Graft-versus-host disease (GVHD) is a major complication of allogeneic hematopoietic stem cell transplant associated with significant morbidity and mortality. This review focuses on the pathophysiology, clinical features, prevention, and treatment of acute GVHD.
Keywords: Peripheral blood stem cells, Immune reconstitution, Transplantation tolerance, Adult hematopoietic stem cells, Adult human bone marrow, Cellular therapy, Hematologic malignancies, Hematopoietic stem cell transplantation
Abstract
Graft-versus-host disease (GVHD) is a major complication of allogeneic hematopoietic stem cell transplant (AHSCT) associated with significant morbidity and mortality. This review focuses on the pathophysiology, clinical features, prevention, and treatment of acute GVHD. Specifically, we explain how new discoveries in immunology have expanded our understanding of GVHD, in which tissue damage from chemotherapy or radiation results in cytokine release, which activates T cells, resulting in proliferation and differentiation, trafficking to target organs, and tissue destruction and inflammation. Insights into the mechanisms of this disease relate directly to the development of preventive strategies and therapies, such as immunosuppression, T-cell depletion, calcineurin inhibitors, CCR5 antagonists, gut decontamination, extracorporeal photopheresis, and more. We also discuss how GVHD affects the gut, liver, and skin, as well as diagnosis, grading, and scoring. We end by examining future directions of treatment, including new immunomodulators and biomarkers. Understanding the immunobiology of GVHD and developing effective preventions and treatments are critical to the continuing success of AHSCT.
Introduction
Allogeneic hematopoietic stem cell transplant (AHSCT) is an effective treatment for many hematologic and genetic diseases. Patients with hematologic malignancies may derive particular benefit: by replacing the patient's bone marrow with the donor's, the new immune system can attack tumor cells, known as graft-versus-tumor (GVT). However, donor-derived cells may also recognize recipient organs as foreign and mount an immune attack against the patient's own tissues, known as graft-versus-host disease (GVHD).
GVHD is a major cause of nonrelapse morbidity and mortality, affecting up to 40%–60% of AHSCT patients [1] and accounting for 15% of deaths after AHSCT [2]. Acute GVHD, typically occurring between the time of engraftment through 100 days after transplant, can have devastating consequences on the skin, gut, and liver. Chronic GVHD typically occurs after 100 days, although this temporal distinction is blurring with strategies such as reduced-intensity conditioning, and an overlap syndrome is recognized that shares features of both. This review focuses on the pathophysiology, clinical features, prevention, and treatment of acute GVHD following AHSCT. Of note, GVHD has also been observed in rare instances after autologous bone marrow transplant [3], as well as after blood transfusion [4]; these lie outside the scope of this review, as does discussion of chronic GVHD.
Pathophysiology
GVHD classically develops over five steps [5] (Fig. 1). First, tissue damage from the conditioning regimen (either radiation or chemotherapy) releases proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1) and danger signals such as adenosine-5′-triphosphate (ATP) and nicotine adenine dinucleotide, as well as extracellular matrix proteins such as biglycan that promote activation and maturation of antigen-presenting cells (APCs) [6]. This is furthered by damage to the gastrointestinal epithelium, allowing translocation of lipopolysaccharide, which can activate innate immunity through Toll-like receptors, furthering the cytokine cascade [7]. Polymorphisms in cytokine genes have been shown to affect the severity of GVHD [8].
Figure 1.
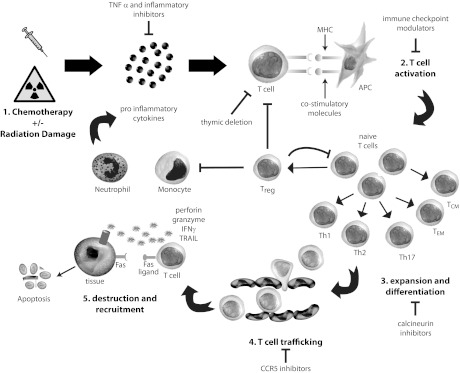
Pathophysiology of graft-versus-host disease (GVHD). (1) Chemotherapy and radiation cause tissue damage, producing proinflammatory cytokines, resulting in (2) T-cell activation through APC-T-cell interaction via MHC-T-cell receptor binding and costimulatory signals, leading to (3) expansion and differentiation into various subtypes of T cells, which (4) traffic through blood vessels to target organs, where they (5) cause tissue destruction and recruitment of other inflammatory cells through pathways such as perforin/granzyme and cytokine release. These inflammatory cells and cytokines can further propagate the cycle of GVHD. This process is internally regulated by Tregs as well as thymic deletion of alloreactive T cells (inside of circle); exogenous means of treating GVHD include inhibitors of inflammation and cytokines, immune checkpoint modulators, calcineurin inhibitors, and CCR5 inhibitors (outside of circle). Abbreviations: APC, antigen-presenting cell; CCR5, chemokine receptor type 5; IFNγ, interferon γ; MHC, major histocompatibility complex; TCM, central memory T cell; TEM, effector memory T cell; TNFα, tumor necrosis factor α; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; Treg, regulatory T-cell.
Second, donor T-cell activation is triggered by recipient antigens presented by host APCs [9] and sustained by donor APCs [10]. This is mediated by human leukocyte antigen (HLA) proteins encoded by the major histocompatibility complex (MHC) found on chromosome 6. MHC compatibility is the most powerful determinant of GVHD, and there is a direct relationship between the frequency of GVHD and mismatch at HLA-A, -B, -C, and -DRB1 (mismatch at HLA-DQ and -DP appears less significant, although still important) [11]. However, despite full 8 of 8 or even 12 of 12 match, 40% of recipients still develop GVHD [12], thought secondary to minor histocompatibility antigens (MiHAs) [13]. MiHAs are also targets for GVT [14].
