The goal of this study was to identify feasible and reproducible in vitro assays to predict mesenchymal stromal cell (MSC) potency. MSC lines were generated from normal human bone marrow samples and characterized by their growth, proliferation, and viability as determined by cell count, bromodeoxyuridine incorporation, and cellular ATP levels, respectively. It was found that high performance in a combination of in vitro tests accurately predicted which lines functioned well in vivo, suggesting that reliable and reproducible in vitro assays may be used to measure the functional potential of MSCs for therapeutic use.
Keywords: Stem cells, Clinical trials, Mesenchymal stem cells, Stem cell transplantation, Tissue regeneration
Abstract
Multipotent mesenchymal stromal cells (MSCs) have the potential to repair and regenerate damaged tissues, making them attractive candidates for cell-based therapies. To maximize efficacy of MSCs, prediction of their therapeutic abilities must be made so that only the best cells will be used. Our goal was to identify feasible and reproducible in vitro assays to predict MSC potency. We generated cell lines from 10 normal human bone marrow samples and used the International Society for Cellular Therapy's minimal criteria to define them as MSCs: plastic adherence, appropriate surface marker expression, and trilineage differentiation. Each MSC line was further characterized by its growth, proliferation, and viability as determined by cell count, bromodeoxyuridine incorporation, and cellular ATP levels, respectively. To determine whether these tests reliably predict the therapeutic aptitude of the MSCs, several lines were implanted in vivo to examine their capacity to engraft and form granulation tissue in a well-established murine wound model using polyvinyl alcohol sponges. Long-term engraftment of MSCs in the sponges was quantified through the presence of the human-specific Alu gene in sponge sections. Sections were also stained for proliferating cells, vascularity, and granulation tissue formation to determine successful engraftment and repair. We found that high performance in a combination of the in vitro tests accurately predicted which lines functioned well in vivo. These findings suggest that reliable and reproducible in vitro assays may be used to measure the functional potential of MSCs for therapeutic use.
Introduction
Mesenchymal stromal cells (MSCs) are multipotent, self-renewing stem cells [1–4]. Transplanted MSCs have been shown to promote healing in myocardium, bone, and soft tissue injuries [5–8]. Although the exact mechanisms have not yet been elucidated, the regenerative properties of MSCs have been largely attributed to paracrine signaling in the wound site [8–12]. The relative ease of MSC isolation from bone marrow aspirates, the remarkable capacity for in vitro expansion, and the broad regenerative potential make MSCs ideal candidates for cell-based therapy for tissue repair [5, 13]. Additionally, MSCs demonstrate unique immune tolerance allowing allogeneic transplantation of a single MSC line into multiple patients [14, 15]. Presently, there are more than 200 clinical trials that involve exogenous human MSCs for a wide variety of indications [16]. However, variations in the isolation, expansion, and characterization of MSCs in clinical use have made it difficult to interpret study outcomes or rigorously assess therapeutic efficacy [17, 18].
The International Society for Cellular Therapy (ISCT) has proposed minimal criteria to define human MSCs [19]. These criteria include adherence to plastic, the presence and absence of specific clusters of differentiation, and the capacity to differentiate into osteoblasts, adipocytes, and chondroblasts. Protocols to isolate and expand human MSCs under good manufacturing practices/good laboratory practices suitable for clinical use are available, including methodologies that examine multilineage in vitro differentiation [20, 21]. However, differences between MSCs due to different donors, tissue sources, and isolation and expansion protocols make interlaboratory comparison of MSCs very difficult [22]. Additionally, ISCT minimum standards only define MSCs; they do not assess MSC potency.
Although the U.S. Food and Drug Administration regulates the safety, purity, and potency of all cell therapy products, currently there is much flexibility, with no consensus on how to assess potency. As a result, many laboratories currently use nucleated cell count, cell viability, differentiation capacity, and colony forming units in conjunction with immunophenotyping to estimate MSC cell line potency [20, 21]. Many of these measurements are primarily supporting the presence of viable MSCs in the sample and provide minimal insight into the efficacy of the cell line. There is a clear need for research to determine complementary, reproducible assays that measure MSC potency in regenerative biology [23]. Such assays would provide clinicians with a way to determine not only whether an individual qualifies for autologous MSC administration but also which lines are best to be mass produced as “universal donor” MSCs for clinical use.
Toward the goal of identifying potential MSC assays that predict functional regenerative potential, the present study compared three standard and reproducible in vitro assays that measure proliferation, growth, and viability of MSCs. Performance on these assays was correlated to the ability of cells to generate vascularized granulation tissue in vivo and to exhibit long-term engraftment in a murine preclinical wound model. As many clinical trials for tissue regeneration use a single, expanded MSC line, identification of assays to predict functional regenerative potential of each of these MSC lines may be valuable in optimizing therapeutic outcomes.
