified. RT-PCR analysis indicated that postdifferentiation the retinal progenitor cell transcription factors Pax6 and Chx10; the early photoreceptor cell transcription factors OTX2, CRX, and NRL; and the mature photoreceptor genes recoverin and rhodopsin were expressed.
Figure 6.

Xeno-free derivation of retinal cells from normal mouse and human fibroblast-derived induced pluripotent stem cells (iPSCs). (A–F): Microscopic analysis of mouse (A–C) and human (D–F) iPSC-retinal precursor cells generated on Synthemax cell culture surfaces. At 33 days postdifferentiation, retinal pigmented epithelium (A, D), photoreceptor (B, E), and neuronal (C, F) morphologies were identified. (G, H): RT-PCR analysis of differentiated mouse (G) and human (H) iPSCs for expression of the retinal specification/photoreceptor genes Pax6, Chx10, Otx2, Crx, NRL, recoverin, and rhodopsin. Magnification, ×20 (A–F). Abbreviation: bp, base pairs.
To further demonstrate the utility of Synthemax cell culture surfaces for production of patient-specific retinal cells, the RP-specific iPSC line described in Figure 4 was analyzed. At 60–90 days postdifferentiation, pigmented RPE cell foci large enough to be mechanically isolated and expanded were present (Fig. 7A). When isolated and plated on fresh Synthemax cell culture surfaces, as previously reported [13–15] these pigmented foci gave rise to cells with a fibroblastic morphology that initially lost pigmentation (Fig. 7B). Upon reestablishment of confluence, cells established a typical RPE morphology and regained pigmentation (Fig. 7C). Confluent RPE cell cultures formed tight junctions as evident by ZO-1 staining (Fig. 7D) and began to acquire proper RPE cell function as evident by dome formation (supplemental online Fig. 2). As determined by immunocytochemical staining targeted against the photoreceptor markers Otx2 (Fig. 7E) and recoverin (Fig. 7F) and the retinal ganglion cell markers NF200 (Fig. 7G) and Brn3B (Fig. 7H), in addition to RPE, at 90 days postdifferentiation photoreceptor and retinal ganglion precursor cells were present.
Figure 7.
Xeno-free derivation of retinal cells from a patient with retinitis pigmentosa (RP-induced pluripotent stem cells [iPSCs]). (A–F): Microscopic analysis of human RP-iPSC-derived retinal pigmented epithelium (RPE) generated on Synthemax cell culture surfaces. At 60–90 days postdifferentiation large pigment RPE cell foci (A) were isolated, subcultured onto fresh Synthemax cell culture surfaces (B), and allowed to reach confluence (C) prior to being stained with the tight junction marker ZO-1 (D). Note that upon reaching confluence, RPE cultures regained pigmentation (see high-magnification inset in [C]). (E–H): Immunocytochemical analysis of differentiated human RP-iPSCs for expression of the retinal photoreceptor markers Otx2 and recoverin and the retinal ganglion cell markers NF200 and Brn3B. (I–L): Immunocytochemical analysis of differentiated human RP-iPSCs plated on ECM-coated (I, J) and Synthemax (K, L) cell culture substrates for expression of the retinal photoreceptor markers Otx2 and recoverin. High-magnification insets are included to depict cellular morphology. (M): Average number of cells expressing the photoreceptor markers OTX2 and recoverin at 90 days postdifferentiation. Scale bars = 100 μm (C, D, G) and 200 μm (A, B, E, F, H–L). **, p < .001. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; ECM, extracellular matrix; Rec, recoverin.
As our ultimate goal is to develop patient-specific photoreceptor precursor cells for transplantation and treatment of retinal degenerative disease, it was useful for us to determine (a) the efficiency of photoreceptor cell differentiation on Synthemax versus traditional extracellular matrix (ECM)-coated dishes, and (b) whether retinal photoreceptor precursor cells could be generated on Synthemax surfaces using a completely xeno-free culture medium. To answer these questions an additional series of experiments were performed in which (a) cells were differentiated on ECM-coated culture surfaces using the previously described differentiation protocol, and (b) cells were differentiated on Synthemax surfaces using a xeno-free differentiation protocol that lacked the recombinant proteins noggin, Dkk-1, IGF-1, and bFGF (depicted in supplemental online Fig. 1).