In addition to the interaction between the T-cell receptor and MHC, T-cell activation requires signaling between costimulatory molecules such as CD28 (present on the T cell) and B7.1 or B7.2 (CD80 or CD86, present on the APC); other T-cell:APC costimulatory signaling pairs include inducible costimulator (ICOS) (CD278):B7H (CD275), OX40 (CD134):OX40L (CD252), CD40L (CD154):CD40, and 4-1BB (CD137):glucocorticoid-induced tumor necrosis factor receptor (GITR) [15]. The absence of these costimulatory signals, particularly CD28:B7.1/B7.2, can lead to anergy; furthermore, this interaction can be blocked by coinhibitory molecules such as CTLA4 (CD152), which competes with CD28 for B7.1/B7.2. Programmed death-1 (PD-1) (CD279):programmed death ligand 1 (B7H1, CD274) are another pair of inhibitory molecules that can induce anergy or tolerance. Models that block these costimulatory or coinhibitory interactions have been shown to reduce or exacerbate GVHD, suggesting possible therapeutic targets [15].
Third, T cells proliferate and differentiate into naïve, effector, memory, regulatory, Th1/Tc1, Th2/Tc2, Th17, and other subsets. Naïve CD44loCD62Lhi T cells appear to be essential to this response [16]; interestingly, CD44hiCD62Llo effector memory and CD44hiCD62Lhi central memory T cells may be able to promote GVT without GVHD [17]. The balance between Th1/Tc1 and Th2/Tc2 subsets as well as other subsets such as Th17 and the productions of cytokines such as IL-4, IL-5, IL-6, IL-12, IL-13, IL-17, IL-21, IL-23, TNF-α, transforming growth factor-β, and interferon-γ (IFN-γ) have been shown to impact the manifestation of GVHD, although the various contributions of each of these elements are still under active investigation [18].
Fourth, activated T cells migrate from secondary lymphoid organs to target tissues (skin, liver, gut) through a combination of chemokine-receptor, selectin-ligand, and integrin-ligand interactions [19]. Selectins and integrins mediate rolling and tethering of lymphocytes along high endothelial venules through interactions with their matching ligands. For example, interactions between L-selectin (CD62L) and α4β7 integrin expressed on T cells and peripheral node addressin and mucosal addressin cell adhesion molecule expressed on secondary lymphoid tissue mediate homing to mesenteric lymph nodes and Peyer patches and induction of gut GVHD [20]. Lymphocyte chemotaxis receptors such as chemokine receptor type 5 (CCR5), CCR6, and CCR7 are also essential to T-cell trafficking between secondary lymphoid tissues and target organs [21–23].
Fifth, once they reach target organs, T cells cause tissue destruction through direct cytotoxic activity as well as recruitment of other leukocytes. Cytotoxic activity is largely mediated by the Fas ligand:Fas and perforin-granzyme pathways [24]. Interestingly, other cytolytic pathways, such as TNF-related apoptosis-inducing ligand, may preferentially mediate GVT but not GVHD [25]. Cytokines such as TNF-α, IFN-γ, IL-2, IL-7, IL-10, and others also appear to be essential to regulating leukocyte recruitment and tissue destruction; these effects are dependent on strength, timing, and other interactions, making the effects of individual cytokines difficult to predict [5].
Although the above discussion focuses on T cells, B cells also play a role in GVHD via antigen presentation, cytokine secretion, and antibody production [26–28]. A retrospective analysis suggested that the concentration of infused B cells may predict the incidence of GVHD [29], and correlations have been reported between high levels of B cell activating factor and chronic GVHD [30]. Furthermore, a preparative regimen including rituximab for patients with follicular lymphoma undergoing AHSCT has been associated with an intriguingly low rate of GVHD (11%) [31], although further studies are needed before recommending rituximab for prophylaxis or treatment, and data from murine studies on the role of recipient B cells in GVHD are mixed [32, 33].
Another important part of immune reconstitution is the induction of tolerance and anergy [34]. These negative feedback mechanisms are crucial to the prevention of excessive tissue destruction and autoimmunity. Central deletion of autoreactive T cells occurs in the thymus, and loss of normal thymic repertoire selection has been shown to contribute to GVHD [35]. Peripherally, FoxP3+ regulatory T cells (Tregs) have been found to suppress GVHD [36]. Coinhibitory signals as noted above also contribute to tolerance and anergy. Further investigation into these regulatory pathways will provide insight into the pathogenesis and treatment of GVHD.
Clinical Features
Acute GVHD primarily affects the skin (81% of patients with GVHD), gastrointestinal tract (54%), and liver (50%) [37]. Skin lesions are usually the first manifestation and arise around the time of white cell engraftment. Affected patients typically have a maculopapular rash that starts around the neck and shoulders and often involves the palms, soles, and ears; however, the scalp is usually spared [12]. Milder forms can look like sunburn, but more severe lesions can blister and ulcerate, with bullae and toxic epidermal necrolysis mimicking Stevens-Johnson syndrome in the most extreme cases (staging is shown in Table 1). The differential also includes drug rash and viral exanthem. Pathologic findings include dyskeratotic epidermal keratinocytes, lymphocytic exocytosis, perivascular lymphocytic infiltration, and apoptosis at the base of crypts [38].
Table 1.
Acute graft-versus-host disease staging
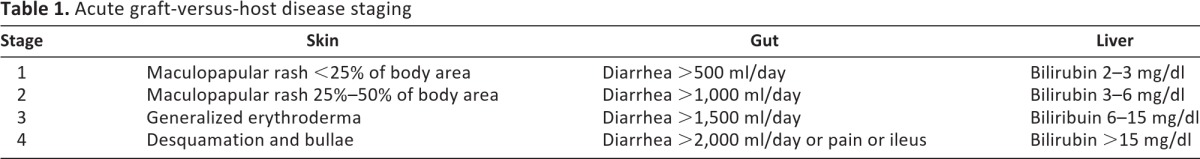
Gastrointestinal (GI) manifestations include abdominal cramping and pain, diarrhea, hematochezia, and ileus (lower GI), as well as anorexia, nausea, and vomiting (upper GI). Severity is determined by the volume of diarrhea (Table 1), which is secretory and may persist despite cessation of oral intake. Hematochezia may result in significant transfusion requirements. The differential includes Clostridium difficile colitis, cytomegalovirus (CMV) enteritis, herpes simplex virus or candida esophagitis, gastritis, ulcers, and postchemoradiation effect. Histologic features include apoptotic bodies in the base of crypts, crypt abscesses, and loss and flattening of surface epithelium [39].
