Rodent and human beta cells are not identical, and knowledge of human beta cells has not progressed as quickly as understanding of rodent beta cells because of the difficulty of accessing unlimited sources of functional human pancreatic beta cells. The main focus of this review concerns recent strategies to generate new sources of human pancreatic beta cells. This knowledge would provide a strong basis for developing new treatments for diabetic patients.
Keywords: Diabetes, Differentiation, Pancreas, Beta cells
Abstract
It is well-established that insulin-producing pancreatic beta cells are central in diabetes. In type 1 diabetes, beta cells are destroyed by an autoimmune mechanism, whereas in type 2 diabetes, there is a decrease in functional beta-cell mass. In this context, studying beta cells is of major importance. Beta cells represent only 1% of total pancreatic cells and are found dispersed in the pancreatic gland. During the past decades, many tools and approaches have been developed to study rodent beta cells that efficiently pushed the field forward. However, rodent and human beta cells are not identical, and our knowledge of human beta cells has not progressed as quickly as our understanding of rodent beta cells. We believe that one of the reasons for this inefficient progress is the difficulty of accessing unlimited sources of functional human pancreatic beta cells. The main focus of this review concerns recent strategies to generate new sources of human pancreatic beta cells.
Introduction
In mammals, insulin is the only physiological hypoglycemic hormone. Insulin is produced in the pancreas by highly specialized cells named beta cells. In the pancreas, beta cells are found associated in small clusters with other endocrine cells (alpha, delta, PP, and epsilon cells, which produce glucagon, somatostatin, pancreatic polypeptide, and ghrelin, respectively). These micro-organs are named the islets of Langerhans. There are approximately 1,000 islets in a mouse pancreas and 1 million islets in a human pancreas. Each islet of Langerhans contains 1,000–3,000 cells, with 50%–80% being beta cells. We can thus estimate that a mouse pancreas contains 106 beta cells (1 mg of beta cells) and a human pancreas contains 109 beta cells (1 g of beta cells). Such cells play a major role in pathophysiology. Beta cells are destroyed by an autoimmune process in patients with type 1 diabetes, and the functional beta-cell mass is insufficient in patients with type 2 diabetes.
During past years, many studies have aimed at understanding how beta cells develop, function, grow, and die. The majority of these studies were performed in rodent models either in vivo or in vitro. In the case of in vitro experiments, both rodent islets and cell lines have been used in a recurrent fashion during the past 40 years. On the other hand, work on human beta cells suffered both from the paucity of available human islet preparations and the lack of functional human beta-cell lines.
In 2011, two groups developed new human beta-cell lines [1, 2]. This review will focus on the search for unlimited sources of functional pancreatic human beta cells as a tool to better study this specific cell type.
Similarities and Differences Between Rodent and Human Insulin-Producing Pancreatic Beta Cells
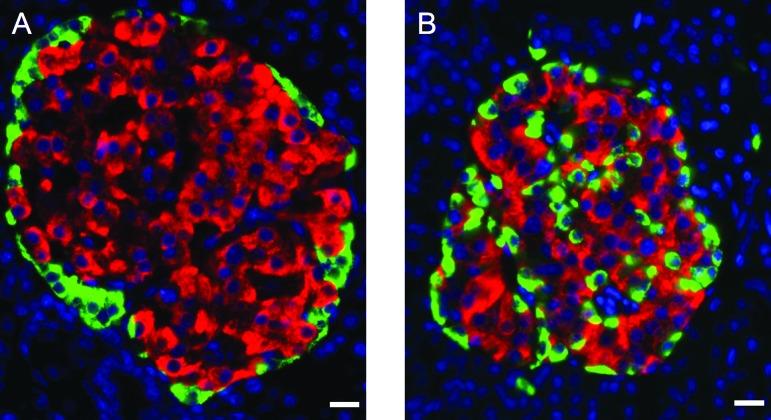
The major tasks of pancreatic beta cells are to produce, store, and finally secrete insulin in response to increased glycemia. Beta cells are highly specialized cells acting as factories that produce a huge amount of insulin. For example, 20% of the mRNA mass in beta cells represents insulin mRNA [3], and total human pancreatic insulin content is approximately 10 mg (1% of beta-cell weight) [4], which corresponds to 10 days of secretion by healthy people [5]. Although rodent and human beta cells share many similarities, a number of data also indicate marked differences between species. First, two genes encode insulin in rodents (rats and mice), although there is only one insulin gene in humans [6]. Second, in murine islets, beta cells are found as a core surrounded by alpha and delta cells. On the other hand, in human islets alpha, beta, and delta cells are dispersed throughout the islet [7] (Fig. 1), an anatomical pattern with functional implications [8]. Third, rodent and human beta cells share many common transcription factors, but some differences are observed. For example, the transcription factor MAFB is present in alpha but absent from adult mouse beta cells, whereas in humans, it is found at equal levels in adult human alpha and beta cells [9]. Fourth, there are a number of subtle functional differences between rodent and human beta cells. As an example, human beta cells differ from rodent cells in several important aspects of voltage-gated ion channels [10], and human beta cells have a lower set point for glucose-stimulated insulin secretion compared with rodent beta cells [11]. Finally, there are major species differences between humans and rodents in the susceptibility to pancreatic beta-cell injury [12].
Figure 1.
