Mesenchymal stromal cells (MSCs) are an interesting candidate to aid in the long-term survival of transplanted kidneys, because of their immunosuppressive and regenerative properties. This phase I study was the first to investigate the effects of MSCs in allograft rejection and fibrosis. Findings support the potential of MSCs as a novel cell therapy to prevent allograft rejection and interstitial fibrosis/tubular atrophy. The observed systemic immune suppression implies that careful monitoring of opportunistic viral infection is needed.
Keywords: Mesenchymal stem cells, Kidney, Immunosuppression, Transplantation
Abstract
Despite excellent short-term results, long-term survival of transplanted kidneys has not improved accordingly. Although alloimmune responses and calcineurin inhibitor-related nephrotoxicity have been identified as main drivers of fibrosis, no effective treatment options have emerged. In this perspective, mesenchymal stromal cells (MSCs) are an interesting candidate because of their immunosuppressive and regenerative properties. Of importance, no other clinical studies have investigated their effects in allograft rejection and fibrosis. We performed a safety and feasibility study in kidney allograft recipients to whom two intravenous infusions (1 million cells per kilogram) of autologous bone marrow (BM) MSCs were given, when a protocol renal biopsy at 4 weeks or 6 months showed signs of rejection and/or an increase in interstitial fibrosis/tubular atrophy (IF/TA). Six patients received MSC infusions. Clinical and immune monitoring was performed up to 24 weeks after MSC infusions. MSCs fulfilled the release criteria, infusions were well-tolerated, and no treatment-related serious adverse events were reported. In two recipients with allograft rejection, we had a clinical indication to perform surveillance biopsies and are able to report on the potential effects of MSCs in rejection. Although maintenance immunosuppression remained unaltered, there was a resolution of tubulitis without IF/TA in both patients. Additionally, three patients developed an opportunistic viral infection, and five of the six patients displayed a donor-specific downregulation of the peripheral blood mononuclear cell proliferation assay, not reported in patients without MSC treatment. Autologous BM MSC treatment in transplant recipients with subclinical rejection and IF/TA is clinically feasible and safe, and the findings are suggestive of systemic immunosuppression.
Introduction
Despite excellent short-term results, long-term survival of transplanted kidneys has not improved accordingly [1]. Mesenchymal stromal cells (MSCs) have anti-inflammatory and antifibrotic properties [2–4] and prevent renal injury in preclinical models [5–8], and they may thus constitute a new therapeutic option in transplant recipients [9–12]. There are, however, only limited data on the effect of MSCs in human solid organ transplantation [13, 14], and the use of MSC treatment for allograft rejection and renal fibrosis has not been studied yet. We report the results of six full human leukocyte antigen (HLA)-DR-mismatched living kidney allograft recipients who received expanded autologous bone marrow-derived MSCs (106 cells per kilogram twice i.v., 7 days apart) because of rejection and/or increased interstitial fibrosis and tubular atrophy (IF/TA) in the 6-month protocol biopsy compared with the renal biopsy 4 weeks after transplantation.
Materials and Methods
Patients
The study was approved by the Medical Ethical Committee of Leiden University Medical Center (LUMC) and the National Central Committee on Research involving Human Subjects (ClinicalTrials.gov NCT00734396). All patients gave written informed consent. The clinical and research activities are consistent with the principles of the Declaration of Helsinki and the Declaration of Istanbul.