Liver disease is due to damage to bile canaliculi, leading to cholestasis with hyperbilirubinemia and elevated alkaline phosphatase; severity is based on serum bilirubin (Table 1). The differential includes sinusoidal obstructive syndrome (also called veno-occlusive disease), drug toxicity, and viral infection. Histologic features of bile damage include bile duct atypia and degeneration, epithelial cell dropout, lymphocytic infiltration of small bile ducts; endothelialitis and pericholangitis may also be observed [40].
The hematopoietic system is also commonly affected with thymic atrophy, cytopenias (particularly thrombocytopenias), and hypogammaglobulinemia (particularly IgA). More rarely affected organs include the eyes (photophobia, hemorrhagic conjunctivitis, lagophthalmos) and kidneys (nephritis, nephrotic syndrome, e.g., membranous nephropathy) [41].
The diagnosis of GVHD is based primarily on clinical criteria, although histopathological changes on biopsy may be helpful. Plasma biomarkers, although not widely adopted, are a promising area of research: elafin (also known as peptidase inhibitor-3, skin-derived antileukoproteinase, or trappin-2) is elevated threefold in skin GVHD [42], and regenerating islet-derived 3-α is increased threefold in patients with GI GVHD [43]. The combination of these two proteins with IL-2 receptor-α, TNF receptor-1, hepatocyte growth factor, and IL-8 form a six-protein biomarker panel that predicted response to GVHD treatment and mortality in a randomized clinical trial [44]. Grading of GVHD is based on dermal, gastrointestinal, and hepatic involvement plus functional impairment; the Glucksberg and International Bone Marrow Transplant Registry systems have both been validated [45, 46] (Tables 2 and 3). Severe GVHD can be associated with significant mortality: 5-year survival for patients with grade III disease is only 25%, and this drops to 5% for patients with grade IV disease [47].
Table 2.
Acute graft-versus-host disease grading: Glucksberg grade [45]
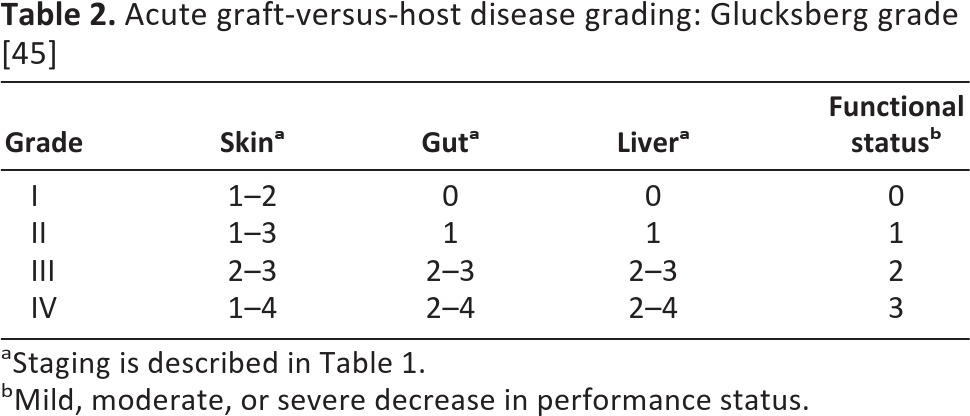
aStaging is described in Table 1.
bMild, moderate, or severe decrease in performance status.
Table 3.
Acute graft-versus-host disease grading: International Bone Marrow Transplant Registry Severity Index [46]
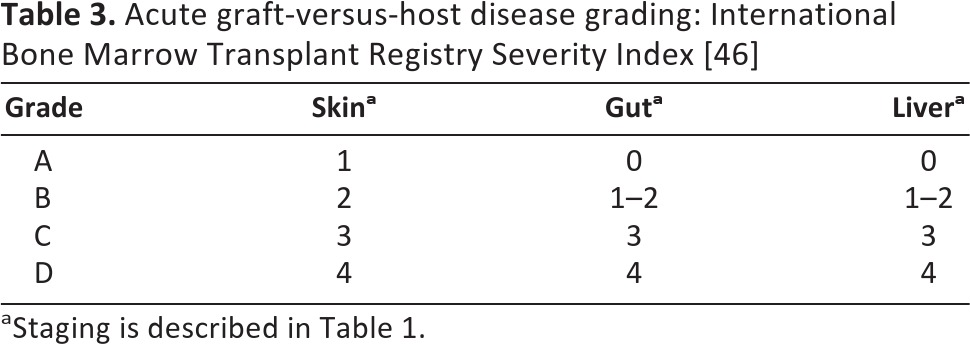
aStaging is described in Table 1.
Predictive Factors
As noted above, HLA mismatch is the strongest determinant of GVHD. Using female donors for male recipients also increases the risk of GVHD; this is thought to be secondary to minor antigen mismatch, which also underlies the increased the risk of GVHD with unrelated donors [48]. Multiparity in donors has also been linked with increased risk of GVHD secondary to maternal alloimmunization [49]. However, in haploidentical transplantation, mismatches for noninherited paternal antigens increase the risk of GVHD compared with noninherited maternal antigens, suggesting that in utero exposure to noninherited maternal antigens may exert more complicated long-lasting immune effects [50]. Interestingly, use of umbilical cord blood appears less likely to cause GVHD, and four of six mismatches can be tolerated with this donor source [51].
In addition to donor characteristics, many other factors have been associated with the risk of GVHD. Reduced intensity conditioning causes less damage and results in less GVHD [52], whereas total body irradiation causes more GVHD [48]. Transplants that result in full donor chimerism (in which all detectable cells are donor in origin) are associated with a higher incidence of GVHD than mixed chimerism (in which a mixed population of donor and recipient cells are detected) [53]. Unfortunately, mixed chimerism is also associated with higher rates of engraftment failure and relapse; attempts to convert mixed to full donor chimerism with donor lymphocyte infusion often increase GVHD [54, 55]. Infections may also play a role: it has been known since 1974 that the intestinal microflora affects GVHD [56], and administration of antibiotics can attenuate the risk [57]. Additionally, if the donor and recipient are both CMV negative, the risk of GVHD is reduced, whereas it is increased if one or both are positive [58]. Older patients are more likely to have GVHD [48], possibly because of increased thymic involution with aging and impaired central deletion of autoreactive T cells. Patients with worse performance status are also at higher risk [58].