As observed on Synthemax surfaces, Otx2 and recoverin were expressed in clusters of cells differentiated on ECM-coated dishes (Fig. 7I, 7J). Analysis of cell number revealed that regardless of substrate (i.e., Synthemax or ECM) within differentiated clusters, approximately 45% of the cells expressed Otx2, whereas approximately 25% of the cells expressed recoverin (Fig. 7M: red bars indicate Synthemax surfaces, green bars indicate ECM-coated surfaces). Although the xeno-free differentiation protocol described above induced extensive cell spreading and morphological differentiation, significantly fewer Otx2 and recoverin-positive cells (approximately 20% and 10% respectively) were identified following termination of this protocol (Fig. 7K–7M, blue bars). Collectively, these data suggest that xeno-free Synthemax cell culture surfaces are useful for the production of retinal cells from adult patient-specific iPSCs.
Discussion
Retinal photoreceptor precursors derived from induced pluripotent stem cells are an attractive cell source for cell replacement therapy. When transplanted subretinally into a murine model of retinal degenerative disease, these cells are capable of providing partial restoration of retinal function [1, 4]. Transplanting human cells exposed to animal-derived products carries an increased risk of infection, disease transmission, immune response, and rejection [16]. For instance, embryonic stem cells cultured on a mouse feeder layer in the presence of bovine serum incorporate the Sialic acid Neu5Gc into their membrane [17]. Incorporation of Neu5Gc was shown to bind naturally circulating antibodies and activate the classic complement pathway targeting the cells for macrophage and natural killer T cell-mediated death in vivo [17]. In light of these findings, cells destined for human transplantation must be derived under xeno-free conditions to be clinically relevant.
In this study, we demonstrated the potential utility of using a synthetic cell culture system for iPSC derivation (using both integrating and nonintegrating systems), maintenance, and subsequent retinal-specific cellular differentiation. In particular, by using Corning Synthemax cell culture plates, we have been able to successfully generate iPSCs from normal adult mouse and human fibroblasts, from fibroblasts isolated from patients with various forms of retinal degenerative disease, and from both keratinocytes and IPEs isolated from elderly individuals. Following mechanical isolation and expansion, these cells express markers of pluripotency and are capable of generating teratomas containing tissues of all three embryonic germ layers. By plating these cells on Synthemax cell culture substrates and following both our previously devised differentiation paradigm and a new xeno-free differentiation protocol developed here, we have been able to successfully generate cells that express numerous retinal progenitor/photoreceptor-specific transcripts and proteins.
Although we have successfully generated patient-specific retinal cells in a xeno-free fashion, it is important to point out that like embryonic stem cells, undifferentiated iPSCs remaining postdifferentiation have the potential to form tumors following transplantation. As such, prior to clinical application it is likely that development/use of negative and or positive selection techniques, such as those described by us and others [1, 18], targeted at removal of undifferentiated cells and purification of differentiated cell types may be required. Likewise one could envision strategies in which suicide genes expressed in transplanted iPSCs could be activated in the event that a donor cell progressed down a tumorigenic pathway.
As published previously, a significant number of undifferentiated cells remain within and around embryoid bodies at the end of our differentiation protocol [1]. As published previously, countermeasures targeted at removal of these potentially tumorigenic cells will be required prior to clinical transplantation (see Discussion) [1].