Comparative organization of endocrine cells in rat and human islets. Pancreatic sections from adult rat (A) and human (B) pancreases were immunostained with antibodies against insulin (red) and glucagon (green). The nuclei were stained with Hoechst 33342 fluorescent stain (blue). Note that in rat islets, glucagon-positive cells surround insulin-positive cells, which is not the case in human islets. Scale bars = 12.5 μm.
Taken together, we have learned a lot during the past years on rodent beta cells. It is also clear that human beta cells are not identical to rodent beta cells. If the objective is to attempt to translate fundamental data to patients with diabetes, it is crucial to find ways to further study human beta cells. In this context, we are asking here the following questions: (a) How did the field efficiently progress in our understanding of rodent beta cells? (b) Why did we not progress as quickly with human beta cells? (c) What are the different strategies to efficiently progress with human beta cells?
How Did the Field Efficiently Progress in Our Understanding of Rodent Beta Cells?
We really know a lot now about rodent beta cells, and this is due to many reasons. First, access to rodents is easy. Second, many years ago protocols were described about how to prepare and culture large quantities of rodent islets in a robust fashion [13]. This is important because islets of Langerhans are small micro-organs, a few thousand cells each, dispersed in the pancreatic gland. Third, many genetically modified mouse models have been developed from which islets were analyzed, giving rise to a better understanding of islet physiology. Fourth, technologies to efficiently purify rodent beta cells from islets started more than 30 years ago [14], allowing the separation and analysis of beta cells in the absence of other islet cell types. Finally, a number of rodent beta-cell lines have been developed as described below in more detail.
Rodent Beta-Cell Lines
Over the past decades, research in the beta-cell field profited from the establishment of insulin-secreting cell lines. The first lines were generated from adult rats and hamsters. RIN lines [15] have been derived from a rat insulinoma induced following sublethal irradiation [16]. Although insulin gene expression decreased with passages and glucose-stimulated insulin secretion was limited, this line remains in use more than 30 years after its publication. For example, a recent work used RIN cells as a tool to link connexins and cell cycle [17]. By following specific culture conditions, other cell lines were next derived from the same rat insulinoma, such as the widely used INS-1 cell line [18]. This line was found to be stable with time, insulin contents were high, and glucose induced insulin secretion. Again, more than 20 years after its first publication, this line remains widely used by the scientific community. As an example, INS1 cells were recently used in mechanistic studies aiming at further characterizing the expression of candidate genes for type 1 diabetes [19]. At the same time as RIN cells were developed, hamster insulinoma cells (HIT cells) were characterized [20]. This line has been widely used and was, for example, important to determine the concept of beta-cell glucotoxicity [21].
More recently, a number of independent mouse pancreatic beta-cell lines were established by targeted oncogenesis from transgenic mice expressing large-T antigen of simian virus 40 (SV40T antigen) under the control of the insulin promoter. The basis of this approach was the demonstration that insulinomas develop in transgenic mice expressing SV40T under the control of the rat insulin promoter [22]. With this approach, S. Efrat and colleagues developed a number of lines named β-TCs [23–25]. Since then, such lines have been widely used for examples in experiments aiming at analyzing the beta-cell function of specific transcription factors such as Dachshund1 [26]. A few years later, other lines such as Min6 cells have been produced using the same approach [27]. Min6 cells and its subclones, such as Min6B1 [28], have been shown to represent useful experimental tools to dissect the function of cell-cell contact in insulin secretion [29].
Summary
Many in vivo and in vitro models have been developed during the past years. Each model by itself surely has some limitations, but by combining such different models, we have now in hand a fine description of rodent beta cells that will be further sharpened in coming years.
Why Did We Not Progress as Quickly on Human Beta-Cell Characterization and Function as Compared With Rodent Beta Cells?
As described above, although the function of rodent and human beta cells is similar (secretion of insulin upon glucose stimulation), rodent and human beta cells are not identical. We clearly know more about rodent beta cells than about human beta cells for a number of reasons.
First, access to human pancreas is difficult because it derives from human deceased donors. Second, although an automated method for isolation of human pancreatic islets was described quite a long time ago [30], human islet preparation remains challenging. The technique is still more traditional than industrial [31], and beta-cell purity varies from one preparation to the other, even in the most professional laboratories [32]. Third, human islets are difficult to handle in culture. In vitro, the proliferation rate of adult human pancreatic beta cells is extremely low (as is the case in vivo) [33], islets are quite difficult to keep alive in vitro for very long [34], and their genetic manipulation remains challenging. Fourth, although it is possible to efficiently purify rodent beta cells from other pancreatic cell types based on autofluorescence-activated cell sorting [14], this approach is less efficient for human beta cells. Of interest, an innovative approach based on cell type-specific surface-reactive antibodies was recently developed that allowed purification by fluorescence-activated cell sorting and performance of gene expression on human pancreatic endocrine cell populations [9]. It will be interesting to determine whether functional analysis will be feasible on such efficiently sorted cells. In conclusion, at this stage, it remains extremely difficult to get access to large quantities of pure human beta cells.
Strategies to Efficiently Progress With Human Beta Cells
Human pancreatic beta cells are scarce and difficult to prepare to homogeneity. To efficiently progress in the characterization of human beta cells, alternative, large, and reproducible beta-cell sources are urgently needed, and some of such sources are described below.