Prevention of GVHD
Immunosuppression has been the primary pharmacologic strategy to prevent GVHD. Methotrexate has been used since the 1950s as a way of shutting down T cells through inhibition of dihydrofolate reductase and production of thymidylate and purines [59]. Post-transplant cyclophosphamide is another method of eliminating rapidly dividing T cells that shows promise in recent clinical trials [60]. The calcineurin inhibitors cyclosporine and tacrolimus inhibit T-cell proliferation; combinations with methotrexate have successfully been used since the 1970s and are the cornerstone of most prophylactic regimens [61]. However, these agents have numerous side effects, including delayed cell count and immunological recovery, thrombotic microangiopathy [62], and posterior reversible encephalopathy syndrome [63]. The inosine monophosphate dehydrogenase inhibitor mycophenolate mofetil and the mammalian target of rapamycin (mTOR) inhibitor sirolimus have been proposed as alternate agents [64, 65]; however, there is no clear consensus on optimal drug combination, dosing, or timing.
Other drugs attempt to target cytokine/chemokine-receptor interactions that appear integral to development of GVHD. Exciting new success has been reported with maraviroc, a CCR5 antagonist that blocks T-cell chemotaxis and dramatically decreased the incidence of gastrointestinal and liver GVHD [66]. At the same time, despite the central role of cytokines IL-1 and TNF-α, drugs that block these pathways (etanercept, infliximab) failed to improve rates of acute GVHD [67, 68].
Alternative methods of reducing GVHD involve dampening the cytokine storm that sets off the cascade. Reduced intensity conditioning causes less tissue damage and has been shown to reduce GVHD [69].
Additionally, gut decontamination with ciprofloxacin and metronidazole has been shown to decrease the risk of acute GVHD compared with ciprofloxacin alone [57]. However, attempts to preserve epithelial integrity with keratinocyte growth factor (palifermin) did not decrease the risk of GVHD, although palifermin does reduce the risk of mucositis [70].
Given the central role of T cells in GVHD, T-cell depletion (TCD) has been studied since the 1980s as a preventative strategy. This can be done with physical techniques, such as ex vivo counterflow centrifugal elutriation or soybean lectin agglutination and E-rosetting, or by immunological methods, such as ex vivo or in vivo administration of anti-sera (anti-thymocyte globulin) or monoclonal antibodies; positive selection techniques can also isolate CD34+ cells ex vivo, allowing T cells to be discarded. Randomized trials have shown that although TCD successfully decreases the risk of GVHD, the risks of graft failure, disease relapse, and opportunistic infections are increased [71, 72]. However, some of these risks can be mitigated (e.g., higher CD34+ cell dose to promote engraftment, antibiotic prophylaxis to prevent opportunistic infections), and more recent single-arm trials have shown 3-year disease-free survival approaching 60% [73, 74]. Furthermore, T-cell depletion strategies such as in vivo administration of the anti-CD52 antibody alemtuzumab can facilitate transplants from HLA-mismatched haploidentical donors without significant GVHD [75], opening up transplant options to patients without HLA-matched donors.
Alternative methods of suppressing T cells are promising. A single-arm trial of soluble CTLA4 targeting the CD28:B7 costimulatory pathway successfully induced anergy and reported a low rate of GVHD [76], although further studies are lacking. Another exciting strategy is infusion of multipotent mesenchymal stromal cells (MSCs, also known as mesenchymal stem cells), which maintain the ability to differentiate into a variety of supportive cells. MSCs exert immunosuppressive effects on both lymphocytes and APCs. In a randomized phase II trial, prophylactic MSC administration resulted in only 5.3% grade II–IV acute GVHD compared with 38.9% in the control arm [77].
Treatment of GVHD
Glucocorticoids are the gold standard for treatment of grade II–IV acute GVHD, even though the main mechanism of action still remains unclear (possibilities include suppression of proinflammatory cytokines, as well as direct lymphotoxic effects) [78]. A randomized clinical trial has suggested that methylprednisolone at 2 mg/kg per day is the ideal starting dose, with escalating doses or the addition of alternative agents if there is no response by 5 days [79]. Acute adverse effects include hyperglycemia and psychosis; chronic changes include immunosuppression and infections, myopathy, osteoporosis and avascular necrosis of bone, cataracts, and fat distribution. Unfortunately, only about half of patients respond [80], and there is no clear second-line agent for steroid-refractory GVHD.
Many single-arm trials have shown benefit with other immunomodulators, such as anti-thymocyte globulin, tacrolimus, sirolimus, or mycophenolate mofetil, or antibody therapy against CD3, CD7, CD52, CD147, IL-2-R, IL-1, and TNF-α; however, none of these agents have proven efficacy in randomized clinical trials [41]. Intravenous immunoglobulin (IVIG) is the only drug that has been shown to reduce the rate of acute GVHD in a large randomized clinical trial (51% in controls vs. 34% in IVIG recipients), although its cost and concern for impaired humoral recovery limit its widespread use [81].
Many novel approaches are currently under investigation. Adoptive transfer of Tregs following in vitro enrichment and expansion has shown safety and decreased rates of GVHD compared with historical controls [82]. Extracorporeal photopheresis involves ex vivo incubation of patient leukocytes with 8-methyoxypsoralen and ultraviolet A (UVA) irradiation, exposure to UVA, and reinfusion, resulting in immunomodulatory effects including lymphocyte apoptosis, increasing Tregs, and shifting from a Th1 to Th2 phenotype [83]; studies are now looking at this strategy for prevention [84]. Denileukin diftitox, a recombinant protein composed of IL-2 fused to diphtheria toxin, is another novel approach that showed promise in a phase II trial [85], as is pentostatin, a purine analog [86]. Phototherapy using UVA irradiation with or without psoralen appears to help cutaneous lesions [87], and oral beclometasone may improve gastrointestinal GVHD [88]. Finally, in addition to use as prophylaxis, mesenchymal stem cells have been used for treatment of GVHD, with promising response rates ranging from 71% to 94% and complete response rates of 55%–74% [89, 90].