Like embryonic stem cells, iPSCs are typically generated and cultured on an inactive layer of mouse embryonic fibroblasts. Generation of these feeder layers is expensive, time consuming, and labor intensive. Likewise, the phenotype of these lines is often variable, potentially introducing inconsistencies in the iPSC derivation and differentiation process. Feeder-free iPSC generation and expansion protocols, which typically use ECM-coated culture substrates, have been developed [19–22]. Typically, Matrigel (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com) and/or Celtrex (Trevigen, Gaithersburg, MD, http://www.trevigen.com), mixtures of ECM molecules secreted by mouse Engelbreth-Holm-Swarm (EHS) sarcoma cells, are used [22–24]. As with iPSCs cultured on these ECM-coated substrates, cells generated and cultured on Synthemax cell culture plates form distinct colonies with tightly packed cells. Although the morphology of these colonies is somewhat different from that originally described for cells plated on inactive feeders, it has been suggested that iPSCs grown in the absence of a feeder layer are genetically more similar to their embryonic stem cell counterparts [19].
Although the above-described ECM mixtures are useful for feeder-free derivation and expansion of iPSCs, they are purified from mouse EHS cells and do not address the problems associated with cellular exposure to animal-derived products. Likewise, these ECM mixtures are often variable; that is, differing ECM compositions and concentrations are often present in each batch. Consequently, other nonanimal ECM components useful for iPSC derivation and culture have been developed. For instance, recently James Thompson's group was able to show that human recombinant vitronectin in combination with defined culture medium could be used to support growth and expansion of human iPSCs [25]. Although promising, production of human recombinant ECM molecules is time consuming and requires a surrogate cell line in which the desired recombinant protein is expressed. As such, synthetic and ready-to-use products such as the one tested in this study would be of benefit.
Conclusion
Synthemax cell culture surfaces provide an ideal surface for the xeno-free production, culture, and differentiation of adult somatic cell-derived iPSCs. These findings demonstrate the potential utility of these surfaces for the production of clinical-grade retinal neurons for transplantation and induction of retinal regeneration.
See www.StemCellsTM.com for supporting information available online.
Acknowledgments
This work was supported by NIH Directors New Innovator Award 1-DP2-OD007483-01, Research to Prevent Blindness, Foundation Fighting Blindness, Grousbeck Family Foundation, and the Corley Research Fund.
Author Contributions
B.A.T., conception and design, financial support, provision of study material or patients, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; K.R.A., collection and/or assembly of data; R.F.M., E.M.S., and M.J.Y., conception and design, financial support, provision of study material or patients, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
M.J.Y. has uncompensated research funding.
References
- 1.Tucker BA, Park IH, Qi SD, et al. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS One. 2011;6:e18992. doi: 10.1371/journal.pone.0018992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tucker BA, Scheetz TE, Mullins RF, et al. Exome sequencing and analysis of induced pluripotent stem cells identify the cilia-related gene male germ cell-associated kinase (MAK) as a cause of retinitis pigmentosa. Proc Natl Acad Sci USA. 2011;108:E569–E576. doi: 10.1073/pnas.1108918108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer JS, Shearer RL, Capowski EE, et al. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci USA. 2009;106:16698–16703. doi: 10.1073/pnas.0905245106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamba DA, McUsic A, Hirata RK, et al. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One. 2010;5:e8763. doi: 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grinnell KL, Bickenbach JR. Skin keratinocytes pre-treated with embryonic stem cell-conditioned medium or BMP4 can be directed to an alternative cell lineage. Cell Prolif. 2007;40:685–705. doi: 10.1111/j.1365-2184.2007.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bickenbach JR. Isolation, characterization, and culture of epithelial stem cells. Methods Mol Biol. 2005;289:97–102. doi: 10.1385/1-59259-830-7:097. [DOI] [PubMed] [Google Scholar]