Beta Cells Derived From Human Embryonic Stem Cells Human-Induced Pluripotent Stem Cells as Alternatives
It has been postulated for many years now that unlimited amounts of functional human beta cells could be derived from human embryonic stem cells (hESCs), taking advantage of the infinite proliferation capabilities of such hESCs. However, since the first claims of beta-cell generation from hESCs [35], the field did not progress as quickly as expected. The first major breakthrough appeared when a California biotech company (ViaCyte, San Diego, CA, http://www.viacyte.com) developed a procedure to convert hESCs into insulin-producing beta cells by mimicking in a tissue culture plate the major steps that take place in vivo during pancreatic organogenesis. With this approach, the team generated pancreatic progenitors that were first claimed to be able to further differentiate in vitro into fetal beta cells [36], but some years later, the interpretation of this last claim remains challenging. However, by further following their approach, the team was able to demonstrate that upon transplantation into immuno-incompetent SCID mice, pancreatic progenitors derived from hESCs could give rise to functional beta cells [37, 38]. This is a big step for the field. Although the hESCs used for such experiments seemed to have been very scarcely distributed, recent experiments using independent hESC clones demonstrated the reproducibility of the approach [39]. However, one limitation of such protocols is that human beta cells derived from hESCs are only produced in vivo in the animal and cannot be used for in vitro purposes such as drug discovery. Interestingly, a number of attempts also aimed at generating functional human beta cells from induced pluripotent stem cells (iPSCs). Although data suggest the feasibility of the process, the in vitro efficiency of the process was extremely low, and more work is needed to generate functional human beta cells from iPSCs [40–42].
Beta Cells Derived From Human Liver or Pancreatic Acinar Cells as Alternatives
In 2000, a work was published that was the first to demonstrate that in mouse, adenovirus-mediated gene transfer of Pdx1, a beta-cell-specific transcription factor, into the liver gave rise to beta-cell formation in this organ [43]. This work was highlighted and viewed as a new approach for diabetes [44]. Interestingly, independent groups were able to reproduce in vitro this type of experiment using human liver cells [45, 46]. It will now be interesting to carefully compare such cells with primary human beta cells and to determine how/whether such cells will be used for in vitro experiments. Interestingly, Melton's group recently identified a specific combination of three transcription factors that reprogram in vivo mouse pancreatic acinar cells into beta cells [47]. It will now be interesting to define whether this approach can be reproduced in vitro and to test it with human pancreatic cells.
Beta Cells Derived From Human Beta Cells Themselves as an Alternative
It is established that the proliferation rate of adult human beta cells is extremely low [33]. Although a number of publications demonstrated that virus-mediated gene transfer of cyclins plus cyclin-dependent kinases induces human beta cells to enter the first phase of the cell cycle [48], more recent work indicates that cell cycle entry could not be sufficient to promote pancreatic beta-cell expansion in human islets [49]. It is thus difficult to imagine that direct amplification of human beta cells will be the solution, as long as the cause of this poor human beta-cell proliferation remains unknown. Unexpectedly, epithelial-to-mesenchymal transition could generate proliferative human islet precursor cells that next give rise to nearly unlimited amount of beta cells [50]. Such results were highly debated but followed by experiments based on lineage-tracing approaches that strongly suggested that in vitro, human beta cells can dedifferentiate, expand, and redifferentiate into functional beta cells [51, 52]. Beta-cell purification, followed by further characterization of redifferentiated cells and comparison with adult human beta cells, will be important for determining the extent of differentiation induced by this type of approach.
Human Beta-Cell Lines as an Alternative
We have learned a lot about rodent beta cells using the different rodent beta-cell lines that were developed during the past 30 years. In comparison, knowledge in the field of human pancreatic beta-cell line production is by far less complete, with some examples of human beta-cell lines derived either from adult or from fetal pancreatic tissue.
Human Beta-Cell Lines Derived From Adult Pancreatic Tissue
Beta-lox5 is an example of a cell line derived from purified human adult beta cells following virus-mediated gene transfer of oncogenes such as SV40T and H-ras [53]. However, further experiments indicated that this line progressively dedifferentiated in culture and lost insulin gene expression [54]. NAKT15 cells are another interesting example [55] that when published seemed to have pushed the field forward [56]. This cell line was generated by oncogene transfer into adult pancreatic beta cells using Moloney-based retroviral vectors. Based on many criteria, this line seemed to resemble primary human beta cells. However, since its publication, we are aware of a limited number of original reports using this line [57]. Of note, our group was unable to generate human insulinomas or to derive human beta lines from human adult islets, even using lentiviral vectors that efficiently transduced human beta cells [1]. Recently, an interesting approach based on cell electrofusion was used to generate new human beta-cell lines. Briefly, the authors performed electrofusion between human islets and a proliferating human pancreatic epithelial cell line, PANC-1, that does not express insulin. They next selected cell hybrids that grew and secreted insulin [2]. Insulin content was stable for at least 40 passages but quite low when compared with primary beta cells. Specifically, we can assume that primary beta cells contain 10 μg of insulin per million cells, whereas the new lines contain approximately 4 ng per million cells, that is, 2,500 times less than primary beta cells. Interestingly, in such new lines some level of regulation of insulin secretion by glucose and by a number of secretagogues could be observed [2]. Thus, by developing and using an innovative electrofusion approach, the authors were able to generate human beta-cell lines from adult islets.