Materials and Methods
Isolation of MSCs from Bone Marrow and Maintenance
Bone marrow samples were obtained from 10 human donors. The donors were otherwise healthy individuals undergoing orthopedic surgeries. Each sample was diluted in Hanks' balanced saline solution (HBSS) and passed through a 70-μm cell filter. The filtered bone marrow solution was layered on Histopaque (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), and the mononuclear cell layer was isolated. Isolated monocytes were washed twice in HBSS, resuspended, plated on 150-mm tissue culture dishes in primary human MSC medium (α-minimal essential medium [αMEM] with 20% fetal calf serum [FCS], 100 U/ml penicillin, and 100 μg/ml streptomycin), and incubated at 37°C with 5% CO2. Medium was replaced every 4 days until the cells reached 80% confluence, at which time they were trypsinized and replated 1:3 in 100-mm tissue culture dishes.
MSC Phenotyping
To confirm proper surface marker expression, all lines of MSCs were assessed between passage 2 and passage 5 using the Human MSC Phenotyping Kit (Macs; Miltenyi Biotec, Auburn, CA, http://www.miltenyibiotec.com). Cells were immunofluorescently stained for CD14, CD20, CD34, CD45, CD73, CD90, and CD105. Analysis occurred on a five-laser LSRII flow cytometer running FACSDiva v5.02 Software (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com).
Trilineage Differentiation
Trilineage differentiation was induced at passage 4 for all lines of MSCs. MSCs from each line were plated in tissue culture slides with fresh medium at a density of 8,000 cells per well. These cell preparations were incubated at 37°C with 5% CO2. Fresh medium was added after 4 days. After 8 days, the medium was changed to one of three differentiation media, each of which was composed of αMEM supplemented with 10% FCS. Osteogenic medium contained 100 μM dexamethasone, 0.1 mM ascorbic acid, and 10 nM β-glycerophosphate. Chrondrogenic medium contained 100 μM dexamethasone and 0.01 μg/ml transforming growth factor-β1. Adipogenic medium contained 1 mM dexamethasone, 5 μl/ml insulin, and 1 μl/ml 3-isobutyl-1-methylxanthine. Fresh differentiation medium was given to the cells every 4 days for a total of 16 days, at which point the cells were fixed and stained.
Alizarin Red, Toluidine Blue, and Von Kossa Staining
Cell preparations incubated in osteogenic and adipogenic media were fixed with 100% ethanol for 15 minutes at room temperature and stained with 0.25% alizarin red and Oil Red O, respectively. Cell preparations incubated in chrondrogenic medium were fixed in 10% formalin and stained with 1% toluidine blue in NaCl. Images of the slides were captured using a CoolSNAP Hq charge-coupled device camera (Photometrics, Tucson, AZ, http://www.photometrics.com).
In Vitro Growth Assay
To assess the in vitro growth rate, MSCs at passage 3 from each line were seeded in duplicate on six-well plates at a density of 20,000 cells per well. These plates were incubated at 37°C with 5% CO2 for 5 days and then counted.
In Vitro Proliferation Assay
Proliferation was analyzed when the cells were at passage 5 by the BrdU Cell Proliferation Assay (QIA58; Calbiochem, San Diego, CA, http://www.emdbiosciences.com) following the manufacturer's protocol. Briefly, 500 cells per well were seeded in triplicate wells of a 96-well plate. After 24 hours of incubation, bromodeoxyuridine (BrdU) label was added to each well. After an 18-hour incubation, the label and medium were removed, and fixative/denaturing solution was added. All incubations after this step were done at room temperature. After 30 minutes, the fixative/denaturing solution was removed, and the wells were incubated for 1 hour with anti-BrdU antibody in dilution buffer. The wells were washed, and horseradish peroxidase conjugate was added to each well for 30 minutes. The wells were washed and then incubated with tetramethylbenzidine substrate solution for 15 minutes in the dark before the reaction was stopped with the stop solution. Immediately following this, the florescence was measured using a spectrophotometric plate reader.
Metabolic Viability
The MSCs' ATP concentration (indicative of metabolic viability) was calculated at passage 7 using the Lumistem assay (KLS-96-A-1; HemoGenix, Colorado Springs, CO, http://www.hemogenix.com) [24]. Briefly, 5,000 cells per well were seeded in triplicate onto the kit's provided 96-well plate and incubated at 37°C with 5% CO2 for 30 minutes. Then, 100 μl of ATP-MR was added to each well and mixed according to the kit's directions. Luminescence of each well was measured on a luminometer. A standard curve was prepared using the standards provided in the kit to quantify the cellular ATP concentration of the MSCs based on luminescence.
In Vivo Wound Model
To assess the healing potential of MSCs in a wound model in vivo, at passage 8, selected lines were loaded onto polyvinyl alcohol sponges and implanted into NOD/SCID immunodeficient mice as previously described [25, 26]. Briefly, sponges were hydrated and sterilized in saline solution. Three sponges per cell line were loaded with 7.5 × 106 cells in 25 μl of phosphate-buffered saline. Each mouse was implanted with three sponges, each containing a different MSC line. Sponges were removed 21 days after implantation and cut in half before being preserved in 10% buffered formalin for 24 hours, after which time the sponges were embedded cut-side-down in paraffin and sectioned for staining.