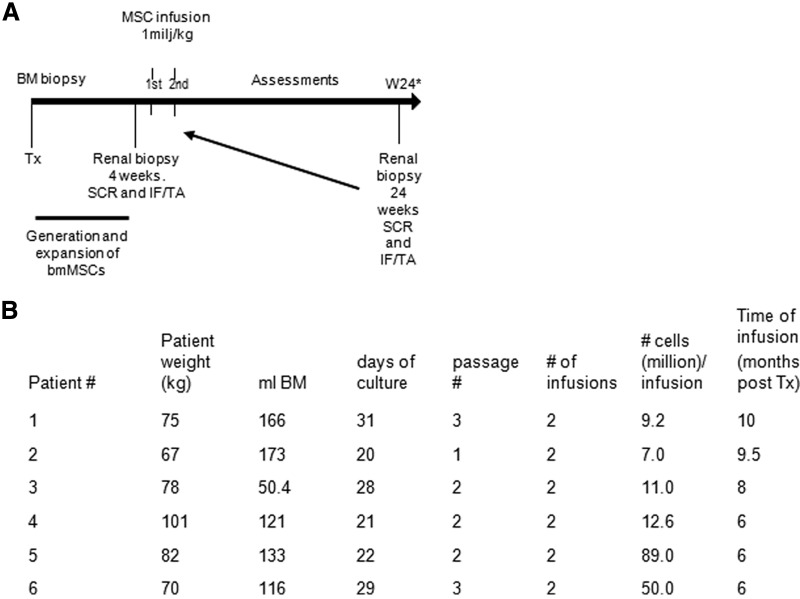
Fifteen renal recipients of two HLA-DR-mismatched living donors, men and women, 18–70 years of age, were recruited from the LUMC renal transplant clinic. Patients with subclinical rejection (SCR) in the renal biopsy at 4 weeks or SCR and/or an increase in IF/TA in the biopsy approximately 6 months after transplantation received two doses of 1–2 × 106 cells per kilogram of body weight of MSCs, 7 days apart. Clinical and immune monitoring was performed up to 24 weeks after MSC infusions (Fig. 1A).
Figure 1.
Study flowchart and MSC characteristics. (A): Study flowchart. Patients with SCR in the protocol biopsy 4 weeks after transplantation or with SCR and/or an increase in IF/TA approximately 6 months after transplantation received MSC infusions. If patients received MSCs because of SCR and/or an increase of IF/TA in the protocol biopsy at 6 months after transplantation, a follow-up renal biopsy was taken only if clinically indicated. (B): Overview of bone marrow collection, time needed for MSC culture, passage number, final MSC product infused, and timing of first MSC infusion. Abbreviations: BM, bone marrow; bmMSC, bone marrow-derived mesenchymal stromal cell; IF/TA, interstitial fibrosis/tubular atrophy; MSC, mesenchymal stromal cell; SCR, subclinical rejection; Tx, transplantation; W, week.
All patients received induction therapy with basiliximab and maintenance immunosuppression with prednisone, calcineurin inhibitor (tacrolimus or cyclosporine), and mycophenolate mofetil (MMF) as described [15]. Patients were treated routinely with oral (tablet) valganciclovir prophylaxis for 3 months, except for a cytomegalovirus (CMV)-negative donor recipient status.
Renal Histology
Ultrasound guided renal biopsies were taken, stained, and analyzed as described previously [15, 16].
MSC Procedures
Heparinized bone marrow (BM) (approximately 100 ml) was aspirated from the iliac crest under general anesthesia, just prior to the renal transplantation. MSC isolation, culture, characterization, and release criteria were as described previously [17].
Functional Immunological Assays
Peripheral blood mononuclear cells (PBMCs) were isolated and multicolor flow cytometry was performed using an LSRII (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com), allowing the discrimination and quantification of the lineages and subsets as indicated (supplemental online Fig. 1; supplemental online data). A mixed lymphocyte reaction (MLR) was performed with recipient and donor PBMCs (1 × 105 cells per well) before and after MSC infusion and proliferation, and different cytokines were measured using the Bio-Plex Pro Human Cytokine 27-Plex, Group I (Bio-Rad, Hercules, CA, http://www.bio-rad.com) as described [18]. HLA-specific antibodies were determined by complement-dependent cytotoxicity assay and enzyme-linked immunosorbent assay.
Results
In total six patients received MSCs and were followed up after the infusion (Table 1; Fig. 1). MSCs fulfilled prespecified release criteria, infusions were well-tolerated, and no treatment-related adverse events were reported. Although maintenance immune suppression remained unaltered, three patients developed an opportunistic viral infection (Table 1). Patient 1 developed a BK-virus associated nephropathy 21 weeks after MSC infusion. Patient 2 developed a late primary CMV infection 2 weeks after MSC infusion (>6 months after prophylactic valganciclovir discontinuation). Both patients recovered uneventfully with antiviral therapy and reduction of immune suppression. In patient 3 a low-grade CMV viral load persisted in the months after MSC infusion, despite reduction of clinical immune suppression.