Human Beta-Cell Lines Derived From Fetal Pancreatic Tissue
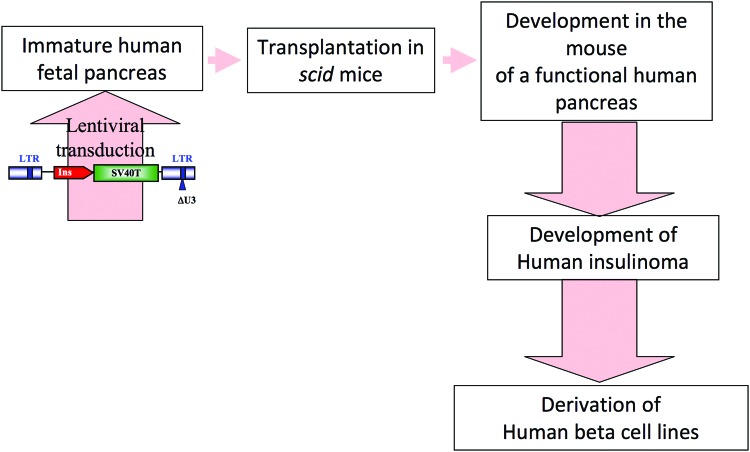
Quite a long time ago, a number of attempts were performed to generate human beta-cell lines from human fetal pancreases. However, selected clones expressed low levels of insulin that was rapidly lost with passages [58, 59]. We, however, reasoned that fetal tissue is frequently more plastic than adult tissue and thus possibly more prone to be transformed and to give rise to cell lines. Interestingly, excellent rodent beta-cell lines had been generated following the transfer into fertilized mouse eggs of SV40T under the control of the rat insulin promoter [23, 27]. Gene transfer into human fertilized egg is not feasible for obvious reasons. Thus to attempt to mimic the strategy used for Min6 or β-TC cell development, we took advantage of a model we had previously validated. Briefly, we had shown that when human fetal pancreas is transplanted into immuno-incompetent SCID mice, the human tissue grows, and endocrine cells differentiate by a mechanism that recapitulated physiological development and mature enough to control the glycemia of mice deficient in endogenous beta cells [60, 61]. In parallel, we developed tools for lentivirus-mediated gene transfer into fetal pancreas [62, 63]. By combining this model of human pancreatic development and expertise in gene transfer, we attempted to develop and validate an alternative transgenic-like approach by performing somatic oncogene transfer into pancreatic progenitors that will develop into beta cells (Fig. 2). Because the expression of the oncogene is under the control of the insulin promoter, insulinoma should develop in transplanted SCID mice and could be explanted and cultured. We first validated this approach using rat fetal pancreases [64] and next transferred each step of the procedure to generate human beta-cell lines. We were successful in generating a number of lines, for example, EndoC-βH1 [1]. This line grows slowly, as expected, for differentiated cells, and the doubling time is in days. The cells express insulin and many other beta-cell-specific markers in a stable fashion for more than 60 passages. Insulin gene expression and content are high when compared with other human beta-cell lines. Insulin gene expression is, for example, 100 times higher than the levels found in human beta-cell lines recently developed by electrofusion [2]. On the other hand, expression remains 10–20 times lower than the one found in primary human beta cells. The beta-cell bottle is thus half full or half empty. Finally, the cells secrete insulin upon glucose stimulation and upon treatment with different secretagogues. One question is whether EndoC-βH1 cells that derived from fetal are mature beta cells. This is important because in pig, for example, immature fetal beta cells respond poorly to glucose [65], whereas beta-cell maturation occurs after birth [66]. We found that glucose induces a two- to threefold increase in insulin secretion by EndoC-βH1, which is in the same range as what has been described by some groups for human islets [67]. It will, however, be important to further study EndoC-βH1 maturity.
Figure 2.
Schematic analysis of the procedure recently used to generate beta cells from human fetal pancreases [1]. Immature human fetal pancreases were transduced with lentiviral vectors that expressed SV40T under the control of the insulin promoter. Transduced pancreases were next transplanted under the kidney capsule of immunoincompetent SCID mice where insulinomas developed, from which human beta-cell lines were derived. Abbreviations: LTR, long terminal repeat; Ins, insulin; SV40T, large-T antigen of simian virus 40.
The Future in the Field of Human Beta-Cell Lines
By definition, human beta-cell lines are proliferating cells and are thus different from primary human beta cells that proliferate inefficiently. This is also true in the case of rodent beta-cell lines. In this last case, Efrat's laboratory developed conditionally transformed pancreatic beta-cell lines that expressed SV40T under control of the tetracycline gene regulatory system. They showed that when tetracycline is added to the culture medium, cell proliferation stopped, insulin content increased, and beta-cell function was maintained for at least 4 weeks [68]. This type of cell line was recently used for important purposes such as screens and characterization of small molecules and peptides that regulate beta-cell proliferation [69, 70], but they seem to be used less frequently than could have been expected. We believe that the development of conditionally transformed human pancreatic beta-cell lines will be largely beneficial for the field, and our preliminary data suggest that these types of lines could be generated from human fetal pancreas.
Unlimited Sources of Functional Pancreatic Human Beta Cells: For Which Purpose?
Taken together, the scientific community has now in hand a number of new tools that should be useful, either independently or in combination, to further dissect what a human beta cell is; to progress in signals that regulate human beta-cell proliferation, function, and survival; and to maybe one day develop cell therapy for diabetics.
Human Pancreatic Beta Cells for Cell Therapy of Diabetes
Cell therapy for type 1 diabetic patients is now a reality [71, 72]. However, human pancreas donors are scarce, and preparing human islets remains difficult, expensive, and time-consuming. Recipients have to be treated with cocktails of immunosuppressive drugs that in some cases could be deleterious for beta cells themselves [73]. Thus, major improvements are needed, and each new source of human beta cells could pave the way for such advances. Here are some examples and ideas.