Immunohistochemistry
To analyze granulation tissue, vascularity and engraftment sections were stained for hematoxylin and eosin (H&E), for CD-31, and for the human-specific Alu gene (PR-1001-01; BioGenex, Fremont, CA, http://www.biogenex.com). Five representative images from each section were taken with a Zeiss microscope (Carl Zeiss, Jena, Germany, http://www.zeiss.com), and staining was quantified using Metamorph (Molecular Devices Corp., Sunnyvale, CA, http://www.moleculardevices.com). The amount of granulation tissue was assessed by H&E staining as the total amount of tissue per total sponge area. The amount of vascularization was determined by percentage of tissue area in the sponge positively stained for CD31. MSC engraftment was calculated as the number of cells positively stained for Alu divided by the number of total cells as determined by positive 4′,6-diamidino-2-phenylindole staining.
Statistical Analysis
The statistical significance between cell lines was calculated by one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test using GraphPad Prism (GraphPad Software, Inc., San Diego, CA, http://www.graphpad.com). Graphs show mean ± SD, and p < .05 was considered statistically significant. The data from the three in vitro experiments were also analyzed for intraclass correlation using STATA software (StataCorp, College Station, TX, http://www.stata.com).
Results
Characterization of MSCs
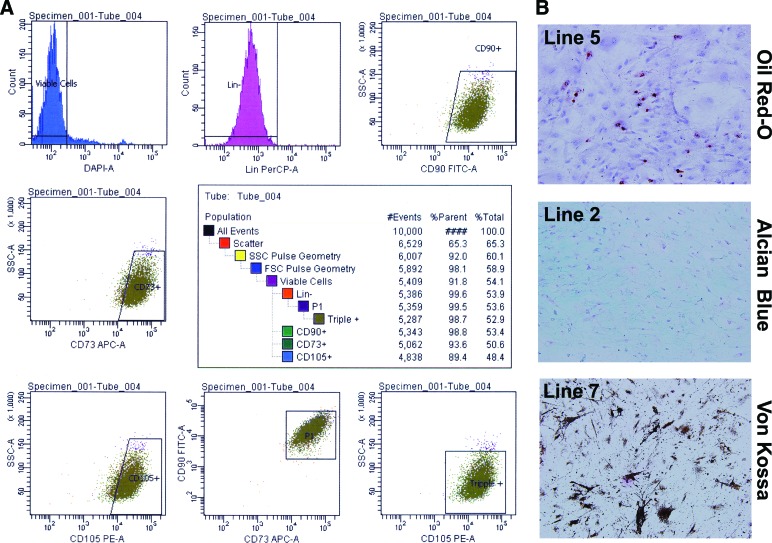
MSCs were isolated from bone marrow donors who ranged in age from 41 to 81 years old, with half of the donors being male and half female. All 10 cell lines were confirmed to be MSCs by the International Society for Cellular Therapy's minimal criteria for defining MSCs. Only plastic-adherent cells were expanded. At passages 2–5, flow cytometry was used to confirm that the MSCs possessed and lacked the appropriate surface markers. Of the viable cells, 96.2 ± 3.8% lacked the lineage markers CD14, CD20, CD34, and CD45. Of these lineage-negative cells, 93.7 ± 6.8% were positive for CD73, CD90, and CD105 (Fig. 1A). At passage 3, trilineage differentiation potential was confirmed for all 10 lines. Representative images show successful differentiation down the adipogenic, osteogenic, and chondrogenic lineages as confirmed by positive staining for Oil Red O (Fig. 1B, top), Alcian blue (Fig. 1B, middle), and von Kossa (Fig. 1B, bottom), respectively.
Figure 1.
Each mesenchymal stromal cell (MSC) line meets the International Society for Cellular Therapy minimal criteria. Shown is immunophenotyping of MSC lines (passages 2–5) by flow cytometry for cell surface expression of lineage markers CD14, CD20, CD34, and CD45 and stemness markers CD73, CD90, and CD105. (A): Representative flow cytometric analysis strategy for one of the lines. (B): Analysis of the differentiation capacity of each line at passage 4. All 10 cell lines showed trilineage differentiation into adipocytes (Oil Red O), chrondroblasts (Alcian blue), and osteoblasts (von Kossa). Abbreviations: APC, allophycocyanin; DAPI, 4′,6-diamidino-2-phenylindole; FITC, fluorescein isothiocyanate; FSC, forward scatter; Lin, lineage cocktail; PE, r-phycoerythrin; PerCP, peridinin chlorphyll protein; SSC, side scatter.