Table 1.
Patients
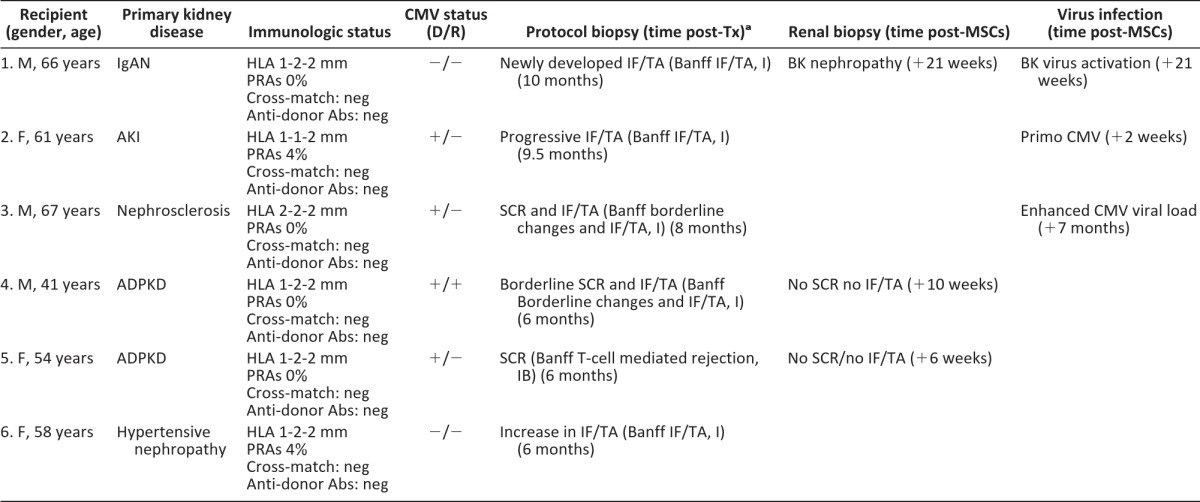
aBanff score according to Banff 97 diagnostic categories for renal allograft biopsies [16].
Abbreviations: Abs, antibodies; ADPKD, adult polycystic kidney disease; AKI, acute kidney injury; CMV, cytomegalovirus; D, donor; F, female; HLA, human leukocyte antigen; IF/TA, interstitial fibrosis tubular atrophy; IgAN, IgA nephropathy; M, male; mm, human leukocyte antigen mismatch; MSC, mesenchymal stromal cell; neg, negative; NODAT, new onset diabetes after transplantation; PRAs, panel reactive antibodies; R, recipient; SCR, subclinical rejection; Tx, transplantation.
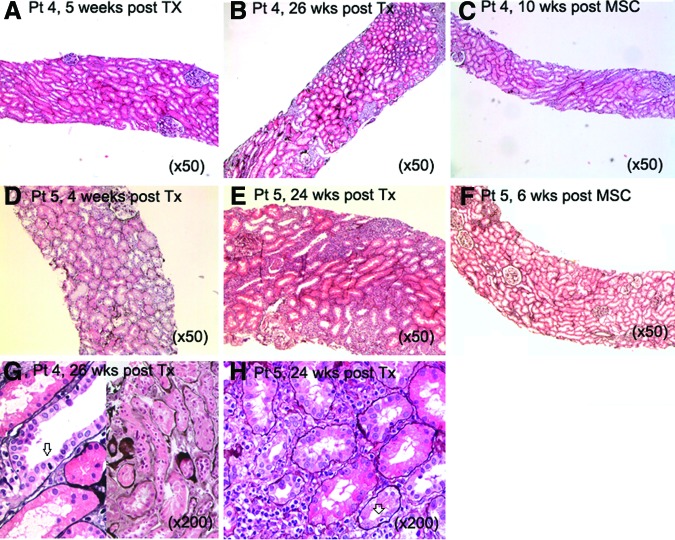
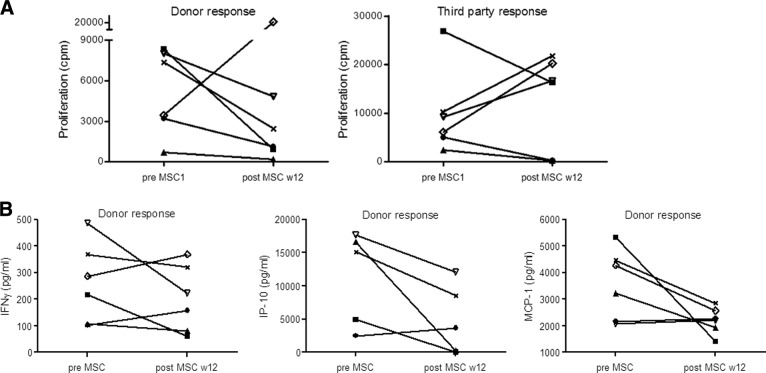
In two recipients with allograft rejection, we had a clinical indication to perform surveillance biopsies after MSC infusions and are able to report for the first time on the potential effects of MSCs in rejection. In patient 4, the 6-month protocol biopsies showed borderline subclinical rejection (Banff <1A) with mild IF/TA, and in patient 5, they showed severe T-cell-mediated acute rejection (Banff 1B) (Fig. 2, as indicated). MSCs were infused and immunosuppressive drugs remained unchanged. Both biopsies after MSC treatment demonstrated resolution of tubulitis without IF/TA (Fig. 2). Given the severity and extension of the abnormalities in the previous biopsy, sampling error was considered not very likely, although it cannot be ruled out. Interestingly, five of the six patients demonstrated a reduction in PBMC proliferation 12 weeks after MSC infusion upon stimulation with donor PBMCs (Fig. 3A). In patient 6, donor-specific proliferation did not decline 12 weeks after MSC infusion; however, cells of this patient showed a high spontaneous medium response (data not shown). Interferon-γ, interferon-γ induced protein (IP)-10 and monocyte chemoattractant protein (MCP)-1 levels measured in the supernatants of the PBMC proliferation assay declined upon stimulation with donor-specific PBMCs in the majority of patients (Fig. 3B). The response to third-party PBMCs was more variable (Fig. 3A), and total numbers and subsets of T cells, B cells, natural killer cells, and monocytes did not demonstrate a consistent change after MSC treatment (supplemental online Fig. 1A–1E).