The most advanced new source of human beta cells is represented by beta cells derived from hESCs. The ViaCyte team has shown that development of functional beta cells from hESCs is feasible [37]. However, they also showed that some ducts and acinar cells developed in parallel, which can give rise to cyst formation and risks of peritonitis [74]. In this context transplantation of pure human beta cells would be safer than progenitor transplantation. Moreover, such protocols based on differentiated cells derived from hESCs give rise to teratomas from undifferentiated cells, and this seems to be quite frequent even when low numbers of cells are transplanted when compared with what will be needed for transplantation in humans [37, 39]. Thus, major progress has been made during recent years, but more work is needed to further enhance beta-cell differentiation.
Human Pancreatic Beta Cells as a Model for Regenerative Medicine
Data from Gershengorn's and Efrat's laboratories suggest that in vitro, human beta cells can be amplified following dedifferentiation, growth, and redifferentiation [50–52]. Whether this process takes place in vivo and can be modulated remains to be demonstrated. However, screening in vitro for molecules that could reproduce this process of amplification would be of major importance. Another application of the above-described models would be to use pancreatic progenitor cell cultures derived from hESCs [37] to screen for factors that can amplify such a pool of progenitors. Indeed, many arguments suggest that in rodents, the beta-cell mass that develops is dependent on the number of progenitors [75–77], and indirect arguments suggest that it should be the same in humans. Some level of human beta-cell amplification could also be obtained by activation of the proliferation of human beta cells as is also the case for rodent beta cells [48, 78]. In this context, it should be interesting to use conditionally transformed human pancreatic beta-cell lines when available both in screens to search for signals that activate their proliferation but also as a model to understand why such cells have a poor proliferation potential.
New Assays to Study Human Beta Cells and to Protect Them From Destruction
As described above, rodent and human beta cells are not completely similar in terms of function [11], and more molecules remain to be discovered that regulate insulin secretion in humans. In this context, functional human beta-cell lines should be useful [1, 2]. Moreover, it is well-established that in type 1 diabetic patients, beta cells are destroyed by an autoimmune mechanism [79], but the exact mechanism of destruction is not fully defined [80]. This is at least in part due to the lack human beta-cell sources. We believe that newly developed human beta cells will be useful to better understand human beta-cell destruction and to develop assays to discover new molecules that can protect beta cells. We also believe that new sources of human beta cells will be useful to design preclinical protocols. As an example, it will be interesting to use conditionally transformed human pancreatic beta cells, when available, in protocols where cells are encapsulated before transplantation [81] and follow their survival and function.
Conclusion
During the past years, we have learned a lot about rodent pancreatic beta cells, and new information is also appearing concerning human beta cells. We believe that such additional knowledge will represent a strong basis for developing new treatments for diabetic patients.
Acknowledgments
Work in the Scharfmann laboratory was supported by Institut National de la Santé et de la Recherche Médicale (INSERM), the seventh Framework Program (BetaCellTherapy no. GA241883), the Innovative Medicines Initiative Joint Undertaking under grant agreement no. 155005 (Innovative Medicines Initiative for Diabetes), the Beta Cell Biology Consortium, the Juvenile Diabetes Research Foundation, and the Agence Nationale de la Recherche. R.S. thanks Dr. C. Pinset and P. Czernichow for discussions. We thank Severine Pechberty for performing the immunostaining used in Figure 1.
Author Contributions
R.S.: conception and design, financial support, manuscript writing, final approval of manuscript; L.R. and P.R.: conception and design, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
R.S. and P.R. are founders of Endo Cells SARL and are compensated for advisory roles but not compensated for patents held.
References
- 1.Ravassard P, Hazhouz Y, Pechberty S, et al. A genetically engineered human pancreatic beta cell line exhibiting glucose-inducible insulin secretion. J Clin Invest. 2011;121:3589–3597. doi: 10.1172/JCI58447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCluskey JT, Hamid M, Guo-Parke H, et al. Development and functional characterization of insulin-releasing human pancreatic beta cell lines produced by electrofusion. J Biol Chem. 2011;286:21982–21992. doi: 10.1074/jbc.M111.226795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Lommel L, Janssens K, Quintens R, et al. Probe-independent and direct quantification of insulin mRNA and growth hormone mRNA in enriched cell preparations. Diabetes. 2006;55:3214–3220. doi: 10.2337/db06-0774. [DOI] [PubMed] [Google Scholar]