Distinct In Vitro Assays to Assess MSCs' Intrinsic Properties
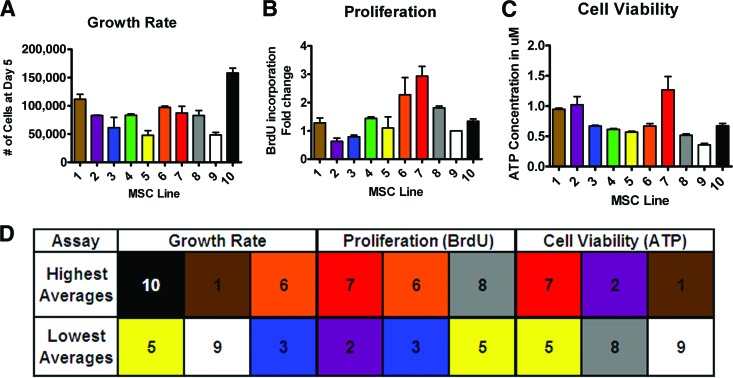
Following trilineage differentiation of MSCs, three distinct in vitro assays to assess MSCs were carried out in a linear fashion. The cell lines' growth was assessed by direct counting after 5 days of growth before any of the cell lines had reached 100% confluence (Fig. 2A). The cell line with the least amount of growth after 5 days was line 5 with 48,000 cells, and the line with the most growth was line 10 with 160,000 cells. This represents a change of more than threefold between the slowest and fastest growing cell lines. There was a significant difference between the two highest scoring lines (10 and 1) and the two lowest scoring lines as indicated by a one-way ANOVA (p ≤ .05). The intraclass correlation coefficient for growth was 0.92653 with a 95% confidence interval of 0.83640–1.01666, showing that variation between the lines was significantly higher than random variation between replicate measurements within each line.
Figure 2.
Distinct in vitro assays to assess MSC robustness indicate some consistent high and low performers. (A): At passage 3, growth rate was determined by the number of cells after 5 days of growth (n = 2). (B): At passage 5, proliferation rate of the MSC lines was quantified by fold change in BrdU incorporation (data were normalized to MSC line 9) (n = 3). (C): At passage 7, cell viability of the MSC lines was determined by cellular ATP concentration (n = 4). (D): The cell lines with the three highest and three lowest averages for each assay are shown. Abbreviations: BrdU, bromodeoxyuridine; MSC, mesenchymal stromal cell.
Next, proliferation was quantified by relative BrdU incorporation by the MSC lines (Fig. 2B). Line 9 was used in each independent proliferation assay as control to compare relative proliferation among lines. Fold change in BrdU incorporation ranged from 0.63-fold (line 2) to 2.9-fold (line 7). This variation represents a 4.5-fold difference in the incorporation of BrdU between the most proliferative and the least proliferative cell line. There was a significant difference between the two lowest performing lines (lines 2 and 3) and the highest performing line (line 7) as indicated by a one-way ANOVA (p ≤ .05). The intraclass correlation coefficient for proliferation was 0.97220 with a 95% confidence interval of 0.74212–1.00227, showing that variation between the lines was significantly higher than random variation between replicate measurements within each line.
Cell viability was measured by cellular ATP concentration (Fig. 2C). The average ATP concentrations of the lines ranged between 0.36 (line 9) and 1.27 (line 7) μM ATP normalized by cell number. These measurements showed an ∼3.5-fold change between the line with the lowest concentration and the one with the highest concentration of intracellular ATP. There was a significant difference between the two lowest performing lines (lines 8 and 9) and the highest performing line (line 7) as indicated by a one-way ANOVA (p ≤ .05). The intraclass correlation coefficient for proliferation was 0.90674 with a 95% confidence interval of 0.81538–0.9981, showing that variation between the lines was significantly higher than random variation between replicate measurements within each line.
Interpretation of the In Vitro Assays
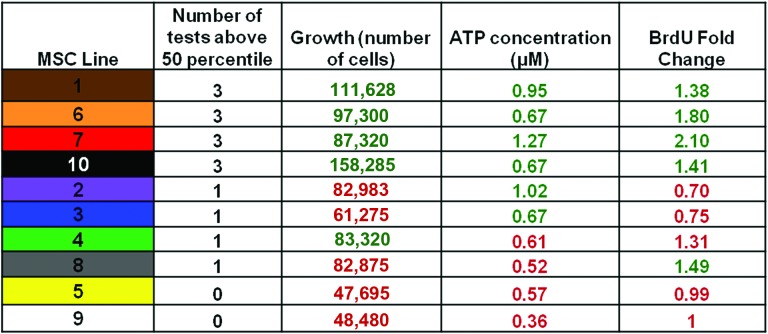
Top performance in any single in vitro test did not necessarily predict superior (above average) performance of a line in a different test (Figs. 2D, 3). For example, line 8 was one of the top three lines for relative BrdU incorporation, but it did not have a high growth rate or ATP concentration (Figs. 2D, 3). There was also a line (line 10) with superior growth rate but not ATP concentration or BrdU incorporation, and a line (line 2) with superior ATP concentration but not growth rate or BrdU incorporation (Fig. 2D). This suggests that the three in vitro assays are independent tests of the potency of the cells. Because of this variation in the tests results we predicted that considering the combination of tests (lines that performed above average in all three assays, i.e., lines 1, 6, 7, and 10 in Fig. 3) would better predict performance in vivo compared with those that performed above average in only one or two (lines 2, 3, 4, 8, 5, and 9 in Fig. 3).