Supportive care is critical for patients with acute GVHD. This includes gut rest, hyperalimentation, and fluid and electrolyte repletion for gastrointestinal GVHD. A single arm trial suggests that octreotide may also help [91]. Prophylaxis against infections and early intervention when infections are suspected are essential, and treatment of hypogammaglobulinemia with IVIG may be helpful. Providers should closely monitor patients for side effects of immunosuppressants, such as diabetes and osteoporosis with steroids and renal impairment and hypertension with calcineurin inhibitors.
Future Directions
Experimental therapies are under development to attack other steps in the GVHD pathway. Preclinical studies suggest that IL-21 blockade may increase FoxP3+ inducible Tregs in vivo and decrease GVHD [92, 93]. Histone deacetylase inhibitors can also enhance Treg function and induce tolerance [94]. ICOS blockade can inhibit expansion of effector T cells in secondary lymphoid organs, thereby reducing GVHD [95]. Other strategies target innate immunity by blocking antigen-presenting cell-derived complement [96]. Administration of CSF-1 can expand host macrophages and decrease GVHD [97]. Ongoing work in biomarkers is essential to identifying GVHD early and initiating treatment before symptoms get worse.
Conclusion
As our ability to prevent and treat GVHD improves, it is important to keep in mind the ultimate goal: curing our patients. As experience with TCD has shown, methods that lower GVHD may also lower GVT, thereby increasing the risk of relapse of hematologic malignancies. Further insights are needed not just to treat GVHD but to treat GVHD while maintaining or maximizing GVT; this separation remains the holy grail of AHSCT.
Acknowledgments
The authors thank Dr. Joel Ross for artistic assistance with the figure. A.D.S. was supported by NIH Grant T32 HL007057-37.
Author Contributions
A.D.S. and N.J.C.: manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Jagasia M, Arora M, Flowers ME, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119:296–307. doi: 10.1182/blood-2011-06-364265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides. 2011. [Accessed 2011]. Available at http://www.cibmtr.org.
- 3.Kline J SS, Lazarus HM, van Besien K. Autologous graft-versus-host disease: Harnessing anti-tumor immunity through impaired self-tolerance. Bone Marrow Transplant. 2008;41:505–513. doi: 10.1038/sj.bmt.1705931. [DOI] [PubMed] [Google Scholar]
- 4.Dwyre DM, Holland PV. Transfusion-associated graft-versus-host disease. Vox Sang. 2008;95:85–93. doi: 10.1111/j.1423-0410.2008.01073.x. [DOI] [PubMed] [Google Scholar]
- 5.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–170. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 6.Zeiser R, Penack O, Holler E, et al. Danger signals activating innate immunity in graft-versus-host disease. J Mol Med (Berl) 2011;89:833–845. doi: 10.1007/s00109-011-0767-x. [DOI] [PubMed] [Google Scholar]
- 7.Hill GR, Crawford JM, Cooke KR, et al. Total body irradiation and acute graft-versus-host disease: The role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–3213. [PubMed] [Google Scholar]
- 8.Dickinson AM, Middleton PG, Rocha V, et al. Genetic polymorphisms predicting the outcome of bone marrow transplants. Br J Haematol. 2004;127:479–490. doi: 10.1111/j.1365-2141.2004.05216.x. [DOI] [PubMed] [Google Scholar]
- 9.Shlomchik WD, Couzens MS, Tang CB, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 10.Matte CC, Liu J, Cormier J, et al. Donor APCs are required for maximal GVHD but not for GVL. Nat Med. 2004;10:987–992. doi: 10.1038/nm1089. [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goulmy E, Schipper R, Pool J, et al. Mismatches of minor histocompatibility antigens between HLA-identical donors and recipients and the development of graft-versus-host disease after bone marrow transplantation. N Engl J Med. 1996;334:281–285. doi: 10.1056/NEJM199602013340501. [DOI] [PubMed] [Google Scholar]
- 14.Bleakley M, Riddell SR. Exploiting T cells specific for human minor histocompatibility antigens for therapy of leukemia. Immunol Cell Biol. 2011;89:396–407. doi: 10.1038/icb.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon B. Intervention with costimulatory pathways as a therapeutic approach for graft-versus-host disease. Exp Mol Med. 2010;42:675–683. doi: 10.3858/emm.2010.42.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Joe G, Hexner E, et al. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med. 2005;11:1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
- 17.Chen BJ, Deoliveira D, Cui X, et al. Inability of memory T cells to induce graft-versus-host disease is a result of an abortive alloresponse. Blood. 2007;109:3115–3123. doi: 10.1182/blood-2006-04-016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi T, Chen Y, Wang L, et al. Reciprocal differentiation and tissue-specific pathogenesis of Th1, Th2, and Th17 cells in graft-versus-host disease. Blood. 2009;114:3101–3112. doi: 10.1182/blood-2009-05-219402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, et al. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191–4199. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dutt S, Ermann J, Tseng D, et al. L-selectin and beta7 integrin on donor CD4 T cells are required for the early migration to host mesenteric lymph nodes and acute colitis of graft-versus-host disease. Blood. 2005;106:4009–4015. doi: 10.1182/blood-2005-06-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer LA, Sale GE, Balogun JI, et al. Chemokine receptor CCR5 mediates alloimmune responses in graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16:311–319. doi: 10.1016/j.bbmt.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weninger W, von Andrian UH. Chemokine regulation of naive T cell traffic in health and disease. Semin Immunol. 2003;15:257–270. doi: 10.1016/j.smim.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Varona R, Cadenas V, Gomez L, et al. CCR6 regulates CD4+ T-cell-mediated acute graft-versus-host disease responses. Blood. 2005;106:18–26. doi: 10.1182/blood-2004-08-2996. [DOI] [PubMed] [Google Scholar]