- 4.Rahier J, Guiot Y, Goebbels RM, et al. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10(suppl 4):32–42. doi: 10.1111/j.1463-1326.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- 5.Ferrannini E. The stunned beta cell: A brief history. Cell Metab. 2010;11:349–352. doi: 10.1016/j.cmet.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Melloul D, Marshak S, Cerasi E. Regulation of insulin gene transcription. Diabetologia. 2002;45:309–326. doi: 10.1007/s00125-001-0728-y. [DOI] [PubMed] [Google Scholar]
- 7.Brissova M, Fowler MJ, Nicholson WE, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53:1087–1097. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- 8.Cabrera O, Berman DM, Kenyon NS, et al. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorrell C, Schug J, Lin CF, et al. Transcriptomes of the major human pancreatic cell types. Diabetologia. 2011;54:2832–2844. doi: 10.1007/s00125-011-2283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun M, Ramracheya R, Bengtsson M, et al. Voltage-gated ion channels in human pancreatic beta-cells: Electrophysiological characterization and role in insulin secretion. Diabetes. 2008;57:1618–1628. doi: 10.2337/db07-0991. [DOI] [PubMed] [Google Scholar]
- 11.Henquin JC, Dufrane D, Nenquin M. Nutrient control of insulin secretion in isolated normal human islets. Diabetes. 2006;55:3470–3477. doi: 10.2337/db06-0868. [DOI] [PubMed] [Google Scholar]
- 12.Eizirik D, Pipeleers D, Ling Z, et al. Major species differences between humans and rodents in the susceptibility to pancreatic β-cell injury. Proc Natl Acad Sci USA. 1994;91:9253–9256. doi: 10.1073/pnas.91.20.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16:35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- 14.Van De Winkel M, Pipeleers D. Autofluorescence-activated cell sorting of pancreatic islet cells: Purification of insulin-containing B-cells according to glucose-induced changes in cellular redox state. Biochem Biophys Res Commun. 1983;114:835–842. doi: 10.1016/0006-291x(83)90857-4. [DOI] [PubMed] [Google Scholar]
- 15.Gazdar A, Chick W, Oie H, et al. Continuous, clonal, insulin- and somatostatin-secreting cell lines established from a transplantable rat islet cell tumor. Proc Natl Acad Sci USA. 1980;77:3519–3523. doi: 10.1073/pnas.77.6.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chick WL, Warren S, Chute RN, et al. A transplantable insulinoma in the rat. Proc Natl Acad Sci USA. 1977;74:628–632. doi: 10.1073/pnas.74.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Good ME, Nelson TK, Simon AM, et al. A functional channel is necessary for growth suppression by Cx37. J Cell Sci. 2011;124:2448–2456. doi: 10.1242/jcs.081695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asfari M, Janjic D, Meda P, et al. Establishment of 2-mercaptoethanol-dependent differentiated insulin secreting cell lines. Endocrinology. 1992;130:167–178. doi: 10.1210/endo.130.1.1370150. [DOI] [PubMed] [Google Scholar]
- 19.Eizirik DL, Sammeth M, Bouckenooghe T, et al. The human pancreatic islet transcriptome: Expression of candidate genes for type 1 diabetes and the impact of pro-inflammatory cytokines. PLoS Genet. 2012;8:e1002552. doi: 10.1371/journal.pgen.1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santerre R, Cook R, Criscl R, et al. Insulin synthesis in a clonal cell line of simian virus 40-transformed hamster pancreatic beta cells. Proc Natl Acad Sci USA. 1981;78:4339–4342. doi: 10.1073/pnas.78.7.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson LK, Redmon JB, Towle HC, et al. Chronic exposure of HIT cells to high glucose concentrations paradoxically decreases insulin gene transcription and alters binding of insulin gene regulatory protein. J Clin Invest. 1993;92:514–519. doi: 10.1172/JCI116596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanahan D. Heritable formation of pancreatic beta-cell tumours in transgenic mice expressing recombinant insulin/simian virus 40 oncogenes. Nature. 1985;315:115–122. doi: 10.1038/315115a0. [DOI] [PubMed] [Google Scholar]
- 23.Efrat S, Linde S, Kofod H, et al. β cell lines derived from transgenic mice expressing a hybrid insulin gene-oncogene. Proc Natl Acad Sci USA. 1988;85:9037–9041. doi: 10.1073/pnas.85.23.9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Efrat S, Leiser M, Surana M, et al. Murine insulinoma cell line with normal glucose-regulated insulin secretion. Diabetes. 1993;42:901–907. doi: 10.2337/diab.42.6.901. [DOI] [PubMed] [Google Scholar]
- 25.Knaack D, Fiore DM, Surana M, et al. Clonal insulinoma cell line that stably maintains correct glucose responsiveness. Diabetes. 1994;43:1413–1417. doi: 10.2337/diab.43.12.1413. [DOI] [PubMed] [Google Scholar]
- 26.Kalousova A, Mavropoulos A, Adams BA, et al. Dachshund homologues play a conserved role in islet cell development. Dev Biol. 2010;348:143–152. doi: 10.1016/j.ydbio.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyazaki J, Araki K, Yamato E, et al. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: Special reference to expression of glucose transporter isoforms. Endocrinology. 1990;127:126–132. doi: 10.1210/endo-127-1-126. [DOI] [PubMed] [Google Scholar]
- 28.Lilla V, Webb G, Rickenbach K, et al. Differential gene expression in well-regulated and dysregulated pancreatic beta-cell (MIN6) sublines. Endocrinology. 2003;144:1368–1379. doi: 10.1210/en.2002-220916. [DOI] [PubMed] [Google Scholar]