Figure 3.
Combination of the in vitro assays to determine lines that are consistently in the top 50th percentile. The chart shows the 10 lines' performances in the three in vitro assays. The results in red show lines that did worse than the 50th percentile, and the results in green show those that did better than the 50th percentile. Abbreviations: BrdU, bromodeoxyuridine; MSC, mesenchymal stromal cell.
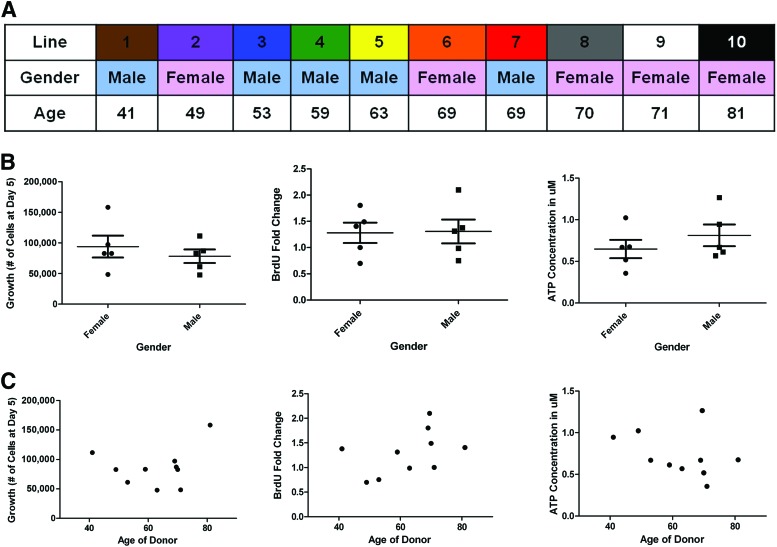
Performance in In Vitro Tests Is Independent of Age and Gender of Donors
The 10 lines came from both male and female donors ranging in age from 41 to 81 years old (Fig. 4A). We compared the average of the results of each of the in vitro assays based on the gender and found no significant difference (Fig. 4B). The correlation plots of each assay versus age of donor also demonstrated no significant relationship (Fig. 4C). We conclude that the performance of the MSC lines in each in vitro assay was independent of the age and gender of the donors.
Figure 4.
Cell growth, proliferation, and viability of the 10 lines are independent of the age and gender of the donors. (A): The donors ranged in age from 41 to 81 years, and half of the donors were male and half female. (B): Plots of the gender of the donor in relation to the average growth rate, fold change in BrdU incorporation, and ATP concentrations (μM) show no correlation between gender and performance in vitro. (C): Plots of age of the donor versus growth rate, fold change in BrdU incorporation, and ATP (μM) concentrations. Abbreviation: BrdU, bromodeoxyuridine.
In Vivo Transplantation in Mouse Wound Model to Assess Whether In Vitro Assay Performance Correlated With Enhanced Regenerative Capacity
In order to determine whether the results of the in vitro assays reliably predicted the potency of MSCs in vivo to generate well-vascularized granulation tissue (i.e., repair) as well as maintain high capacity for long-term survival in a wound bed, we used the polyvinyl alcohol sponge model of repair stimulation [25]. The model is widely used to study granulation tissue deposition in healing by secondary intention [25]. MSC engraftment (long-term viability in vivo) and vascular density of the granulation tissue generated by MSC lines were evaluated after 3 weeks, at which point MSC-loaded sponges generate mature, well-organized and vascularized granulation tissue [27]. Selected cell lines representing high and low scorers for the in vitro tests were chosen for the in vivo analysis. We performed two independent experiments, each with three animals. Lines 1, 6, and 7 were in the top 50th percentile for all three tests. Line 5 was in the bottom 50th percentile for all three tests. Line 4 was in the top 50th percentile for growth but the bottom 50th for ATP and BrdU, whereas line 8 was in the top 50th percentile for BrdU but the bottom 50th for the other two tests.
The relative amount of granulation tissue generated by each line was measured morphometrically by quantifying the surface area of the sponge section containing organized, cellular tissue. Cellularity (measured by counting Ki67+ cells in random fields) was also not statically significant. Qualitative assessment of tissue organization and collagen composition did not identify any differences among the lines.