- 24.Braun MY, Lowin B, French L, et al. Cytotoxic T cells deficient in both functional fas ligand and perforin show residual cytolytic activity yet lose their capacity to induce lethal acute graft-versus-host disease. J Exp Med. 1996;183:657–661. doi: 10.1084/jem.183.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmaltz C, Alpdogan O, Kappel BJ, et al. T cells require TRAIL for optimal graft-versus-tumor activity. Nat Med. 2002;8:1433–1437. doi: 10.1038/nm1202-797. [DOI] [PubMed] [Google Scholar]
- 26.Rowe V, Banovic T, MacDonald KP, et al. Host B cells produce IL-10 following TBI and attenuate acute GVHD after allogeneic bone marrow transplantation. Blood. 2006;108:2485–2492. doi: 10.1182/blood-2006-04-016063. [DOI] [PubMed] [Google Scholar]
- 27.von Bergwelt-Baildon MS, Vonderheide RH, Maecker B, et al. Human primary and memory cytotoxic T lymphocyte responses are efficiently induced by means of CD40-activated B cells as antigen-presenting cells: Potential for clinical application. Blood. 2002;99:3319–3325. doi: 10.1182/blood.v99.9.3319. [DOI] [PubMed] [Google Scholar]
- 28.Miklos DB, Kim HT, Zorn E, et al. Antibody response to DBY minor histocompatibility antigen is induced after allogeneic stem cell transplantation and in healthy female donors. Blood. 2004;103:353–359. doi: 10.1182/blood-2003-03-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iori AP, Torelli GF, De Propris MS, et al. B-cell concentration in the apheretic product predicts acute graft-versus-host disease and treatment-related mortality of allogeneic peripheral blood stem cell transplantation. Transplantation. 2008;85:386–390. doi: 10.1097/TP.0b013e3181622e36. [DOI] [PubMed] [Google Scholar]
- 30.Sarantopoulos S, Stevenson KE, Kim HT, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113:3865–3874. doi: 10.1182/blood-2008-09-177840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khouri IF, McLaughlin P, Saliba RM, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111:5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schultz KR, Paquet J, Bader S, et al. Requirement for B cells in T cell priming to minor histocompatibility antigens and development of graft-versus-host disease. Bone Marrow Transplant. 1995;16:289–295. [PubMed] [Google Scholar]
- 33.Li H, Demetris AJ, McNiff J, et al. Profound depletion of host conventional dendritic cells, plasmacytoid dendritic cells, and B cells does not prevent graft-versus-host disease induction. J Immunol. 2012;188:3804–3811. doi: 10.4049/jimmunol.1102795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hess AD. Reconstitution of self-tolerance after hematopoietic stem cell transplantation. Immunol Res. 2010;47:143–152. doi: 10.1007/s12026-009-8145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holländer GA, Widmer B, Burakoff SJ. Loss of normal thymic repertoire selection and persistence of autoreactive T cells in graft vs host disease. J Immunol. 1994;152:1609–1617. [PubMed] [Google Scholar]
- 36.Beres AJ, Haribhai D, Chadwick AC, et al. CD8+ Foxp3+ regulatory T cells are induced during graft-versus-host disease and mitigate disease severity. J Immunol. 2012;189:464–474. doi: 10.4049/jimmunol.1200886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin PJ, Schoch G, Fisher L, et al. A retrospective analysis of therapy for acute graft-versus-host disease: Initial treatment. Blood. 1990;76:1464–1472. [PubMed] [Google Scholar]
- 38.Darmstadt GL, Donnenberg AD, Vogelsang GB, et al. Clinical, laboratory, and histopathologic indicators of the development of progressive acute graft-versus-host disease. J Invest Dermatol. 1992;99:397–402. doi: 10.1111/1523-1747.ep12616112. [DOI] [PubMed] [Google Scholar]
- 39.Snover DC, Weisdorf SA, Vercellotti GM, et al. A histopathologic study of gastric and small intestinal graft-versus-host disease following allogeneic bone marrow transplantation. Hum Pathol. 1985;16:387–392. doi: 10.1016/s0046-8177(85)80232-x. [DOI] [PubMed] [Google Scholar]
- 40.Snover DC, Weisdorf SA, Ramsay NK, et al. Hepatic graft versus host disease: A study of the predictive value of liver biopsy in diagnosis. Hepatology. 1984;4:123–130. doi: 10.1002/hep.1840040122. [DOI] [PubMed] [Google Scholar]
- 41.Sullivan K. Graft-vs.-host disease. In: Blume KG, FS, Appelbaum FR, editors. Thomas' Hematopoietic Cell Transplantation. 3rd ed. Oxford, U.K.: Blackwell Publishing; 2004. pp. 635–64. [Google Scholar]
- 42.Paczesny S, Braun TM, Levine JE, et al. Elafin is a biomarker of graft-versus-host disease of the skin. Sci Transl Med. 2010;2:13ra2. doi: 10.1126/scitranslmed.3000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferrara JL, Harris AC, Greenson JK, et al. Regenerating islet-derived 3-alpha is a biomarker of gastrointestinal graft-versus-host disease. Blood. 2011;118:6702–6708. doi: 10.1182/blood-2011-08-375006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levine JE, Logan BR, Wu J, et al. Acute graft-versus-host disease biomarkers measured during therapy can predict treatment outcomes: A Blood and Marrow Transplant Clinical Trials Network study. Blood. 2012;119:3854–3860. doi: 10.1182/blood-2012-01-403063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: Retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 47.Cahn JY, Klein JP, Lee SJ, et al. Prospective evaluation of 2 acute graft-versus-host (GVHD) grading systems: A joint Societe Francaise de Greffe de Moelle et Therapie Cellulaire (SFGM-TC), Dana Farber Cancer Institute (DFCI), and International Bone Marrow Transplant Registry (IBMTR) prospective study. Blood. 2005;106:1495–1500. doi: 10.1182/blood-2004-11-4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flowers ME, Inamoto Y, Carpenter PA, et al. Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood. 2011;117:3214–3219. doi: 10.1182/blood-2010-08-302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flowers ME, Pepe MS, Longton G, et al. Previous donor pregnancy as a risk factor for acute graft-versus-host disease in patients with aplastic anaemia treated by allogeneic marrow transplantation. Br J Haematol. 1990;74:492–496. doi: 10.1111/j.1365-2141.1990.tb06340.x. [DOI] [PubMed] [Google Scholar]