- 29.Jaques F, Jousset H, Tomas A, et al. Dual effect of cell-cell contact disruption on cytosolic calcium and insulin secretion. Endocrinology. 2008;149:2494–2505. doi: 10.1210/en.2007-0974. [DOI] [PubMed] [Google Scholar]
- 30.Ricordi C, Lacy PE, Finke EH, et al. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 31.Vantyghem MC, Balavoine AS, Caiazzo R, et al. Diabetes cell therapy: A decade later. Minerva Endocrinol. 2011;36:23–39. [PubMed] [Google Scholar]
- 32.Movahedi B, Gysemans C, Jacobs-Tulleneers-Thevissen D, et al. Pancreatic duct cells in human islet cell preparations are a source of angiogenic cytokines interleukin-8 and vascular endothelial growth factor. Diabetes. 2008;57:2128–2136. doi: 10.2337/db07-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parnaud G, Bosco D, Berney T, et al. Proliferation of sorted human and rat beta cells. Diabetologia. 2008;51:91–100. doi: 10.1007/s00125-007-0855-1. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen JH. Beta-cell function in isolated human pancreatic islets in long-term tissue culture. Acta Biol Med Ger. 1981;40:55–60. [PubMed] [Google Scholar]
- 35.Assady S, Maor G, Amit M, et al. Insulin production by human embryonic stem cells. Diabetes. 2001;50:1691–1697. doi: 10.2337/diabetes.50.8.1691. [DOI] [PubMed] [Google Scholar]
- 36.D'Amour KA, Bang AG, Eliazer S, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 37.Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 38.Kelly OG, Chan MY, Martinson LA, et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol. 2011;29:750–756. doi: 10.1038/nbt.1931. [DOI] [PubMed] [Google Scholar]
- 39.Rezania A, Bruin JE, Riedel MJ, et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61:2016–2029. doi: 10.2337/db11-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bar-Nur O, Russ HA, Efrat S, et al. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell. 2011;9:17–23. doi: 10.1016/j.stem.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Maehr R, Chen S, Snitow M, et al. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci USA. 2009;106:15768–15773. doi: 10.1073/pnas.0906894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tateishi K, He J, Taranova O, et al. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem. 2008;283:31601–31607. doi: 10.1074/jbc.M806597200. [DOI] [PubMed] [Google Scholar]
- 43.Ferber S, Halkin A, Cohen H, et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6:568–572. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- 44.Kahn A. Converting hepatocytes to beta-cells: A new approach for diabetes? Nat Med. 2000;6:505–506. doi: 10.1038/74980. [DOI] [PubMed] [Google Scholar]
- 45.Zalzman M, Anker-Kitai L, Efrat S. Differentiation of human liver-derived, insulin-producing cells toward the beta-cell phenotype. Diabetes. 2005;54:2568–2575. doi: 10.2337/diabetes.54.9.2568. [DOI] [PubMed] [Google Scholar]
- 46.Sapir T, Shternhall K, Meivar-Levy I, et al. Cell-replacement therapy for diabetes: Generating functional insulin-producing tissue from adult human liver cells. Proc Natl Acad Sci USA. 2005;102:7964–7969. doi: 10.1073/pnas.0405277102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Q, Brown J, Kanarek A, et al. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kulkarni RN, Mizrachi EB, Ocana AG, et al. Human beta-cell proliferation and intracellular signaling: Driving in the dark without a road map. Diabetes. 2012;61:2205–2213. doi: 10.2337/db12-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rieck S, Zhang J, Li Z, et al. Overexpression of hepatocyte nuclear factor-4alpha initiates cell cycle entry, but is not sufficient to promote beta-cell expansion in human islets. Mol Endocrinol. 2012;26:1590–1602. doi: 10.1210/me.2012-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gershengorn MC, Hardikar AA, Wei C, et al. Epithelial-to-mesenchymal transition generates proliferative human islet precursor cells. Science. 2004;306:2261–2264. doi: 10.1126/science.1101968. [DOI] [PubMed] [Google Scholar]
- 51.Russ HA, Bar Y, Ravassard P, et al. In vitro proliferation of cells derived from adult human beta-cells revealed by cell-lineage tracing. Diabetes. 2008;57:1575–1583. doi: 10.2337/db07-1283. [DOI] [PubMed] [Google Scholar]
- 52.Russ HA, Sintov E, Anker-Kitai L, et al. Insulin-producing cells generated from dedifferentiated human pancreatic beta cells expanded in vitro. PLoS One. 2011;6:e25566. doi: 10.1371/journal.pone.0025566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de la Tour D, Halvorsen T, Demeterco C, et al. Beta-cell differentiation from a human pancreatic cell line in vitro and in vivo. Mol Endocrinol. 2001;15:476–483. doi: 10.1210/mend.15.3.0604. [DOI] [PubMed] [Google Scholar]
- 54.Ball AJ, Abrahamsson AE, Tyrberg B, et al. HES6 reverses nuclear reprogramming of insulin-producing cells following cell fusion. Biochem Biophys Res Commun. 2007;355:331–337. doi: 10.1016/j.bbrc.2007.01.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Narushima M, Kobayashi N, Okitsu T, et al. A human beta-cell line for transplantation therapy to control type 1 diabetes. Nat Biotechnol. 2005;23:1274–1282. doi: 10.1038/nbt1145. [DOI] [PubMed] [Google Scholar]
- 56.Hohmeier HE, Newgard CB. Islets for all? Nat Biotechnol. 2005;23:1231–1232. doi: 10.1038/nbt1005-1231. [DOI] [PubMed] [Google Scholar]