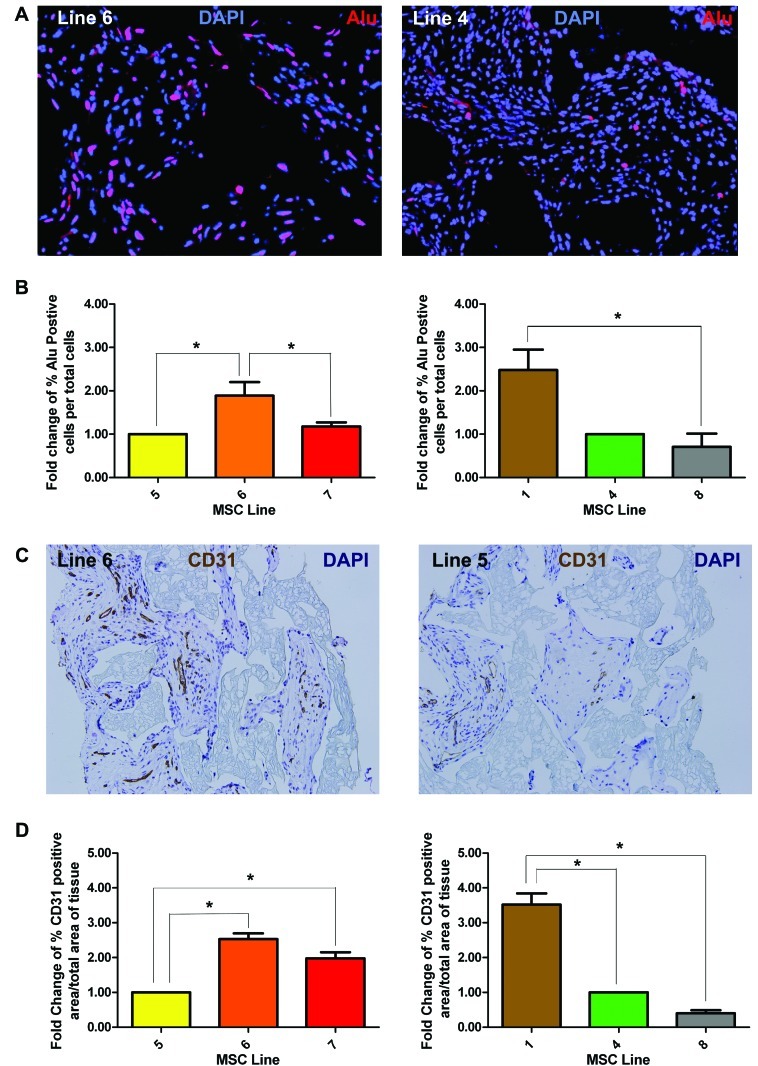
Engraftment of the MSCs was assessed by quantifying the percentage of cells in the sponge area that stained positive for the human-specific Alu gene by immunofluorescence (Fig. 5A, 5B). In experiment 1, line 6 (high performing line) had a significantly higher percentage of Alu-positive cells than line 5 or line 7 (low performers; Fig. 5B, p < .05). In experiment 2, line 1 (also high performer) had significantly more Alu-positive cells than line 8 (low performer; Fig. 5B, p < .05).
Figure 5.
MSCs with higher scores for in vitro tests show higher engraftment and vascularity in mouse wound model. Selected high-scoring and low-scoring MSC lines were tested in polyvinyl alcohol sponges in an in vivo granulation tissue formation assay to correlate in vitro performance with in vivo function. (A): Representative images (lines 6 and 4) stained for Alu (human-specific marker, red) expression to assess degree of donor MSC content (i.e., engraftment) at day 21 after subcutaneous implantation. (B): Graph of the averages of the number of Alu-positive cells (red in [A]) normalized to total number of cells (DAPI, blue in [A]). (C): Representative immunostained sections (lines 5 and 6) using anti-PECAM-1 (CD31, brown) to designate vascular density of MSC-derived granulation tissue. (D): Vascular density graphed as a percentage of immunopositive CD31 area/total tissue area in histologic sections from sponges. Engraftment and vascular density graphed data represent averages of multiple ×40 fields (n = 5). A given experimental cohort of n = 3 for each line compared lines 5, 6, and 7 or lines 1, 4, and 8. *, p < .05 was considered statistically significant. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; MSC, mesenchymal stromal cell.
Granulation tissue vascularity was assessed by quantitative analysis of generated granulation tissues stained with anti-CD31 (PECAM-1) (Fig. 5C, 5D). In experiment 1, line 6 (p < .001) and line 7 (p < .05) both had higher vascular density than line 5 (Fig. 5C, 5D, left panel). In experiment 2, line 1 had significantly more (Fig. 5D, right panel, p < .001) vascular density than lines 4 and 8.
As predicted, lines 1, 6, and 7, which performed above average in all three in vitro assays, also performed relatively better in vivo than line 5 (which performed below average in all three assays). The two lines (lines 4 and 8) that only did well in a single in vitro assay fared statistically worse in engraftment and vascular density in vivo than lines that performing above average in all three assays (supplemental online Fig. 1). These results confirm our hypothesis that good performance in all three tests correlates with good performance in vivo.
Discussion
We used three quantitative assays—growth rate, proliferation, and measurement of cellular ATP—to compare the potency of MSCs isolated from bone marrow from 10 otherwise normal individuals undergoing orthopedic surgeries (knee or hip replacement). Variability in the preparation procedure was minimized by careful adherence to standard process by a single staff member and by processing all 10 samples within 1 week. Additionally, each assay was performed at the same passage in order to avoid variation due to differences in passage number [17].