Figure 2.
Renal histology in two patients before and after autologous bone marrow-derived MSC infusions. In patient 4 the protocol renal biopsy 5 weeks post-transplantation demonstrated only small areas of interstitial fibrosis/tubular atrophy (IF/TA) without signs of tubulitis (A). The renal biopsy 26 weeks post-transplantation showed multiple foci of tubulitis and mild IF/TA (B, G) (in [G], left panel shows tubulitis, right panel shows IF/TA; arrow indicates infiltrating lymphocyte). A third renal biopsy 10 weeks after MSC infusions showed a complete normal renal histology (hematoxylin & eosin [H&E] stain) (C). In patient 5 the protocol biopsy 4 weeks post-transplantation showed no signs of allograft rejection (D). The standard renal biopsy at 24 weeks post-transplantation showed T-cell-mediated rejection with severe tubulitis (E, H) (in [H], arrow indicates infiltrating lymphocyte), which was resolved without signs of IF/TA 6 weeks after the MSC infusion (F) (H&E stain). Abbreviations: MSC, mesenchymal stromal cell; Pt, patient; TX, transplantation; wks post Tx; weeks after renal transplantation; wks post MSC, weeks after first mesenchymal stromal cell infusion.
Fig. 3.
Immune monitoring in MSC-treated patients. Twelve weeks after MSC treatment, proliferation of patient peripheral blood mononuclear cells (PBMCs) to donor antigen, but not third-party antigen, was reduced in five of the six patients as compared with the proliferation before MSC infusions (A). IFN-γ, IP-10, and MCP-1 levels measured in the supernatants of the PBMC proliferation assay declined upon stimulation with donor-specific PBMCs in the majority of the patients (B). ●, patient 1; ■, patient 2; ▴, patient 3; ×, patient 4; ▿, patient 5; ◊, patient 6. Abbreviations: cpm, counts per minute; IFN-γ, interferon γ; IP-10, interferon-γ induced protein-10; MCP-1, monocyte chemoattractant protein-1; MSC, mesenchymal stromal cell; w, weeks.