- 57.Jin J, Park J, Kim K, et al. Detection of differential proteomes of human beta-cells during islet-like differentiation using iTRAQ labeling. J Proteome Res. 2009;8:1393–1403. doi: 10.1021/pr800765t. [DOI] [PubMed] [Google Scholar]
- 58.Wang S, Beattie GM, Mally MI, et al. Isolation and characterization of a cell line from the epithelial cells of the human fetal pancreas. Cell Transplant. 1997;6:59–67. doi: 10.1177/096368979700600110. [DOI] [PubMed] [Google Scholar]
- 59.Wang S, Beattie GM, Mally MI, et al. Analysis of a human fetal pancreatic islet cell line. Transplant Proc. 1997;29:2219. doi: 10.1016/s0041-1345(97)00306-0. [DOI] [PubMed] [Google Scholar]
- 60.Castaing M, Duvillie B, Quemeneur E, et al. Ex vivo analysis of acinar and endocrine cell development in the human embryonic pancreas. Dev Dyn. 2005;234:339–345. doi: 10.1002/dvdy.20547. [DOI] [PubMed] [Google Scholar]
- 61.Castaing M, Peault B, Basmaciogullari A, et al. Blood glucose normalization upon transplantation of human embryonic pancreas into beta-cell-deficient SCID mice. Diabetologia. 2001;44:2066–2076. doi: 10.1007/s001250100012. [DOI] [PubMed] [Google Scholar]
- 62.Castaing M, Guerci A, Mallet J, et al. Efficient restricted gene expression in beta cells by lentivirus-mediated gene transfer into pancreatic stem/progenitor cells. Diabetologia. 2005;48:709–719. doi: 10.1007/s00125-005-1694-6. [DOI] [PubMed] [Google Scholar]
- 63.Scharfmann R, Xiao X, Heimberg H, et al. Beta cells within single human islets originate from multiple progenitors. PLoS One. 2008;3:e3559. doi: 10.1371/journal.pone.0003559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ravassard P, Emilie B-N, Hazhouz Y, et al. A new strategy to generate functional insulin-producing cell lines by somatic gene transfer into pancreatic progenitors. PLoS One. 2009;4:e4731. doi: 10.1371/journal.pone.0004731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Korsgren O, Jansson L, Eizirik D, et al. Functional and morphological differentiation of fetal porcine islet-like cell clusters after transplantation into nude mice. Diabetologia. 1991;34:379–386. doi: 10.1007/BF00403174. [DOI] [PubMed] [Google Scholar]
- 66.Kühl C, Hornnes PJ, Jensen SL, et al. Gastric inhibitory polypeptide and insulin: Response to intraduodenal and intravenous glucose infusions in fetal and neonatal pigs. Endocrinology. 1980;107:1446–1450. doi: 10.1210/endo-107-5-1446. [DOI] [PubMed] [Google Scholar]
- 67.Lukowiak B, Vandewalle B, Riachy R, et al. Identification and purification of functional human beta-cells by a new specific zinc-fluorescent probe. J Histochem Cytochem. 2001;49:519–528. doi: 10.1177/002215540104900412. [DOI] [PubMed] [Google Scholar]
- 68.Fleischer N, Chen C, Surana M, et al. Functional analysis of a conditionally transformed pancreatic beta-cell line. Diabetes. 1998;47:1419–1425. doi: 10.2337/diabetes.47.9.1419. [DOI] [PubMed] [Google Scholar]
- 69.Wang W, Walker JR, Wang X, et al. Identification of small-molecule inducers of pancreatic beta-cell expansion. Proc Natl Acad Sci USA. 2009;106:1427–1432. doi: 10.1073/pnas.0811848106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klinger S, Poussin C, Debril MB, et al. Increasing GLP-1-induced beta-cell proliferation by silencing the negative regulators of signaling cAMP response element modulator-alpha and DUSP14. Diabetes. 2008;57:584–593. doi: 10.2337/db07-1414. [DOI] [PubMed] [Google Scholar]
- 71.Keymeulen B, Gillard P, Mathieu C, et al. Correlation between beta cell mass and glycemic control in type 1 diabetic recipients of islet cell graft. Proc Natl Acad Sci USA. 2006;103:17444–17449. doi: 10.1073/pnas.0608141103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 73.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest. 2007;117:2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schulz TC, Young HY, Agulnick AD, et al. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLoS One. 2012;7:e37004. doi: 10.1371/journal.pone.0037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Attali M, Stetsyuk V, Basmaciogullari A, et al. Control of beta-cell differentiation by the pancreatic mesenchyme. Diabetes. 2007;56:1248–1258. doi: 10.2337/db06-1307. [DOI] [PubMed] [Google Scholar]
- 76.Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature. 2007;445:886–891. doi: 10.1038/nature05537. [DOI] [PubMed] [Google Scholar]
- 77.Rachdi L, Aiello V, Duvillie B, et al. L-Leucine alters pancreatic beta-cell differentiation and function via the mTor signaling pathway. Diabetes. 2012;61:409–417. doi: 10.2337/db11-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nir T, Dor Y. How to make pancreatic beta cells: Prospects for cell therapy in diabetes. Curr Opin Biotechnol. 2005;16:524–529. doi: 10.1016/j.copbio.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 79.Mathis D, Vence L, Benoist C. Beta-cell death during progression to diabetes. Nature. 2001;414:792–798. doi: 10.1038/414792a. [DOI] [PubMed] [Google Scholar]
- 80.Brezar V, Carel JC, Boitard C, et al. Beyond the hormone: Insulin as an autoimmune target in type 1 diabetes. Endocr Rev. 2011;32:623–669. doi: 10.1210/er.2011-0010. [DOI] [PubMed] [Google Scholar]
- 81.O'Sullivan ES, Vegas A, Anderson DG, et al. Islets transplanted in immunoisolation devices: A review of the progress and the challenges that remain. Endocr Rev. 2011;32:827–844. doi: 10.1210/er.2010-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]