- 7.Hu DN, Ritch R, McCormick SA, et al. Isolation and cultivation of human iris pigment epithelium. Invest Ophthalmol Vis Sci. 1992;33:2443–2453. [PubMed] [Google Scholar]
- 8.Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, et al. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell. 2001;1:423–434. doi: 10.1016/s1534-5807(01)00041-7. [DOI] [PubMed] [Google Scholar]
- 9.Anderson RM, Lawrence AR, Stottmann RW, et al. Chordin and noggin promote organizing centers of forebrain development in the mouse. Development. 2002;129:4975–4987. doi: 10.1242/dev.129.21.4975. [DOI] [PubMed] [Google Scholar]
- 10.Lamb TM, Knecht AK, Smith WC, et al. Neural induction by the secreted polypeptide noggin. Science. 1993;262:713–718. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- 11.Pera EM, Wessely O, Li SY, et al. Neural and head induction by insulin-like growth factor signals. Dev Cell. 2001;1:655–665. doi: 10.1016/s1534-5807(01)00069-7. [DOI] [PubMed] [Google Scholar]
- 12.Jadhav AP, Mason HA, Cepko CL. Notch 1 inhibits photoreceptor production in the developing mammalian retina. Development. 2006;133:913–923. doi: 10.1242/dev.02245. [DOI] [PubMed] [Google Scholar]
- 13.Hu Q, Friedrich AM, Johnson LV, et al. Memory in induced pluripotent stem cells: Reprogrammed human retinal-pigmented epithelial cells show tendency for spontaneous redifferentiation. Stem Cells. 2010;28:1981–1991. doi: 10.1002/stem.531. [DOI] [PubMed] [Google Scholar]
- 14.Carr AJ, Vugler AA, Hikita ST, et al. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One. 2009;4:e8152. doi: 10.1371/journal.pone.0008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchholz DE, Hikita ST, Rowland TJ, et al. Derivation of functional retinal pigmented epithelium from induced pluripotent stem cells. Stem Cells. 2009;27:2427–2434. doi: 10.1002/stem.189. [DOI] [PubMed] [Google Scholar]
- 16.Lei T, Jacob S, Ajil-Zaraa I, et al. Xeno-free derivation and culture of human embryonic stem cells: Current status, problems and challenges. Cell Res. 2007;17:682–688. doi: 10.1038/cr.2007.61. [DOI] [PubMed] [Google Scholar]
- 17.Martin MJ, Muotri A, Gage F, et al. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228–232. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- 18.Lakowski J, Han YT, Pearson RA, et al. Effective transplantation of photoreceptor precursor cells selected via cell surface antigen expression. Stem Cells. 2011;29:1391–1404. doi: 10.1002/stem.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung HC, Lin RC, Logan GJ, et al. Human induced pluripotent stem cells derived under feeder-free conditions display unique cell cycle and DNA replication gene profiles. Stem Cells Dev. 2012;21:206–216. doi: 10.1089/scd.2010.0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin J, Fernandez I, Roy K. Development of feeder-free culture systems for generation of ckit+sca1+ progenitors from mouse iPS cells. Stem Cell Rev. 2011;7:736–747. doi: 10.1007/s12015-010-9215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baharvand H, Totonchi M, Taei A, et al. Human-induced pluripotent stem cells: Derivation, propagation, and freezing in serum- and feeder layer-free culture conditions. Methods Mol Biol. 2010;584:425–443. doi: 10.1007/978-1-60761-369-5_23. [DOI] [PubMed] [Google Scholar]
- 22.Sun N, Panetta NJ, Gupta DM, et al. Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc Natl Acad Sci USA. 2009;106:15720–15725. doi: 10.1073/pnas.0908450106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugii S, Kida Y, Berggren WT, et al. Feeder-dependent and feeder-independent iPS cell derivation from human and mouse adipose stem cells. Nat Protoc. 2011;6:346–358. doi: 10.1038/nprot.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludwig T, Thomson JA. Defined, feeder-independent medium for human embryonic stem cell culture. Curr Protoc Stem Cell Biol. 2007 doi: 10.1002/9780470151808.sc01c02s2. Chapter 1:Unit 1C.2. [DOI] [PubMed] [Google Scholar]
- 25.Chen G, Gulbranson DR, Hou Z, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]