Discussion
Our study demonstrates that autologous BM MSC administration in renal recipients with SCR and IF/TA is feasible and well-tolerated. Although the design of the study does not allow conclusions on efficacy, there was a suggestion of immunosuppression after MSC infusions, not described in previous transplant studies involving MSC treatment. Three patients developed an opportunistic infection, and five of the six patients displayed a downregulation of the MLR to the donor. In addition, in two recipients with allograft rejection, renal biopsies after MSC treatment demonstrated resolution of tubulitis without IF/TA, whereas maintenance immune suppression remained unaltered.
The only available clinical data on the use of MSCs in solid organ transplantation are two studies on the use of autologous MSCs in the early period after renal transplantation [13, 14]. In one study among patients undergoing renal transplantation, the use of autologous MSCs at kidney reperfusion and 2 weeks later was compared with anti-interleukin-2 receptor antibody induction therapy. This resulted in a better estimated renal function at 1 year, a lower incidence of acute rejection, and decreased opportunistic infections in the MSC-treated patient groups. Of importance, CMV donor recipient status was negative in 151 of 154 patients, probably explaining the low incidence of CMV infections in this study. In the other study, two patients were given T-cell-depleting induction therapy, maintenance immunosuppressants with cyclosporine, and MMF and MSC infusion 7 days after transplantation [14]. Administration of MSCs resulted in (transient) renal impairment that was attributed to an engraftment reaction, where the inflammatory milieu early after kidney transplantation may have altered MSC function [6]. In our study, where MSCs were administered beyond 6 months after transplantation, these unexpected deleterious short-term effects were not observed (supplemental online Fig. 2).
Although no phenotypical changes in tolerogenic cell populations were observed, we did find an inhibition of donor-specific immunity in five of the six MSC-treated patients, as proliferation of patient PBMC to donor-PBMC was reduced compared with the proliferation before MSC infusions. In three patients the third-party response was reduced, with low proliferation in two of these patients, indicating a more general immunosuppressive effect. This observation seems in line with the observed systemic viral reactivation in these patients, although the mechanisms of immune regulation need further investigation. In the current study we have included a short follow-up of the MSC-treated patients. In future studies, long-term follow-up to better understand mechanisms of MSC-based treatment and to monitor unwanted side effects is of greatest importance, especially since transplant recipients are already at increased risk for (opportunistic) infections and malignancies.
Conclusion
These first clinical observations support the potential of MSCs as a novel cell therapy to prevent allograft rejection and IF/TA. The observed systemic immune suppression implies that careful monitoring of opportunistic viral infection is needed.
Acknowledgments
This study was sponsored by the Dutch Organisation for Sciences (NWO/ZonMW; TAS and Veni) and the European Community's Seventh Framework Programme (FP7/2007-13, HEALTH-F5-2008-223007 STAR-T REK).