- 50.van Rood JJ, Loberiza FR, Jr., Zhang MJ, et al. Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease after bone marrow transplantation from a parent or an HLA-haploidentical sibling. Blood. 2002;99:1572–1577. doi: 10.1182/blood.v99.5.1572. [DOI] [PubMed] [Google Scholar]
- 51.Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373–381. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 52.Mielcarek M, Martin PJ, Leisenring W, et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003;102:756–762. doi: 10.1182/blood-2002-08-2628. [DOI] [PubMed] [Google Scholar]
- 53.Svenberg P, Mattsson J, Ringden O, et al. Allogeneic hematopoietic SCT in patients with non-malignant diseases, and importance of chimerism. Bone Marrow Transplant. 2009;44:757–763. doi: 10.1038/bmt.2009.82. [DOI] [PubMed] [Google Scholar]
- 54.Dey BR, McAfee S, Colby C, et al. Impact of prophylactic donor leukocyte infusions on mixed chimerism, graft-versus-host disease, and antitumor response in patients with advanced hematologic malignancies treated with nonmyeloablative conditioning and allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2003;9:320–329. doi: 10.1016/s1083-8791(03)00077-6. [DOI] [PubMed] [Google Scholar]
- 55.Bader P, Kreyenberg H, Hoelle W, et al. Increasing mixed chimerism is an important prognostic factor for unfavorable outcome in children with acute lymphoblastic leukemia after allogeneic stem-cell transplantation: Possible role for pre-emptive immunotherapy? J Clin Oncol. 2004;22:1696–1705. doi: 10.1200/JCO.2004.05.198. [DOI] [PubMed] [Google Scholar]
- 56.van Bekkum DW, Roodenburg J, Heidt PJ, et al. Mitigation of secondary disease of allogeneic mouse radiation chimeras by modification of the intestinal microflora. J Natl Cancer Inst. 1974;52:401–404. doi: 10.1093/jnci/52.2.401. [DOI] [PubMed] [Google Scholar]
- 57.Beelen DW, Elmaagacli A, Muller KD, et al. Influence of intestinal bacterial decontamination using metronidazole and ciprofloxacin or ciprofloxacin alone on the development of acute graft-versus-host disease after marrow transplantation in patients with hematologic malignancies: Final results and long-term follow-up of an open-label prospective randomized trial. Blood. 1999;93:3267–3275. [PubMed] [Google Scholar]
- 58.Hahn T, McCarthy PL, Jr., Zhang MJ, et al. Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. J Clin Oncol. 2008;26:5728–5734. doi: 10.1200/JCO.2008.17.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uphoff DE. Alteration of homograft reaction by A-methopterin in lethally irradiated mice treated with homologous marrow. Proc Soc Exp Biol Med. 1958;99:651–653. doi: 10.3181/00379727-99-24450. [DOI] [PubMed] [Google Scholar]
- 60.Luznik L, Fuchs EJ. High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol Res. 2010;47:65–77. doi: 10.1007/s12026-009-8139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Storb R, Antin JH, Cutler C. Should methotrexate plus calcineurin inhibitors be considered standard of care for prophylaxis of acute graft-versus-host disease? Biol Blood Marrow Transplant. 2010;16:S18–S27. doi: 10.1016/j.bbmt.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laskin BL, Goebel J, Davies SM, et al. Small vessels, big trouble in the kidneys and beyond: Hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Blood. 2011;118:1452–1462. doi: 10.1182/blood-2011-02-321315. [DOI] [PubMed] [Google Scholar]
- 63.Wong R, Beguelin GZ, de Lima M, et al. Tacrolimus-associated posterior reversible encephalopathy syndrome after allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2003;122:128–134. doi: 10.1046/j.1365-2141.2003.04447.x. [DOI] [PubMed] [Google Scholar]
- 64.Cutler C, Antin JH. Sirolimus immunosuppression for graft-versus-host disease prophylaxis and therapy: An update. Curr Opin Hematol. 2010;17:500–504. doi: 10.1097/MOH.0b013e32833e5b2e. [DOI] [PubMed] [Google Scholar]
- 65.Nash RA, Johnston L, Parker P, et al. A phase I/II study of mycophenolate mofetil in combination with cyclosporine for prophylaxis of acute graft-versus-host disease after myeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2005;11:495–505. doi: 10.1016/j.bbmt.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 66.Reshef R, Luger SM, Hexner EO, et al. Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N Engl J Med. 2012;367:135–145. doi: 10.1056/NEJMoa1201248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Antin JH, Weisdorf D, Neuberg D, et al. Interleukin-1 blockade does not prevent acute graft-versus-host disease: Results of a randomized, double-blind, placebo-controlled trial of interleukin-1 receptor antagonist in allogeneic bone marrow transplantation. Blood. 2002;100:3479–3482. doi: 10.1182/blood-2002-03-0985. [DOI] [PubMed] [Google Scholar]
- 68.Hamadani M, Hofmeister CC, Jansak B, et al. Addition of infliximab to standard acute graft-versus-host disease prophylaxis following allogeneic peripheral blood cell transplantation. Biol Blood Marrow Transplant. 2008;14:783–789. doi: 10.1016/j.bbmt.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Couriel DR, Saliba RM, Giralt S, et al. Acute and chronic graft-versus-host disease after ablative and nonmyeloablative conditioning for allogeneic hematopoietic transplantation. Biol Blood Marrow Transplant. 2004;10:178–185. doi: 10.1016/j.bbmt.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 70.Levine JE, Blazar BR, DeFor T, et al. Long-term follow-up of a phase I/II randomized, placebo-controlled trial of palifermin to prevent graft-versus-host disease (GVHD) after related donor allogeneic hematopoietic cell transplantation (HCT) Biol Blood Marrow Transplant. 2008;14:1017–1021. doi: 10.1016/j.bbmt.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wagner JE, Thompson JS, Carter SL, et al. Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell Depletion Trial): A multi-centre, randomised phase II-III trial. Lancet. 2005;366:733–741. doi: 10.1016/S0140-6736(05)66996-6. [DOI] [PubMed] [Google Scholar]
- 72.Mitsuyasu RT, Champlin RE, Gale RP, et al. Treatment of donor bone marrow with monoclonal anti-T-cell antibody and complement for the prevention of graft-versus-host disease. A prospective, randomized, double-blind trial. Ann Intern Med. 1986;105:20–26. doi: 10.7326/0003-4819-105-1-20. [DOI] [PubMed] [Google Scholar]