As anticipated, we found significant variation among the cell lines in assay performance. These variations were not influenced by age or gender of the donor. As these assays measure aspects of cell growth, we were surprised at the lack of consensus among the assays to identify top performing lines. Because of this lack of consistency in performance in any given assay, we anticipated that a combination of all three assays (i.e., an assay matrix) would provide the best available prediction of in vivo performance.
To establish a relationship between these surrogate measurements and biological activity, we used an in vivo animal model of sponge granulation tissue formation. The assessment of granulation tissue quantity, its vascular density, and its organization by this assay system has been shown to correlate with more physiological models of cutaneous repair [25, 28, 29]. Importantly, the ability to generate well-vascularized granulation tissue in the sponge model has also been shown to correlate with MSC regenerative capacity in a murine model of myocardial infarction [27, 30]. Six of the 10 MSC lines representing high and low performers in the in vitro assays were chosen to perform in vivo experiments. We found that cell lines that performed above average in all three assays were able to create better vascularized granulation tissue and more long-term MSC engraftment compared with cell lines with below-average performance in any single assay.
MSC activities in mediating tissue repair are complex and incompletely understood [23]. Because of this complexity, it is difficult to develop relevant and meaningful potency measurements. We used the sponge granulation tissue model as a highly reproducible in vivo model of MSC-driven repair, tissue vascularity, and MSC tissue engraftment, correlating with the ability of MSCs improve cardiac regeneration and clinical response.
Although this is the first study to directly compare the importance of stem cell line properties in relation to tissue repair, it has been shown that clones of single cells from a sample of MSCs do not all have the same differentiation capabilities, and that trilineage differentiation capacity is associated with a higher proliferation potential [31–35]. There are also many studies that have sorted MSCs based on surface markers and the side population assay to select the purest and most potent populations [36]. A population of cells purified for their differentiation potential and sorted by flow cytometry, and then analyzed by the assays described in the study, could be used to select the best cells for repair and regeneration.
Our study has some limitations. First, we were limited to 10 donors (and further limited to 6 donors for in vivo studies). These findings will need to be validated on a larger cohort. In addition, none of the assays used in this study assess immunomodulatory properties of MSCs. Finally, there are no studies that have examined whether the regenerative and immunomodulatory biological activities of MSCs are related. It is likely that different potency assays may be necessary for different clinical applications.
Conclusion
The ability to produce large quantities of MSCs with predictable quality and quantifiable potency is necessary for successful clinical use. Especially in developing MSCs for autologous use, the time to test the product is limited, making routine in vivo testing for functional ability difficult. Hence, using animal models as routine potency tests is not feasible. Our results support the use of three standardized assays to measure MSC characteristics associated with quality. When used together, these results correlate with a relevant biologic activity. Although our study does not encompass assays to measure the multiple complex functions of MSCs such as immune modulation or paracrine factor secretion, this study based on viability and growth serves as a springboard to develop a more comprehensive assay matrix.
Acknowledgments
This work was supported by NIH Grants R01-HL088424, HL08842402S1, and R01-GM081635; a Veterans Affairs merit award (to P.P.Y.); and Vanderbilt University Medical Center CTSA Grant UL1 RR024975-01 from the National Center for Research Resources, NIH. We thank the Vanderbilt Department of Biostatistics Clinic staff for their helpful analysis advice.
Author Contributions
D.L.D.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; D.B.: collection and/or assembly of data, data analysis and interpretation, manuscript writing; S.S.: conception and design, collection and/or assembly of data; A.S. and G.E.H.: provision of study materials or patients; P.P.Y.: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
A.S. is a compensated consultant for Smith and Nephew on revision hip replacement components.
References
- 1.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 3.Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113:1161–1166. doi: 10.1242/jcs.113.7.1161. [DOI] [PubMed] [Google Scholar]
- 4.Smith JR, Pochampally R, Perry A, et al. Isolation of a highly clonogenic and multipotential subfraction of adult stem cells from bone marrow stroma. Stem Cells. 2004;22:823–831. doi: 10.1634/stemcells.22-5-823. [DOI] [PubMed] [Google Scholar]
- 5.Horwitz EM, Prockop DJ, Fitzpatrick LA, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 6.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 7.Iyer SS, Rojas M. Anti-inflammatory effects of mesenchymal stem cells: Novel concept for future therapies. Expert Opin Biol Ther. 2008;8:569–581. doi: 10.1517/14712598.8.5.569. [DOI] [PubMed] [Google Scholar]
- 8.Silva GV, Litovsky S, Assad JA, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 9.Tang YL, Zhao Q, Zhang YC, et al. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept. 2004;117:3–10. doi: 10.1016/j.regpep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 11.Sun Y, Li W, Lu Z, et al. Rescuing replication and osteogenesis of aged mesenchymal stem cells by exposure to a young extracellular matrix. FASEB J. 2011;25:1474–1485. doi: 10.1096/fj.10-161497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Poll D, Parekkadan B, Borel Rinkes IHM, et al. Mesenchymal stem cell therapy for protection and repair of injured vital organs. Cell Mol Bioeng. 2008;1:42–50. [Google Scholar]
- 13.Deans RJ, Moseley AB. Mesenchymal stem cells: Biology and potential clinical uses. Exp Hematol. 2000;28:875–884. doi: 10.1016/s0301-472x(00)00482-3. [DOI] [PubMed] [Google Scholar]
- 14.Le Blanc K. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biology Blood Marrow Transplant. 2005;11:321–334. doi: 10.1016/j.bbmt.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Ringdén O, Uzunel M, Sundberg B, et al. Tissue repair using allogeneic mesenchymal stem cells for hemorrhagic cystitis, pneumomediastinum and perforated colon. Leukemia. 2007;21:2271–2276. doi: 10.1038/sj.leu.2404833. [DOI] [PubMed] [Google Scholar]
- 16.U.S. National Library of Medicine, U.S. National Institutes of Health, U.S. Department of Health and Human Services. ClinicalTrials.gov, Lester Hill National Center for Biomedical Communications.