Author Contributions
M.E.J.R.: research design, performance of research, manuscript preparation, final approval of manuscript; J.W.d.F. and C.v.K: research design, data analysis, manuscript preparation; H.R. and A.F.S.: research design, performance of research; I.M.B. and F.H.J.C.: data analysis; D.K.d.V. and P.P.M.C.v.M.: performance of research; D.L.R.: performance of research, data analysis; W.E.F.: research design, data analysis; T.J.R.: research design, data analysis, manuscript preparation, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Pascual M, Theruvath T, Kawai T, et al. Strategies to improve long-term outcomes after renal transplantation. N Engl J Med. 2002;346:580–590. doi: 10.1056/NEJMra011295. [DOI] [PubMed] [Google Scholar]
- 2.Tolar J, Le Blanc K, Keating A, et al. Concise review: Hitting the right spot with mesenchymal stromal cells. Stem Cells. 2010;28:1446–1455. doi: 10.1002/stem.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franquesa M, Herrero E, Torras J, et al. Mesenchymal stem cell therapy prevents interstitial fibrosis and tubular atrophy in a rat kidney allograft model. Stem Cells Dev. 2012;21:3125–3135. doi: 10.1089/scd.2012.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ninichuk V, Gross O, Segerer S, et al. Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney Int. 2006;70:121–129. doi: 10.1038/sj.ki.5001521. [DOI] [PubMed] [Google Scholar]
- 5.Morigi M, Introna M, Imberti B, et al. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26:2075–2082. doi: 10.1634/stemcells.2007-0795. [DOI] [PubMed] [Google Scholar]
- 6.Casiraghi F, Azzollini N, Todeschini M, et al. Localization of mesenchymal stromal cells dictates their immune or proinflammatory effects in kidney transplantation. Am J Transplant. 2012;12:2373–2383. doi: 10.1111/j.1600-6143.2012.04115.x. [DOI] [PubMed] [Google Scholar]
- 7.Inoue S, Popp FC, Koehl GE, et al. Immunomodulatory effects of mesenchymal stem cells in a rat organ transplant model. Transplantation. 2006;81:1589–1595. doi: 10.1097/01.tp.0000209919.90630.7b. [DOI] [PubMed] [Google Scholar]
- 8.Ge W, Jiang J, Baroja ML, et al. Infusion of mesenchymal stem cells and rapamycin synergize to attenuate alloimmune responses and promote cardiac allograft tolerance. Am J Transplant. 2009;9:1760–1772. doi: 10.1111/j.1600-6143.2009.02721.x. [DOI] [PubMed] [Google Scholar]
- 9.English K, French A, Wood KJ. Mesenchymal stromal cells: Facilitators of successful transplantation? Cell Stem Cell. 2010;7:431–442. doi: 10.1016/j.stem.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Salem HK, Thiemermann C. Mesenchymal stromal cells: Current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoogduijn MJ, Popp FC, Grohnert A, et al. Advancement of mesenchymal stem cell therapy in solid organ transplantation (MISOT) Transplantation. 2010;90:124–126. doi: 10.1097/TP.0b013e3181ea4240. [DOI] [PubMed] [Google Scholar]
- 12.Reinders ME, Fibbe WE, Rabelink TJ. Multipotent mesenchymal stromal cell therapy in renal disease and kidney transplantation. Nephrol Dial Transplant. 2010;25:17–24. doi: 10.1093/ndt/gfp552. [DOI] [PubMed] [Google Scholar]
- 13.Tan J, Wu W, Xu X, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: A randomized controlled trial. JAMA. 2012;307:1169–1177. doi: 10.1001/jama.2012.316. [DOI] [PubMed] [Google Scholar]
- 14.Perico N, Casiraghi F, Introna M, et al. Autologous mesenchymal stromal cells and kidney transplantation: A pilot study of safety and clinical feasibility. Clin J Am Soc Nephrol. 2011;6:412–422. doi: 10.2215/CJN.04950610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roos-van Groningen MC, Scholten EM, Lelieveld PM, et al. Molecular comparison of calcineurin inhibitor-induced fibrogenic responses in protocol renal transplant biopsies. J Am Soc Nephrol. 2006;17:881–888. doi: 10.1681/ASN.2005080891. [DOI] [PubMed] [Google Scholar]
- 16.Solez K, Colvin RB, Racusen LC, et al. Banff '05 Meeting Report: Differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’) Am J Transplant. 2007;7:518–526. doi: 10.1111/j.1600-6143.2006.01688.x. [DOI] [PubMed] [Google Scholar]
- 17.Duijvestein M, Vos AC, Roelofs H, et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn's disease: Results of a phase I study. Gut. 2010;59:1662–1669. doi: 10.1136/gut.2010.215152. [DOI] [PubMed] [Google Scholar]
- 18.Lashley LE, van der Hoorn ML, van der Mast BJ, et al. Changes in cytokine production and composition of peripheral blood leukocytes during pregnancy are not associated with a difference in the proliferative immune response to the fetus. Hum Immunol. 2011;72:805–811. doi: 10.1016/j.humimm.2011.05.027. [DOI] [PubMed] [Google Scholar]