- 73.Devine SM, Carter S, Soiffer RJ, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: Results of the blood and marrow transplant clinical trials network protocol 0303. Biol Blood Marrow Transplant. 2011;17:1343–1351. doi: 10.1016/j.bbmt.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jakubowski AA, Small TN, Kernan NA, et al. T cell-depleted unrelated donor stem cell transplantation provides favorable disease-free survival for adults with hematologic malignancies. Biol Blood Marrow Transplant. 2011;17:1335–1342. doi: 10.1016/j.bbmt.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rizzieri DA, Koh LP, Long GD, et al. Partially matched, nonmyeloablative allogeneic transplantation: Clinical outcomes and immune reconstitution. J Clin Oncol. 2007;25:690–697. doi: 10.1200/JCO.2006.07.0953. [DOI] [PubMed] [Google Scholar]
- 76.Guinan EC, Boussiotis VA, Neuberg D, et al. Transplantation of anergic histoincompatible bone marrow allografts. N Engl J Med. 1999;340:1704–1714. doi: 10.1056/NEJM199906033402202. [DOI] [PubMed] [Google Scholar]
- 77.Kuzmina LA, Petinati NA, Parovichnikova EN, et al. Multipotent mesenchymal stromal cells for the prophylaxis of acute graft-versus-host disease: A phase II study. Stem Cells Int. 2012;2012:968213. doi: 10.1155/2012/968213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thomas E, Storb R, Clift RA, et al. Bone-marrow transplantation. New Engl J Med. 1975;292:832–843. 895–902. doi: 10.1056/NEJM197504172921605. [DOI] [PubMed] [Google Scholar]
- 79.Van Lint MT, Uderzo C, Locasciulli A, et al. Early treatment of acute graft-versus-host disease with high- or low-dose 6-methylprednisolone: A multicenter randomized trial from the Italian Group for Bone Marrow Transplantation. Blood. 1998;92:2288–2293. [PubMed] [Google Scholar]
- 80.MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: Comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–394. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 81.Sullivan KM, Kopecky KJ, Jocom J, et al. Immunomodulatory and antimicrobial efficacy of intravenous immunoglobulin in bone marrow transplantation. N Engl J Med. 1990;323:705–712. doi: 10.1056/NEJM199009133231103. [DOI] [PubMed] [Google Scholar]
- 82.Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: Safety profile and detection kinetics. Blood. 2011;117:1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perfetti P, Carlier P, Strada P, et al. Extracorporeal photopheresis for the treatment of steroid refractory acute GVHD. Bone Marrow Transplant. 2008;42:609–617. doi: 10.1038/bmt.2008.221. [DOI] [PubMed] [Google Scholar]
- 84.Shaughnessy PJ, Bolwell BJ, van Besien K, et al. Extracorporeal photopheresis for the prevention of acute GVHD in patients undergoing standard myeloablative conditioning and allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45:1068–1076. doi: 10.1038/bmt.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shaughnessy PJ, Bachier C, Grimley M, et al. Denileukin diftitox for the treatment of steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11:188–193. doi: 10.1016/j.bbmt.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 86.Bolaños-Meade J, Jacobsohn DA, Margolis J, et al. Pentostatin in steroid-refractory acute graft-versus-host disease. J Clin Oncol. 2005;23:2661–2668. doi: 10.1200/JCO.2005.06.130. [DOI] [PubMed] [Google Scholar]
- 87.Furlong T, Leisenring W, Storb R, et al. Psoralen and ultraviolet A irradiation (PUVA) as therapy for steroid-resistant cutaneous acute graft-versus-host disease. Biol Blood Marrow Transplant. 2002;8:206–212. doi: 10.1053/bbmt.2002.v8.pm12014809. [DOI] [PubMed] [Google Scholar]
- 88.Hockenbery DM, Cruickshank S, Rodell TC, et al. A randomized, placebo-controlled trial of oral beclomethasone dipropionate as a prednisone-sparing therapy for gastrointestinal graft-versus-host disease. Blood. 2007;109:4557–4563. doi: 10.1182/blood-2006-05-021139. [DOI] [PubMed] [Google Scholar]
- 89.Kebriaei P, Isola L, Bahceci E, et al. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:804–811. doi: 10.1016/j.bbmt.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 90.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 91.Ippoliti C, Champlin R, Bugazia N, et al. Use of octreotide in the symptomatic management of diarrhea induced by graft-versus-host disease in patients with hematologic malignancies. J Clin Oncol. 1997;15:3350–3354. doi: 10.1200/JCO.1997.15.11.3350. [DOI] [PubMed] [Google Scholar]
- 92.Bucher C, Koch L, Vogtenhuber C, et al. IL-21 blockade reduces graft-versus-host disease mortality by supporting inducible T regulatory cell generation. Blood. 2009;114:5375–5384. doi: 10.1182/blood-2009-05-221135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hanash AM, Kappel LW, Yim NL, et al. Abrogation of donor T-cell IL-21 signaling leads to tissue-specific modulation of immunity and separation of GVHD from GVL. Blood. 2011;118:446–455. doi: 10.1182/blood-2010-07-294785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang L, Tao R, Hancock WW. Using histone deacetylase inhibitors to enhance Foxp3(+) regulatory T-cell function and induce allograft tolerance. Immunol Cell Biol. 2009;87:195–202. doi: 10.1038/icb.2008.106. [DOI] [PubMed] [Google Scholar]
- 95.Taylor PA, Panoskaltsis-Mortari A, Freeman GJ, et al. Targeting of inducible costimulator (ICOS) expressed on alloreactive T cells down-regulates graft-versus-host disease (GVHD) and facilitates engraftment of allogeneic bone marrow (BM) Blood. 2005;105:3372–3380. doi: 10.1182/blood-2004-10-3869. [DOI] [PubMed] [Google Scholar]
- 96.Kwan WH, Hashimoto D, Paz-Artal E, et al. Antigen-presenting cell-derived complement modulates graft-versus-host disease. J Clin invest. 2012;122:2234–2238. doi: 10.1172/JCI61019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hashimoto D, Chow A, Greter M, et al. Pretransplant CSF-1 therapy expands recipient macrophages and ameliorates GVHD after allogeneic hematopoietic cell transplantation. J Exp Med. 2011;208:1069–1082. doi: 10.1084/jem.20101709. [DOI] [PMC free article] [PubMed] [Google Scholar]