- 17.Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: Paradoxes of passaging. Exp Hematol. 2004;32:414–425. doi: 10.1016/j.exphem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010;16:203–209. doi: 10.1016/j.molmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells: The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 20.Bieback K, Schallmoser K, Kluter H, et al. Clinical protocols for the isolation and expansion of mesenchymal stromal cells. Transfus Med Hemother. 2008;35:286–294. doi: 10.1159/000141567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sotiropoulou PA, Perez SA, Papamichail M. Clinical grade expansion of human bone marrow mesenchymal stem cells. Methods Mol Biol. 2007;407:245–263. doi: 10.1007/978-1-59745-536-7_17. [DOI] [PubMed] [Google Scholar]
- 22.Chamberlain G, Fox J, Ashton B, et al. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 23.Stroncek DF, Jin P, Wang E, et al. Potency analysis of cellular therapies: The emerging role of molecular assays. J Transl Med. 2007;5:24. doi: 10.1186/1479-5876-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanley PE. A review of bioluminescent ATP techniques in rapid microbiology. J Biolumin Chemilumin. 1989;4:375–380. doi: 10.1002/bio.1170040151. [DOI] [PubMed] [Google Scholar]
- 25.Deskins DL, Ardestani S, Young PP. The polyvinyl alcohol sponge model implantation. J Vis Exp. 2012;(62):3885. doi: 10.3791/3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alfaro MP, Vincent A, Saraswati S, et al. sFRP2 suppression of bone morphogenic protein (BMP) and Wnt signaling mediates mesenchymal stem cell (MSC) self-renewal promoting engraftment and myocardial repair. J Biol Chem. 2010;285:35645–35653. doi: 10.1074/jbc.M110.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alfaro MP, Pagni M, Vincent A, et al. A Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proc Natl Acad Sci USA. 2008;105:18366–18371. doi: 10.1073/pnas.0803437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Efron DT, Barbul A. Subcutaneous sponge models. Methods Mol Med. 2003;78:83–93. doi: 10.1385/1-59259-332-1:083. [DOI] [PubMed] [Google Scholar]
- 29.Andrade SP, Ferreira MA. The sponge implant model of angiogenesis. Methods Mol Biol. 2009;467:295–304. doi: 10.1007/978-1-59745-241-0_18. [DOI] [PubMed] [Google Scholar]
- 30.Saraswati S, Alfaro MP, Thorne CA, et al. Pyrvinium, a potent small molecule Wnt inhibitor, promotes wound repair and post-MI cardiac remodeling. PLoS One. 2010;5:e15521. doi: 10.1371/journal.pone.0015521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell KC, Lacey MR, Gilliam JK, et al. Clonal analysis of the proliferation potential of human bone marrow mesenchymal stem cells as a function of potency. Biotechnol Bioeng. 2011;108:2716–2726. doi: 10.1002/bit.23193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell KC, Phinney DG, Lacey MR, et al. In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells. 2010;28:788–798. doi: 10.1002/stem.312. [DOI] [PubMed] [Google Scholar]
- 33.Lee CC, Christensen JE, Yoder MC, et al. Clonal analysis and hierarchy of human bone marrow mesenchymal stem and progenitor cells. Exp Hematol. 2010;38:46–54. doi: 10.1016/j.exphem.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee RH, Hsu SC, Munoz J, et al. A subset of human rapidly self-renewing marrow stromal cells preferentially engraft in mice. Blood. 2006;107:2153–2161. doi: 10.1182/blood-2005-07-2701. [DOI] [PubMed] [Google Scholar]
- 35.Digirolamo CM, Stokes D, Colter D, et al. Propagation and senescence of human marrow stromal cells in culture: A simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999;107:275–281. doi: 10.1046/j.1365-2141.1999.01715.x. [DOI] [PubMed] [Google Scholar]
- 36.Golebiewska A, Brons NH, Bjerkvig R, et al. Critical appraisal of the side population assay in stem cell and cancer stem cell research. Cell Stem Cell. 2011;8:136–147. doi: 10.1016/j.stem.2011.01.007. [DOI] [PubMed] [Google Scholar]